Weight Management Effectiveness and Predictors of Dropout in Breast Cancer Survivors: A Retrospective Study
Abstract
:Simple Summary
Abstract
1. Introduction
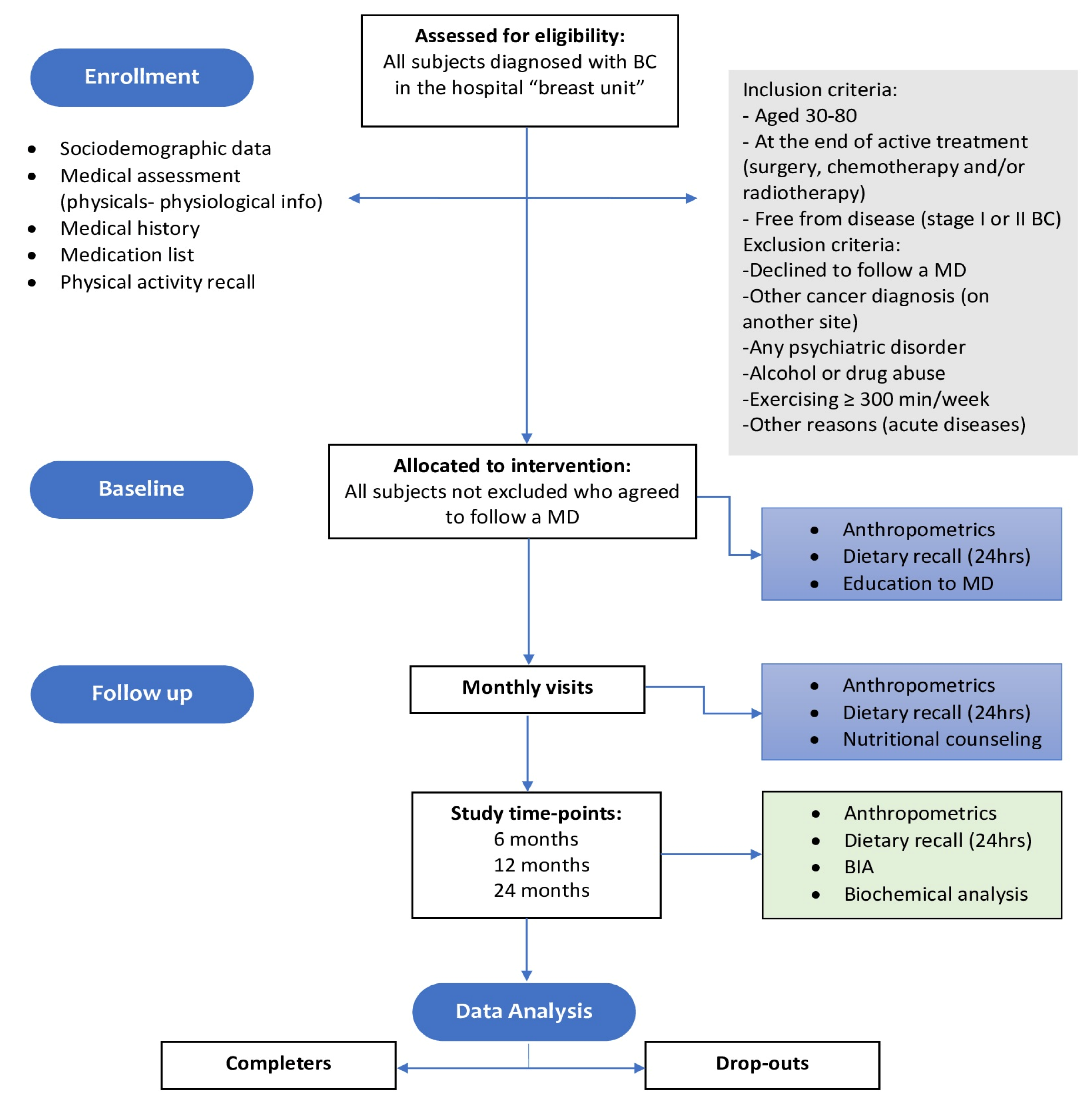
2. Materials and Methods
2.1. Patients
2.2. Intervention and Dietary Characteristics of the Traditional Mediterranean Diet
2.3. Anthropometric and Biochemical Measurements
2.4. Statistical Analysis
3. Results
- During our study period, dietary intervention was provided to 182 female patients, out of which 20 were normal weight (NW), thus receiving a normocaloric MD, while 59 were overweight (OW) and 103 were obese (OB), thus receiving a hypocaloric MD according to their needs. In the whole sample, the mean age was 53.9 years, and the mean BMI was 31.6 Kg/m2. Table 1 illustrates demographic, lifestyle, and therapy characteristics of participants at the beginning of the study, while Table 2 shows relevant anthropometric and biochemical parameters, with statistical differences analyzed between BMI subgroups.
