Salvage High-Dose-Rate Interventional Radiotherapy (Brachytherapy) Combined with Surgery for Regionally Relapsed Head and Neck Cancers
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Interventional Radiotherapy (Brachytherapy)
2.3. Follow-Up
2.4. Statistical Analysis
2.5. Ethics Committee/Informed Consent
3. Results
3.1. Patient and Tumor Characteristics
3.2. Initial Treatments
3.3. Salvage Treatment
3.4. HDR-IRT Treatment Characteristics
3.5. Treatment Outcome
3.6. Treatment-Related Toxicities
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chang, J.H.; Wu, C.C.; Yuan, K.S.P.; Wu, A.T.H.; Wu, S.Y. Locoregionally Recurrent Head and Neck Squamous Cell Carcinoma: Incidence, Survival, Prognostic Factors, and Treatment Outcomes. Oncotarget 2017, 8, 55600. [Google Scholar] [CrossRef] [PubMed]
- Brockstein, B.; Haraf, D.J.; Rademaker, A.W.; Kies, M.S.; Stenson, K.M.; Rosen, F.; Mittal, B.B.; Pelzer, H.; Fung, B.B.; Witt, M.E.; et al. Patterns of Failure, Prognostic Factors and Survival in Locoregionally Advanced Head and Neck Cancer Treated with Concomitant Chemoradiotherapy: A 9-Year, 337-Patient, Multi-Institutional Experience. Ann. Oncol. 2004, 15, 1179–1186. [Google Scholar] [CrossRef]
- Bourhis, J.; Le Maître, A.; Baujat, B.; Audry, H.; Pignon, J.P. Individual Patients’ Data Meta-Analyses in Head and Neck Cancer. Curr. Opin. Oncol. 2007, 19, 188–194. [Google Scholar] [CrossRef] [PubMed]
- Forastiere, A.; Weber, R.; Ang, K. Treatment of Head and Neck Cancer. N. Engl. J. Med. 2008, 358, 1076. [Google Scholar] [CrossRef] [PubMed]
- Posner, M.R.; Hershock, D.M.; Blajman, C.R.; Mickiewicz, E.; Winquist, E.; Gorbounova, V.; Tjulandin, S.; Shin, D.M.; Cullen, K.; Ervin, T.J.; et al. Cisplatin and Fluorouracil Alone or with Docetaxel in Head and Neck Cancer. N. Engl. J. Med. 2007, 357, 1705–1715. [Google Scholar] [CrossRef] [PubMed]
- Dive, A.M.; Bodhade, A.S.; Mishra, M.S.; Upadhyaya, N. Histological Patterns of Head and Neck Tumors: An Insight to Tumor Histology. J. Oral. Maxillofac. Pathol. 2014, 18, 58. [Google Scholar] [CrossRef]
