Clinicopathological Factors Associated with Oncotype DX Risk Group in Patients with ER+/HER2- Breast Cancer
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient and Variable Selection
2.2. Risk Stratification by the ODX RS
2.3. Statistical Analysis
2.4. Ethical Approval
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lei, S.; Zheng, R.; Zhang, S.; Wang, S.; Chen, R.; Sun, K.; Zeng, H.; Zhou, J.; Wei, W. Global patterns of breast cancer incidence and mortality: A population-based cancer registry data analysis from 2000 to 2020. Cancer Commun. 2021, 41, 1183–1194. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.Y.; Kim, Y.S.; Kim, Z.; Kim, H.Y.; Kim, H.J.; Park, S.; Bae, S.Y.; Yoon, K.H.; Lee, S.B.; Lee, S.K.; et al. Breast Cancer Statistics in Korea in 2017: Data from a Breast Cancer Registry. J. Breast Cancer 2020, 23, 115–128. [Google Scholar] [CrossRef] [PubMed]
- Fujii, T.; Le Du, F.; Xiao, L.; Kogawa, T.; Barcenas, C.H.; Alvarez, R.H.; Valero, V.; Shen, Y.; Ueno, N.T. Effectiveness of an Adjuvant Chemotherapy Regimen for Early-Stage Breast Cancer: A Systematic Review and Network Meta-analysis. JAMA Oncol. 2015, 1, 1311–1318. [Google Scholar] [CrossRef] [PubMed]
- Early Breast Cancer Trialists’ Collaborative Group; Peto, R.; Davies, C.; Godwin, J.; Gray, R.; Pan, H.C.; Clarke, M.; Cutter, D.; Mcgale, P.; Taylor, C.; et al. Comparisons between different polychemotherapy regimens for early breast cancer: Meta-analyses of long-term outcome among 100,000 women in 123 randomised trials. Lancet 2012, 379, 432–444. [Google Scholar]
- Van der Hage, J.A.; Cooperating Investigators of the European Organization for Research and Treatment of Cancer; Mieog, J.S.D.; van de Vijver, M.J.; van de Velde, C.J. Efficacy of adjuvant chemotherapy according to hormone receptor status in young patients with breast cancer: A pooled analysis. Breast Cancer Res. 2007, 9, R70. [Google Scholar] [CrossRef]
- De Angelis, C.; Di Maio, M.; Crispo, A.; Giuliano, M.; Schettini, F.; Bonotto, M.; Gerratana, L.; Iacono, D.; Cinausero, M.; Riccardi, F.; et al. Luminal-like HER2-negative stage IA breast cancer: A multicenter retrospective study on long-term outcome with propensity score analysis. Oncotarget 2017, 8, 112816–112824. [Google Scholar] [CrossRef]
- Paik, S.; Shak, S.; Tang, G.; Kim, C.; Baker, J.; Cronin, M.; Baehner, F.L.; Walker, M.G.; Watson, D.; Park, T.; et al. A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. N. Engl. J. Med. 2004, 351, 2817–2826. [Google Scholar] [CrossRef] [PubMed]
- Habel, L.A.; Shak, S.; Jacobs, M.K.; Capra, A.; Alexander, C.; Pho, M.; Baker, J.; Walker, M.; Watson, D.; Hackett, J.; et al. A population-based study of tumor gene expression and risk of breast cancer death among lymph node-negative patients. Breast Cancer Res. 2006, 8, R25. [Google Scholar] [CrossRef]
- Syed, Y.Y. Oncotype DX Breast Recurrence Score((R)): A Review of its Use in Early-Stage Breast Cancer. Mol. Diagn. Ther. 2020, 24, 621–632. [Google Scholar] [CrossRef] [PubMed]
- Paik, S.; Tang, G.; Shak, S.; Kim, C.; Baker, J.; Kim, W.; Cronin, M.; Baehner, F.L.; Watson, D.; Bryant, J.; et al. Gene expression and benefit of chemotherapy in women with node-negative, estrogen receptor-positive breast cancer. J. Clin. Oncol. 2006, 24, 3726–3734. [Google Scholar] [CrossRef] [PubMed]
- Sparano, J.A. TAILORx: Trial assigning individualized options for treatment (Rx). Clin. Breast Cancer 2006, 7, 347–350. [Google Scholar] [CrossRef] [PubMed]
- Sparano, J.A.; Gray, R.J.; Makower, D.F.; Pritchard, K.I.; Albain, K.S.; Hayes, D.F.; Geyer, C.E., Jr.; Dees, E.C.; Perez, E.A.; Olson, J.A., Jr.; et al. Prospective Validation of a 21-Gene Expression Assay in Breast Cancer. N. Engl. J. Med. 2015, 373, 2005–2014. [Google Scholar] [CrossRef] [PubMed]
- Sparano, J.A.; Gray, R.J.; Makower, D.F.; Pritchard, K.I.; Albain, K.S.; Hayes, D.F.; Geyer, C.E., Jr.; Dees, E.C.; Goetz, M.P.; Olson, J.A., Jr.; et al. Adjuvant Chemotherapy Guided by a 21-Gene Expression Assay in Breast Cancer. N. Engl. J. Med. 2018, 379, 111–121. [Google Scholar] [CrossRef]
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: Breast Cancer, Version 2. 2022. Available online: https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf (accessed on 20 December 2021).