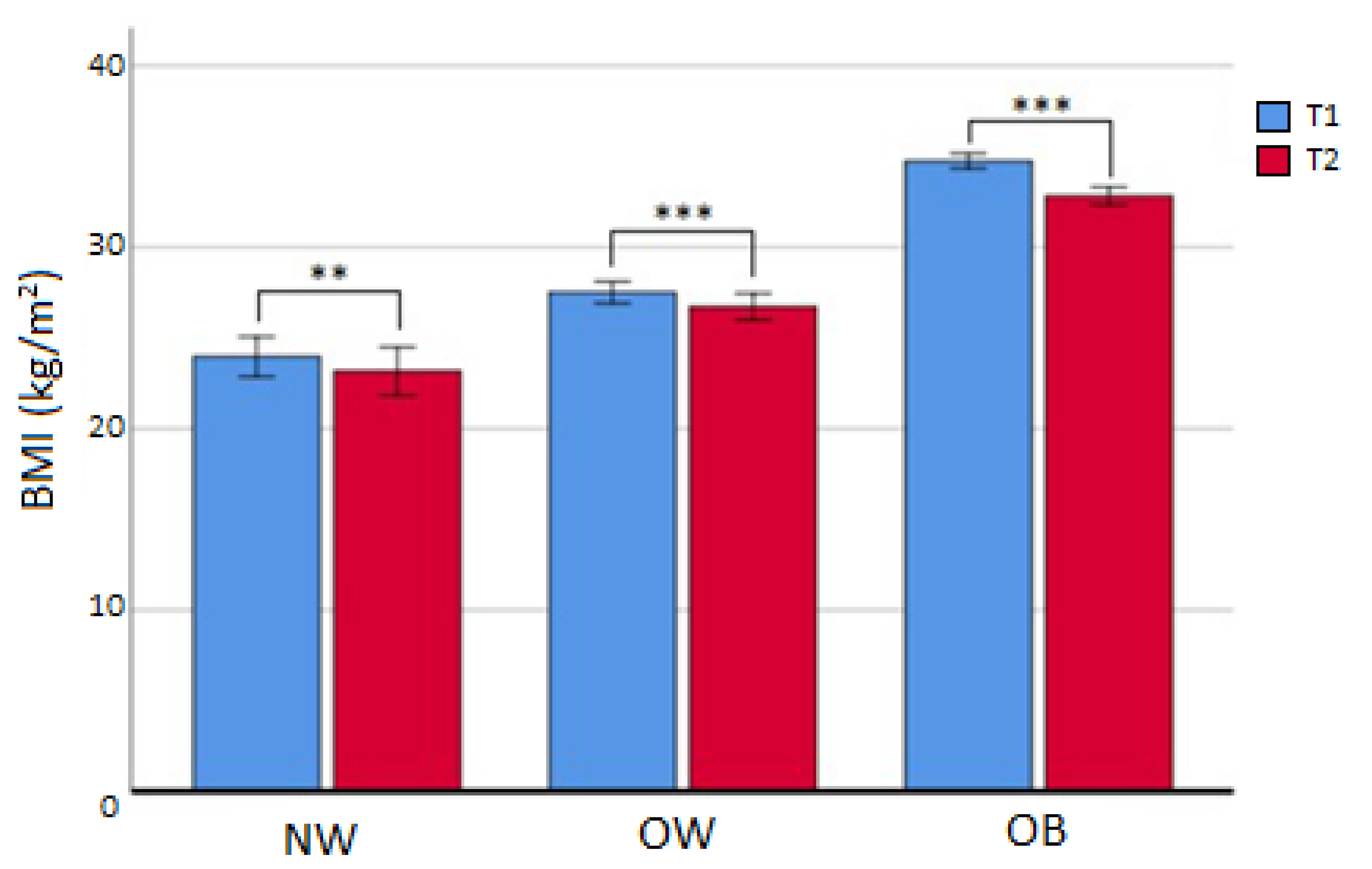
- After the first 6 months (T2), 77 subjects (42.3%) had discontinued treatment and 4 patients were deceased. Out of the 101 that continued, BMI was reduced in all subgroups (Table 3, Figure 2), in particular for OW (from median value 27.4 to median value 26.6 kg/m2; p < 0.001) and OB (from median value 33.5 kg/m2 to median value 31.8 kg/m2; p < 0.001). In the OB subgroup, a reduction in FM% compared to baseline (Δ = −3.30 ± 3.22 %; p < 0.05), an increase in HDL cholesterol (p < 0.05), and a decrease in triglycerides (p < 0.05) were also registered. No difference was seen in the dropout rate at 6 months between different initial BMI subgroups (χ2 = 3.099; p = 0.212). The prevalence of NW/OW/OB and attrition rates are displayed in Table 4. BMI was not independently linked to attrition, and after dividing the whole sample into completers and dropouts, the investigation of attrition differences showed no statistically significant results (Table 5).
