Antitumor Activity of Metformin Combined with Locoregional Therapy for Liver Cancer: Evidence and Future Directions
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Literature Search and Strategy
2.2. Eligibility and Search Criteria
2.3. Data Extraction
2.4. Statistical Analysis
3. Results
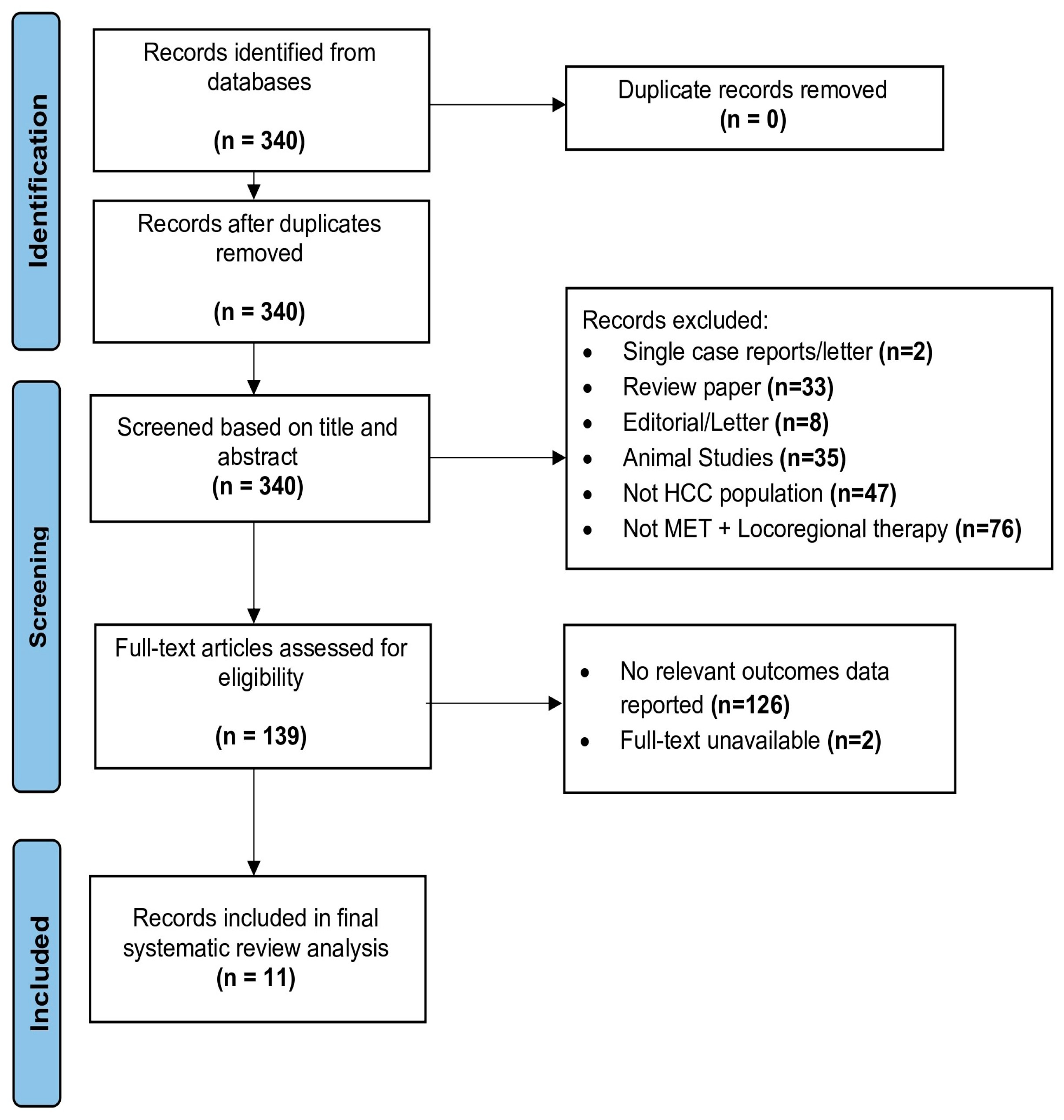
3.1. Study Selection
3.2. Study and Patient Characteristics
3.3. Effect of Metformin and Locoregional Therapy on Survival in Patients
3.4. Tumor Recurrence
3.5. Tumor Growth and Size
3.6. Tumor Proliferation, Migration, and Invasion
3.7. Tumor Apoptosis, DNA Damage, and Cell Cycle Arrest
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Yang, J.D.; Hainaut, P.; Gores, G.J.; Amadou, A.; Plymoth, A.; Roberts, L.R. A global view of hepatocellular carcinoma: Trends, risk, prevention and management. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 589–604. [Google Scholar] [CrossRef]
- Rich, N.E.; Yopp, A.C.; Singal, A.G.; Murphy, C.C. Hepatocellular Carcinoma Incidence Is Decreasing Among Younger Adults in the United States. Clin. Gastroenterol. Hepatol. 2020, 18, 242–248.e245. [Google Scholar] [CrossRef]
- Petrick, J.L.; Kelly, S.P.; Altekruse, S.F.; McGlynn, K.A.; Rosenberg, P.S. Future of Hepatocellular Carcinoma Incidence in the United States Forecast Through 2030. J. Clin. Oncol. 2016, 34, 1787–1794. [Google Scholar] [CrossRef]
- Rawla, P.; Sunkara, T.; Muralidharan, P.; Raj, J.P. Update in global trends and aetiology of hepatocellular carcinoma. Współczesna Onkol. 2018, 22, 141–150. [Google Scholar] [CrossRef]
- Mittal, S.; Sada, Y.H.; El-Serag, H.B.; Kanwal, F.; Duan, Z.; Temple, S.; May, S.B.; Kramer, J.R.; Richardson, P.A.; Davila, J.A. Temporal trends of nonalcoholic fatty liver disease-related hepatocellular carcinoma in the veteran affairs population. Clin. Gastroenterol. Hepatol. 2015, 13, 594–601.e591. [Google Scholar] [CrossRef]
- Kallwitz, E.R.; Daviglus, M.L.; Allison, M.A.; Emory, K.T.; Zhao, L.; Kuniholm, M.H.; Chen, J.; Gouskova, N.; Pirzada, A.; Talavera, G.A.; et al. Prevalence of suspected nonalcoholic fatty liver disease in Hispanic/Latino individuals differs by heritage. Clin. Gastroenterol. Hepatol. 2015, 13, 569–576. [Google Scholar] [CrossRef]
- Chang, K.C.; Wu, Y.Y.; Hung, C.H.; Lu, S.N.; Lee, C.M.; Chiu, K.W.; Tsai, M.C.; Tseng, P.L.; Huang, C.M.; Cho, C.L.; et al. Clinical-guide risk prediction of hepatocellular carcinoma development in chronic hepatitis C patients after interferon-based therapy. Br. J. Cancer 2013, 109, 2481–2488. [Google Scholar] [CrossRef]
- Meredith, S.; Shiels, T.R.O.B. Recent Decline in Hepatocellular Carcinoma Rates in the United States. Gastroenterology 2020, 158, 1503–1505.e2. [Google Scholar]
- Medavaram, S.; Zhang, Y. Emerging therapies in advanced hepatocellular carcinoma. Exp. Hematol. Oncol. 2018, 7, 17. [Google Scholar] [CrossRef]
- McGlynn, K.A.; Petrick, J.L.; London, W.T. Global Epidemiology of Hepatocellular Carcinoma. Clin. Liver Dis. 2015, 19, 223–238. [Google Scholar] [CrossRef]
- Mittal, S.; El-Serag, H.B. Epidemiology of Hepatocellular Carcinoma. J. Clin. Gastroenterol. 2013, 47, S2–S6. [Google Scholar] [CrossRef]
- Raza, A. Hepatocellular carcinoma review: Current treatment, and evidence-based medicine. World J. Gastroenterol. 2014, 20, 4115. [Google Scholar] [CrossRef]
- Lewandowski, R.J.; Kulik, L.M.; Riaz, A.; Senthilnathan, S.; Mulcahy, M.F.; Ryu, R.K.; Ibrahim, S.M.; Sato, K.T.; Baker, T.; Miller, F.H.; et al. A Comparative Analysis of Transarterial Downstaging for Hepatocellular Carcinoma: Chemoembolization Versus Radioembolization. Am. J. Transpl. 2009, 9, 1920–1928. [Google Scholar] [CrossRef]
- Dimitroulis, D.; Damaskos, C.; Valsami, S.; Davakis, S.; Garmpis, N.; Spartalis, E.; Athanasiou, A.; Moris, D.; Sakellariou, S.; Kykalos, S.; et al. From diagnosis to treatment of hepatocellular carcinoma: An epidemic problem for both developed and developing world. World J. Gastroenterol. 2017, 23, 5282. [Google Scholar] [CrossRef]
- Daher, S.; Massarwa, M.; Benson, A.A.; Khoury, T. Current and Future Treatment of Hepatocellular Carcinoma: An Updated Comprehensive Review. J. Clin. Transl. Hepatol. 2018, 6, 69–78. [Google Scholar] [CrossRef]