- Andre, F.; Ismaila, N.; Henry, N.L.; Somerfield, M.R.; Bast, R.C.; Barlow, W.; Collyar, D.E.; Hammond, M.E.; Kuderer, N.M.; Liu, M.C.; et al. Use of Biomarkers to Guide Decisions on Adjuvant Systemic Therapy for Women with Early-Stage Invasive Breast Cancer: ASCO Clinical Practice Guideline Update-Integration of Results From TAILORx. J. Clin. Oncol. 2019, 37, 1956–1964. [Google Scholar] [CrossRef] [PubMed]
- Reed, S.D.; Dinan, M.A.; Schulman, K.A.; Lyman, G.H. Cost-effectiveness of the 21-gene recurrence score assay in the context of multifactorial decision making to guide chemotherapy for early-stage breast cancer. Genet. Med. 2013, 15, 203–211. [Google Scholar] [CrossRef] [PubMed]
- Albanell, J.; Svedman, C.; Gligorov, J.; Holt, S.D.; Bertelli, G.; Blohmer, J.U.; Rouzier, R.; Lluch, A.; Eiermann, W. Pooled analysis of prospective European studies assessing the impact of using the 21-gene Recurrence Score assay on clinical decision making in women with oestrogen receptor-positive, human epidermal growth factor receptor 2-negative early-stage breast cancer. Eur. J. Cancer 2016, 66, 104–113. [Google Scholar]
- Orucevic, A.; Heidel, R.E.; Bell, J.L. Utilization and impact of 21-gene recurrence score assay for breast cancer in clinical practice across the United States: Lessons learned from the 2010 to 2012 National Cancer Data Base analysis. Breast Cancer Res. Treat. 2016, 157, 427–435. [Google Scholar] [CrossRef]
- Ramsey, S.D.; Barlow, W.E.; Gonzalez-Angulo, A.M.; Tunis, S.; Baker, L.; Crowley, J.; Deverka, P.; Veenstra, D.; Hortobagyi, G.N. Integrating comparative effectiveness design elements and endpoints into a phase III, randomized clinical trial (SWOG S1007) evaluating oncotypeDX-guided management for women with breast cancer involving lymph nodes. Contemp. Clin. Trials 2013, 34, 1–9. [Google Scholar] [CrossRef]
- Sparano, J.A.; Gray, R.J.; Ravdin, P.M.; Makower, D.F.; Pritchard, K.I.; Albain, K.S.; Hayes, D.F.; Geyer, C.E., Jr.; Dees, E.C.; Goetz, M.P.; et al. Clinical and Genomic Risk to Guide the Use of Adjuvant Therapy for Breast Cancer. N. Engl. J. Med. 2019, 380, 2395–2405. [Google Scholar] [CrossRef]
- Chen, M.; Yang, Y.; Xu, K.; Li, L.; Huang, J.; Qiu, F. Androgen Receptor in Breast Cancer: From Bench to Bedside. Front. Endocrinol. 2020, 11, 573. [Google Scholar] [CrossRef]
- Anestis, A.; Zoi, I.; Papavassiliou, A.G.; Karamouzis, M.V. Androgen Receptor in Breast Cancer-Clinical and Preclinical Research Insights. Molecules 2020, 25, 358. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, A.; Ziolkowski, P.; Grzebieniak, Z.; Jelen, M.; Bobinski, P.; Agrawal, S. Expression of Androgen Receptor in Estrogen Receptor-positive Breast Cancer. Appl. Immunohistochem. Mol. Morphol. 2016, 24, 550–555. [Google Scholar] [CrossRef] [PubMed]
