Three Dimensional Models of Endocrine Organs and Target Tissues Regulated by the Endocrine System
Abstract
Simple Summary
Abstract
1. Introduction
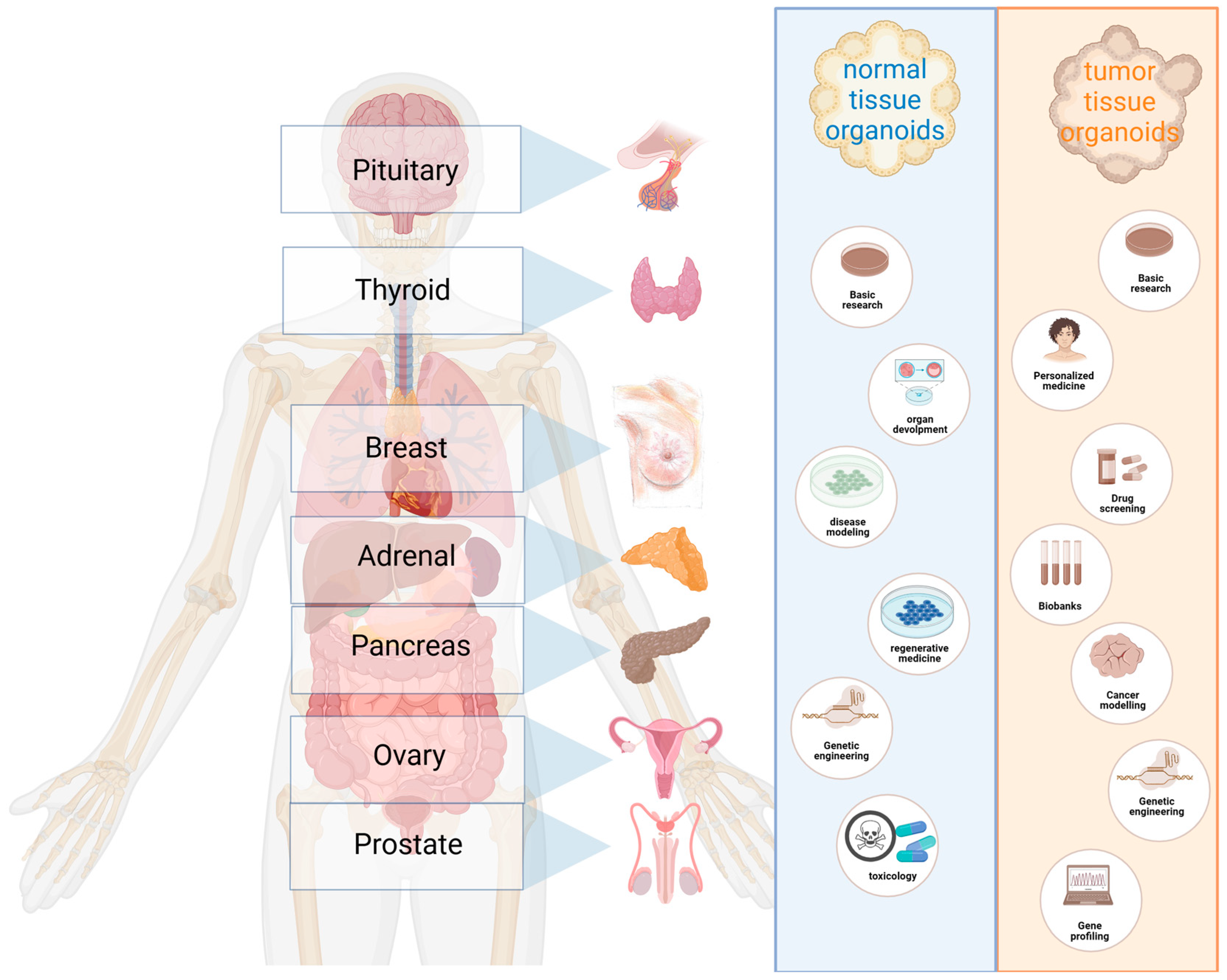
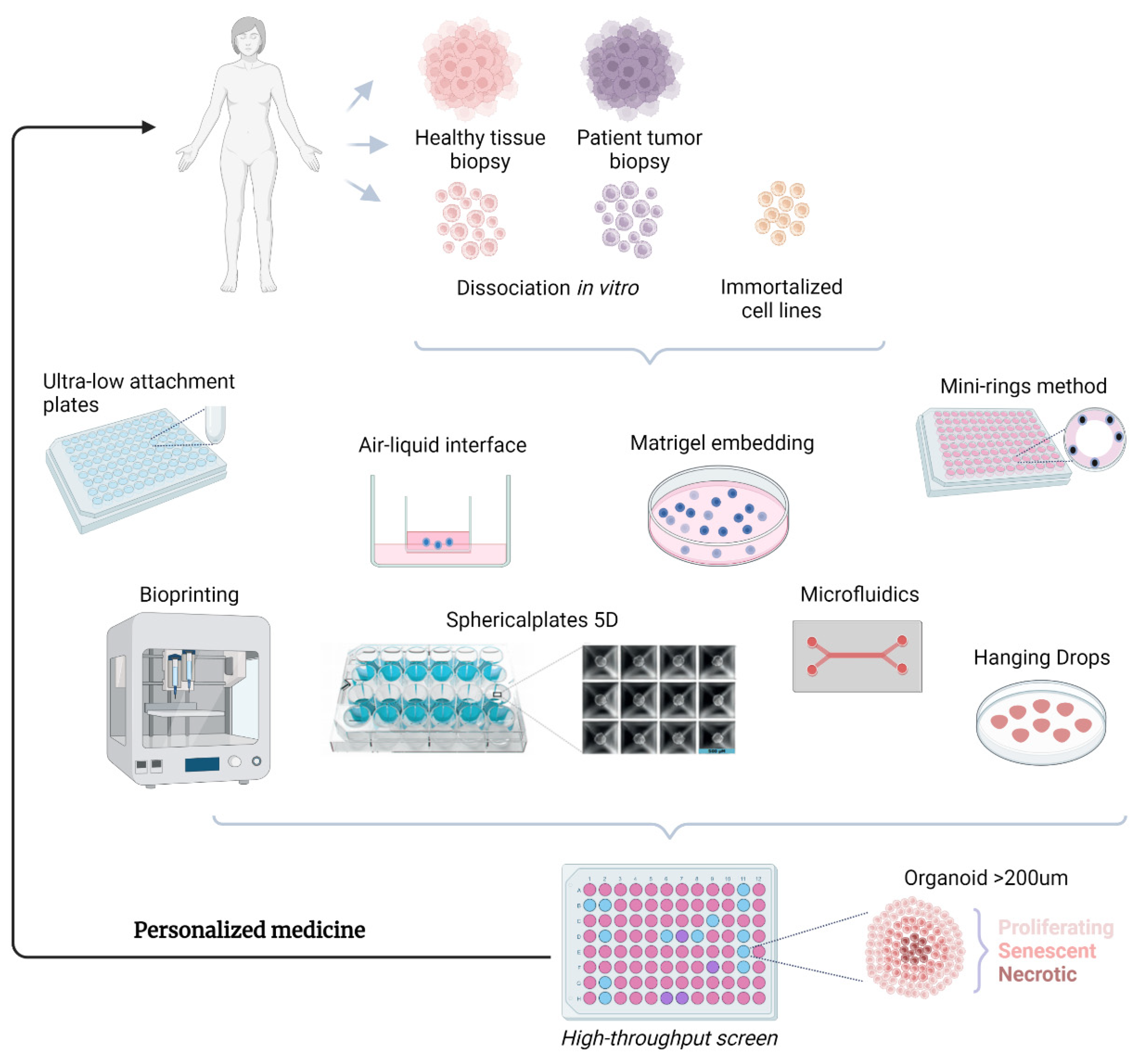
2. Advantages of Spheroids/Organoids
3. Pituitary
4. Thyroid
5. Adrenal
6. Pancreas
7. Ovaries
8. Breast
9. Prostate
10. Future Directions
11. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Asa, S.L.; Mete, O. Endocrine pathology: Past, present and future. Pathology 2018, 50, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Brassard, J.A.; Lutolf, M.P. Engineering Stem Cell Self-organization to Build Better Organoids. Cell Stem Cell 2019, 24, 860–876. [Google Scholar] [CrossRef] [PubMed]
- Kasai, T.; Suga, H.; Sakakibara, M.; Ozone, C.; Matsumoto, R.; Kano, M.; Mitsumoto, K.; Ogawa, K.; Kodani, Y.; Nagasaki, H.; et al. Hypothalamic Contribution to Pituitary Functions Is Recapitulated In vitro Using 3D-Cultured Human iPS Cells. Cell Rep. 2020, 30, 18–24 e15. [Google Scholar] [CrossRef] [PubMed]
- Lancaster, M.A.; Knoblich, J.A. Organogenesis in a dish: Modeling development and disease using organoid technologies. Science 2014, 345, 1247125. [Google Scholar] [CrossRef] [PubMed]
- Fong, E.L.; Harrington, D.A.; Farach-Carson, M.C.; Yu, H. Heralding a new paradigm in 3D tumor modeling. Biomaterials 2016, 108, 197–213. [Google Scholar] [CrossRef]
- Yu, Y.; Gamble, A.; Pawlick, R.; Pepper, A.R.; Salama, B.; Toms, D.; Razian, G.; Ellis, C.; Bruni, A.; Gala-Lopez, B.; et al. Bioengineered human pseudoislets form efficiently from donated tissue, compare favourably with native islets in vitro and restore normoglycaemia in mice. Diabetologia 2018, 61, 2016–2029. [Google Scholar] [CrossRef]
- Zuellig, R.A.; Cavallari, G.; Gerber, P.; Tschopp, O.; Spinas, G.A.; Moritz, W.; Lehmann, R. Improved physiological properties of gravity-enforced reassembled rat and human pancreatic pseudo-islets. J. Tissue Eng. Regen. Med. 2017, 11, 109–120. [Google Scholar] [CrossRef]
- Hollenberg, A.N.; Choi, J.; Serra, M.; Kotton, D.N. Regenerative therapy for hypothyroidism: Mechanisms and possibilities. Mol. Cell Endocrinol. 2017, 445, 35–41. [Google Scholar] [CrossRef]
- Ogundipe, V.M.L.; Plukker, J.T.M.; Links, T.P.; Coppes, R.P. Thyroid Gland Organoids: Current Models and Insights for Application in Tissue Engineering. Tissue Eng. Part. A 2022, 28, 500–510. [Google Scholar] [CrossRef]
- Zhao, J.; Ren, Y.; Ge, Z.; Zhao, X.; Li, W.; Wang, H.; Jiang, M. Thyroid organoids: Advances and applications. Endokrynol. Pol. 2023, 74, 121–127. [Google Scholar] [CrossRef]
- D’Agosto, S.; Andreani, S.; Scarpa, A.; Corbo, V. Preclinical Modelling of PDA: Is Organoid the New Black? Int. J. Mol. Sci. 2019, 20, 2766. [Google Scholar] [CrossRef]
- Yang, L.; Yang, S.; Li, X.; Li, B.; Li, Y.; Zhang, X.; Ma, Y.; Peng, X.; Jin, H.; Fan, Q.; et al. Tumor organoids: From inception to future in cancer research. Cancer Lett. 2019, 454, 120–133. [Google Scholar] [CrossRef]
- Mehta, G.; Hsiao, A.Y.; Ingram, M.; Luker, G.D.; Takayama, S. Opportunities and challenges for use of tumor spheroids as models to test drug delivery and efficacy. J. Control. Release 2012, 164, 192–204. [Google Scholar] [CrossRef]
- Ney, A.; Canciani, G.; Hsuan, J.J.; Pereira, S.P. Modelling Pancreatic Neuroendocrine Cancer: From Bench Side to Clinic. Cancers 2020, 12, 3170. [Google Scholar] [CrossRef]
- Silveira, E.; Cavalcante, I.P.; Kremer, J.L.; de Mendonca, P.O.R.; Lotfi, C.F.P. The tyrosine kinase inhibitor nilotinib is more efficient than mitotane in decreasing cell viability in spheroids prepared from adrenocortical carcinoma cells. Cancer Cell Int. 2018, 18, 29. [Google Scholar] [CrossRef]
- Heredia-Soto, V.; Redondo, A.; Berjon, A.; Miguel-Martin, M.; Diaz, E.; Crespo, R.; Hernandez, A.; Yebenes, L.; Gallego, A.; Feliu, J.; et al. High-throughput 3-dimensional culture of epithelial ovarian cancer cells as preclinical model of disease. Oncotarget 2018, 9, 21893–21903. [Google Scholar] [CrossRef] [PubMed]
- Bornstein, S.; Shapiro, I.; Malyukov, M.; Zullig, R.; Luca, E.; Gelfgat, E.; Beuschlein, F.; Nolting, S.; Berruti, A.; Sigala, S.; et al. Innovative multidimensional models in a high-throughput-format for different cell types of endocrine origin. Cell Death Dis. 2022, 13, 648. [Google Scholar] [CrossRef] [PubMed]
- Raghavan, S.; Winter, P.S.; Navia, A.W.; Williams, H.L.; DenAdel, A.; Lowder, K.E.; Galvez-Reyes, J.; Kalekar, R.L.; Mulugeta, N.; Kapner, K.S.; et al. Microenvironment drives cell state, plasticity, and drug response in pancreatic cancer. Cell 2021, 184, 6119–6137.e26. [Google Scholar] [CrossRef]
- Brown, J.M.; Wilson, W.R. Exploiting tumour hypoxia in cancer treatment. Nat. Rev. Cancer 2004, 4, 437–447. [Google Scholar] [CrossRef]
- Chen, Z.; Han, F.; Du, Y.; Shi, H.; Zhou, W. Hypoxic microenvironment in cancer: Molecular mechanisms and therapeutic interventions. Signal Transduct. Target. Ther. 2023, 8, 70. [Google Scholar] [CrossRef] [PubMed]
- Singleton, D.C.; Macann, A.; Wilson, W.R. Therapeutic targeting of the hypoxic tumour microenvironment. Nat. Rev. Clin. Oncol. 2021, 18, 751–772. [Google Scholar] [CrossRef] [PubMed]
- Bredel-Geissler, A.; Karbach, U.; Walenta, S.; Vollrath, L.; Mueller-Klieser, W. Proliferation-associated oxygen consumption and morphology of tumor cells in monolayer and spheroid culture. J. Cell Physiol. 1992, 153, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Kunz-Schughart, L.A.; Groebe, K.; Mueller-Klieser, W. Three-dimensional cell culture induces novel proliferative and metabolic alterations associated with oncogenic transformation. Int. J. Cancer 1996, 66, 578–586. [Google Scholar] [CrossRef]
- Wartenberg, M.; Donmez, F.; Ling, F.C.; Acker, H.; Hescheler, J.; Sauer, H. Tumor-induced angiogenesis studied in confrontation cultures of multicellular tumor spheroids and embryoid bodies grown from pluripotent embryonic stem cells. FASEB J. 2001, 15, 995–1005. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, A.; Huang, Y.; Krajina, B.A.; McBirney, M.; Doak, A.E.; Qu, S.; Wang, C.L.; Haffner, M.C.; Cheung, K.J. Metastasis from the tumor interior and necrotic core formation are regulated by breast cancer-derived angiopoietin-like 7. Proc. Natl. Acad. Sci. USA 2023, 120, e2214888120. [Google Scholar] [CrossRef]
- Zhao, Z.; Chen, X.; Dowbaj, A.M.; Sljukic, A.; Bratlie, K.; Lin, L.; Fong, E.L.S.; Balachander, G.M.; Chen, Z.; Soragni, A.; et al. Organoids. Nat. Rev. Methods Primers 2022, 2, 94. [Google Scholar] [CrossRef]
- Bresciani, G.; Hofland, L.J.; Dogan, F.; Giamas, G.; Gagliano, T.; Zatelli, M.C. Evaluation of Spheroid 3D Culture Methods to Study a Pancreatic Neuroendocrine Neoplasm Cell Line. Front. Endocrinol. 2019, 10, 682. [Google Scholar] [CrossRef]
- Misun, P.M.; Yesildag, B.; Forschler, F.; Neelakandhan, A.; Rousset, N.; Biernath, A.; Hierlemann, A.; Frey, O. In vitro Platform for Studying Human Insulin Release Dynamics of Single Pancreatic Islet Microtissues at High Resolution. Adv. Biosyst. 2020, 4, e1900291. [Google Scholar] [CrossRef]
- Ootani, A.; Li, X.; Sangiorgi, E.; Ho, Q.T.; Ueno, H.; Toda, S.; Sugihara, H.; Fujimoto, K.; Weissman, I.L.; Capecchi, M.R.; et al. Sustained in vitro intestinal epithelial culture within a Wnt-dependent stem cell niche. Nat. Med. 2009, 15, 701–706. [Google Scholar] [CrossRef] [PubMed]
- Neal, J.T.; Li, X.; Zhu, J.; Giangarra, V.; Grzeskowiak, C.L.; Ju, J.; Liu, I.H.; Chiou, S.H.; Salahudeen, A.A.; Smith, A.R.; et al. Organoid Modeling of the Tumor Immune Microenvironment. Cell 2018, 175, 1972–1988.e16. [Google Scholar] [CrossRef]
- Phan, N.; Hong, J.J.; Tofig, B.; Mapua, M.; Elashoff, D.; Moatamed, N.A.; Huang, J.; Memarzadeh, S.; Damoiseaux, R.; Soragni, A. A simple high-throughput approach identifies actionable drug sensitivities in patient-derived tumor organoids. Commun. Biol. 2019, 2, 78. [Google Scholar] [CrossRef]
- Tebon, P.J.; Wang, B.; Markowitz, A.L.; Davarifar, A.; Krawczuk, P.; Murray, G.; Nguyen, H.T.L.; Tavanaie, N.; Nguyen, T.L.; Boutros, P.C.; et al. Drug screening at single-organoid resolution via bioprinting and interferometry. Nat. Commun. 2023, 14, 3168. [Google Scholar] [CrossRef]
- Weeber, F.; Ooft, S.N.; Dijkstra, K.K.; Voest, E.E. Tumor Organoids as a Pre-clinical Cancer Model for Drug Discovery. Cell Chem. Biol. 2017, 24, 1092–1100. [Google Scholar] [CrossRef]