- Lee, M.W.; Lim, H.K. Management of sub-centimeter recurrent hepatocellular carcinoma after curative treatment: Current status and future. World J. Gastroenterol. 2018, 24, 5215–5222. [Google Scholar] [CrossRef]
- Proneth, A.; Zeman, F.; Schlitt, H.J.; Schnitzbauer, A.A. Is resection or transplantation the ideal treatment in patients with hepatocellular carcinoma in cirrhosis if both are possible? A systematic review and metaanalysis. Ann. Surg. Oncol. 2014, 21, 3096–3107. [Google Scholar] [CrossRef]
- Schwartz, M. Liver transplantation in patients with hepatocellular carcinoma. Liver Transpl. 2004, 10, S81–S85. [Google Scholar] [CrossRef]
- Mazzaferro, V.; Bhoori, S.; Sposito, C.; Bongini, M.; Langer, M.; Miceli, R.; Mariani, L. Milan criteria in liver transplantation for hepatocellular carcinoma: An evidence-based analysis of 15 years of experience. Liver Transpl. 2011, 17 (Suppl. 2), S44–S57. [Google Scholar] [CrossRef]
- Higgins, H.; Berger, D.L. RFA for liver tumors: Does it really work? Oncologist 2006, 11, 801–808. [Google Scholar] [CrossRef]
- Salem, R.; Lewandowski, R.J.; Mulcahy, M.F.; Riaz, A.; Ryu, R.K.; Ibrahim, S.; Atassi, B.; Baker, T.; Gates, V.; Miller, F.H.; et al. Radioembolization for hepatocellular carcinoma using Yttrium-90 microspheres: A comprehensive report of long-term outcomes. Gastroenterology 2010, 138, 52–64. [Google Scholar] [CrossRef]
- Tohme, S.; Sukato, D.; Chen, H.W.; Amesur, N.; Zajko, A.B.; Humar, A.; Geller, D.A.; Marsh, J.W.; Tsung, A. Yttrium-90 radioembolization as a bridge to liver transplantation: A single-institution experience. J. Vasc. Interv. Radiol. 2013, 24, 1632–1638. [Google Scholar] [CrossRef]
- Kallini, J.R.; Gabr, A.; Salem, R.; Lewandowski, R.J. Transarterial Radioembolization with Yttrium-90 for the Treatment of Hepatocellular Carcinoma. Adv. Ther. 2016, 33, 699–714. [Google Scholar] [CrossRef]
- Mohamed, M.; Katz, A.W.; Tejani, M.A.; Sharma, A.K.; Kashyap, R.; Noel, M.S.; Qiu, H.; Hezel, A.F.; Ramaraju, G.A.; Dokus, M.K.; et al. Comparison of outcomes between SBRT, yttrium-90 radioembolization, transarterial chemoembolization, and radiofrequency ablation as bridge to transplant for hepatocellular carcinoma. Adv. Radiat. Oncol 2016, 1, 35–42. [Google Scholar] [CrossRef]
- Fidelman, N.; Kerlan, R.K. Transarterial Chemoembolization and90Y Radioembolization for Hepatocellular Carcinoma: Review of Current Applications Beyond Intermediate-Stage Disease. Am. J. Roentgenol. 2015, 205, 742–752. [Google Scholar] [CrossRef]
- Lee, J.J.X.; Choo, S.P. Locoregional therapy in hepatocullar carcinoma: When to start and when to stop and when to revisit. ESMO Open 2021, 6, 100129. [Google Scholar] [CrossRef]
- Ren, N.; Qin, S.; Ding, L.; Jia, E.; Xue, J. Comparison of Transarterial Y90 Radioembolization and Conventional Transarterial Chemoembolization in Hepatocarcinoma Patients: A Meta-analysis. Indian J. Pharm. Sci. 2020, 82, 76–81. [Google Scholar] [CrossRef]
- Yang, Y.; Si, T. Yttrium-90 transarterial radioembolization versus conventional transarterial chemoembolization for patients with hepatocellular carcinoma: A systematic review and meta-analysis. Cancer Biol. Med. 2018, 15, 299–310. [Google Scholar] [CrossRef]
- Vouche, M.; Habib, A.; Ward, T.J.; Kim, E.; Kulik, L.; Ganger, D.; Mulcahy, M.; Baker, T.; Abecassis, M.; Sato, K.T.; et al. Unresectable solitary hepatocellular carcinoma not amenable to radiofrequency ablation: Multicenter radiology-pathology correlation and survival of radiation segmentectomy. Hepatology 2014, 60, 192–201. [Google Scholar] [CrossRef]
- Kis, B.; El-Haddad, G.; Sheth, R.A.; Parikh, N.S.; Ganguli, S.; Shyn, P.B.; Choi, J.; Brown, K.T. Liver-Directed Therapies for Hepatocellular Carcinoma and Intrahepatic Cholangiocarcinoma. Cancer Control 2017, 24, 1073274817729244. [Google Scholar] [CrossRef]
- Viveiros, P.; Riaz, A.; Lewandowski, R.J.; Mahalingam, D. Current State of Liver-Directed Therapies and Combinatory Approaches with Systemic Therapy in Hepatocellular Carcinoma (HCC). Cancers 2019, 11, 1085. [Google Scholar] [CrossRef]