- Galea, M.H.; Blamey, R.W.; Elston, C.E.; Ellis, I.O. The Nottingham Prognostic Index in primary breast cancer. Breast Cancer Res. Treat. 1992, 22, 207–219. [Google Scholar] [CrossRef]
- Phung, M.T.; Tin, S.T.; Elwood, J.M. Prognostic models for breast cancer: A systematic review. BMC Cancer 2019, 19, 230. [Google Scholar] [CrossRef]
- Rejali, M.; Tazhibi, M.; Mokarian, F.; Gharanjik, N.; Mokarian, R. The Performance of the Nottingham Prognosis Index and the Adjuvant Online Decision Making Tool for Prognosis in Early-stage Breast Cancer Patients. Int. J. Prev. Med. 2015, 6, 93. [Google Scholar] [PubMed]
- Henson, D.E.; Ries, L.; Freedman, L.S.; Carriaga, M. Relationship among outcome, stage of disease, and histologic grade for 22,616 cases of breast cancer. The basis for a prognostic index. Cancer 1991, 68, 2142–2149. [Google Scholar] [CrossRef]
- Van Dooijeweert, C.; van Diest, P.J.; Ellis, I.O. Grading of invasive breast carcinoma: The way forward. Virchows Arch. 2021, 480, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Nelson, D.R.; Brown, J.; Morikawa, A.; Method, M. Breast cancer-specific mortality in early breast cancer as defined by high-risk clinical and pathologic characteristics. PLoS ONE 2022, 17, e0264637. [Google Scholar] [CrossRef]
- Li, J.-P.; Zhang, X.-M.; Zhang, Z.; Zheng, L.-H.; Jindal, S.; Liu, Y.-J. Association of p53 expression with poor prognosis in patients with triple-negative breast invasive ductal carcinoma. Medicine 2019, 98, e15449. [Google Scholar] [CrossRef]
- Yang, P.; Du, C.W.; Kwan, M.; Liang, S.X.; Zhang, G.J. The impact of p53 in predicting clinical outcome of breast cancer patients with visceral metastasis. Sci. Rep. 2013, 3, 2246. [Google Scholar] [CrossRef]
- Pekarek, L.; Cendra, A.S.; Cervantes, E.D.R.; Cendra, C.S.; Fraile-Martinez, O.; García-Montero, C.; Diaz-Pedrero, R.; Torres-Carranza, D.; Lopez-Gonzalez, L.; Aguado-Henche, S.; et al. Clinical and Translational Applications of Serological and Histopathological Biomarkers in Metastatic Breast Cancer: A Comprehensive Review. Int. J. Mol. Sci. 2023, 24, 8396. [Google Scholar] [CrossRef] [PubMed]
- Eom, Y.H.; Kim, H.S.; Lee, A.; Song, B.J.; Chae, B.J. BCL2 as a Subtype-Specific Prognostic Marker for Breast Cancer. J. Breast Cancer 2016, 19, 252–260. [Google Scholar] [CrossRef] [PubMed]
- Denkert, C.; Loibl, S.; Noske, A.; Roller, M.; Müller, B.M.; Komor, M.; Budczies, J.; Darb-Esfahani, S.; Kronenwett, R.; Hanusch, C.; et al. Tumor-associated lymphocytes as an independent predictor of response to neoadjuvant chemotherapy in breast cancer. J. Clin. Oncol. 2010, 28, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Duque, G.; Manterola, C.; Otzen, T.; Arias, C.; Galindo, B.; Mora, M.; Guerrero, E.; García, N. Clinical utility of liquid biopsy in breast cancer: A systematic review. Clin. Genet. 2021, 101, 285–295. [Google Scholar] [CrossRef] [PubMed]