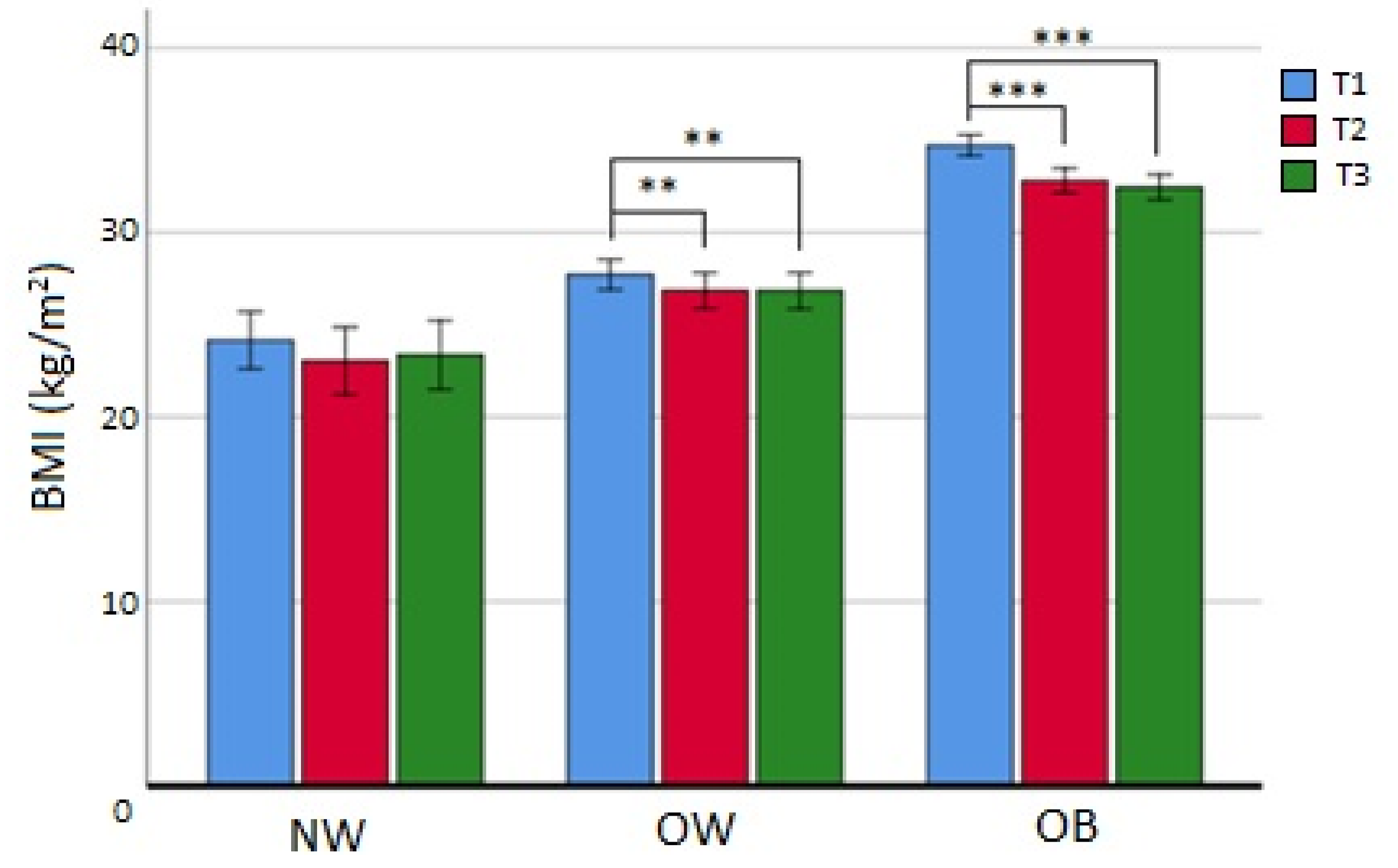
- At 12 months (T3), 61 patients (33.9% of the initial sample) had continued the study, and 40 (21.9% of the initial sample and 34.2% of all dropouts) were lost at follow-up. Patients that completed T3 and were in the NW subgroup, contrarily to T2 completers, did not reach statistical significance for BMI reduction, while OW patients maintained the same BMI between T2 and T3 (p = ns). Finally, subjects in the OB group, even if the difference between medians was not statistically significant, appeared to have additionally reduced their BMI (from T1 median value BMIOB 33.7 to T3 median value BMIOB 31.9 kg/m2; p = 0.062) (Figure 3). Also in this situation, there were no observed differences in the dropout rate at 12 months among the BMI subgroups (χ2 = 1.120; p = 0.571). Nevertheless, the analysis of differences at baseline according to T3 completers and dropouts showed that age (p < 0.05), menopausal status (p < 0.01), HDL cholesterol (p < 0.05), and diastolic blood pressure (DBP) (p < 0.05) were statistically different (Table 6), while weight loss or BMI loss was not different in any BMI subgroup (p = ns). Finally, multivariate logistic analysis evidenced that lower age (in interaction with menopausal status) (ORage/mp=no = 0.91; CI95%: 0.80–1.01; p = 0.092; ORage/mp=yes = 0.91; CI95%: 0.82–1.0.99; p < 0.05) and higher DBP (OR = 1.07; CI95%: 1.02–1.12; p < 0.01) at baseline were significant predictors of dropout at 12 months.