- Bahrambeigi, S.; Shafiei-Irannejad, V. Immune-mediated anti-tumor effects of metformin; targeting metabolic reprogramming of T cells as a new possible mechanism for anti-cancer effects of metformin. Biochem. Pharmacol. 2020, 174, 113787. [Google Scholar] [CrossRef]
- Guo, J.; Li, L.; Guo, B.; Liu, D.; Shi, J.; Wu, C.; Chen, J.; Zhang, X.; Wu, J. Mechanisms of resistance to chemotherapy and radiotherapy in hepatocellular carcinoma. Transl. Cancer Res. 2018, 7, 765–781. [Google Scholar] [CrossRef]
- Mukherjee, K.; Elsayed, M.; Choksi, E.; Loya, M.F.; Duszak, R.; Akce, M.; Majdalany, B.S.; Bercu, Z.L.; Cristescu, M.; Kokabi, N. Use of Metformin and Survival in Patients with Hepatocellular Carcinoma (HCC) Undergoing Liver Directed Therapy: Analysis of a Nationwide Cancer Registry. Cardiovasc. Interv. Radiol. 2023, 46, 870–879. [Google Scholar] [CrossRef]
- Facciorusso, A.; Del Prete, V.; Crucinio, N.; Muscatiello, N.; Carr, B.I.; Di Leo, A.; Barone, M. Angiotensin receptor blockers improve survival outcomes after radiofrequency ablation in hepatocarcinoma patients. J. Gastroenterol. Hepatol. 2015, 30, 1643–1650. [Google Scholar] [CrossRef]
- Yoshiji, H.; Kuriyama, S.; Fukui, H. Blockade of renin-angiotensin system in antifibrotic therapy. J. Gastroenterol. Hepatol. 2007, 22 (Suppl. 1), S93–S95. [Google Scholar] [CrossRef]
- Yoshiji, H.; Noguchi, R.; Toyohara, M.; Ikenaka, Y.; Kitade, M.; Kaji, K.; Yamazaki, M.; Yamao, J.; Mitoro, A.; Sawai, M.; et al. Combination of vitamin K2 and angiotensin-converting enzyme inhibitor ameliorates cumulative recurrence of hepatocellular carcinoma. J. Hepatol. 2009, 51, 315–321. [Google Scholar] [CrossRef]
- Yoshiji, H.; Noguchi, R.; Ikenaka, Y.; Kaji, K.; Aihara, Y.; Yamazaki, M.; Yamao, J.; Toyohara, M.; Mitoro, A.; Sawai, M.; et al. Combination of branched-chain amino acids and angiotensin-converting enzyme inhibitor suppresses the cumulative recurrence of hepatocellular carcinoma: A randomized control trial. Oncol. Rep. 2011, 26, 1547–1553. [Google Scholar] [CrossRef]
- Cabibbo, G.; Celsa, C.; Calvaruso, V.; Petta, S.; Cacciola, I.; Cannavò, M.R.; Madonia, S.; Rossi, M.; Magro, B.; Rini, F.; et al. Direct-acting antivirals after successful treatment of early hepatocellular carcinoma improve survival in HCV-cirrhotic patients. J. Hepatol. 2019, 71, 265–273. [Google Scholar] [CrossRef]
- Huang, G.; Lau, W.Y.; Wang, Z.G.; Pan, Z.Y.; Yuan, S.X.; Shen, F.; Zhou, W.P.; Wu, M.C. Antiviral therapy improves postoperative survival in patients with hepatocellular carcinoma: A randomized controlled trial. Ann. Surg. 2015, 261, 56–66. [Google Scholar] [CrossRef]
- Okamura, Y.; Sugiura, T.; Ito, T.; Yamamoto, Y.; Ashida, R.; Ohgi, K.; Uesaka, K. The Achievement of a Sustained Virological Response Either Before or After Hepatectomy Improves the Prognosis of Patients with Primary Hepatitis C Virus-Related Hepatocellular Carcinoma. Ann. Surg. Oncol. 2019, 26, 4566–4575. [Google Scholar] [CrossRef]
- Singal, A.G.; Rich, N.E.; Mehta, N.; Branch, A.D.; Pillai, A.; Hoteit, M.; Volk, M.; Odewole, M.; Scaglione, S.; Guy, J.; et al. Direct-Acting Antiviral Therapy for Hepatitis C Virus Infection Is Associated With Increased Survival in Patients With a History of Hepatocellular Carcinoma. Gastroenterology 2019, 157, 1253–1263.e1252. [Google Scholar] [CrossRef]
- Singal, A.K.; Salameh, H.; Kuo, Y.F.; Fontana, R.J. Meta-analysis: The impact of oral anti-viral agents on the incidence of hepatocellular carcinoma in chronic hepatitis B. Aliment. Pharmacol. Ther. 2013, 38, 98–106. [Google Scholar] [CrossRef]
- Wang, C.; Wang, X.; Su, Z.; Fei, H.; Liu, X.; Pan, Q. The novel mTOR inhibitor Torin-2 induces autophagy and downregulates the expression of UHRF1 to suppress hepatocarcinoma cell growth. Oncol. Rep. 2015, 34, 1708–1716. [Google Scholar] [CrossRef]
- Yin, J.; Li, N.; Han, Y.; Xue, J.; Deng, Y.; Shi, J.; Guo, W.; Zhang, H.; Wang, H.; Cheng, S.; et al. Effect of Antiviral Treatment With Nucleotide/Nucleoside Analogs on Postoperative Prognosis of Hepatitis B Virus–Related Hepatocellular Carcinoma: A Two-Stage Longitudinal Clinical Study. J. Clin. Oncol. 2013, 31, 3647–3655. [Google Scholar] [CrossRef]