- Pai, S.I.; Westra, W.H. Molecular Pathology of Head and Neck Cancer: Implications for Diagnosis, Prognosis, and Treatment. Annu. Rev. Pathol. 2009, 4, 49. [Google Scholar] [CrossRef]
- Goodwin, W.J. Salvage Surgery for Patients with Recurrent Squamous Cell Carcinoma of the Upper Aerodigestive Tract: When Do the Ends Justify the Means? Laryngoscope 2000, 110, 1–18. [Google Scholar] [CrossRef]
- Jeong, S.; Yoo, E.J.; Kim, J.Y.; Han, C.W.; Kim, K.J.; Kay, C.S. Re-Irradiation of Unresectable Recurrent Head and Neck Cancer: Using Helical Tomotherapy as Image-Guided Intensity-Modulated Radiotherapy. Radiat. Oncol. J. 2013, 31, 206–215. [Google Scholar] [CrossRef]
- Tortochaux, J.; Tao, Y.; Tournay, E.; Lapeyre, M.; Lesaunier, F.; Bardet, E.; Janot, F.; Lusinchi, A.; Benhamou, E.; Bontemps, P.; et al. Randomized Phase III Trial (GORTEC 98-03) Comparing Re-Irradiation plus Chemotherapy versus Methotrexate in Patients with Recurrent or a Second Primary Head and Neck Squamous Cell Carcinoma, Treated with a Palliative Intent. Radiother. Oncol. 2011, 100, 70–75. [Google Scholar] [CrossRef]
- Alterio, D.; Zaffaroni, M.; Bossi, P.; Dionisi, F.; Elicin, O.; Falzone, A.; Ferrari, A.; Jereczek-Fossa, B.A.; Sanguineti, G.; Szturz, P.; et al. Reirradiation of Head and Neck Squamous Cell Carcinomas: A Pragmatic Approach, Part II: Radiation Technique and Fractionations. Radiol. Med. 2023, 128, 1007–1021. [Google Scholar] [CrossRef] [PubMed]
- Haraf, D.J.; Weichselbaum, R.R.; Yokes, E.E. Re-Irradiation with Concomitant Chemotherapy of Unresectable Recurrent Head and Neck Cancer: A Potentially Curable Disease. Ann. Oncol. 1996, 7, 913–918. [Google Scholar] [CrossRef] [PubMed]
- Biagioli, M.C.; Harvey, M.; Roman, E.; Raez, L.E.; Wolfson, A.H.; Mutyala, S.; Han, H.S.; Markoe, A. Intensity-Modulated Radiotherapy with Concurrent Chemotherapy for Previously Irradiated, Recurrent Head and Neck Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2007, 69, 1067–1073. [Google Scholar] [CrossRef] [PubMed]