- Orucevic, A.; Bell, J.L.; McNabb, A.P.; Heidel, R.E. Oncotype DX breast cancer recurrence score can be predicted with a novel nomogram using clinicopathologic data. Breast Cancer Res. Treat. 2017, 163, 51–61. [Google Scholar] [CrossRef]
- Lee, S.B.; Kim, J.; Sohn, G.; Kim, J.; Chung, I.Y.; Kim, H.J.; Ko, B.S.; Son, B.H.; Ahn, S.-H.; Lee, J.W.; et al. A Nomogram for Predicting the Oncotype DX Recurrence Score in Women with T1-3N0-1miM0 Hormone ReceptorPositive, Human Epidermal Growth Factor 2 (HER2) Negative Breast Cancer. Cancer Res. Treat. 2019, 51, 1073–1085. [Google Scholar] [CrossRef]
- Yoo, S.H.; Kim, T.-Y.; Kim, M.; Lee, K.-H.; Lee, E.; Lee, H.-B.; Moon, H.-G.; Han, W.; Noh, D.-Y.; Han, S.-W.; et al. Development of a Nomogram to Predict the Recurrence Score of 21-Gene Prediction Assay in Hormone Receptor-Positive Early Breast Cancer. Clin. Breast Cancer 2020, 20, 98–107.e1. [Google Scholar] [CrossRef]
- Geradts, J.; Bean, S.M.; Bentley, R.C.; Barry, W.T. The oncotype DX recurrence score is correlated with a composite index including routinely reported pathobiologic features. Cancer Investig. 2010, 28, 969–977. [Google Scholar] [CrossRef]
- Mattes, M.D.; Mann, J.M.; Ashamalla, H.; Tejwani, A. Routine histopathologic characteristics can predict oncotype DX(TM) recurrence score in subsets of breast cancer patients. Cancer Investig. 2013, 31, 604–606. [Google Scholar] [CrossRef]
- Allison, K.H.; Kandalaft, P.L.; Sitlani, C.M.; Dintzis, S.M.; Gown, A.M. Routine pathologic parameters can predict Oncotype DX recurrence scores in subsets of ER positive patients: Who does not always need testing? Breast Cancer Res Treat. 2012, 131, 413–424. [Google Scholar] [CrossRef]
- Ingoldsby, H.; Webber, M.; Wall, D.; Scarrott, C.; Newell, J.; Callagy, G. Prediction of Oncotype DX and TAILORx risk categories using histopathological and immunohistochemical markers by classification and regression tree (CART) analysis. Breast 2013, 22, 879–886. [Google Scholar] [CrossRef] [PubMed]
- Gage, M.M.; Rosman, M.; Mylander, W.C.; Giblin, E.; Kim, H.-S.; Cope, L.; Umbricht, C.; Wolff, A.C.; Tafra, L. A Validated Model for Identifying Patients Unlikely to Benefit From the 21-Gene Recurrence Score Assay. Clin. Breast Cancer 2015, 15, 467–472. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.L.; Nolan, M.E.; Silverstein, M.J.; Mihm, M.C.; Sober, A.J.; Tanabe, K.K.; Smith, B.L.; Younger, J.; Michaelson, J.S. The impact of primary tumor size, lymph node status, and other prognostic factors on the risk of cancer death. Cancer 2009, 115, 5071–5083. [Google Scholar] [CrossRef]