- Yuan, P.; Chen, P.; Qian, Y. Evaluation of Antiviral Therapy Performed after Curative Therapy in Patients with HBV-Related Hepatocellular Carcinoma: An Updated Meta-Analysis. Can. J. Gastroenterol. Hepatol. 2016, 2016, 5234969. [Google Scholar] [CrossRef][Green Version]
- Wang, G.; Duan, Z. Guidelines for Prevention and Treatment of Chronic Hepatitis B. J. Clin. Transl. Hepatol. 2021, 9, 769–791. [Google Scholar] [CrossRef]
- Zhong, J.H.; Mo, X.S.; Xiang, B.D.; Yuan, W.P.; Jiang, J.F.; Xie, G.S.; Li, L.Q. Postoperative use of the chemopreventive vitamin K2 analog in patients with hepatocellular carcinoma. PLoS ONE 2013, 8, e58082. [Google Scholar] [CrossRef]
- Hitomi, M.; Nonomura, T.; Yokoyama, F.; Yoshiji, H.; Ogawa, M.; Nakai, S.; Deguchi, A.; Masaki, T.; Inoue, H.; Kimura, Y.; et al. In vitro and in vivo antitumor effects of vitamin K5 on hepatocellular carcinoma. Int. J. Oncol. 2005, 26, 1337–1344. [Google Scholar] [CrossRef]
- Hitomi, M.; Yokoyama, F.; Kita, Y.; Nonomura, T.; Masaki, T.; Yoshiji, H.; Inoue, H.; Kinekawa, F.; Kurokohchi, K.; Uchida, N.; et al. Antitumor effects of vitamins K1, K2 and K3 on hepatocellular carcinoma in vitro and in vivo. Int. J. Oncol. 2005, 26, 713–720. [Google Scholar] [CrossRef]
- Hotta, N.; Ayada, M.; Sato, K.; Ishikawa, T.; Okumura, A.; Matsumoto, E.; Ohashi, T.; Kakumu, S. Effect of vitamin K2 on the recurrence in patients with hepatocellular carcinoma. Hepatogastroenterology 2007, 54, 2073–2077. [Google Scholar]
- Kakizaki, S.; Sohara, N.; Sato, K.; Suzuki, H.; Yanagisawa, M.; Nakajima, H.; Takagi, H.; Naganuma, A.; Otsuka, T.; Takahashi, H.; et al. Preventive effects of vitamin K on recurrent disease in patients with hepatocellular carcinoma arising from hepatitis C viral infection. J. Gastroenterol. Hepatol. 2007, 22, 518–522. [Google Scholar] [CrossRef]
- Mizuta, T.; Ozaki, I.; Eguchi, Y.; Yasutake, T.; Kawazoe, S.; Fujimoto, K.; Yamamoto, K. The effect of menatetrenone, a vitamin K2 analog, on disease recurrence and survival in patients with hepatocellular carcinoma after curative treatment: A pilot study. Cancer 2006, 106, 867–872. [Google Scholar] [CrossRef]
- Yoshida, H.; Shiratori, Y.; Kudo, M.; Shiina, S.; Mizuta, T.; Kojiro, M.; Yamamoto, K.; Koike, Y.; Saito, K.; Koyanagi, N.; et al. Effect of vitamin K2 on the recurrence of hepatocellular carcinoma. Hepatology 2011, 54, 532–540. [Google Scholar] [CrossRef]
- Xue, L.; Chen, F.; Yue, F.; Camacho, L.; Kothapalli, S.; Wei, G.; Huang, S.; Mo, Q.; Ma, F.; Li, Y.; et al. Metformin and an insulin/IGF-1 receptor inhibitor are synergistic in blocking growth of triple-negative breast cancer. Breast Cancer Res. Treat. 2021, 185, 73–84. [Google Scholar] [CrossRef]
- Wang, Y.; An, H.; Liu, T.; Qin, C.; Sesaki, H.; Guo, S.; Radovick, S.; Hussain, M.; Maheshwari, A.; Wondisford, F.E.; et al. Metformin Improves Mitochondrial Respiratory Activity through Activation of AMPK. Cell Rep. 2019, 29, 1511–1523.e1515. [Google Scholar] [CrossRef]
- Leprivier, G.; Rotblat, B. How does mTOR sense glucose starvation? AMPK is the usual suspect. Cell Death Discov. 2020, 6, 27. [Google Scholar] [CrossRef]
- Li, L.; Wang, T.; Hu, M.; Zhang, Y.; Chen, H.; Xu, L. Metformin Overcomes Acquired Resistance to EGFR TKIs in EGFR-Mutant Lung Cancer via AMPK/ERK/NF-κB Signaling Pathway. Front. Oncol. 2020, 10, 1605. [Google Scholar] [CrossRef]
- Lv, Z.; Guo, Y. Metformin and Its Benefits for Various Diseases. Front. Endocrinol. 2020, 11, 191. [Google Scholar] [CrossRef]
- Zhou, Z.; Liu, Y.; Song, W.; Jiang, X.; Deng, Z.; Xiong, W.; Shen, J. Metabolic reprogramming mediated PD-L1 depression and hypoxia reversion to reactivate tumor therapy. J. Control. Release 2022, 352, 793–812. [Google Scholar] [CrossRef]
- Evans, J.M.M.; Donnelly, L.A.; Emslie-Smith, A.M.; Alessi, D.R.; Morris, A.D. Metformin and reduced risk of cancer in diabetic patients. BMJ 2005, 330, 1304–1305. [Google Scholar] [CrossRef]
- Bowker, S.L.; Majumdar, S.R.; Veugelers, P.; Johnson, J.A. Increased Cancer-Related Mortality for Patients With Type 2 Diabetes Who Use Sulfonylureas or Insulin. Diabetes Care 2006, 29, 254–258. [Google Scholar] [CrossRef]