- Driehuis, E.; van Hoeck, A.; Moore, K.; Kolders, S.; Francies, H.E.; Gulersonmez, M.C.; Stigter, E.C.A.; Burgering, B.; Geurts, V.; Gracanin, A.; et al. Pancreatic cancer organoids recapitulate disease and allow personalized drug screening. Proc. Natl. Acad. Sci. USA 2019, 116, 26580–26590. [Google Scholar] [CrossRef]
- Vlachogiannis, G.; Hedayat, S.; Vatsiou, A.; Jamin, Y.; Fernandez-Mateos, J.; Khan, K.; Lampis, A.; Eason, K.; Huntingford, I.; Burke, R.; et al. Patient-derived organoids model treatment response of metastatic gastrointestinal cancers. Science 2018, 359, 920–926. [Google Scholar] [CrossRef] [PubMed]
- Verduin, M.; Hoeben, A.; De Ruysscher, D.; Vooijs, M. Patient-Derived Cancer Organoids as Predictors of Treatment Response. Front. Oncol. 2021, 11, 641980. [Google Scholar] [CrossRef] [PubMed]
- Tiriac, H.; Plenker, D.; Baker, L.A.; Tuveson, D.A. Organoid models for translational pancreatic cancer research. Curr. Opin. Genet. Dev. 2019, 54, 7–11. [Google Scholar] [CrossRef]
- de Witte, C.J.; Espejo Valle-Inclan, J.; Hami, N.; Lohmussaar, K.; Kopper, O.; Vreuls, C.P.H.; Jonges, G.N.; van Diest, P.; Nguyen, L.; Clevers, H.; et al. Patient-Derived Ovarian Cancer Organoids Mimic Clinical Response and Exhibit Heterogeneous Inter- and Intrapatient Drug Responses. Cell Rep. 2020, 31, 107762. [Google Scholar] [CrossRef] [PubMed]
- van de Wetering, M.; Francies, H.E.; Francis, J.M.; Bounova, G.; Iorio, F.; Pronk, A.; van Houdt, W.; van Gorp, J.; Taylor-Weiner, A.; Kester, L.; et al. Prospective derivation of a living organoid biobank of colorectal cancer patients. Cell 2015, 161, 933–945. [Google Scholar] [CrossRef]
- Drost, J.; Clevers, H. Organoids in cancer research. Nat. Rev. Cancer 2018, 18, 407–418. [Google Scholar] [CrossRef]
- Tiriac, H.; Belleau, P.; Engle, D.D.; Plenker, D.; Deschenes, A.; Somerville, T.D.D.; Froeling, F.E.M.; Burkhart, R.A.; Denroche, R.E.; Jang, G.H.; et al. Organoid Profiling Identifies Common Responders to Chemotherapy in Pancreatic Cancer. Cancer Discov. 2018, 8, 1112–1129. [Google Scholar] [CrossRef]
- Perez-Castro, C.; Renner, U.; Haedo, M.R.; Stalla, G.K.; Arzt, E. Cellular and molecular specificity of pituitary gland physiology. Physiol. Rev. 2012, 92, 1–38. [Google Scholar] [CrossRef]
- Laporte, E.; Vennekens, A.; Vankelecom, H. Pituitary Remodeling Throughout Life: Are Resident Stem Cells Involved? Front. Endocrinol. 2020, 11, 604519. [Google Scholar] [CrossRef]
- Cox, B.; Laporte, E.; Vennekens, A.; Kobayashi, H.; Nys, C.; Van Zundert, I.; Uji, I.H.; Vercauteren Drubbel, A.; Beck, B.; Roose, H.; et al. Organoids from pituitary as a novel research model toward pituitary stem cell exploration. J. Endocrinol. 2019, 240, 287–308. [Google Scholar] [CrossRef]
- Ozaki, H.; Suga, H.; Arima, H. Hypothalamic-pituitary organoid generation through the recapitulation of organogenesis. Dev. Growth Differ. 2021, 63, 154–165. [Google Scholar] [CrossRef]
- Ozone, C.; Suga, H.; Eiraku, M.; Kadoshima, T.; Yonemura, S.; Takata, N.; Oiso, Y.; Tsuji, T.; Sasai, Y. Functional anterior pituitary generated in self-organizing culture of human embryonic stem cells. Nat. Commun. 2016, 7, 10351. [Google Scholar] [CrossRef] [PubMed]
- Asa, S.L.; Mete, O.; Cusimano, M.D.; McCutcheon, I.E.; Perry, A.; Yamada, S.; Nishioka, H.; Casar-Borota, O.; Uccella, S.; La Rosa, S.; et al. Pituitary neuroendocrine tumors: A model for neuroendocrine tumor classification. Mod. Pathol. 2021, 34, 1634–1650. [Google Scholar] [CrossRef] [PubMed]
- Asa, S.L.; Mete, O.; Perry, A.; Osamura, R.Y. Overview of the 2022 WHO Classification of Pituitary Tumors. Endocr. Pathol. 2022, 33, 6–26. [Google Scholar] [CrossRef] [PubMed]
- Kikuchi, Y.; Seki, K.; Momose, E.; Kizawa, I.; Oomori, K.; Shima, K.; Mukai, K.; Kato, K. Establishment and characterization of a new human cultured cell line from a prolactin-secreting pituitary adenoma. Cancer Res. 1985, 45, 5722–5727. [Google Scholar]
- Chomczynski, P.; Soszynski, P.A.; Frohman, L.A. Stimulatory effect of thyroid hormone on growth hormone gene expression in a human pituitary cell line. J. Clin. Endocrinol. Metab. 1993, 77, 281–285. [Google Scholar] [CrossRef]
- Jin, L.; Kulig, E.; Qian, X.; Scheithauer, B.W.; Eberhardt, N.L.; Lloyd, R.V. A human pituitary adenoma cell line proliferates and maintains some differentiated functions following expression of SV40 large T-antigen. Endocr. Pathol. 1998, 9, 169–184. [Google Scholar] [CrossRef]
- Danila, D.C.; Zhang, X.; Zhou, Y.; Dickersin, G.R.; Fletcher, J.A.; Hedley-Whyte, E.T.; Selig, M.K.; Johnson, S.R.; Klibanski, A. A human pituitary tumor-derived folliculostellate cell line. J. Clin. Endocrinol. Metab. 2000, 85, 1180–1187. [Google Scholar] [CrossRef] [PubMed]
- Fazekas, I.; Hegedus, B.; Bacsy, E.; Kerekes, E.; Slowik, F.; Balint, K.; Pasztor, E. Characterization of human pituitary adenomas in cell cultures by light and electron microscopic morphology and immunolabeling. Folia Histochem. Cytobiol. 2005, 43, 81–90. [Google Scholar] [PubMed]
- Ishibashi, M.; Yamaji, T. Direct effects of catecholamines, thyrotropin-releasing hormone, and somatostatin on growth hormone and prolactin secretion from adenomatous and nonadenomatous human pituitary cells in culture. J. Clin. Investig. 1984, 73, 66–78. [Google Scholar] [CrossRef]
- Ishiwata, I.; Ishiwata, C.; Iguchi, M.; Soma, M.; Sato, Y.; Sonobe, M.; Kiguchi, K.; Tachibana, T.; Ishikawa, H. Biological characteristics of cultured cells derived from various types of human brain tumors. Hum. Cell 2004, 17, 117–124. [Google Scholar] [CrossRef]
- Melmed, S.; Odenheimer, D.; Carlson, H.E.; Hershman, J.M. Establishment of functional human pituitary tumor cell cultures. Vitr. Cell. Dev. Biol.-Plant 1982, 18, 35–42. [Google Scholar] [CrossRef]
- Nys, C.; Lee, Y.L.; Roose, H.; Mertens, F.; De Pauw, E.; Kobayashi, H.; Sciot, R.; Bex, M.; Versyck, G.; De Vleeschouwer, S.; et al. Exploring stem cell biology in pituitary tumors and derived organoids. Endocr. Relat. Cancer 2022, 29, 427–450. [Google Scholar] [CrossRef]
- Chakrabarti, J.; Pandey, R.; Churko, J.M.; Eschbacher, J.; Mallick, S.; Chen, Y.; Hermes, B.; Mallick, P.; Stansfield, B.N.; Pond, K.W.; et al. Development of Human Pituitary Neuroendocrine Tumor Organoids to Facilitate Effective Targeted Treatments of Cushing’s Disease. Cells 2022, 11, 3344. [Google Scholar] [CrossRef]
- Mallick, S.; Chakrabarti, J.; Eschbacher, J.; Moraitis, A.G.; Greenstein, A.E.; Churko, J.; Pond, K.W.; Livolsi, A.; Thorne, C.A.; Little, A.S.; et al. Genetically engineered human pituitary corticotroph tumor organoids exhibit divergent responses to glucocorticoid receptor modulators. Transl. Res. 2023, 256, 56–72. [Google Scholar] [CrossRef]
- Nilsson, M.; Fagman, H. Development of the thyroid gland. Development 2017, 144, 2123–2140. [Google Scholar] [CrossRef]
- Kameda, Y.; Nishimaki, T.; Chisaka, O.; Iseki, S.; Sucov, H.M. Expression of the epithelial marker E-cadherin by thyroid C cells and their precursors during murine development. J. Histochem. Cytochem. 2007, 55, 1075–1088. [Google Scholar] [CrossRef] [PubMed]
- Visciano, C.; Prevete, N.; Liotti, F.; Marone, G. Tumor-Associated Mast Cells in Thyroid Cancer. Int. J. Endocrinol. 2015, 2015, 705169. [Google Scholar] [CrossRef] [PubMed]
- Prete, A.; Borges de Souza, P.; Censi, S.; Muzza, M.; Nucci, N.; Sponziello, M. Update on Fundamental Mechanisms of Thyroid Cancer. Front. Endocrinol. 2020, 11, 102. [Google Scholar] [CrossRef] [PubMed]
- Sondorp, L.H.J.; Ogundipe, V.M.L.; Groen, A.H.; Kelder, W.; Kemper, A.; Links, T.P.; Coppes, R.P.; Kruijff, S. Patient-Derived Papillary Thyroid Cancer Organoids for Radioactive Iodine Refractory Screening. Cancers 2020, 12, 3212. [Google Scholar] [CrossRef] [PubMed]
- Saiselet, M.; Floor, S.; Tarabichi, M.; Dom, G.; Hebrant, A.; van Staveren, W.C.; Maenhaut, C. Thyroid cancer cell lines: An overview. Front. Endocrinol. 2012, 3, 133. [Google Scholar] [CrossRef]
- Chen, D.; Tan, Y.; Li, Z.; Li, W.; Yu, L.; Chen, W.; Liu, Y.; Liu, L.; Guo, L.; Huang, W.; et al. Organoid Cultures Derived From Patients With Papillary Thyroid Cancer. J. Clin. Endocrinol. Metab. 2021, 106, 1410–1426. [Google Scholar] [CrossRef]
- Pecce, V.; Sponziello, M.; Bini, S.; Grani, G.; Durante, C.; Verrienti, A. Establishment and maintenance of thyroid organoids from human cancer cells. STAR Protoc. 2022, 3, 101393. [Google Scholar] [CrossRef]