- Kim, J.-M.; Ryu, J.M.; Kim, I.; Choi, H.J.; Nam, S.J.; Kim, S.W.; Yu, J.; Lee, S.K.; Lee, J.E. Verification of a Western Nomogram for Predicting Oncotype DX Recurrence Scores in Korean Patients with Breast Cancer. J. Breast Cancer 2018, 21, 222–226. [Google Scholar] [CrossRef] [PubMed]
- Orucevic, A.; Bell, J.L.; King, M.; McNabb, A.P.; Heidel, R.E. Nomogram update based on TAILORx clinical trial results—Oncotype DX breast cancer recurrence score can be predicted using clinicopathologic data. Breast 2019, 46, 116–125. [Google Scholar] [CrossRef]
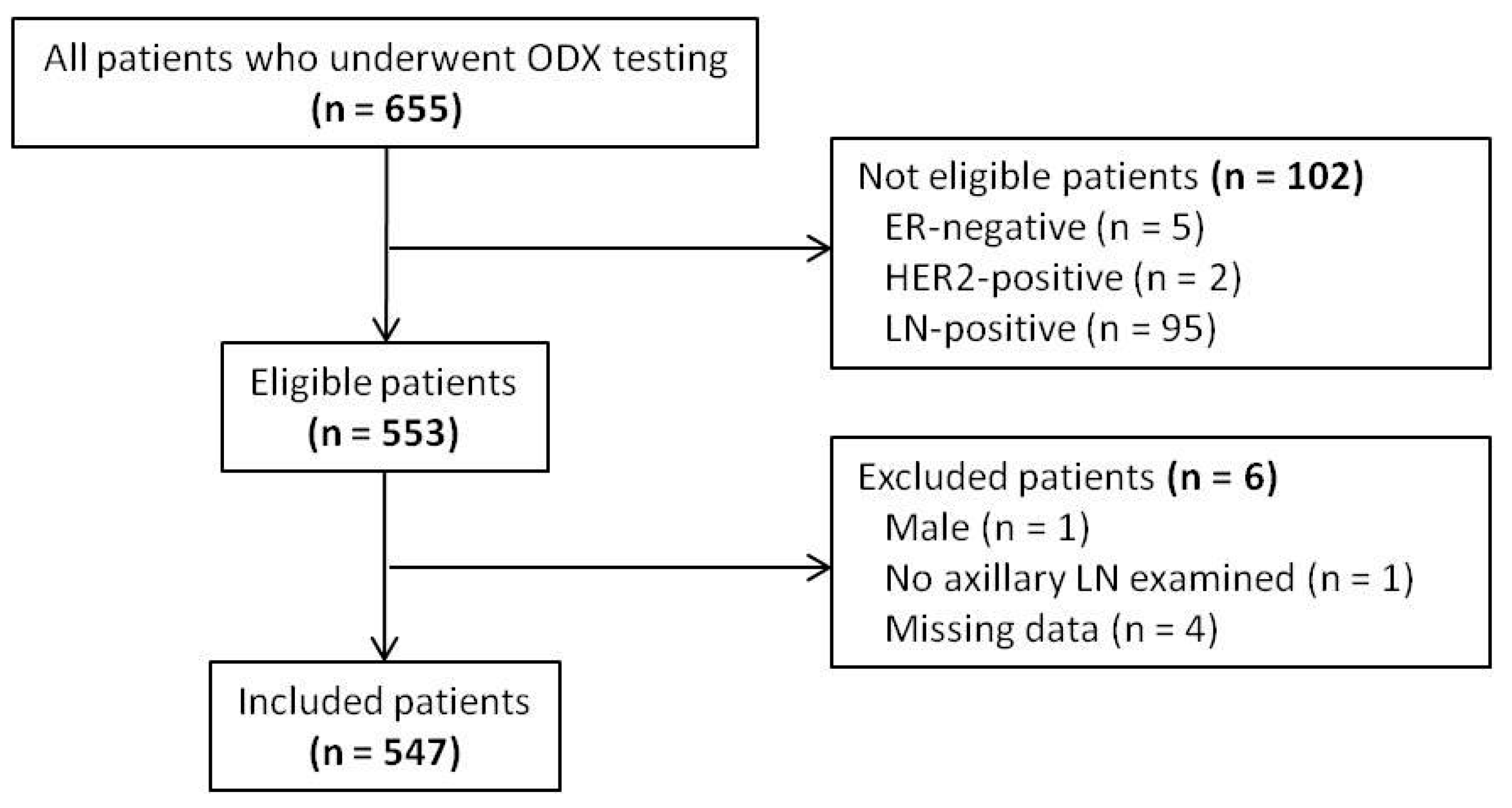
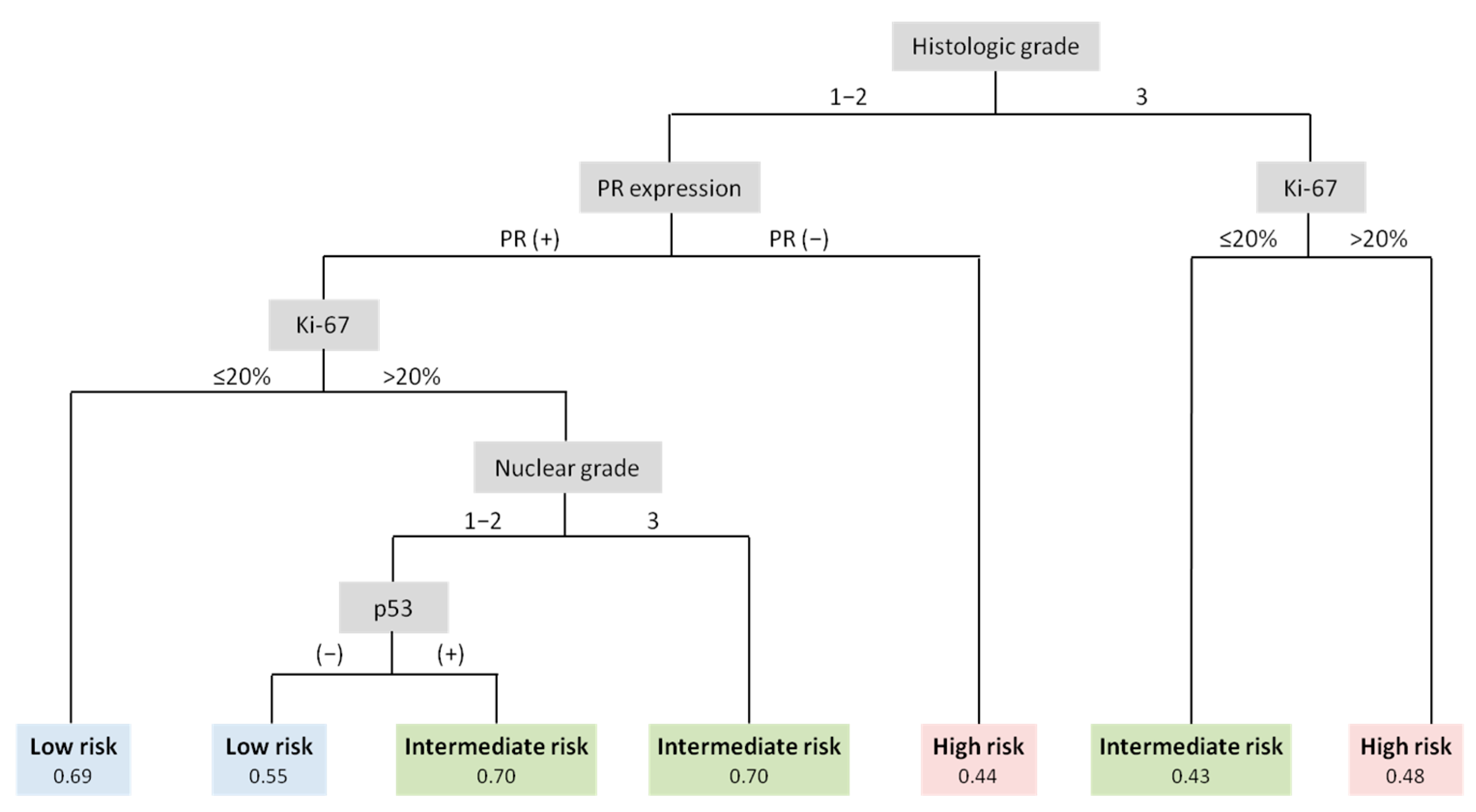


| Characteristics | n (%) | RS 0–15 | RS 16–25 | RS 26–100 | p |
|---|---|---|---|---|---|
| n (%) | 547 | 292 (53.4%) | 188 (34.4%) | 67 (12.2%) | - |
| Age (years) | 0.3615 | ||||
| Mean ± SD | 47.5 ± 7.8 | 47.1 ± 7.7 | 47.7 ± 7.5 | 48.9 ± 8.8 | |
| ≤50 | 379 (69.3%) | 209 (71.6%) | 127 (67.6%) | 43 (64.2%) | 0.4054 |
| >50 | 168 (30.7%) | 83 (28.4%) | 61 (32.5%) | 24 (35.8%) | |
| ) | 0.3904 | ||||
| Mean ± SD | 23.3 ± 3.4 | 23.2 ± 3.2 | 23.2 ± 3.4 | 23.8 ± 3.7 | |
| Menopausal status | 0.1384 | ||||
| Premenopausal | 403 (73.7%) | 222 (76%) | 138 (73.4%) | 43 (64.2%) | |
| Postmenopausal | 144 (26.3%) | 70 (24%) | 50 (26.6%) | 24 (35.8%) | |
| Tumor size (cm) | |||||
| ≤1 cm | 52 (9.5%) | 30 (10.3%) | 16 (8.5%) | 6 (9%) | 0.8158 |
| >1 cm, ≤2 cm | 315 (57.6%) | 167 (57.2%) | 114 (60.6%) | 34 (50.8%) | |
| >2 cm, ≤5 cm | 173 (31.6%) | 91 (31.2%) | 56 (29.8%) | 26 (38.8%) | |
| >5 cm | 7 (1.3%) | 4 (1.4%) | 2 (1.1%) | 1 (1.5%) | |
| Histologic type | 0.0842 | ||||
| IDC | 449 (82.1%) | 230 (78.8%) | 158 (84%) | 61 (91%) | |
| ILC | 52 (9.5%) | 31 (10.6%) | 19 (10.1%) | 2 (3%) | |
| others | 46 (8.4%) | 31 (10.6%) | 11 (5.9%) | 4 (6%) | |
| Histologic grade | <0.0001 | ||||
| 1 | 76 (13.9%) | 50 (17.1%) | 22 (11.7%) | 4 (6%) | |
| 2 | 398 (72.8%) | 223 (76.4%) | 142 (75.5%) | 33 (49.3%) | |
| 3 | 73 (13.4%) | 19 (6.5%) | 24 (12.8%) | 30 (44.8%) | |
| Nuclear grade | <0.0001 | ||||
| Low | 12 (2.2%) | 10 (2.8%) | 1 (0.8%) | 1 (1.5%) | |
| Intermediate | 447 (81.7%) | 308 (87.3%) | 103 (81.1%) | 36 (53.7%) | |
| High | 88 (16.1%) | 35 (9.9%) | 23 (18.1%) | 30 (44.8%) | |
| LVI | 0.6422 | ||||
| Negative | 319 (58.4%) | 176 (60.3%) | 106 (56.4%) | 37 (56.1%) | |
| Positive | 227 (41.6%) | 116 (39.7%) | 82 (43.6%) | 29 (43.9%) | |
| PR expression | |||||
| Negative | 49 (9%) | 12 (4.1%) | 19 (10.1%) | 18 (26.9%) | <0.0001 |
| Positive | 498 (91%) | 280 (95.9%) | 169 (89.9%) | 49 (73.1%) | |
| AR expression (n = 290) | |||||
| Negative | 14 (4.8%) | 5 (3%) | 5 (5.7%) | 4 (11.4%) | 0.0848 |
| Positive | 276 (95.2%) | 162 (97%) | 83 (94.3%) | 31 (88.6%) | |
| Ki-67 (%) | <0.0001 | ||||
| Median (min-max) | 16 (1–87) | 13.5 (1–76) | 17 (1–67) | 27 (1–87) | |
| p53 expression | <0.0001 | ||||
| Negative | 482 (88.1%) | 278 (95.2%) | 160 (85.1%) | 44 (65.7%) | |