- Donadon, V.; Balbi, M.; Ghersetti, M.; Grazioli, S.; Perciaccante, A.; Della Valentina, G.; Gardenal, R.; Dal Mas, M.; Casarin, P.; Zanette, G.; et al. Antidiabetic therapy and increased risk of hepatocellular carcinoma in chronic liver disease. World J. Gastroenterol. 2009, 15, 2506–2511. [Google Scholar] [CrossRef]
- Hassan, M.M.; Curley, S.A.; Li, D.; Kaseb, A.; Davila, M.; Abdalla, E.K.; Javle, M.; Moghazy, D.M.; Lozano, R.D.; Abbruzzese, J.L.; et al. Association of diabetes duration and diabetes treatment with the risk of hepatocellular carcinoma. Cancer 2010, 116, 1938–1946. [Google Scholar] [CrossRef]
- Landman, G.W.; van Hateren, K.J.; Kleefstra, N.; Groenier, K.H.; Gans, R.O.; Bilo, H.J. The relationship between glycaemic control and mortality in patients with type 2 diabetes in general practice (ZODIAC-11). Br. J. Gen. Pract. 2010, 60, 172–175. [Google Scholar] [CrossRef]
- Lee, M.S.; Hsu, C.C.; Wahlqvist, M.L.; Tsai, H.N.; Chang, Y.H.; Huang, Y.C. Type 2 diabetes increases and metformin reduces total, colorectal, liver and pancreatic cancer incidences in Taiwanese: A representative population prospective cohort study of 800,000 individuals. BMC Cancer 2011, 11, 20. [Google Scholar] [CrossRef]
- Lai, S.W.; Chen, P.C.; Liao, K.F.; Muo, C.H.; Lin, C.C.; Sung, F.C. Risk of hepatocellular carcinoma in diabetic patients and risk reduction associated with anti-diabetic therapy: A population-based cohort study. Am. J. Gastroenterol. 2012, 107, 46–52. [Google Scholar] [CrossRef]
- Deperalta, D.K.; Wei, L.; Ghoshal, S.; Schmidt, B.; Lauwers, G.Y.; Lanuti, M.; Chung, R.T.; Tanabe, K.K.; Fuchs, B.C. Metformin prevents hepatocellular carcinoma development by suppressing hepatic progenitor cell activation in a rat model of cirrhosis. Cancer 2016, 122, 1216–1227. [Google Scholar] [CrossRef]
- Kasmari, A.J.; Welch, A.; Liu, G.; Leslie, D.; McGarrity, T.; Riley, T. Independent of Cirrhosis, Hepatocellular Carcinoma Risk Is Increased with Diabetes and Metabolic Syndrome. Am. J. Med. 2017, 130, 746.e1–746.e7. [Google Scholar] [CrossRef]
- Yu, H.; Yin, L.; Jiang, X.; Sun, X.; Wu, J.; Tian, H.; Gao, X.; He, X. Effect of Metformin on Cancer Risk and Treatment Outcome of Prostate Cancer: A Meta-Analysis of Epidemiological Observational Studies. PLoS ONE 2014, 9, e116327. [Google Scholar] [CrossRef]
- Stopsack, K.H.; Ziehr, D.R.; Rider, J.R.; Giovannucci, E.L. Metformin and prostate cancer mortality: A meta-analysis. Cancer Causes Control 2016, 27, 105–113. [Google Scholar] [CrossRef]
- Nevadunsky, N.S.; Van Arsdale, A.; Strickler, H.D.; Moadel, A.; Kaur, G.; Frimer, M.; Conroy, E.; Goldberg, G.L.; Einstein, M.H. Metformin use and endometrial cancer survival. Gynecol. Oncol. 2014, 132, 236–240. [Google Scholar] [CrossRef]
- Tarhini, Z.; Manceur, K.; Magne, J.; Mathonnet, M.; Jost, J.; Christou, N. The effect of metformin on the survival of colorectal cancer patients with type 2 diabetes mellitus. Sci. Rep. 2022, 12, 12374. [Google Scholar] [CrossRef]
- Rêgo, D.F.; Pavan, L.M.; Elias, S.T.; De Luca Canto, G.; Guerra, E.N. Effects of metformin on head and neck cancer: A systematic review. Oral Oncol. 2015, 51, 416–422. [Google Scholar] [CrossRef]
- Shankaraiah, R.C.; Callegari, E.; Guerriero, P.; Rimessi, A.; Pinton, P.; Gramantieri, L.; Silini, E.M.; Sabbioni, S.; Negrini, M. Metformin prevents liver tumourigenesis by attenuating fibrosis in a transgenic mouse model of hepatocellular carcinoma. Oncogene 2019, 38, 7035–7045. [Google Scholar] [CrossRef]
- Cai, X.; Hu, X.; Cai, B.; Wang, Q.; Li, Y.; Tan, X.; Hu, H.; Chen, X.; Huang, J.; Cheng, J.; et al. Metformin suppresses hepatocellular carcinoma cell growth through induction of cell cycle G1/G0 phase arrest and p21CIP and p27KIP expression and downregulation of cyclin D1 in vitro and in vivo. Oncol. Rep. 2013, 30, 2449–2457. [Google Scholar] [CrossRef]
- Bhalla, K.; Hwang, B.J.; Dewi, R.E.; Twaddel, W.; Goloubeva, O.G.; Wong, K.-K.; Saxena, N.K.; Biswal, S.; Girnun, G.D. Metformin Prevents Liver Tumorigenesis by Inhibiting Pathways Driving Hepatic Lipogenesis. Cancer Prev. Res. 2012, 5, 544–552. [Google Scholar] [CrossRef]