- Antonica, F.; Kasprzyk, D.F.; Opitz, R.; Iacovino, M.; Liao, X.H.; Dumitrescu, A.M.; Refetoff, S.; Peremans, K.; Manto, M.; Kyba, M.; et al. Generation of functional thyroid from embryonic stem cells. Nature 2012, 491, 66–71. [Google Scholar] [CrossRef]
- Romitti, M.; Tourneur, A.; de Faria da Fonseca, B.; Doumont, G.; Gillotay, P.; Liao, X.H.; Eski, S.E.; Van Simaeys, G.; Chomette, L.; Lasolle, H.; et al. Transplantable human thyroid organoids generated from embryonic stem cells to rescue hypothyroidism. Nat. Commun. 2022, 13, 7057. [Google Scholar] [CrossRef]
- Kurmann, A.A.; Serra, M.; Hawkins, F.; Rankin, S.A.; Mori, M.; Astapova, I.; Ullas, S.; Lin, S.; Bilodeau, M.; Rossant, J.; et al. Regeneration of Thyroid Function by Transplantation of Differentiated Pluripotent Stem Cells. Cell Stem Cell 2015, 17, 527–542. [Google Scholar] [CrossRef]
- Bulanova, E.A.; Koudan, E.V.; Degosserie, J.; Heymans, C.; Pereira, F.D.; Parfenov, V.A.; Sun, Y.; Wang, Q.; Akhmedova, S.A.; Sviridova, I.K.; et al. Bioprinting of a functional vascularized mouse thyroid gland construct. Biofabrication 2017, 9, 034105. [Google Scholar] [CrossRef] [PubMed]
- Nanba, K.; Blinder, A.R.; Rainey, W.E. Primary Cultures and Cell Lines for In vitro Modeling of the Human Adrenal Cortex. Tohoku J. Exp. Med. 2021, 253, 217–232. [Google Scholar] [CrossRef] [PubMed]
- Poli, G.; Sarchielli, E.; Guasti, D.; Benvenuti, S.; Ballerini, L.; Mazzanti, B.; Armignacco, R.; Cantini, G.; Lulli, M.; Chortis, V.; et al. Human fetal adrenal cells retain age-related stem- and endocrine-differentiation potential in culture. FASEB J. 2019, 33, 2263–2277. [Google Scholar] [CrossRef] [PubMed]
- Melau, C.; Nielsen, J.E.; Perlman, S.; Lundvall, L.; Langhoff Thuesen, L.; Juul Hare, K.; Schou Hammerum, M.; Frederiksen, H.; Mitchell, R.T.; Juul, A.; et al. Establishment of a Novel Human Fetal Adrenal Culture Model that Supports de Novo and Manipulated Steroidogenesis. J. Clin. Endocrinol. Metab. 2021, 106, 843–857. [Google Scholar] [CrossRef]
- Sigala, S.; Rossini, E.; Abate, A.; Tamburello, M.; Bornstein, S.R.; Hantel, C. An update on adrenocortical cell lines of human origin. Endocrine 2022, 77, 432–437. [Google Scholar] [CrossRef] [PubMed]
- Sedlack, A.J.H.; Hatfield, S.J.; Kumar, S.; Arakawa, Y.; Roper, N.; Sun, N.Y.; Nilubol, N.; Kiseljak-Vassiliades, K.; Hoang, C.D.; Bergsland, E.K.; et al. Preclinical Models of Adrenocortical Cancer. Cancers 2023, 15, 2873. [Google Scholar] [CrossRef] [PubMed]
- Ayala-Ramirez, M.; Jasim, S.; Feng, L.; Ejaz, S.; Deniz, F.; Busaidy, N.; Waguespack, S.G.; Naing, A.; Sircar, K.; Wood, C.G.; et al. Adrenocortical carcinoma: Clinical outcomes and prognosis of 330 patients at a tertiary care center. Eur. J. Endocrinol. 2013, 169, 891–899. [Google Scholar] [CrossRef]
- Gazdar, A.F.; Oie, H.K.; Shackleton, C.H.; Chen, T.R.; Triche, T.J.; Myers, C.E.; Chrousos, G.P.; Brennan, M.F.; Stein, C.A.; La Rocca, R.V. Establishment and characterization of a human adrenocortical carcinoma cell line that expresses multiple pathways of steroid biosynthesis. Cancer Res. 1990, 50, 5488–5496. [Google Scholar]
- Hantel, C.; Shapiro, I.; Poli, G.; Chiapponi, C.; Bidlingmaier, M.; Reincke, M.; Luconi, M.; Jung, S.; Beuschlein, F. Targeting heterogeneity of adrenocortical carcinoma: Evaluation and extension of preclinical tumor models to improve clinical translation. Oncotarget 2016, 7, 79292–79304. [Google Scholar] [CrossRef]
- Kiseljak-Vassiliades, K.; Zhang, Y.; Bagby, S.M.; Kar, A.; Pozdeyev, N.; Xu, M.; Gowan, K.; Sharma, V.; Raeburn, C.D.; Albuja-Cruz, M.; et al. Development of new preclinical models to advance adrenocortical carcinoma research. Endocr. Relat. Cancer 2018, 25, 437–451. [Google Scholar] [CrossRef]
- Landwehr, L.S.; Schreiner, J.; Appenzeller, S.; Kircher, S.; Herterich, S.; Sbiera, S.; Fassnacht, M.; Kroiss, M.; Weigand, I. A novel patient-derived cell line of adrenocortical carcinoma shows a pathogenic role of germline MUTYH mutation and high tumour mutational burden. Eur. J. Endocrinol. 2021, 184, 823–835. [Google Scholar] [CrossRef]
- Sigala, S.; Bothou, C.; Penton, D.; Abate, A.; Peitzsch, M.; Cosentini, D.; Tiberio, G.A.M.; Bornstein, S.R.; Berruti, A.; Hantel, C. A Comprehensive Investigation of Steroidogenic Signaling in Classical and New Experimental Cell Models of Adrenocortical Carcinoma. Cells 2022, 11, 1439. [Google Scholar] [CrossRef]
- Brenner, T.; O’Shaughnessy, K.M. Both TASK-3 and TREK-1 two-pore loop K channels are expressed in H295R cells and modulate their membrane potential and aldosterone secretion. Am. J. Physiol. Endocrinol. Metab. 2008, 295, E1480–E1486. [Google Scholar] [CrossRef]
- Kurlbaum, M.; Sbiera, S.; Kendl, S.; Martin Fassnacht, M.; Kroiss, M. Steroidogenesis in the NCI-H295 Cell Line Model is Strongly Affected By Culture Conditions and Substrain. Exp. Clin. Endocrinol. Diabetes 2020, 128, 672–680. [Google Scholar] [CrossRef] [PubMed]
- Canton, R.F.; Sanderson, J.T.; Nijmeijer, S.; Bergman, A.; Letcher, R.J.; van den Berg, M. In vitro effects of brominated flame retardants and metabolites on CYP17 catalytic activity: A novel mechanism of action? Toxicol. Appl. Pharmacol. 2006, 216, 274–281. [Google Scholar] [CrossRef]
- Gracia, T.; Hilscherova, K.; Jones, P.D.; Newsted, J.L.; Higley, E.B.; Zhang, X.; Hecker, M.; Murphy, M.B.; Yu, R.M.; Lam, P.K.; et al. Modulation of steroidogenic gene expression and hormone production of H295R cells by pharmaceuticals and other environmentally active compounds. Toxicol. Appl. Pharmacol. 2007, 225, 142–153. [Google Scholar] [CrossRef] [PubMed]
- Karmaus, A.L.; Toole, C.M.; Filer, D.L.; Lewis, K.C.; Martin, M.T. High-Throughput Screening of Chemical Effects on Steroidogenesis Using H295R Human Adrenocortical Carcinoma Cells. Toxicol. Sci. 2016, 150, 323–332. [Google Scholar] [CrossRef] [PubMed]
- Germano, A.; Rapa, I.; Volante, M.; Lo Buono, N.; Carturan, S.; Berruti, A.; Terzolo, M.; Papotti, M. Cytotoxic activity of gemcitabine, alone or in combination with mitotane, in adrenocortical carcinoma cell lines. Mol. Cell Endocrinol. 2014, 382, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Kroiss, M.; Sbiera, S.; Kendl, S.; Kurlbaum, M.; Fassnacht, M. Drug Synergism of Proteasome Inhibitors and Mitotane by Complementary Activation of ER Stress in Adrenocortical Carcinoma Cells. Horm. Cancer 2016, 7, 345–355. [Google Scholar] [CrossRef]
- Mariniello, B.; Rosato, A.; Zuccolotto, G.; Rubin, B.; Cicala, M.V.; Finco, I.; Iacobone, M.; Frigo, A.C.; Fassina, A.; Pezzani, R.; et al. Combination of sorafenib and everolimus impacts therapeutically on adrenocortical tumor models. Endocr. Relat. Cancer 2012, 19, 527–539. [Google Scholar] [CrossRef] [PubMed]
- Poli, G.; Cantini, G.; Armignacco, R.; Fucci, R.; Santi, R.; Canu, L.; Nesi, G.; Mannelli, M.; Luconi, M. Metformin as a new anti-cancer drug in adrenocortical carcinoma. Oncotarget 2016, 7, 49636–49648. [Google Scholar] [CrossRef]
- Lichtenauer, U.D.; Shapiro, I.; Osswald, A.; Meurer, S.; Kulle, A.; Reincke, M.; Riepe, F.; Beuschlein, F. Characterization of NCI-H295R cells as an in vitro model of hyperaldosteronism. Horm. Metab. Res. 2013, 45, 124–129. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Nilubol, N.; Zhang, L.; Shen, M.; Zhang, Y.Q.; He, M.; Austin, C.P.; Kebebew, E. Four clinically utilized drugs were identified and validated for treatment of adrenocortical cancer using quantitative high-throughput screening. J. Transl. Med. 2012, 10, 198. [Google Scholar] [CrossRef] [PubMed]
- Krokker, L.; Szabo, B.; Nemeth, K.; Tohati, R.; Sarkadi, B.; Meszaros, K.; Patocs, A.; Butz, H. Three Dimensional Cell Culturing for Modeling Adrenal and Pituitary Tumors. Pathol. Oncol. Res. 2021, 27, 640676. [Google Scholar] [CrossRef] [PubMed]
- Cerquetti, L.; Bucci, B.; Raffa, S.; Amendola, D.; Maggio, R.; Lardo, P.; Petrangeli, E.; Torrisi, M.R.; Toscano, V.; Pugliese, G.; et al. Effects of Sorafenib, a Tyrosin Kinase Inhibitor, on Adrenocortical Cancer. Front. Endocrinol. 2021, 12, 667798. [Google Scholar] [CrossRef] [PubMed]
- Berruti, A.; Sperone, P.; Ferrero, A.; Germano, A.; Ardito, A.; Priola, A.M.; De Francia, S.; Volante, M.; Daffara, F.; Generali, D.; et al. Phase II study of weekly paclitaxel and sorafenib as second/third-line therapy in patients with adrenocortical carcinoma. Eur. J. Endocrinol. 2012, 166, 451–458. [Google Scholar] [CrossRef]