- Sulman, E.P.; Schwartz, D.L.; Le, T.T.; Ang, K.K.; Morrison, W.H.; Rosenthal, D.I.; Ahamad, A.; Kies, M.; Glisson, B.; Weber, R.; et al. IMRT Reirradiation of Head and Neck Cancer-Disease Control and Morbidity Outcomes. Int. J. Radiat. Oncol. Biol. Phys. 2009, 73, 399–409. [Google Scholar] [CrossRef] [PubMed]
- Duprez, F.; Madani, I.; Bonte, K.; Boterberg, T.; Vakaet, L.; Derie, C.; De Gersem, W.; De Neve, W. Intensity-Modulated Radiotherapy for Recurrent and Second Primary Head and Neck Cancer in Previously Irradiated Territory. Radiother. Oncol. 2009, 93, 563–569. [Google Scholar] [CrossRef]
- Chen, A.M.; Farwell, D.G.; Luu, Q.; Cheng, S.; Donald, P.J.; Purdy, J.A. Prospective Trial of High-Dose Reirradiation Using Daily Image Guidance with Intensity-Modulated Radiotherapy for Recurrent and Second Primary Head-and-Neck Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2011, 80, 669–676. [Google Scholar] [CrossRef]
- Chen, A.M.; Phillips, T.L.; Lee, N.Y. Practical Considerations in the Re-Irradiation of Recurrent and Second Primary Head-and-Neck Cancer: Who, Why, How, and How Much? Int. J. Radiat. Oncol. Biol. Phys. 2011, 81, 1211–1219. [Google Scholar] [CrossRef]
- Baliga, S.; Kabarriti, R.; Ohri, N.; Haynes–Lewis, H.; Yaparpalvi, R.; Kalnicki, S.; Garg, M.K. Stereotactic Body Radiotherapy for Recurrent Head and Neck Cancer: A Critical Review. Head. Neck 2017, 39, 595–601. [Google Scholar] [CrossRef]
- Lee, J.; Kim, T.H.; Kim, Y.S.; Kim, M.; Park, J.W.; Kim, S.H.; Kim, H.J.; Lee, C.G. Intensity-Modulated Radiotherapy-Based Reirradiation for Head and Neck Cancer: A Multi-Institutional Study by Korean Radiation Oncology Group (KROG 1707). Cancer Res. Treat. 2020, 52, 1031–1040. [Google Scholar] [CrossRef]
- McDonald, M.W.; Zolali-Meybodi, O.; Lehnert, S.J.; Estabrook, N.C.; Liu, Y.; Cohen-Gadol, A.A.; Moore, M.G. Reirradiation of Recurrent and Second Primary Head and Neck Cancer With Proton Therapy. Int. J. Radiat. Oncol. Biol. Phys. 2016, 96, 808–819. [Google Scholar] [CrossRef]