- Guo, W.J.; Yu, E.X.; Liu, L.M.; Li, J.; Chen, Z.; Lin, J.H.; Meng, Z.Q.; Feng, Y. Comparison between chemoembolization combined with radiotherapy and chemoembolization alone for large hepatocellular carcinoma. World J. Gastroenterol. 2003, 9, 1697–1701. [Google Scholar] [CrossRef]
- Facciuto, M.E.; Singh, M.K.; Rochon, C.; Sharma, J.; Gimenez, C.; Katta, U.; Moorthy, C.R.; Bentley-Hibbert, S.; Rodriguez-Davalos, M.; Wolf, D.C. Stereotactic body radiation therapy in hepatocellular carcinoma and cirrhosis: Evaluation of radiological and pathological response. J. Surg. Oncol. 2012, 105, 692–698. [Google Scholar] [CrossRef]
- Hozo, S.P.; Djulbegovic, B.; Hozo, I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med. Res. Methodol. 2005, 5, 13. [Google Scholar] [CrossRef]
- Chen, T.M.; Lin, C.C.; Huang, P.T.; Wen, C.F. Metformin associated with lower mortality in diabetic patients with early stage hepatocellular carcinoma after radiofrequency ablation. J. Gastroenterol. Hepatol. 2011, 26, 858–865. [Google Scholar] [CrossRef]
- Chen, M.L.; Wu, C.X.; Zhang, J.B.; Zhang, H.; Sun, Y.D.; Tian, S.L.; Han, J.J. Transarterial chemoembolization combined with metformin improves the prognosis of hepatocellular carcinoma patients with type 2 diabetes. Front. Endocrinol. 2022, 13, 996228. [Google Scholar] [CrossRef]
- Elsayed, M.; Wagstaff, W.; Behbahani, K.; Villalobos, A.; Bercu, Z.; Majdalany, B.S.; Akce, M.; Schuster, D.M.; Mao, H.; Kokabi, N. Improved Tumor Response in Patients on Metformin Undergoing Yttrium-90 Radioembolization Segmentectomy for Hepatocellular Carcinoma. Cardiovasc. Interv. Radiol. 2021, 44, 1937–1944. [Google Scholar] [CrossRef]
- Jang, W.I.; Kim, M.S.; Lim, J.S.; Yoo, H.J.; Seo, Y.S.; Han, C.J.; Park, S.C.; Kay, C.S.; Kim, M.; Jang, H.S.; et al. Survival Advantage Associated with Metformin Usage in Hepatocellular Carcinoma Patients Receiving Radiotherapy: A Propensity Score Matching Analysis. Anticancer Res. 2015, 35, 5047–5054. [Google Scholar]
- Jung, W.J.; Jang, S.; Choi, W.J.; Park, J.; Choi, G.H.; Jang, E.S.; Jeong, S.H.; Choi, W.S.; Lee, J.H.; Yoon, C.J.; et al. Metformin administration is associated with enhanced response to transarterial chemoembolization for hepatocellular carcinoma in type 2 diabetes patients. Sci. Rep. 2022, 12, 14482. [Google Scholar] [CrossRef]
- Kim, E.H.; Kim, M.S.; Cho, C.K.; Jung, W.G.; Jeong, Y.K.; Jeong, J.H. Low and high linear energy transfer radiation sensitization of HCC cells by metformin. J. Radiat. Res. 2014, 55, 432–442. [Google Scholar] [CrossRef]
- Liu, J.; Hou, M.; Yuan, T.; Yi, G.; Zhang, S.; Shao, X.; Chen, J.; Jia, X.; He, Z. Enhanced cytotoxic effect of low doses of metformin combined with ionizing radiation on hepatoma cells via ATP deprivation and inhibition of DNA repair. Oncol. Rep. 2012, 28, 1406–1412. [Google Scholar] [CrossRef]
- Tian, Y.; Tang, B.; Wang, C.; Sun, D.; Zhang, R.; Luo, N.; Han, Z.; Liang, R.; Gao, Z.; Wang, L. Metformin mediates resensitivity to 5-fluorouracil in hepatocellular carcinoma via the suppression of YAP. Oncotarget 2016, 7, 46230–46241. [Google Scholar] [CrossRef]
- Zhang, Q.; Kong, J.; Dong, S.; Xu, W.; Sun, W. Metformin exhibits the anti-proliferation and anti-invasion effects in hepatocellular carcinoma cells after insufficient radiofrequency ablation. Cancer Cell Int. 2017, 17, 48. [Google Scholar] [CrossRef]
- Zhang, K.F.; Wang, J.; Guo, J.; Huang, Y.Y.; Huang, T.R. Metformin enhances radiosensitivity in hepatocellular carcinoma by inhibition of specificity protein 1 and epithelial-to-mesenchymal transition. J. Cancer Res. Ther. 2019, 15, 1603–1610. [Google Scholar] [CrossRef]
- Xin, H.W.; Ambe, C.M.; Miller, T.C.; Chen, J.Q.; Wiegand, G.W.; Anderson, A.J.; Ray, S.; Mullinax, J.E.; Hari, D.M.; Koizumi, T.; et al. Liver Label Retaining Cancer Cells Are Relatively Resistant to the Reported Anti-Cancer Stem Cell Drug Metformin. J. Cancer 2016, 7, 1142–1151. [Google Scholar] [CrossRef]