| Positive | 60 (11%) | 11 (3.8%) | 27 (14.4%) | 22 (32.8%) | |
| Unknown | 5 (0.9%) | 3 (1%) | 1 (0.5%) | 1 (1.5%) | |
| Type of surgery | 0.1526 | ||||
| BCS | 440 (80.4%) | 226 (77.4%) | 157 (83.5%) | 57 (85.1%) | |
| Mastectomy | 107 (19.6%) | 66 (22.6%) | 31 (16.5%) | 10 (14.9%) | |
| Anti-hormonal therapy | 0.0729 | ||||
| No | 3 (0.6%) | 1 (0.3%) | 0 (0%) | 2 (3%) | |
| Yes | 544 (99.5%) | 291 (99.7%) | 188 (100%) | 65 (97%) | |
| Chemotherapy | <0.0001 | ||||
| No | 442 (80.8%) | 288 (98.6%) | 147 (78.2%) | 7 (10.5%) | |
| Yes | 105 (19.2%) | 4 (1.4%) | 41 (21.8%) | 60 (89.6%) | |
| Radiation therapy | 0.7773 | ||||
| No | 107 (19.6%) | 62 (21.2%) | 34 (18.1%) | 11 (16.4%) | |
| Yes | 440 (80.4%) | 230 (78.8%) | 154 (81.9%) | 56 (83.6%) |
| Characteristics | Age ≤ 50 Years | Age > 50 Years | |||||
|---|---|---|---|---|---|---|---|
| RS 0–15 | RS 16–25 | RS 26–100 | p | RS 0–25 | RS 26–100 | p | |
| n (%) | 209 (55.2%) | 127 (33.5%) | 43 (11.4%) | 144 (85.7%) | 24 (14.3%) | ||
| BMI (kg/) | 0.5409 | 0.5179 | |||||
| Mean ± SD | 22.9 ± 3.1 | 22.7 ± 3.1 | 23.3 ± 3.7 | 24.2 ± 3.6 | 24.7 ± 3.4 | ||
| Menopausal status | 0.7644 | 0.1332 | |||||
| Premenopausal | 199 (95.2%) | 119 (93.7%) | 40 (93%) | 42 (29.2%) | 3 (12.5%) | ||
| Postmenopausal | 10 (4.8%) | 8 (6.3%) | 3 (7%) | 102 (70.8%) | 21 (87.5%) | ||
| Tumor size (cm) | |||||||
| ≤1 cm | 25 (12%) | 12 (9.5%) | 5 (11.6%) | 0.9282 | 9 (6.3%) | 1 (4.2%) | 0.0967 |
| >1 cm, ≤2 cm | 122 (58.4%) | 71 (55.9%) | 24 (55.8%) | 88 (61.1%) | 10 (41.7%) | ||
| >2 cm, ≤5 cm | 59 (28.2%) | 42 (33.1%) | 14 (32.6%) | 46 (31.9%) | 12 (50%) | ||
| >5 cm | 3 (1.4%) | 2 (1.6%) | 0 (0%) | 1 (0.7%) | 1 (4.2%) | ||
| Histologic type | 0.0891 | 0.6442 | |||||
| IDC | 162 (77.5%) | 104 (81.9%) | 41 (95.4%) | 122 (84.7%) | 20 (83.3%) | ||
| ILC | 24 (11.5%) | 14 (11%) | 1 (2.3%) | 12 (8.3%) | 1 (4.2%) | ||
| Others | 23 (11%) | 9 (7.1%) | 1 (2.3%) | 10 (6.9%) | 3 (12.5%) | ||
| Histologic grade | <0.0001 | 0.0055 | |||||
| 1 | 39 (18.7%) | 14 (11%) | 3 (7%) | 19 (13.2%) | 1 (4.2%) | ||
| 2 | 155 (74.2%) | 95 (74.8%) | 17 (39.5%) | 115 (79.9%) | 16 (66.7%) | ||
| 3 | 15 (7.2%) | 18 (14.2%) | 23 (53.5%) | 10 (6.9%) | 7 (29.2%) | ||
| Nuclear grade | <0.0001 | 0.0021 | |||||
| Low | 6 (2.9%) | 1 (0.8%) | 1 (2.3%) | 4 (2.8%) | 0 (0%) | ||
| Intermediate | 181 (86.6%) | 103 (81.1%) | 21 (48.8%) | 127 (88.2%) | 15 (62.5%) | ||
| High | 22 (10.5%) | 23 (18.1%) | 21 (48.8%) | 13 (9.0%) | 9 (37.5%) | ||
| LVI | (n = 378) | 0.8834 | 0.3711 | ||||
| Negative | 124 (59.3%) | 72 (56.7%) | 25 (59.5%) | 86 (59.7%) | 12 (50%) | ||
| Positive | 85 (40.7%) | 55 (43.3%) | 17 (40.5%) | 58 (40.3%) | 12 (50%) | ||
| PR expression | |||||||
| Negative | 4 (1.9%) | 5 (3.9%) | 12 (27.9%) | <0.0001 | 22 (15.3%) | 6 (25%) | 0.2431 |