- Romesser, P.B.; Cahlon, O.; Scher, E.D.; Hug, E.B.; Sine, K.; Deselm, C.; Fox, J.L.; Mah, D.; Garg, M.K.; Han-Chih Chang, J.; et al. Proton Beam Reirradiation for Recurrent Head and Neck Cancer: Multi-Institutional Report on Feasibility and Early Outcomes. Int. J. Radiat. Oncol. Biol. Phys. 2016, 95, 386–395. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, H.; Demizu, Y.; Okimoto, T.; Ogita, M.; Himei, K.; Nakamura, S.; Suzuki, G.; Yoshida, K.; Kotsuma, T.; Yoshioka, Y.; et al. Reirradiation for Recurrent Head and Neck Cancers Using Charged Particle or Photon Radiotherapy. Strahlenther. Onkol. 2017, 193, 525–533. [Google Scholar] [CrossRef] [PubMed]
- Soror, T.; Melchert, C.; Rades, D.; Merseburger, A.S.; Kovács, G. Salvage High-Dose-Rate Interventional Radiotherapy (Brachytherapy) for Locally Relapsed Prostate Cancer after Radical Prostatectomy and Subsequent External Irradiation. J. Contemp. Brachytherapy 2023, 15, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Soror, T.; Kovács, G.; Fürschke, V.; Ismail, M.; Badakhshi, H. Salvage Treatment with Sole High-Dose-Rate Endobronchial Interventional Radiotherapy (Brachytherapy) for Isolated Endobronchial Tumor Recurrence in Non-Small-Cell Lung Cancer Patients: A 20-Year Experience. Brachytherapy 2019, 18, 727–732. [Google Scholar] [CrossRef]
- Soror, T.; Banys-Paluchowski, M.; Melchert, C.; Rades, D.; Rody, A.; Muras, K.; Xie, M.; Kovács, G. Salvage Perioperative Interstitial High-Dose-Rate Interventional Radiotherapy (Brachytherapy) for Local Recurrences of the Chest Wall Following Mastectomy and Previous External Irradiation. Cancers 2023, 15, 614. [Google Scholar] [CrossRef] [PubMed]
- Teudt, I.U.; Kovàcs, G.; Ritter, M.; Melchert, C.; Soror, T.; Wollenberg, B.; Meyer, J.E. Intensity Modulated Perioperative HDR Brachytherapy for Recurrent and/or Advanced Head and Neck Metastases. Eur. Arch. Otorhinolaryngol. 2016, 273, 2707–2715. [Google Scholar] [CrossRef]
- Hannoun-Levi, J.-M.; Gal, J.; Polgar, C.; Strnad, V.; Loessl, M.; Polat, B.; Kauer-Domer, D.; Schiappa, R.; Gutierrez, C.; GEC-ESTRO Breast Cancer Working Group. Second Conservative Treatment for Local Recurrence Breast Cancer: A GEC-ESTRO Oncological Outcome and Prognostic Factor Analysis. Int. J. Radiat. Oncol. Biol. Phys. 2023. [Google Scholar] [CrossRef]