- Haider, M.S.; Schreiner, J.; Kendl, S.; Kroiss, M.; Luxenhofer, R. A Micellar Mitotane Formulation with High Drug-Loading and Solubility: Physico-Chemical Characterization and Cytotoxicity Studies in 2D and 3D In vitro Tumor Models. Macromol. Biosci. 2020, 20, e1900178. [Google Scholar] [CrossRef]
- Langer, C.; Koll-Weber, M.; Holzer, M.; Hantel, C.; Suss, R. Mitotane Nanocarriers for the Treatment of Adrenocortical Carcinoma: Evaluation of Albumin-Stabilized Nanoparticles and Liposomes in a Preclinical In vitro Study with 3D Spheroids. Pharmaceutics 2022, 14, 1891. [Google Scholar] [CrossRef]
- Avena, P.; De Luca, A.; Chimento, A.; Nocito, M.C.; Sculco, S.; La Padula, D.; Zavaglia, L.; Giulietti, M.; Hantel, C.; Sirianni, R.; et al. Estrogen Related Receptor Alpha (ERRalpha) a Bridge between Metabolism and Adrenocortical Cancer Progression. Cancers 2022, 14, 3885. [Google Scholar] [CrossRef]
- Baregamian, N.; Sekhar, K.R.; Krystofiak, E.S.; Vinogradova, M.; Thomas, G.; Mannoh, E.; Solorzano, C.C.; Kiernan, C.M.; Mahadevan-Jansen, A.; Abumrad, N.; et al. Engineering functional 3-dimensional patient-derived endocrine organoids for broad multiplatform applications. Surgery 2023, 173, 67–75. [Google Scholar] [CrossRef]
- Amar, L.; Servais, A.; Gimenez-Roqueplo, A.P.; Zinzindohoue, F.; Chatellier, G.; Plouin, P.F. Year of diagnosis, features at presentation, and risk of recurrence in patients with pheochromocytoma or secreting paraganglioma. J. Clin. Endocrinol. Metab. 2005, 90, 2110–2116. [Google Scholar] [CrossRef]
- Edstrom Elder, E.; Hjelm Skog, A.L.; Hoog, A.; Hamberger, B. The management of benign and malignant pheochromocytoma and abdominal paraganglioma. Eur. J. Surg. Oncol. 2003, 29, 278–283. [Google Scholar] [CrossRef]
- Goldstein, R.E.; O’Neill, J.A., Jr.; Holcomb, G.W., 3rd; Morgan, W.M., 3rd; Neblett, W.W., 3rd; Oates, J.A.; Brown, N.; Nadeau, J.; Smith, B.; Page, D.L.; et al. Clinical experience over 48 years with pheochromocytoma. Ann. Surg. 1999, 229, 755–764. [Google Scholar] [CrossRef]
- Patel, D.; Phay, J.E.; Yen, T.W.F.; Dickson, P.V.; Wang, T.S.; Garcia, R.; Yang, A.D.; Solorzano, C.C.; Kim, L.T. Update on Pheochromocytoma and Paraganglioma from the SSO Endocrine/Head and Neck Disease-Site Work Group. Part 1 of 2: Advances in Pathogenesis and Diagnosis of Pheochromocytoma and Paraganglioma. Ann. Surg. Oncol. 2020, 27, 1329–1337. [Google Scholar] [CrossRef] [PubMed]
- Proye, C.A.; Vix, M.; Jansson, S.; Tisell, L.E.; Dralle, H.; Hiller, W. “The” pheochromocytoma: A benign, intra-adrenal, hypertensive, sporadic unilateral tumor. Does it exist? World J. Surg. 1994, 18, 467–472. [Google Scholar] [CrossRef] [PubMed]
- Remine, W.H.; Chong, G.C.; Van Heerden, J.A.; Sheps, S.G.; Harrison, E.G., Jr. Current management of pheochromocytoma. Ann. Surg. 1974, 179, 740–748. [Google Scholar] [CrossRef] [PubMed]
- Burnichon, N.; Vescovo, L.; Amar, L.; Libe, R.; de Reynies, A.; Venisse, A.; Jouanno, E.; Laurendeau, I.; Parfait, B.; Bertherat, J.; et al. Integrative genomic analysis reveals somatic mutations in pheochromocytoma and paraganglioma. Hum. Mol. Genet. 2011, 20, 3974–3985. [Google Scholar] [CrossRef]
- Fishbein, L.; Leshchiner, I.; Walter, V.; Danilova, L.; Robertson, A.G.; Johnson, A.R.; Lichtenberg, T.M.; Murray, B.A.; Ghayee, H.K.; Else, T.; et al. Comprehensive Molecular Characterization of Pheochromocytoma and Paraganglioma. Cancer Cell 2017, 31, 181–193. [Google Scholar] [CrossRef]
- Gieldon, L.; William, D.; Hackmann, K.; Jahn, W.; Jahn, A.; Wagner, J.; Rump, A.; Bechmann, N.; Nolting, S.; Knosel, T.; et al. Optimizing Genetic Workup in Pheochromocytoma and Paraganglioma by Integrating Diagnostic and Research Approaches. Cancers 2019, 11, 809. [Google Scholar] [CrossRef]
- Jiang, J.; Zhang, J.; Pang, Y.; Bechmann, N.; Li, M.; Monteagudo, M.; Calsina, B.; Gimenez-Roqueplo, A.P.; Nolting, S.; Beuschlein, F.; et al. Sino-European Differences in the Genetic Landscape and Clinical Presentation of Pheochromocytoma and Paraganglioma. J. Clin. Endocrinol. Metab. 2020, 105, 3295–3307. [Google Scholar] [CrossRef]
- Jochmanova, I.; Pacak, K. Genomic Landscape of Pheochromocytoma and Paraganglioma. Trends Cancer 2018, 4, 6–9. [Google Scholar] [CrossRef]
- Luchetti, A.; Walsh, D.; Rodger, F.; Clark, G.; Martin, T.; Irving, R.; Sanna, M.; Yao, M.; Robledo, M.; Neumann, H.P.; et al. Profiling of somatic mutations in phaeochromocytoma and paraganglioma by targeted next generation sequencing analysis. Int. J. Endocrinol. 2015, 2015, 138573. [Google Scholar] [CrossRef] [PubMed]
- Nolting, S.; Bechmann, N.; Taieb, D.; Beuschlein, F.; Fassnacht, M.; Kroiss, M.; Eisenhofer, G.; Grossman, A.; Pacak, K. Personalized Management of Pheochromocytoma and Paraganglioma. Endocr. Rev. 2022, 43, 199–239. [Google Scholar] [CrossRef] [PubMed]
- Greene, L.A.; Tischler, A.S. Establishment of a noradrenergic clonal line of rat adrenal pheochromocytoma cells which respond to nerve growth factor. Proc. Natl. Acad. Sci. USA 1976, 73, 2424–2428. [Google Scholar] [CrossRef]
- Powers, J.F.; Cochran, B.; Baleja, J.D.; Sikes, H.D.; Pattison, A.D.; Zhang, X.; Lomakin, I.; Shepard-Barry, A.; Pacak, K.; Moon, S.J.; et al. A xenograft and cell line model of SDH-deficient pheochromocytoma derived from Sdhb+/− rats. Endocr. Relat. Cancer 2020, 27, 337–354. [Google Scholar] [CrossRef] [PubMed]
- Powers, J.F.; Evinger, M.J.; Tsokas, P.; Bedri, S.; Alroy, J.; Shahsavari, M.; Tischler, A.S. Pheochromocytoma cell lines from heterozygous neurofibromatosis knockout mice. Cell Tissue Res. 2000, 302, 309–320. [Google Scholar] [CrossRef]
- Martiniova, L.; Lai, E.W.; Elkahloun, A.G.; Abu-Asab, M.; Wickremasinghe, A.; Solis, D.C.; Perera, S.M.; Huynh, T.T.; Lubensky, I.A.; Tischler, A.S.; et al. Characterization of an animal model of aggressive metastatic pheochromocytoma linked to a specific gene signature. Clin. Exp. Metastasis 2009, 26, 239–250. [Google Scholar] [CrossRef]
- Bayley, J.P.; Rebel, H.G.; Scheurwater, K.; Duesman, D.; Zhang, J.; Schiavi, F.; Korpershoek, E.; Jansen, J.C.; Schepers, A.; Devilee, P. Long-term in vitro 2D-culture of SDHB and SDHD-related human paragangliomas and pheochromocytomas. PLoS ONE 2022, 17, e0274478. [Google Scholar] [CrossRef]
- Fankhauser, M.; Bechmann, N.; Lauseker, M.; Goncalves, J.; Favier, J.; Klink, B.; William, D.; Gieldon, L.; Maurer, J.; Spottl, G.; et al. Synergistic Highly Potent Targeted Drug Combinations in Different Pheochromocytoma Models Including Human Tumor Cultures. Endocrinology 2019, 160, 2600–2617. [Google Scholar] [CrossRef]
- Wang, K.; Schutze, I.; Gulde, S.; Bechmann, N.; Richter, S.; Helm, J.; Lauseker, M.; Maurer, J.; Reul, A.; Spoettl, G.; et al. Personalized drug testing in human pheochromocytoma/paraganglioma primary cultures. Endocr. Relat. Cancer 2022, 29, 285–306. [Google Scholar] [CrossRef]
- Calucho, M.; Cheng, Z.; Nguyen, H.T.L.; Shihabi, A.A.; Gonzalez-Cantu, H.; Guo, Q.; Thaker, M.; Bechmann, N.; Eisenhofer, G.; Ding, Y.; et al. Establishment and validation of pheochromocytoma organoids for high-throughput drug screening. Cancer Res. 2023, 87 (Suppl. 7), 195. [Google Scholar] [CrossRef]
- Arutyunyan, I.V.; Fatkhudinov, T.K.; Makarov, A.V.; Elchaninov, A.V.; Sukhikh, G.T. Regenerative medicine of pancreatic islets. World J. Gastroenterol. 2020, 26, 2948–2966. [Google Scholar] [CrossRef]
- Stanger, B.Z.; Hebrok, M. Control of cell identity in pancreas development and regeneration. Gastroenterology 2013, 144, 1170–1179. [Google Scholar] [CrossRef] [PubMed]
- Barreto, S.G.; Carati, C.J.; Toouli, J.; Saccone, G.T. The islet-acinar axis of the pancreas: More than just insulin. Am. J. Physiol. Gastrointest. Liver Physiol. 2010, 299, G10–G22. [Google Scholar] [CrossRef]
- Walma, D.A.C.; Yamada, K.M. The extracellular matrix in development. Development 2020, 147, dev175596. [Google Scholar] [CrossRef] [PubMed]
- Ulrich, A.B.; Schmied, B.M.; Standop, J.; Schneider, M.B.; Pour, P.M. Pancreatic cell lines: A review. Pancreas 2002, 24, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Szczerbinska, I.; Tessitore, A.; Hansson, L.K.; Agrawal, A.; Ragel Lopez, A.; Helenius, M.; Malinowski, A.R.; Gilboa, B.; Ruby, M.A.; Gupta, R.; et al. Large-Scale Functional Genomics Screen to Identify Modulators of Human beta-Cell Insulin Secretion. Biomedicines 2022, 10, 103. [Google Scholar] [CrossRef]
- Lehmann, R.; Zuellig, R.A.; Kugelmeier, P.; Baenninger, P.B.; Moritz, W.; Perren, A.; Clavien, P.A.; Weber, M.; Spinas, G.A. Superiority of small islets in human islet transplantation. Diabetes 2007, 56, 594–603. [Google Scholar] [CrossRef]