| Positive | 205 (98.1%) | 122 (96.1%) | 31 (72.1%) | 122 (84.7%) | 18 (75%) | ||
| AR expression | (n = 191) | (n = 99) | |||||
| Negative | 5 (4.2%) | 3 (5.9%) | 4 (20%) | 0.0377 | 2 (2.4%) | 0 (0%) | >0.9999 |
| Positive | 115 (95.8%) | 48 (94.1%) | 16 (80%) | 82 (97.6%) | 15 (100%) | ||
| Ki-67 (%) | |||||||
| Median (min-max) | 14 (1–76) | 18 (1–67) | 33 (1–87) | <0.0001 | 13 (1–58) | 22 (4–80) | 0.0001 |
| p53 expression | (n = 375) | ||||||
| Negative | 196 (95.2%) | 109 (85.8%) | 29 (69.1%) | <0.0001 | 133 (93%) | 15 (62.5%) | 0.0002 |
| Positive | 10 (4.9%) | 18 (14.2%) | 13 (31%) | 10 (7%) | 9 (37.5%) | ||
| Characteristics | Univariable (n = 379) | Multivariable (n = 375) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Type 3 Analysis of Effects p-Value | RS 16–25 (Ref: RS 0–15) | RS 26–100 (Ref: RS 0–15) | Type 3 Analysis of Effects p-Value | RS 16–25 (Ref: RS 0–15) | RS 26–100 (Ref: RS 0–15) | |||||||||
| OR | 95% CI | p | OR | 95% CI | p | OR | 95% CI | p | OR | 95% CI | p | |||
| BMI (kg/) | 0.5404 | 0.984 | 0.917–1.056 | 0.6597 | 1.044 | 0.948–1.149 | 0.3863 | |||||||
| Menopausal status | 0.7660 | |||||||||||||
| Premenopausal | 1 (ref) | 1 (ref) | ||||||||||||
| Postmenopausal | 1.338 | 0.514–3.484 | 0.5512 | 1.493 | 0.393–5.667 | 0.5562 | ||||||||
| Tumor size (cm) | 0.6306 | |||||||||||||
| ≤2 cm | 1 (ref) | 1 (ref) | ||||||||||||
| >2 cm | 1.257 | 0.785–2.013 | 0.3412 | 1.145 | 0.566–2.313 | 0.7067 | ||||||||
| Histologic type | 0.0454 | |||||||||||||
| IDC | 1 (ref) | 1 (ref) | ||||||||||||
| non-IDC | 0.762 | 0.437–1.329 | 0.3388 | 0.168 | 0.039–0.721 | 0.0164 | ||||||||
| Histologic grade | <0.0001 | 0.0002 | ||||||||||||
| 1–2 | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) | ||||||||||
| 3 | 2.136 | 1.035–4.406 | 0.04 | 14.873 | 6.704–32.997 | <0.0001 | 1.467 | 0.681–3.160 | 0.3274 | 8.021 | 2.942–21.864 | <0.0001 | ||
| Nuclear grade | <0.0001 | |||||||||||||
| Low/Intermediate | 1 (ref) | 1 (ref) | ||||||||||||
| High | 1.879 | 0.999–3.534 | 0.0504 | 8.112 | 3.857–17.06 | <0.0001 | ||||||||
| LVI | 0.8834 | |||||||||||||
| Negative | 1 (ref) | 1 (ref) | ||||||||||||
| Positive | 1.114 | 0.713–1.742 | 0.6345 | 0.992 | 0.505–1.949 | 0.9814 | ||||||||
| PR expression | <0.0001 | <0.0001 | ||||||||||||
| Positive | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) | ||||||||||
| Negative | 2.100 | 0.553–7.971 | 0.2755 | 19.838 | 6.017–65.403 | <0.0001 | 2.732 | 0.705–10.594 | 0.146 | 79.673 | 17.23–368.423 | <0.0001 | ||
| AR expression | 0.0472 | |||||||||||||
| Positive | 1 (ref) | 1 (ref) | ||||||||||||
| Negative | 1.438 | 0.330–6.255 | 0.6285 | 5.749 | 1.397–23.666 | 0.0154 | ||||||||
| Ki-67 (%) | <0.0001 | 1.026 | 1.008–1.043 | 0.0034 | 1.075 | 1.052–1.099 | <0.0001 | <0.0001 | 1.026 | 1.007–1.044 | 0.0063 | 1.086 | 1.055–1.117 | <0.0001 |