- Chen, C.P.; Weinberg, V.; Shinohara, K.; Roach, M.; Nash, M.; Gottschalk, A.; Chang, A.J.; Hsu, I.C. Salvage HDR Brachytherapy for Recurrent Prostate Cancer after Previous Definitive Radiation Therapy: 5-Year Outcomes. Int. J. Radiat. Oncol. Biol. Phys. 2013, 86, 324–329. [Google Scholar] [CrossRef]
- Quivrin, M.; Peignaux-Casasnovas, K.; Martin; Rouffiac, M.; Thibouw, D.; Chevalier, C.; Vulquin, N.; Aubignac, L.; Truc, G.; Créhange, G. Salvage Brachytherapy as a Modern Reirradiation Technique for Local Cancer Failure: The Phoenix Is Reborn from Its Ashes. Cancer Radiother. 2018, 22, 372–381. [Google Scholar] [CrossRef]
- Rodin, J.; Bar-Ad, V.; Cognetti, D.; Curry, J.; Johnson, J.; Zender, C.; Doyle, L.; Kutler, D.; Leiby, B.; Keane, W.; et al. A Systematic Review of Treating Recurrent Head and Neck Cancer: A Reintroduction of Brachytherapy with or without Surgery. J. Contemp. Brachytherapy 2018, 10, 454–462. [Google Scholar] [CrossRef]
- Strnad, V.; Lotter, M.; Kreppner, S.; Fietkau, R. Reirradiation for Recurrent Head and Neck Cancer with Salvage Interstitial Pulsed-Dose-Rate Brachytherapy: Long-Term Results. Strahlenther. Onkol. 2015, 191, 495–500. [Google Scholar] [CrossRef] [PubMed]
- Puthawala, A.; Nisar Syed, A.M.; Gamie, S.; Chen, Y.J.; Londrc, A.; Nixon, V. Interstitial Low-Dose-Rate Brachytherapy as a Salvage Treatment for Recurrent Head-and-Neck Cancers: Long-Term Results. Int. J. Radiat. Oncol. Biol. Phys. 2001, 51, 354–362. [Google Scholar] [CrossRef] [PubMed]
- Hepel, J.T.; Syed, A.M.N.; Puthawala, A.; Sharma, A.; Frankel, P. Salvage High-Dose-Rate (HDR) Brachytherapy for Recurrent Head-and-Neck Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2005, 62, 1444–1450. [Google Scholar] [CrossRef] [PubMed]
- Glatzel, M.; Büntzel, J.; Schröder, D.; Küttner, K.; Fröhlich, D. High-Dose-Rate Brachytherapy in the Treatment of Recurrent and Residual Head and Neck Cancer. Laryngoscope 2002, 112, 1366–1371. [Google Scholar] [CrossRef]
- Meng, N.; Jiang, Y.L.; Wang, J.J.; Ran, W.Q.; Yuan, H.S.; Qu, A.; Jiang, P.; Yang, R.J. Permanent Implantation of Iodine-125 Seeds as a Salvage Therapy for Recurrent Head and Neck Carcinoma after Radiotherapy. Cancer Investig. 2012, 30, 236–242. [Google Scholar] [CrossRef]