- Wassmer, C.H.; Lebreton, F.; Bellofatto, K.; Perez, L.; Cottet-Dumoulin, D.; Andres, A.; Bosco, D.; Berney, T.; Othenin-Girard, V.; Martinez De Tejada, B.; et al. Bio-Engineering of Pre-Vascularized Islet Organoids for the Treatment of Type 1 Diabetes. Transpl. Int. 2021, 35, 10214. [Google Scholar] [CrossRef]
- Honarpisheh, M.; Lei, Y.; Zhang, Y.; Pehl, M.; Kemter, E.; Kraetzl, M.; Lange, A.; Wolf, E.; Wolf-van Buerck, L.; Seissler, J.; et al. Formation of Re-Aggregated Neonatal Porcine Islet Clusters Improves In vitro Function and Transplantation Outcome. Transpl. Int. 2022, 35, 10697. [Google Scholar] [CrossRef]
- Boj, S.F.; Hwang, C.I.; Baker, L.A.; Chio, I.I.C.; Engle, D.D.; Corbo, V.; Jager, M.; Ponz-Sarvise, M.; Tiriac, H.; Spector, M.S.; et al. Organoid models of human and mouse ductal pancreatic cancer. Cell 2015, 160, 324–338. [Google Scholar] [CrossRef]
- Hennig, A.; Wolf, L.; Jahnke, B.; Polster, H.; Seidlitz, T.; Werner, K.; Aust, D.E.; Hampe, J.; Distler, M.; Weitz, J.; et al. CFTR Expression Analysis for Subtyping of Human Pancreatic Cancer Organoids. Stem Cells Int. 2019, 2019, 1024614. [Google Scholar] [CrossRef]
- Huang, L.; Holtzinger, A.; Jagan, I.; BeGora, M.; Lohse, I.; Ngai, N.; Nostro, C.; Wang, R.; Muthuswamy, L.B.; Crawford, H.C.; et al. Ductal pancreatic cancer modeling and drug screening using human pluripotent stem cell- and patient-derived tumor organoids. Nat. Med. 2015, 21, 1364–1371. [Google Scholar] [CrossRef]
- Romero-Calvo, I.; Weber, C.R.; Ray, M.; Brown, M.; Kirby, K.; Nandi, R.K.; Long, T.M.; Sparrow, S.M.; Ugolkov, A.; Qiang, W.; et al. Human Organoids Share Structural and Genetic Features with Primary Pancreatic Adenocarcinoma Tumors. Mol. Cancer Res. 2019, 17, 70–83. [Google Scholar] [CrossRef]
- Walsh, A.J.; Castellanos, J.A.; Nagathihalli, N.S.; Merchant, N.B.; Skala, M.C. Optical Imaging of Drug-Induced Metabolism Changes in Murine and Human Pancreatic Cancer Organoids Reveals Heterogeneous Drug Response. Pancreas 2016, 45, 863–869. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Seino, T.; Kawasaki, S.; Shimokawa, M.; Tamagawa, H.; Toshimitsu, K.; Fujii, M.; Ohta, Y.; Matano, M.; Nanki, K.; Kawasaki, K.; et al. Human Pancreatic Tumor Organoids Reveal Loss of Stem Cell Niche Factor Dependence during Disease Progression. Cell Stem Cell 2018, 22, 454–467.e6. [Google Scholar] [CrossRef]
- Baker, L.A.; Tiriac, H.; Tuveson, D.A. Generation and Culture of Human Pancreatic Ductal Adenocarcinoma Organoids from Resected Tumor Specimens. Methods Mol. Biol. 2019, 1882, 97–115. [Google Scholar] [CrossRef] [PubMed]
- Bian, B.; Juiz, N.A.; Gayet, O.; Bigonnet, M.; Brandone, N.; Roques, J.; Cros, J.; Wang, N.; Dusetti, N.; Iovanna, J. Pancreatic Cancer Organoids for Determining Sensitivity to Bromodomain and Extra-Terminal Inhibitors (BETi). Front. Oncol. 2019, 9, 475. [Google Scholar] [CrossRef]
- Gendoo, D.M.A.; Denroche, R.E.; Zhang, A.; Radulovich, N.; Jang, G.H.; Lemire, M.; Fischer, S.; Chadwick, D.; Lungu, I.M.; Ibrahimov, E.; et al. Whole genomes define concordance of matched primary, xenograft, and organoid models of pancreas cancer. PLoS Comput. Biol. 2019, 15, e1006596. [Google Scholar] [CrossRef] [PubMed]
- Burkhart, R.A.; Baker, L.A.; Tiriac, H. Testing Susceptibility of Patient-Derived Organoid Cultures to Therapies: Pharmacotyping. Methods Mol. Biol. 2018, 1787, 253–261. [Google Scholar] [CrossRef]
- Assarzadegan, N.; Montgomery, E. What is New in the 2019 World Health Organization (WHO) Classification of Tumors of the Digestive System: Review of Selected Updates on Neuroendocrine Neoplasms, Appendiceal Tumors, and Molecular Testing. Arch. Pathol. Lab. Med. 2021, 145, 664–677. [Google Scholar] [CrossRef] [PubMed]
- Mafficini, A.; Scarpa, A. Genomic landscape of pancreatic neuroendocrine tumours: The International Cancer Genome Consortium. J. Endocrinol. 2018, 236, R161–R167. [Google Scholar] [CrossRef]
- Rinke, A.; Auernhammer, C.J.; Bodei, L.; Kidd, M.; Krug, S.; Lawlor, R.; Marinoni, I.; Perren, A.; Scarpa, A.; Sorbye, H.; et al. Treatment of advanced gastroenteropancreatic neuroendocrine neoplasia, are we on the way to personalised medicine? Gut 2021, 70, 1768–1781. [Google Scholar] [CrossRef] [PubMed]
- Gulde, S.; Foscarini, A.; April-Monn, S.L.; Genio, E.; Marangelo, A.; Satam, S.; Helbling, D.; Falconi, M.; Toledo, R.A.; Schrader, J.; et al. Combined Targeting of Pathogenetic Mechanisms in Pancreatic Neuroendocrine Tumors Elicits Synergistic Antitumor Effects. Cancers 2022, 14, 5481. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Rainey, W.E. Human adrenocortical carcinoma cell lines. Mol. Cell Endocrinol. 2012, 351, 58–65. [Google Scholar] [CrossRef]
- Falletta, S.; Partelli, S.; Rubini, C.; Nann, D.; Doria, A.; Marinoni, I.; Polenta, V.; Di Pasquale, C.; Degli Uberti, E.; Perren, A.; et al. mTOR inhibitors response and mTOR pathway in pancreatic neuroendocrine tumors. Endocr. Relat. Cancer 2016, 23, 883–891. [Google Scholar] [CrossRef]
- Mohamed, A.; Blanchard, M.P.; Albertelli, M.; Barbieri, F.; Brue, T.; Niccoli, P.; Delpero, J.R.; Monges, G.; Garcia, S.; Ferone, D.; et al. Pasireotide and octreotide antiproliferative effects and sst2 trafficking in human pancreatic neuroendocrine tumor cultures. Endocr. Relat. Cancer 2014, 21, 691–704. [Google Scholar] [CrossRef]
- Mohamed, A.; Romano, D.; Saveanu, A.; Roche, C.; Albertelli, M.; Barbieri, F.; Brue, T.; Niccoli, P.; Delpero, J.R.; Garcia, S.; et al. Anti-proliferative and anti-secretory effects of everolimus on human pancreatic neuroendocrine tumors primary cultures: Is there any benefit from combination with somatostatin analogs? Oncotarget 2017, 8, 41044–41063. [Google Scholar] [CrossRef]
- Moreno, A.; Akcakanat, A.; Munsell, M.F.; Soni, A.; Yao, J.C.; Meric-Bemstam, F. Antitumor activity of rapamycin and octreotide as single agents or in combination in neuroendocrine tumors. Endocr.-Relat. Cancer 2008, 15, 257–266. [Google Scholar] [CrossRef]
- Kawasaki, K.; Toshimitsu, K.; Matano, M.; Fujita, M.; Fujii, M.; Togasaki, K.; Ebisudani, T.; Shimokawa, M.; Takano, A.; Takahashi, S.; et al. An Organoid Biobank of Neuroendocrine Neoplasms Enables Genotype-Phenotype Mapping. Cell 2020, 183, 1420–1435. [Google Scholar] [CrossRef] [PubMed]
- April-Monn, S.L.; Wiedmer, T.; Skowronska, M.; Maire, R.; Schiavo Lena, M.; Trippel, M.; Di Domenico, A.; Muffatti, F.; Andreasi, V.; Capurso, G.; et al. Three-Dimensional Primary Cell Culture: A Novel Preclinical Model for Pancreatic Neuroendocrine Tumors. Neuroendocrinology 2021, 111, 273–287. [Google Scholar] [CrossRef]
- Simon, L.E.; Kumar, T.R.; Duncan, F.E. In vitro ovarian follicle growth: A comprehensive analysis of key protocol variablesdagger. Biol. Reprod. 2020, 103, 455–470. [Google Scholar] [CrossRef]
- Desai, N.; Alex, A.; AbdelHafez, F.; Calabro, A.; Goldfarb, J.; Fleischman, A.; Falcone, T. Three-dimensional in vitro follicle growth: Overview of culture models, biomaterials, design parameters and future directions. Reprod. Biol. Endocrinol. 2010, 8, 119. [Google Scholar] [CrossRef] [PubMed]
- Wycherley, G.; Downey, D.; Kane, M.T.; Hynes, A.C. A novel follicle culture system markedly increases follicle volume, cell number and oestradiol secretion. Reproduction 2004, 127, 669–677. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yin, H.; Kristensen, S.G.; Jiang, H.; Rasmussen, A.; Andersen, C.Y. Survival and growth of isolated pre-antral follicles from human ovarian medulla tissue during long-term 3D culture. Hum. Reprod. 2016, 31, 1531–1539. [Google Scholar] [CrossRef] [PubMed]
- McLaughlin, M.; Albertini, D.F.; Wallace, W.H.B.; Anderson, R.A.; Telfer, E.E. Metaphase II oocytes from human unilaminar follicles grown in a multi-step culture system. Mol. Hum. Reprod. 2018, 24, 135–142. [Google Scholar] [CrossRef]
- Levanon, K.; Ng, V.; Piao, H.Y.; Zhang, Y.; Chang, M.C.; Roh, M.H.; Kindelberger, D.W.; Hirsch, M.S.; Crum, C.P.; Marto, J.A.; et al. Primary ex vivo cultures of human fallopian tube epithelium as a model for serous ovarian carcinogenesis. Oncogene 2010, 29, 1103–1113. [Google Scholar] [CrossRef]
- Kessler, M.; Hoffmann, K.; Brinkmann, V.; Thieck, O.; Jackisch, S.; Toelle, B.; Berger, H.; Mollenkopf, H.J.; Mangler, M.; Sehouli, J.; et al. The Notch and Wnt pathways regulate stemness and differentiation in human fallopian tube organoids. Nat. Commun. 2015, 6, 8989. [Google Scholar] [CrossRef]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Orr, B.; Edwards, R.P. Diagnosis and Treatment of Ovarian Cancer. Hematol. Oncol. Clin. North. Am. 2018, 32, 943–964. [Google Scholar] [CrossRef]