- Kim, H.S.; El-Serag, H.B. The Epidemiology of Hepatocellular Carcinoma in the USA. Curr. Gastroenterol. Rep. 2019, 21, 17. [Google Scholar] [CrossRef]
- Li, J.; Hernanda, P.Y.; Bramer, W.M.; Peppelenbosch, M.P.; van Luijk, J.; Pan, Q. Anti-tumor effects of metformin in animal models of hepatocellular carcinoma: A systematic review and meta-analysis. PLoS ONE 2015, 10, e0127967. [Google Scholar] [CrossRef]
- Coyle, C.; Cafferty, F.H.; Vale, C.; Langley, R.E. Metformin as an adjuvant treatment for cancer: A systematic review and meta-analysis. Ann. Oncol. 2016, 27, 2184–2195. [Google Scholar] [CrossRef]
- Ng, C.W.; Jiang, A.A.; Toh, E.M.S.; Ng, C.H.; Ong, Z.H.; Peng, S.; Tham, H.Y.; Sundar, R.; Chong, C.S.; Khoo, C.M. Metformin and colorectal cancer: A systematic review, meta-analysis and meta-regression. Int. J. Color. Dis. 2020, 35, 1501–1512. [Google Scholar] [CrossRef]
- Zhang, K.; Bai, P.; Dai, H.; Deng, Z. Metformin and risk of cancer among patients with type 2 diabetes mellitus: A systematic review and meta-analysis. Prim. Care Diabetes 2021, 15, 52–58. [Google Scholar] [CrossRef]
- Chen, Y.H.; Wang, P.H.; Chen, P.N.; Yang, S.F.; Hsiao, Y.H. Molecular and Cellular Mechanisms of Metformin in Cervical Cancer. Cancers 2021, 13, 2545. [Google Scholar] [CrossRef]
- Tan, X.; Li, Y.; Hou, Z.; Zhang, M.; Li, L.; Wei, J. Combination therapy with PD-1 inhibition plus rapamycin and metformin enhances anti-tumor efficacy in triple negative breast cancer. Exp. Cell Res. 2023, 429, 113647. [Google Scholar] [CrossRef]
- Zhou, Z.; Liu, Y.; Jiang, X.; Zheng, C.; Luo, W.; Xiang, X.; Qi, X.; Shen, J. Metformin modified chitosan as a multi-functional adjuvant to enhance cisplatin-based tumor chemotherapy efficacy. Int. J. Biol. Macromol. 2023, 224, 797–809. [Google Scholar] [CrossRef]
- Cheaib, B.; Auguste, A.; Leary, A. The PI3K/Akt/mTOR pathway in ovarian cancer: Therapeutic opportunities and challenges. Chin. J. Cancer 2015, 34, 4–16. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Marcus, A.I.; Vertino, P.M. Dysregulation of mTOR activity through LKB1 inactivation. Chin. J. Cancer 2013, 32, 427–433. [Google Scholar] [CrossRef]
- Karuman, P.; Gozani, O.; Odze, R.D.; Zhou, X.C.; Zhu, H.; Shaw, R.; Brien, T.P.; Bozzuto, C.D.; Ooi, D.; Cantley, L.C.; et al. The Peutz-Jegher gene product LKB1 is a mediator of p53-dependent cell death. Mol. Cell 2001, 7, 1307–1319. [Google Scholar] [CrossRef] [PubMed]
- Algire, C.; Amrein, L.; Zakikhani, M.; Panasci, L.; Pollak, M. Metformin blocks the stimulative effect of a high-energy diet on colon carcinoma growth in vivo and is associated with reduced expression of fatty acid synthase. Endocr. Relat. Cancer 2010, 17, 351–360. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, T.; Yamashita, T.; Okada, H.; Oishi, N.; Sunagozaka, H.; Nio, K.; Hayashi, T.; Hara, Y.; Asahina, Y.; Yoshida, M.; et al. A Novel mTOR Inhibitor; Anthracimycin for the Treatment of Human Hepatocellular Carcinoma. Anticancer Res. 2017, 37, 3397–3403. [Google Scholar] [CrossRef]
- Snima, K.S.; Jayakumar, R.; Unnikrishnan, A.G.; Nair, S.V.; Lakshmanan, V.K. O-carboxymethyl chitosan nanoparticles for metformin delivery to pancreatic cancer cells. Carbohydr. Polym. 2012, 89, 1003–1007. [Google Scholar] [CrossRef]
- Verdura, S.; Cuyàs, E.; Martin-Castillo, B.; Menendez, J.A. Metformin as an archetype immuno-metabolic adjuvant for cancer immunotherapy. OncoImmunology 2019, 8, e1633235. [Google Scholar] [CrossRef]
| Author, Year | Study Type | Location of Study | No. of Patients 1 | Sex, M:F Ratio | Mean Age (Years) | Type of HCC Intervention 2 | Control Group (# of Patients) 3 | Experimental Group (# of Patients) 3 |
|---|---|---|---|---|---|---|---|---|
| Chen 2011 [82] | Human clinical | Taiwan | 135 | 84:51 | 63.0 ± 9.7 | LDT | RFA (114) | RFA + MET (21) |
| Chen 2022 [83] | Human clinical | China | 123 | 108:15 | - | LDT | TACE (73) | TACE + MET (50) |
| Elsayed 2021 [84] | Human clinical | USA | 103 | 72:34 | 64.2 ± 8.9 | LDT | Y-90 RS (93) | Y-90 RS + MET (19) |
| Jang 2015 [85] | Human clinical | Korea | 217 | 57:160 | - | EBRT | SBRT or HypoRT (198) | SBRT or HypoRT + MET (10) |