- Narayana, A.; Cohen, G.N.; Zaider, M.; Chan, K.; Lee, N.; Wong, R.J.; Boyle, J.; Shaha, A.; Kraus, D.; Shah, J.; et al. High-Dose-Rate Interstitial Brachytherapy in Recurrent and Previously Irradiated Head and Neck Cancers—Preliminary Results. Brachytherapy 2007, 6, 157–163. [Google Scholar] [CrossRef]
- Rudzianskas, V.; Inciura, A.; Juozaityte, E.; Rudzianskiene, M.; Kubilius, R.; Vaitkus, S.; Kaseta, M.; Adliene, D. Reirradiation of Recurrent Head and Neck Cancer High-Dose-Rate Brachytherapy. Acta Otorhinolaryngol. Ital. 2012, 32, 297. [Google Scholar]
- Tagliaferri, L.; Bussu, F.; Fionda, B.; Catucci, F.; Rigante, M.; Gambacorta, M.A.; Autorino, R.; Mattiucci, G.C.; Miccichè, F.; Placidi, E.; et al. Perioperative HDR Brachytherapy for Reirradiation in Head and Neck Recurrences: Single-Institution Experience and Systematic Review. Tumori 2017, 103, 516–524. [Google Scholar] [CrossRef]
- Martínez-Fernández, M.I.; Alcalde, J.; Cambeiro, M.; Peydró, G.V.; Martínez-Monge, R. Perioperative High Dose Rate Brachytherapy (PHDRB) in Previously Irradiated Head and Neck Cancer: Results of a Phase I/II Reirradiation Study. Radiother. Oncol. 2017, 122, 255–259. [Google Scholar] [CrossRef]
- Kasperts, N.; Slotman, B.; Leemans, C.R.; Langendijk, J.A. A Review on Re-Irradiation for Recurrent and Second Primary Head and Neck Cancer. Oral. Oncol. 2005, 41, 225–243. [Google Scholar] [CrossRef]
- Ritter, M.; Teudt, I.U.; Meyer, J.E.; Schröder, U.; Kovács, G.; Wollenberg, B. Second-Line Treatment of Recurrent HNSCC: Tumor Debulking in Combination with High-Dose-Rate Brachytherapy and a Simultaneous Cetuximab-Paclitaxel Protocol. Radiat. Oncol. 2016, 11, 6. [Google Scholar] [CrossRef] [PubMed]
- Schiefke, F.; Hildebrandt, G.; Pohlmann, S.; Heinicke, F.; Hemprich, A.; Frerich, B. Combination of Surgical Resection and HDR-Brachytherapy in Patients with Recurrent or Advanced Head and Neck Carcinomas. J. Craniomaxillofac. Surg. 2008, 36, 285–292. [Google Scholar] [CrossRef] [PubMed]