- Vaughan, S.; Coward, J.I.; Bast, R.C., Jr.; Berchuck, A.; Berek, J.S.; Brenton, J.D.; Coukos, G.; Crum, C.C.; Drapkin, R.; Etemadmoghadam, D.; et al. Rethinking ovarian cancer: Recommendations for improving outcomes. Nat. Rev. Cancer 2011, 11, 719–725. [Google Scholar] [CrossRef]
- Kopper, O.; de Witte, C.J.; Lohmussaar, K.; Valle-Inclan, J.E.; Hami, N.; Kester, L.; Balgobind, A.V.; Korving, J.; Proost, N.; Begthel, H.; et al. An organoid platform for ovarian cancer captures intra- and interpatient heterogeneity. Nat. Med. 2019, 25, 838–849. [Google Scholar] [CrossRef]
- Anglesio, M.S.; Wiegand, K.C.; Melnyk, N.; Chow, C.; Salamanca, C.; Prentice, L.M.; Senz, J.; Yang, W.; Spillman, M.A.; Cochrane, D.R.; et al. Type-specific cell line models for type-specific ovarian cancer research. PLoS ONE 2013, 8, e72162. [Google Scholar] [CrossRef]
- Barnes, B.M.; Nelson, L.; Tighe, A.; Burghel, G.J.; Lin, I.H.; Desai, S.; McGrail, J.C.; Morgan, R.D.; Taylor, S.S. Distinct transcriptional programs stratify ovarian cancer cell lines into the five major histological subtypes. Genome Med. 2021, 13, 140. [Google Scholar] [CrossRef]
- Beaufort, C.M.; Helmijr, J.C.; Piskorz, A.M.; Hoogstraat, M.; Ruigrok-Ritstier, K.; Besselink, N.; Murtaza, M.; van, I.W.F.; Heine, A.A.; Smid, M.; et al. Ovarian cancer cell line panel (OCCP): Clinical importance of in vitro morphological subtypes. PLoS ONE 2014, 9, e103988. [Google Scholar] [CrossRef] [PubMed]
- Domcke, S.; Sinha, R.; Levine, D.A.; Sander, C.; Schultz, N. Evaluating cell lines as tumour models by comparison of genomic profiles. Nat. Commun. 2013, 4, 2126. [Google Scholar] [CrossRef] [PubMed]
- Papp, E.; Hallberg, D.; Konecny, G.E.; Bruhm, D.C.; Adleff, V.; Noe, M.; Kagiampakis, I.; Palsgrove, D.; Conklin, D.; Kinose, Y.; et al. Integrated Genomic, Epigenomic, and Expression Analyses of Ovarian Cancer Cell Lines. Cell Rep. 2018, 25, 2617–2633. [Google Scholar] [CrossRef] [PubMed]
- Nowacka, M.; Sterzynska, K.; Andrzejewska, M.; Nowicki, M.; Januchowski, R. Drug resistance evaluation in novel 3D in vitro model. Biomed. Pharmacother. 2021, 138, 111536. [Google Scholar] [CrossRef] [PubMed]
- Casagrande, N.; Borghese, C.; Agostini, F.; Durante, C.; Mazzucato, M.; Colombatti, A.; Aldinucci, D. In Ovarian Cancer Multicellular Spheroids, Platelet Releasate Promotes Growth, Expansion of ALDH+ and CD133+ Cancer Stem Cells, and Protection against the Cytotoxic Effects of Cisplatin, Carboplatin and Paclitaxel. Int. J. Mol. Sci. 2021, 22, 3019. [Google Scholar] [CrossRef] [PubMed]
- Sodek, K.L.; Ringuette, M.J.; Brown, T.J. Compact spheroid formation by ovarian cancer cells is associated with contractile behavior and an invasive phenotype. Int. J. Cancer 2009, 124, 2060–2070. [Google Scholar] [CrossRef] [PubMed]
- Novak, C.M.; Horst, E.N.; Lin, E.; Mehta, G. Compressive Stimulation Enhances Ovarian Cancer Proliferation, Invasion, Chemoresistance, and Mechanotransduction via CDC42 in a 3D Bioreactor. Cancers 2020, 12, 1521. [Google Scholar] [CrossRef] [PubMed]
- Maenhoudt, N.; Vankelecom, H. Protocol for establishing organoids from human ovarian cancer biopsies. STAR Protoc. 2021, 2, 100429. [Google Scholar] [CrossRef]
- Maru, Y.; Tanaka, N.; Itami, M.; Hippo, Y. Efficient use of patient-derived organoids as a preclinical model for gynecologic tumors. Gynecol. Oncol. 2019, 154, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Psilopatis, I.; Sykaras, A.G.; Mandrakis, G.; Vrettou, K.; Theocharis, S. Patient-Derived Organoids: The Beginning of a New Era in Ovarian Cancer Disease Modeling and Drug Sensitivity Testing. Biomedicines 2022, 11, 1. [Google Scholar] [CrossRef]
- Hill, S.J.; Decker, B.; Roberts, E.A.; Horowitz, N.S.; Muto, M.G.; Worley, M.J., Jr.; Feltmate, C.M.; Nucci, M.R.; Swisher, E.M.; Nguyen, H.; et al. Prediction of DNA Repair Inhibitor Response in Short-Term Patient-Derived Ovarian Cancer Organoids. Cancer Discov. 2018, 8, 1404–1421. [Google Scholar] [CrossRef] [PubMed]
- Jabs, J.; Zickgraf, F.M.; Park, J.; Wagner, S.; Jiang, X.; Jechow, K.; Kleinheinz, K.; Toprak, U.H.; Schneider, M.A.; Meister, M.; et al. Screening drug effects in patient-derived cancer cells links organoid responses to genome alterations. Mol. Syst. Biol. 2017, 13, 955. [Google Scholar] [CrossRef] [PubMed]
- Bi, J.; Newtson, A.M.; Zhang, Y.; Devor, E.J.; Samuelson, M.I.; Thiel, K.W.; Leslie, K.K. Successful Patient-Derived Organoid Culture of Gynecologic Cancers for Disease Modeling and Drug Sensitivity Testing. Cancers 2021, 13, 2901. [Google Scholar] [CrossRef]
- Nanki, Y.; Chiyoda, T.; Hirasawa, A.; Ookubo, A.; Itoh, M.; Ueno, M.; Akahane, T.; Kameyama, K.; Yamagami, W.; Kataoka, F.; et al. Patient-derived ovarian cancer organoids capture the genomic profiles of primary tumours applicable for drug sensitivity and resistance testing. Sci. Rep. 2020, 10, 12581. [Google Scholar] [CrossRef]
- Yamawaki, K.; Mori, Y.; Sakai, H.; Kanda, Y.; Shiokawa, D.; Ueda, H.; Ishiguro, T.; Yoshihara, K.; Nagasaka, K.; Onda, T.; et al. Integrative analyses of gene expression and chemosensitivity of patient-derived ovarian cancer spheroids link G6PD-driven redox metabolism to cisplatin chemoresistance. Cancer Lett. 2021, 521, 29–38. [Google Scholar] [CrossRef]
- Chen, H.; Gotimer, K.; De Souza, C.; Tepper, C.G.; Karnezis, A.N.; Leiserowitz, G.S.; Chien, J.; Smith, L.H. Short-term organoid culture for drug sensitivity testing of high-grade serous carcinoma. Gynecol. Oncol. 2020, 157, 783–792. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, K.; Berger, H.; Kulbe, H.; Thillainadarasan, S.; Mollenkopf, H.J.; Zemojtel, T.; Taube, E.; Darb-Esfahani, S.; Mangler, M.; Sehouli, J.; et al. Stable expansion of high-grade serous ovarian cancer organoids requires a low-Wnt environment. EMBO J. 2020, 39, e104013. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, V.; Huycke, T.R.; Phong, K.T.; Gartner, Z.J. Organoid models for mammary gland dynamics and breast cancer. Curr. Opin. Cell Biol. 2020, 66, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Macias, H.; Hinck, L. Mammary gland development. Wiley Interdiscip. Rev. Dev. Biol. 2012, 1, 533–557. [Google Scholar] [CrossRef]
- Jena, M.K.; Jaswal, S.; Kumar, S.; Mohanty, A.K. Molecular mechanism of mammary gland involution: An update. Dev. Biol. 2019, 445, 145–155. [Google Scholar] [CrossRef]
- Zwick, R.K.; Rudolph, M.C.; Shook, B.A.; Holtrup, B.; Roth, E.; Lei, V.; Van Keymeulen, A.; Seewaldt, V.; Kwei, S.; Wysolmerski, J.; et al. Adipocyte hypertrophy and lipid dynamics underlie mammary gland remodeling after lactation. Nat. Commun. 2018, 9, 3592. [Google Scholar] [CrossRef]
- Kessenbrock, K.; Smith, P.; Steenbeek, S.C.; Pervolarakis, N.; Kumar, R.; Minami, Y.; Goga, A.; Hinck, L.; Werb, Z. Diverse regulation of mammary epithelial growth and branching morphogenesis through noncanonical Wnt signaling. Proc. Natl. Acad. Sci. USA 2017, 114, 3121–3126. [Google Scholar] [CrossRef]
- Shackleton, M.; Vaillant, F.; Simpson, K.J.; Stingl, J.; Smyth, G.K.; Asselin-Labat, M.L.; Wu, L.; Lindeman, G.J.; Visvader, J.E. Generation of a functional mammary gland from a single stem cell. Nature 2006, 439, 84–88. [Google Scholar] [CrossRef]
- Rosenbluth, J.M.; Schackmann, R.C.J.; Gray, G.K.; Selfors, L.M.; Li, C.M.; Boedicker, M.; Kuiken, H.J.; Richardson, A.; Brock, J.; Garber, J.; et al. Organoid cultures from normal and cancer-prone human breast tissues preserve complex epithelial lineages. Nat. Commun. 2020, 11, 1711. [Google Scholar] [CrossRef]
- Sachs, N.; de Ligt, J.; Kopper, O.; Gogola, E.; Bounova, G.; Weeber, F.; Balgobind, A.V.; Wind, K.; Gracanin, A.; Begthel, H.; et al. A Living Biobank of Breast Cancer Organoids Captures Disease Heterogeneity. Cell 2018, 172, 373–386.e10. [Google Scholar] [CrossRef]
- Barcellos-Hoff, M.H.; Aggeler, J.; Ram, T.G.; Bissell, M.J. Functional differentiation and alveolar morphogenesis of primary mammary cultures on reconstituted basement membrane. Development 1989, 105, 223–235. [Google Scholar] [CrossRef]
- Darcy, K.M.; Black, J.D.; Hahm, H.A.; Ip, M.M. Mammary organoids from immature virgin rats undergo ductal and alveolar morphogenesis when grown within a reconstituted basement membrane. Exp. Cell Res. 1991, 196, 49–65. [Google Scholar] [CrossRef]