| p53 expression | <0.0001 | 0.0227 | ||||||||||||
| Negative | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) | ||||||||||
| Positive | 3.237 | 1.443–7.259 | 0.0044 | 8.786 | 3.530–21.871 | <0.0001 | 2.635 | 1.145–6.064 | 0.0227 | 4.260 | 1.346–13.487 | 0.0137 | ||
| Characteristics | RS > 25 (Ref: RS ≤ 25) | |||||
|---|---|---|---|---|---|---|
| Univariable | Multivariable | |||||
| OR | 95% CI | p | OR | 95% CI | p | |
| ) | 1.039 | 0.927–1.164 | 0.516 | |||
| Menopausal status | ||||||
| Premenopausal | 1 (ref) | |||||
| Postmenopausal | 2.882 | 0.816–10.179 | 0.1002 | |||
| Tumor size (cm) | ||||||
| ≤2 cm | 1 (ref) | 1 (ref) | ||||
| >2 cm | 2.439 | 1.017–5.852 | 0.0459 | 3.421 | 1.192–9.821 | 0.0223 |
| Histologic type | ||||||
| IDC | 1 (ref) | |||||
| non-IDC | 1.109 | 0.346–3.558 | 0.8618 | |||
| Histologic grade | ||||||
| 1–2 | 1 (ref) | |||||
| 3 | 5.518 | 1.856–16.408 | 0.0021 | |||
| Nuclear grade | ||||||
| Low/Intermediate | 1 (ref) | |||||
| High | 6.046 | 2.216–16.499 | 0.0004 | |||
| LVI | ||||||
| Negative | 1 (ref) | |||||
| Positive | 1.483 | 0.623–3.528 | 0.373 | |||
| PR expression | ||||||
| Positive | 1 (ref) | |||||
| Negative | 1.848 | 0.66–5.175 | 0.2421 | |||
| AR expression | ||||||
| Positive | N/A | |||||
| Negative | ||||||
| Ki-67 (%) | 1.070 | 1.035–1.107 | <0.0001 | 1.061 | 1.025–1.099 | 0.0008 |
| p53 expression | ||||||
| Negative | 1 (ref) | 1 (ref) | ||||
| Positive | 7.980 | 2.801–22.733 | 0.0001 | 7.33 | 2.201–24.411 | 0.0012 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, R.; Lee, D.-E.; Lee, E.-G.; Lee, S.; Kang, H.-S.; Han, J.H.; Lee, K.S.; Sim, S.H.; Chae, H.; Kwon, Y.; et al. Clinicopathological Factors Associated with Oncotype DX Risk Group in Patients with ER+/HER2- Breast Cancer. Cancers 2023, 15, 4451. https://doi.org/10.3390/cancers15184451
Song R, Lee D-E, Lee E-G, Lee S, Kang H-S, Han JH, Lee KS, Sim SH, Chae H, Kwon Y, et al. Clinicopathological Factors Associated with Oncotype DX Risk Group in Patients with ER+/HER2- Breast Cancer. Cancers. 2023; 15(18):4451. https://doi.org/10.3390/cancers15184451
Chicago/Turabian StyleSong, Ran, Dong-Eun Lee, Eun-Gyeong Lee, Seeyoun Lee, Han-Sung Kang, Jai Hong Han, Keun Seok Lee, Sung Hoon Sim, Heejung Chae, Youngmee Kwon, and et al. 2023. "Clinicopathological Factors Associated with Oncotype DX Risk Group in Patients with ER+/HER2- Breast Cancer" Cancers 15, no. 18: 4451. https://doi.org/10.3390/cancers15184451
APA StyleSong, R., Lee, D.-E., Lee, E.-G., Lee, S., Kang, H.-S., Han, J. H., Lee, K. S., Sim, S. H., Chae, H., Kwon, Y., Woo, J., & Jung, S.-Y. (2023). Clinicopathological Factors Associated with Oncotype DX Risk Group in Patients with ER+/HER2- Breast Cancer. Cancers, 15(18), 4451. https://doi.org/10.3390/cancers15184451