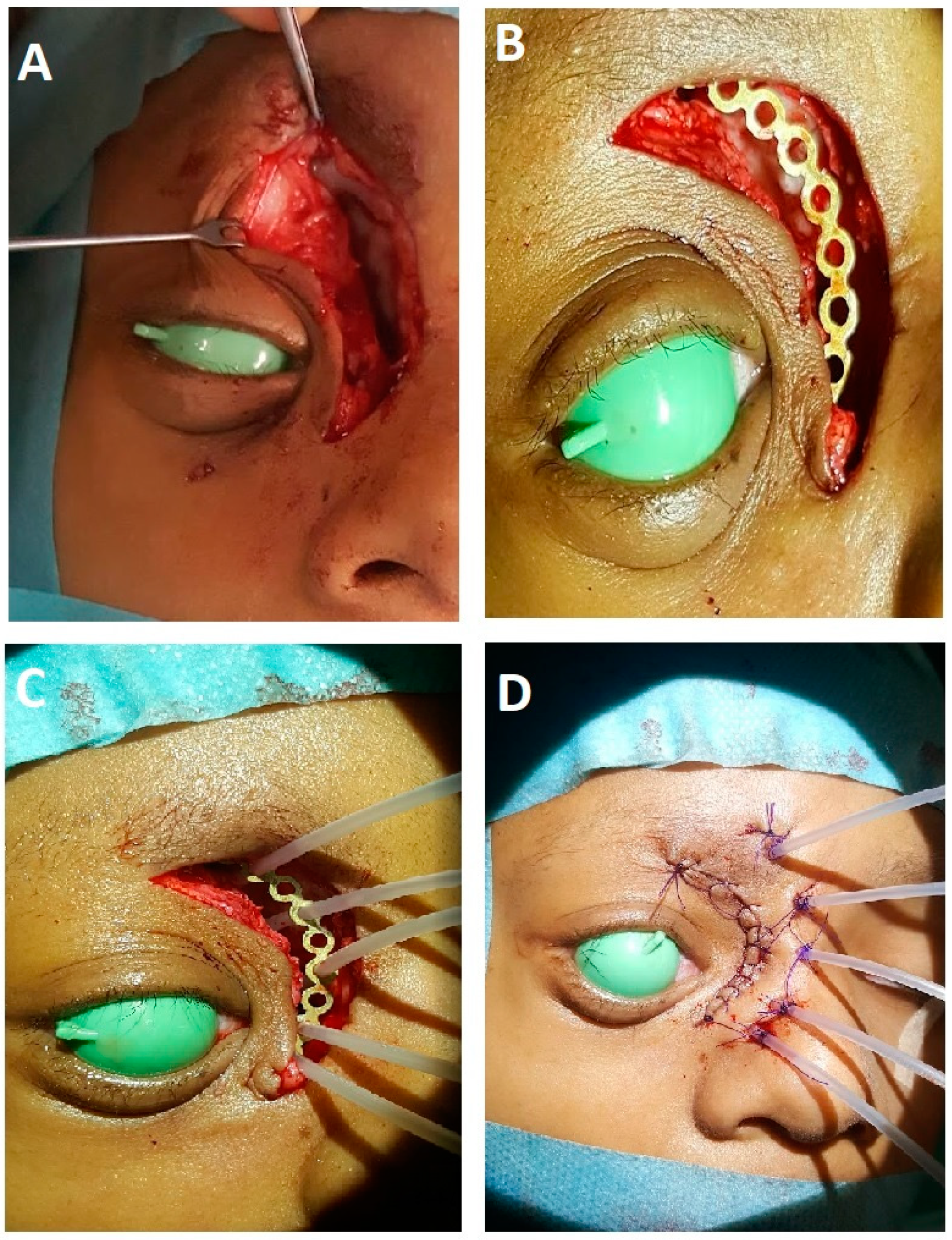
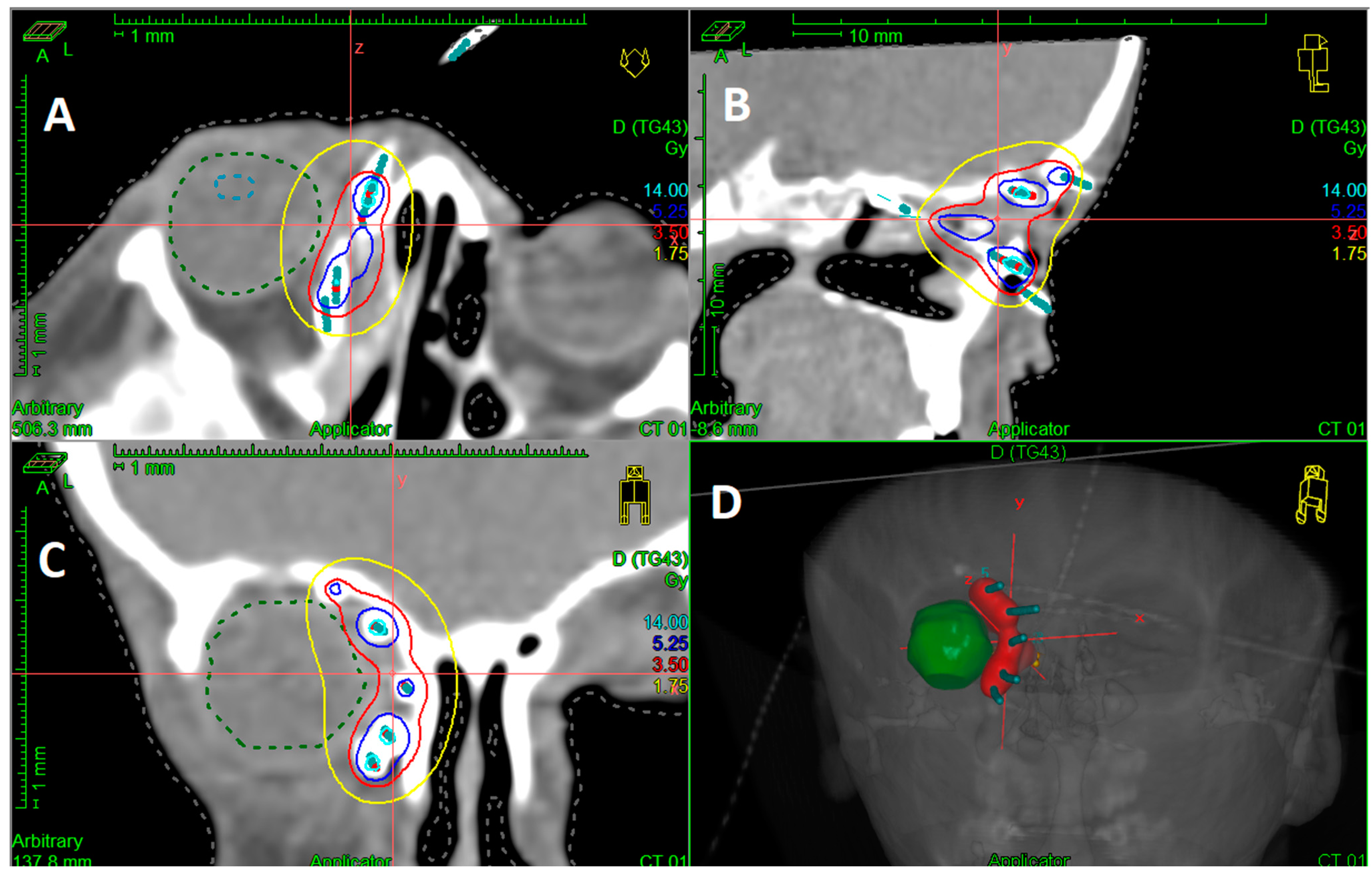
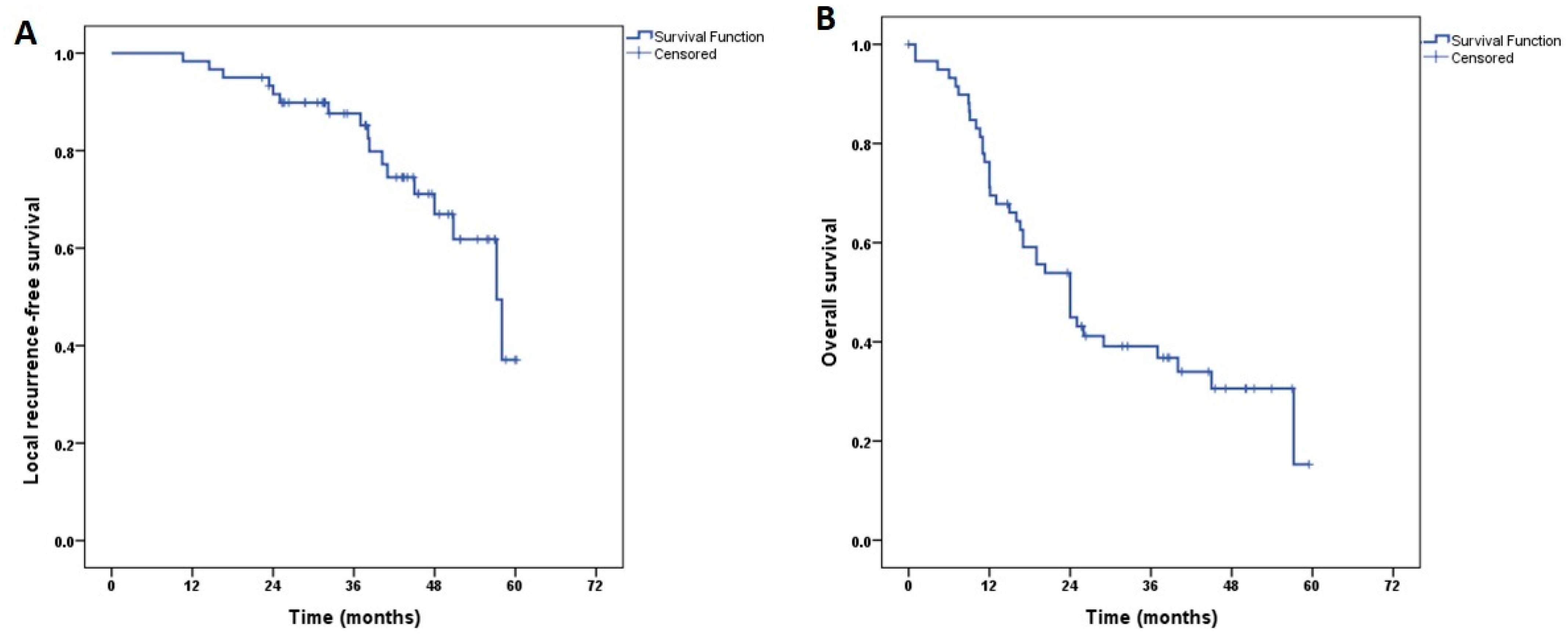
| Characteristics | Value (Range; %) |
|---|---|
| Median age at salvage treatment (range) | 65.6 years (15.4–92.7) |
| Gender | |
| Males | 46 (76.7%) |
| Females | 14 (23.3%) |
| Primary site | |
| Oral cavity | 14 (23.3%) |
| Oropharynx | 15 (25%) |
| Hypopharynx | 3 (5%) |
| Sinonasal/Orbit | 8 (13.3%) |
| Nasopharynx | 2 (3.3%) |
| Lymph nodes | 14 (23.3%) |
| Ear | 2 (3.3%) |
| Recurrence Site | |
| Local recurrence | 41 (68.3%) |
| Lymph nodes | 14(23.3%) |
| Secondary site | 5 (8.3%) |
| Initial T stage | |
| T0 | 9 (15%) |
| T1 | 12 (20%) |
| T2 | 12 (20%) |
| T3 | 11 (18.3%) |
| T4 | 14 (23.3%) |
| Tx | 2 (3.3%) |
| Initial lymph-node status | |
| Positive | 30 (50%) |
| Negative | 30 (50%) |
| Prior neck dissection | |
| Yes | 20 (33.3%) |
| No | 40 (66.7%) |
| Prior Chemotherapy | |
| Yes | 27 (45%) |
| No | 33 (55%) |
| Prior EBRT | |
| Yes | 42 (70%) |
| No | 18 (30%) |
| Median dose of prior EBRT * (range) | 60 Gy (32–70) |
| Salvage surgery | |
| Radical excision | 51 (85%) |
| Organ-preservation | 9 (15%) |
| Pathology | |
| Squamous cell carcinoma | 54 (90.0%) |
| Adenocarcinoma | 5 (8.3%) |
| Other | 1 (1.7%) |
| Grade | |
| Grade 1 | 2 (3.3%) |
| Grade 2 | 38 (63.3%) |
| Grade 3 | 18 (30%) |
| Unknown | 2 (3.3%) |
| LVSI */PNI * | |
| Yes | 44 (73.3%) |
| No | 16 (26.7%) |
| Margin status | |
| Negative | 19 (31.7%) |
| Close margin | 4 (5%) |
| Positive margin | 11 (18.3%) |
| Gross residual (debulking surgery) | 27 (45%) |
| Variable | Value (%; Range) |
|---|---|
| Techniuqe | |
| HDR-IRT * | 53 (88.3%) |
| HDR-IRT + EBRT * | 7 (11.7%) |
| Median HDR-IRT dose | 30 Gy (12–40) |
| Median HDR-IRT dose/fraction | 3 Gy (2.5–5) |
| Median EBRT dose | 30 Gy (30–50) |
| Acute Toxicities | Late Toxicities | ||
|---|---|---|---|
| No. of Patients (%) | No. of Patients (%) | ||
| Pain | Pain | ||
| Grade 1–2 | 15 (25%) | Grade 1–2 | 11 (18.3%) |
| Grade 3–4 | 10 (16.7%) | Grade 3–4 | 5 (8.3%) |
| Mucositis | Mucositis | ||