- Caruso, M.; Huang, S.; Mourao, L.; Scheele, C. A Mammary Organoid Model to Study Branching Morphogenesis. Front. Physiol. 2022, 13, 826107. [Google Scholar] [CrossRef]
- Sumbal, J.; Chiche, A.; Charifou, E.; Koledova, Z.; Li, H. Primary Mammary Organoid Model of Lactation and Involution. Front Cell. Dev. Biol. 2020, 8, 68. [Google Scholar] [CrossRef] [PubMed]
- Frohlich, E. The Variety of 3D Breast Cancer Models for the Study of Tumor Physiology and Drug Screening. Int. J. Mol. Sci. 2023, 24, 7116. [Google Scholar] [CrossRef] [PubMed]
- Azamjah, N.; Soltan-Zadeh, Y.; Zayeri, F. Global Trend of Breast Cancer Mortality Rate: A 25-Year Study. Asian Pac. J. Cancer Prev. 2019, 20, 2015–2020. [Google Scholar] [CrossRef] [PubMed]
- Gallo, M.; Campione, S.; Di Vito, V.; Fortunati, N.; Lo Calzo, F.; Messina, E.; Ruggeri, R.M.; Faggiano, A.; Colao, A.A.L. Primary Neuroendocrine Neoplasms of the Breast: Still Open Issues. Front. Endocrinol. 2020, 11, 610230. [Google Scholar] [CrossRef]
- Dai, X.; Cheng, H.; Bai, Z.; Li, J. Breast Cancer Cell Line Classification and Its Relevance with Breast Tumor Subtyping. J. Cancer 2017, 8, 3131–3141. [Google Scholar] [CrossRef]
- Froehlich, K.; Haeger, J.D.; Heger, J.; Pastuschek, J.; Photini, S.M.; Yan, Y.; Lupp, A.; Pfarrer, C.; Mrowka, R.; Schleussner, E.; et al. Generation of Multicellular Breast Cancer Tumor Spheroids: Comparison of Different Protocols. J. Mammary Gland. Biol. Neoplasia 2016, 21, 89–98. [Google Scholar] [CrossRef]
- Keller, F.; Rudolf, R.; Hafner, M. Towards optimized breast cancer 3D spheroid mono- and co-culture models for pharmacological research and screening. J. Cell. Biotechnol. 2019, 5, 89–101. [Google Scholar] [CrossRef]
- Anastasov, N.; Hofig, I.; Radulovic, V.; Strobel, S.; Salomon, M.; Lichtenberg, J.; Rothenaigner, I.; Hadian, K.; Kelm, J.M.; Thirion, C.; et al. A 3D-microtissue-based phenotypic screening of radiation resistant tumor cells with synchronized chemotherapeutic treatment. BMC Cancer 2015, 15, 466. [Google Scholar] [CrossRef] [PubMed]
- Dittmer, A.; Dittmer, J. Carcinoma-Associated Fibroblasts Promote Growth of Sox2-Expressing Breast Cancer Cells. Cancers 2020, 12, 3435. [Google Scholar] [CrossRef] [PubMed]
- Yakavets, I.; Francois, A.; Benoit, A.; Merlin, J.L.; Bezdetnaya, L.; Vogin, G. Advanced co-culture 3D breast cancer model for investigation of fibrosis induced by external stimuli: Optimization study. Sci. Rep. 2020, 10, 21273. [Google Scholar] [CrossRef]
- Kaur, P.; Ward, B.; Saha, B.; Young, L.; Groshen, S.; Techy, G.; Lu, Y.; Atkinson, R.; Taylor, C.R.; Ingram, M.; et al. Human breast cancer histoid: An in vitro 3-dimensional co-culture model that mimics breast cancer tissue. J. Histochem. Cytochem. 2011, 59, 1087–1100. [Google Scholar] [CrossRef] [PubMed]
- Crippa, M.; Talo, G.; Lamouline, A.; Bolis, S.; Arrigoni, C.; Bersini, S.; Moretti, M. A microfluidic model of human vascularized breast cancer metastasis to bone for the study of neutrophil-cancer cell interactions. Mater. Today Bio 2022, 17, 100460. [Google Scholar] [CrossRef]
- Fischer, L.; Nosratlo, M.; Hast, K.; Karakaya, E.; Strohlein, N.; Esser, T.U.; Gerum, R.; Richter, S.; Engel, F.B.; Detsch, R.; et al. Calcium supplementation of bioinks reduces shear stress-induced cell damage during bioprinting. Biofabrication 2022, 14, 045005. [Google Scholar] [CrossRef]
- Lee, G.; Kim, S.J.; Park, J.K. Bioprinting of heterogeneous and multilayered cell-hydrogel constructs using continuous multi-material printing and aerosol-based crosslinking. STAR Protoc. 2022, 3, 101303. [Google Scholar] [CrossRef]
- Song, K.; Zu, X.; Du, Z.; Hu, Z.; Wang, J.; Li, J. Diversity Models and Applications of 3D Breast Tumor-on-a-Chip. Micromachines 2021, 12, 814. [Google Scholar] [CrossRef]
- Tevis, K.M.; Cecchi, R.J.; Colson, Y.L.; Grinstaff, M.W. Mimicking the tumor microenvironment to regulate macrophage phenotype and assessing chemotherapeutic efficacy in embedded cancer cell/macrophage spheroid models. Acta Biomater. 2017, 50, 271–279. [Google Scholar] [CrossRef]
- Sato, T.; Clevers, H. SnapShot: Growing Organoids from Stem Cells. Cell 2015, 161, 1700–1700.e1. [Google Scholar] [CrossRef]
- Dekkers, J.F.; van Vliet, E.J.; Sachs, N.; Rosenbluth, J.M.; Kopper, O.; Rebel, H.G.; Wehrens, E.J.; Piani, C.; Visvader, J.E.; Verissimo, C.S.; et al. Long-term culture, genetic manipulation and xenotransplantation of human normal and breast cancer organoids. Nat. Protoc. 2021, 16, 1936–1965. [Google Scholar] [CrossRef]
- Tsai, K.K.; Huang, S.S.; Northey, J.J.; Liao, W.Y.; Hsu, C.C.; Cheng, L.H.; Werner, M.E.; Chuu, C.P.; Chatterjee, C.; Lakins, J.N.; et al. Screening of organoids derived from patients with breast cancer implicates the repressor NCOR2 in cytotoxic stress response and antitumor immunity. Nat. Cancer 2022, 3, 734–752. [Google Scholar] [CrossRef]
- Davaadelger, B.; Choi, M.R.; Singhal, H.; Clare, S.E.; Khan, S.A.; Kim, J.J. BRCA1 mutation influences progesterone response in human benign mammary organoids. Breast Cancer Res. 2019, 21, 124. [Google Scholar] [CrossRef]
- Pan, B.; Zhao, D.; Liu, Y.; Li, N.; Song, C.; Li, N.; Li, X.; Zhao, Z. Breast cancer organoids from malignant pleural effusion-derived tumor cells as an individualized medicine platform. Vitr. Cell. Dev. Biol.-Anim. 2021, 57, 510–518. [Google Scholar] [CrossRef] [PubMed]
- Berry, P.A.; Maitland, N.J.; Collins, A.T. Androgen receptor signalling in prostate: Effects of stromal factors on normal and cancer stem cells. Mol. Cell Endocrinol. 2008, 288, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Lang, S.H.; Stark, M.; Collins, A.; Paul, A.B.; Stower, M.J.; Maitland, N.J. Experimental prostate epithelial morphogenesis in response to stroma and three-dimensional matrigel culture. Cell Growth Differ. 2001, 12, 631–640. [Google Scholar]
- Garraway, I.P.; Sun, W.; Tran, C.P.; Perner, S.; Zhang, B.; Goldstein, A.S.; Hahm, S.A.; Haider, M.; Head, C.S.; Reiter, R.E.; et al. Human prostate sphere-forming cells represent a subset of basal epithelial cells capable of glandular regeneration in vivo. Prostate 2010, 70, 491–501. [Google Scholar] [CrossRef] [PubMed]
- Chua, C.W.; Shibata, M.; Lei, M.; Toivanen, R.; Barlow, L.J.; Bergren, S.K.; Badani, K.K.; McKiernan, J.M.; Benson, M.C.; Hibshoosh, H.; et al. Single luminal epithelial progenitors can generate prostate organoids in culture. Nat. Cell Biol. 2014, 16, 951–961. [Google Scholar] [CrossRef]
- Karthaus, W.R.; Iaquinta, P.J.; Drost, J.; Gracanin, A.; van Boxtel, R.; Wongvipat, J.; Dowling, C.M.; Gao, D.; Begthel, H.; Sachs, N.; et al. Identification of multipotent luminal progenitor cells in human prostate organoid cultures. Cell 2014, 159, 163–175. [Google Scholar] [CrossRef] [PubMed]
- Drost, J.; Karthaus, W.R.; Gao, D.; Driehuis, E.; Sawyers, C.L.; Chen, Y.; Clevers, H. Organoid culture systems for prostate epithelial and cancer tissue. Nat. Protoc. 2016, 11, 347–358. [Google Scholar] [CrossRef] [PubMed]
- Richards, Z.; McCray, T.; Marsili, J.; Zenner, M.L.; Manlucu, J.T.; Garcia, J.; Kajdacsy-Balla, A.; Murray, M.; Voisine, C.; Murphy, A.B.; et al. Prostate Stroma Increases the Viability and Maintains the Branching Phenotype of Human Prostate Organoids. iScience 2019, 12, 304–317. [Google Scholar] [CrossRef]
- Kruslin, B.; Ulamec, M.; Tomas, D. Prostate cancer stroma: An important factor in cancer growth and progression. Bosn. J. Basic. Med. Sci. 2015, 15, 1–8. [Google Scholar] [CrossRef]
- Fizazi, K.; Tran, N.; Fein, L.; Matsubara, N.; Rodriguez-Antolin, A.; Alekseev, B.Y.; Ozguroglu, M.; Ye, D.; Feyerabend, S.; Protheroe, A.; et al. Abiraterone plus Prednisone in Metastatic, Castration-Sensitive Prostate Cancer. N. Engl. J. Med. 2017, 377, 352–360. [Google Scholar] [CrossRef]
- James, N.D.; de Bono, J.S.; Spears, M.R.; Clarke, N.W.; Mason, M.D.; Dearnaley, D.P.; Ritchie, A.W.S.; Amos, C.L.; Gilson, C.; Jones, R.J.; et al. Abiraterone for Prostate Cancer Not Previously Treated with Hormone Therapy. N. Engl. J. Med. 2017, 377, 338–351. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, R.; Huang, J.; Alumkal, J.J.; Zhang, L.; Feng, F.Y.; Thomas, G.V.; Weinstein, A.S.; Friedl, V.; Zhang, C.; Witte, O.N.; et al. Clinical and Genomic Characterization of Treatment-Emergent Small-Cell Neuroendocrine Prostate Cancer: A Multi-institutional Prospective Study. J. Clin. Oncol. 2018, 36, 2492–2503. [Google Scholar] [CrossRef]