| Jung 2022 [86] | Human, clinical | South Korea | 164 | 75:89 | 69.0 ± 16.4 | LDT | TACE (73) | TACE + MET (91) |
| Kim 2014 [87] | Human pre-clinical | South Korea | - | - | - | EBRT | y-ray radiation and neutron radiation | y-ray radiation/neutron radiation + MET |
| Liu 2012 [88] | Human pre-clinical | China | - | - | - | EBRT | Ionizing radiation | Ionizing radiation + MET |
| Tian 2016 [89] | Human pre-clinical/clinical | China | 63 | 11:52 | - | LDT | TACE (30) | TACE + MET (33) |
| Zhang 2017 [90] | Human pre-clinical | China | - | - | - | LDT | RFA | RFA + MET |
| Zhang 2019 [91] | Human pre-clinical | China | - | - | - | EBRT | Gamma ray irradiation | Gamma ray irradiation + MET |
| Study | Overall Survival (Control) | Overall Survival (Experimental) | Progression-Free Survival (Control) | Progression Free Survival (Experimental) | Results of Study 1 | Metformin Dosage |
|---|---|---|---|---|---|---|
| Jung 2022 [86] | - | - | 74 months | 79 months | Metformin use associated with higher ORR, lower LTR, better IR, and lower recurrence | - |
| Elsayed 2021 [84] | 39.7 months | 33.7 months | - | - | MET + Y-90 RS had greater reduction in TTD, number of lesions, and % TTD | - |
| Jang 2015 [85] | 37% 2 years | 76% 2 years | 16% 2 years | 46% 2 years | Use of metformin in patients receiving radiotherapy was associated with higher OS | 1000 mg/day |
| Chen 2011 [82] | 93.9% 1 year; 80.2% 3 years; 64.7% 5 years | 95.0% 1 year; 69.2% 3 years; 60.5% 5 years | 74.5% 1 year; 44.8% 3 years; 26.2% 5 years | 95% 1 year; 69.2% 3 years; 60.5% 5 years | Metformin users among diabetic patients with HCC undergoing RFA had favorable overall survival compared with patients without metformin | 750 mg/day |
| Chen 2022 [83] | 32 months | 42 months | 7 months | 12 months | Metformin increased long-term survival time and significantly prevented recurrence after TACE | 2 g/day |
| Tian 2016 [89] | 75 months | 79 months | - | - | Metformin + TACE combination resulted in increased OS | - |
| Study | Cell Type Used | Results of Study 1 | Metformin Dosage |
|---|---|---|---|
| Zhang 2017 [90] | HepG2 and SMMC7721 | MET + RFA inhibited proliferation, migration, and invasion of Hep G2 cells after insufficient RFA. MET also blocked growth of HepG2 cells | 0.5 mg/mL |
| Zhang 2019 [91] | SMMC-7721 | Metformin demonstrated enhanced radiosensitivity and inhibition of EMT in HCC cells. | 0, 1, 5, 10, and 20 mM |
| Tian 2016 [89] | Bel7402 and Bel7402-5 fluorouracil | 5-FU + MET increased chemotherapeutic sensitivity, decreased cell proliferation, increased apoptosis, and arrested cell cycle at G0/G1 | 2 mg/kg |
| Liu 2012 [88] | HepG2 and Bel-7402 cells | Combination of Met + ionizing radiation inhibited cell survival, cell cycle progression, and increased DNA damage more than monotherapies | - |
| Kim 2014 [87] | Huh7, HepG2 and Hep3B | Combination of y-ray radiation + MET enhanced radiation efficacy, increased apoptosis, increased DNA damage, and stopped G2/M cell cycle in HCC cells | - |
| Xin 2016 [92] | PLC/PRF/5, SK-Hep-1, Huh7 | MET increased effects of Sorafenib and significantly decreased viability/proliferation of HCC cells | 200 μM |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choksi, E.J.; Elsayed, M.; Kokabi, N. Antitumor Activity of Metformin Combined with Locoregional Therapy for Liver Cancer: Evidence and Future Directions. Cancers 2023, 15, 4538. https://doi.org/10.3390/cancers15184538
Choksi EJ, Elsayed M, Kokabi N. Antitumor Activity of Metformin Combined with Locoregional Therapy for Liver Cancer: Evidence and Future Directions. Cancers. 2023; 15(18):4538. https://doi.org/10.3390/cancers15184538
Chicago/Turabian StyleChoksi, Eshani J., Mohammad Elsayed, and Nima Kokabi. 2023. "Antitumor Activity of Metformin Combined with Locoregional Therapy for Liver Cancer: Evidence and Future Directions" Cancers 15, no. 18: 4538. https://doi.org/10.3390/cancers15184538
APA StyleChoksi, E. J., Elsayed, M., & Kokabi, N. (2023). Antitumor Activity of Metformin Combined with Locoregional Therapy for Liver Cancer: Evidence and Future Directions. Cancers, 15(18), 4538. https://doi.org/10.3390/cancers15184538