| Grade 1–2 | 13 (21.7%) | Grade 1–2 | 6 (10%) |
| Grade 3–4 | 1 (1.7%) | Grade 3–4 | 3 (5%) |
| Dysphagia | Dysphagia | ||
| Grade 1–2 | 8 (13.3%) | Grade 1–2 | 10 (16.7%) |
| Grade 3–4 | 12 (20%) | Grade 3–4 | 6 (10%) |
| Dryness of mouth | Dryness of mouth | ||
| Grade 1–2 | 9 (15%) | Grade 1–2 | 19 (31.7%) |
| Grade 3–4 | 6 (10%) | Grade 3–4 | 8 (13.3%) |
| Loss of smell | Loss of smell | ||
| Grade 1–2 | 2 (3.3%) | Grade 1–2 | 2 (3.3%) |
| Grade 3–4 | 2 (3.3%) | Grade 3–4 | 3 (5%) |
| Loss of taste | Loss of taste | ||
| Grade 1–2 | 5 (8.3%) | Grade 1–2 | 9 (15%) |
| Grade 3–4 | 1 (1.7%) | Grade 3–4 | 2 (3.3%) |
| Bleeding | 2 (3.3%) | Osteonecrosis | 1 (1.7%) |
| Respiratory infection | 2 (3.3%) | Soft tissue necrosis | 1 (1.7%) |
| Local infection | 2 (3.3%) | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soror, T.; Paul, J.; Melchert, C.; Idel, C.; Rades, D.; Bruchhage, K.-L.; Kovács, G.; Leichtle, A. Salvage High-Dose-Rate Interventional Radiotherapy (Brachytherapy) Combined with Surgery for Regionally Relapsed Head and Neck Cancers. Cancers 2023, 15, 4549. https://doi.org/10.3390/cancers15184549
Soror T, Paul J, Melchert C, Idel C, Rades D, Bruchhage K-L, Kovács G, Leichtle A. Salvage High-Dose-Rate Interventional Radiotherapy (Brachytherapy) Combined with Surgery for Regionally Relapsed Head and Neck Cancers. Cancers. 2023; 15(18):4549. https://doi.org/10.3390/cancers15184549
Chicago/Turabian StyleSoror, Tamer, Justina Paul, Corinna Melchert, Christian Idel, Dirk Rades, Karl-Ludwig Bruchhage, György Kovács, and Anke Leichtle. 2023. "Salvage High-Dose-Rate Interventional Radiotherapy (Brachytherapy) Combined with Surgery for Regionally Relapsed Head and Neck Cancers" Cancers 15, no. 18: 4549. https://doi.org/10.3390/cancers15184549
APA StyleSoror, T., Paul, J., Melchert, C., Idel, C., Rades, D., Bruchhage, K.-L., Kovács, G., & Leichtle, A. (2023). Salvage High-Dose-Rate Interventional Radiotherapy (Brachytherapy) Combined with Surgery for Regionally Relapsed Head and Neck Cancers. Cancers, 15(18), 4549. https://doi.org/10.3390/cancers15184549