- Ku, S.Y.; Rosario, S.; Wang, Y.; Mu, P.; Seshadri, M.; Goodrich, Z.W.; Goodrich, M.M.; Labbe, D.P.; Gomez, E.C.; Wang, J.; et al. Rb1 and Trp53 cooperate to suppress prostate cancer lineage plasticity, metastasis, and antiandrogen resistance. Science 2017, 355, 78–83. [Google Scholar] [CrossRef] [PubMed]
- Mu, P.; Zhang, Z.; Benelli, M.; Karthaus, W.R.; Hoover, E.; Chen, C.C.; Wongvipat, J.; Ku, S.Y.; Gao, D.; Cao, Z.; et al. SOX2 promotes lineage plasticity and antiandrogen resistance in TP53- and RB1-deficient prostate cancer. Science 2017, 355, 84–88. [Google Scholar] [CrossRef] [PubMed]
- Beltran, H.; Oromendia, C.; Danila, D.C.; Montgomery, B.; Hoimes, C.; Szmulewitz, R.Z.; Vaishampayan, U.; Armstrong, A.J.; Stein, M.; Pinski, J.; et al. A Phase II Trial of the Aurora Kinase A Inhibitor Alisertib for Patients with Castration-resistant and Neuroendocrine Prostate Cancer: Efficacy and Biomarkers. Clin. Cancer Res. 2019, 25, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.T.; Yao, Y.H.; Li, B.G.; Tang, Y.; Chang, J.W.; Zhang, J. Neuroendocrine Prostate Cancer (NEPC) progressing from conventional prostatic adenocarcinoma: Factors associated with time to development of NEPC and survival from NEPC diagnosis-a systematic review and pooled analysis. J. Clin. Oncol. 2014, 32, 3383–3390. [Google Scholar] [CrossRef]
- Toivanen, R.; Taylor, R.A.; Pook, D.W.; Ellem, S.J.; Risbridger, G.P. Breaking through a roadblock in prostate cancer research: An update on human model systems. J. Steroid Biochem. Mol. Biol. 2012, 131, 122–131. [Google Scholar] [CrossRef]
- Horoszewicz, J.S.; Leong, S.S.; Kawinski, E.; Karr, J.P.; Rosenthal, H.; Chu, T.M.; Mirand, E.A.; Murphy, G.P. LNCaP model of human prostatic carcinoma. Cancer Res. 1983, 43, 1809–1818. [Google Scholar] [PubMed]
- Kaighn, M.E.; Lechner, J.F.; Narayan, K.S.; Jones, L.W. Prostate carcinoma: Tissue culture cell lines. Natl. Cancer Inst. Monogr. 1978, 78, 17–21. [Google Scholar]
- Stone, K.R.; Mickey, D.D.; Wunderli, H.; Mickey, G.H.; Paulson, D.F. Isolation of a human prostate carcinoma cell line (DU 145). Int. J. Cancer 1978, 21, 274–281. [Google Scholar] [CrossRef] [PubMed]
- Korenchuk, S.; Lehr, J.E.; MClean, L.; Lee, Y.G.; Whitney, S.; Vessella, R.; Lin, D.L.; Pienta, K.J. VCaP, a cell-based model system of human prostate cancer. In Vivo 2001, 15, 163–168. [Google Scholar] [PubMed]
- Navone, N.M.; Olive, M.; Ozen, M.; Davis, R.; Troncoso, P.; Tu, S.M.; Johnston, D.; Pollack, A.; Pathak, S.; von Eschenbach, A.C.; et al. Establishment of two human prostate cancer cell lines derived from a single bone metastasis. Clin. Cancer Res. 1997, 3, 2493–2500. [Google Scholar]
- Mertz, K.D.; Setlur, S.R.; Dhanasekaran, S.M.; Demichelis, F.; Perner, S.; Tomlins, S.; Tchinda, J.; Laxman, B.; Vessella, R.L.; Beroukhim, R.; et al. Molecular characterization of TMPRSS2-ERG gene fusion in the NCI-H660 prostate cancer cell line: A new perspective for an old model. Neoplasia 2007, 9, 200–206. [Google Scholar] [CrossRef] [PubMed]
- Zhau, H.E.; Goodwin, T.J.; Chang, S.M.; Baker, T.L.; Chung, L.W. Establishment of a three-dimensional human prostate organoid coculture under microgravity-simulated conditions: Evaluation of androgen-induced growth and PSA expression. Vitr. Cell. Dev. Biol. Anim. 1997, 33, 375–380. [Google Scholar] [CrossRef]
- Wang, R.; Xu, J.; Juliette, L.; Castilleja, A.; Love, J.; Sung, S.Y.; Zhau, H.E.; Goodwin, T.J.; Chung, L.W. Three-dimensional co-culture models to study prostate cancer growth, progression, and metastasis to bone. Semin. Cancer Biol. 2005, 15, 353–364. [Google Scholar] [CrossRef]
- Chambers, K.F.; Mosaad, E.M.; Russell, P.J.; Clements, J.A.; Doran, M.R. 3D Cultures of prostate cancer cells cultured in a novel high-throughput culture platform are more resistant to chemotherapeutics compared to cells cultured in monolayer. PLoS ONE 2014, 9, e111029. [Google Scholar] [CrossRef]
- Mosaad, E.O.; Chambers, K.F.; Futrega, K.; Clements, J.A.; Doran, M.R. The Microwell-mesh: A high-throughput 3D prostate cancer spheroid and drug-testing platform. Sci. Rep. 2018, 8, 253. [Google Scholar] [CrossRef]
- Neuwirt, H.; Bouchal, J.; Kharaishvili, G.; Ploner, C.; Johrer, K.; Pitterl, F.; Weber, A.; Klocker, H.; Eder, I.E. Cancer-associated fibroblasts promote prostate tumor growth and progression through upregulation of cholesterol and steroid biosynthesis. Cell Commun. Signal 2020, 18, 11. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; White, T.A.; MacKenzie, A.P.; Clegg, N.; Lee, C.; Dumpit, R.F.; Coleman, I.; Ng, S.B.; Salipante, S.J.; Rieder, M.J.; et al. Exome sequencing identifies a spectrum of mutation frequencies in advanced and lethal prostate cancers. Proc. Natl. Acad. Sci. USA 2011, 108, 17087–17092. [Google Scholar] [CrossRef]
- Nguyen, H.M.; Vessella, R.L.; Morrissey, C.; Brown, L.G.; Coleman, I.M.; Higano, C.S.; Mostaghel, E.A.; Zhang, X.; True, L.D.; Lam, H.M.; et al. Prostate 2017, 77, 654–671. [CrossRef]
- Beshiri, M.L.; Tice, C.M.; Tran, C.; Nguyen, H.M.; Sowalsky, A.G.; Agarwal, S.; Jansson, K.H.; Yang, Q.; McGowen, K.M.; Yin, J.; et al. A PDX/Organoid Biobank of Advanced Prostate Cancers Captures Genomic and Phenotypic Heterogeneity for Disease Modeling and Therapeutic Screening. Clin. Cancer Res. 2018, 24, 4332–4345. [Google Scholar] [CrossRef] [PubMed]
- Fong, E.L.; Wan, X.; Yang, J.; Morgado, M.; Mikos, A.G.; Harrington, D.A.; Navone, N.M.; Farach-Carson, M.C. A 3D in vitro model of patient-derived prostate cancer xenograft for controlled interrogation of in vivo tumor-stromal interactions. Biomaterials 2016, 77, 164–172. [Google Scholar] [CrossRef]
- Risbridger, G.P.; Clark, A.K.; Porter, L.H.; Toivanen, R.; Bakshi, A.; Lister, N.L.; Pook, D.; Pezaro, C.J.; Sandhu, S.; Keerthikumar, S.; et al. The MURAL collection of prostate cancer patient-derived xenografts enables discovery through preclinical models of uro-oncology. Nat. Commun. 2021, 12, 5049. [Google Scholar] [CrossRef] [PubMed]
- Gao, D.; Vela, I.; Sboner, A.; Iaquinta, P.J.; Karthaus, W.R.; Gopalan, A.; Dowling, C.; Wanjala, J.N.; Undvall, E.A.; Arora, V.K.; et al. Organoid cultures derived from patients with advanced prostate cancer. Cell 2014, 159, 176–187. [Google Scholar] [CrossRef]
- Puca, L.; Bareja, R.; Prandi, D.; Shaw, R.; Benelli, M.; Karthaus, W.R.; Hess, J.; Sigouros, M.; Donoghue, A.; Kossai, M.; et al. Patient derived organoids to model rare prostate cancer phenotypes. Nat. Commun. 2018, 9, 2404. [Google Scholar] [CrossRef]
- Mosquera, M.J.; Kim, S.; Bareja, R.; Fang, Z.; Cai, S.; Pan, H.; Asad, M.; Martin, M.L.; Sigouros, M.; Rowdo, F.M.; et al. Extracellular Matrix in Synthetic Hydrogel-Based Prostate Cancer Organoids Regulate Therapeutic Response to EZH2 and DRD2 Inhibitors. Adv. Mater. 2022, 34, e2100096. [Google Scholar] [CrossRef]
- Pamarthy, S.; Sabaawy, H.E. Patient derived organoids in prostate cancer: Improving therapeutic efficacy in precision medicine. Mol. Cancer 2021, 20, 125. [Google Scholar] [CrossRef]
- Servant, R.; Garioni, M.; Vlajnic, T.; Blind, M.; Pueschel, H.; Muller, D.C.; Zellweger, T.; Templeton, A.J.; Garofoli, A.; Maletti, S.; et al. Prostate cancer patient-derived organoids: Detailed outcome from a prospective cohort of 81 clinical specimens. J. Pathol. 2021, 254, 543–555. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luca, E.; Zitzmann, K.; Bornstein, S.; Kugelmeier, P.; Beuschlein, F.; Nölting, S.; Hantel, C. Three Dimensional Models of Endocrine Organs and Target Tissues Regulated by the Endocrine System. Cancers 2023, 15, 4601. https://doi.org/10.3390/cancers15184601
Luca E, Zitzmann K, Bornstein S, Kugelmeier P, Beuschlein F, Nölting S, Hantel C. Three Dimensional Models of Endocrine Organs and Target Tissues Regulated by the Endocrine System. Cancers. 2023; 15(18):4601. https://doi.org/10.3390/cancers15184601
Chicago/Turabian StyleLuca, Edlira, Kathrin Zitzmann, Stefan Bornstein, Patrick Kugelmeier, Felix Beuschlein, Svenja Nölting, and Constanze Hantel. 2023. "Three Dimensional Models of Endocrine Organs and Target Tissues Regulated by the Endocrine System" Cancers 15, no. 18: 4601. https://doi.org/10.3390/cancers15184601
APA StyleLuca, E., Zitzmann, K., Bornstein, S., Kugelmeier, P., Beuschlein, F., Nölting, S., & Hantel, C. (2023). Three Dimensional Models of Endocrine Organs and Target Tissues Regulated by the Endocrine System. Cancers, 15(18), 4601. https://doi.org/10.3390/cancers15184601