- At 24 months, only 26 patients (14.6% of the initial sample) continued the study for the last visit. Only 2 patients were part of the NW group (7.7% of the whole sample and 10.5% of the NW original group), while OW and OB groups had 7 and 17 women continuing till 24 months, respectively (26.9% and 65.4% respectively of the whole sample, and 12.3% and 16.7% of their original group). Due to the high attrition, we did not analyze data from these patients as the results would be underpowered.
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Steck, S.E.; Murphy, E.A. Dietary patterns and cancer risk. Nat. Rev. Cancer 2019, 20, 125–138. [Google Scholar] [PubMed]
- Cava, E.; Marzullo, P.; Farinelli, D.; Gennari, A.; Saggia, C.; Riso, S.; Prodam, F. Breast Cancer Diet “BCD”: A Review of Healthy Dietary Patterns to Prevent Breast Cancer Recurrence and Reduce Mortality. Nutrients 2022, 14, 476. [Google Scholar] [PubMed]
- Nimptsch, K.; Pischon, T. Obesity Biomarkers, Metabolism and Risk of Cancer: An Epidemiological Perspective. Recent Results Cancer Res. 2016, 208, 199–217. [Google Scholar] [PubMed]
- Gunter, M.J.; Xie, X.; Xue, X.; Kabat, G.C.; Rohan, T.E.; Wassertheil-Smoller, S.; Ho, G.Y.F.; Wylie-Rosett, J.; Greco, T.; Yu, H.; et al. Breast cancer risk in metabolically healthy but overweight postmenopausal women. Cancer Res. 2015, 75, 270–274. [Google Scholar]
- Berrino, F.; Villarini, A.; Traina, A.; Bonanni, B.; Panico, S.; Mano, M.P.; Mercandino, A.; Galasso, R.; Barbero, M.; Simeoni, M.; et al. Metabolic syndrome and breast cancer prognosis. Breast Cancer Res. Treat. 2014, 147, 159–165. [Google Scholar] [PubMed]
- Capasso, I.; Esposito, E.; Pentimalli, F.; Crispo, A.; Montella, M.; Grimaldi, M.; De Marco, M.; Cavalcanti, E.; D’Aiuto, M.; Fucito, A.; et al. Metabolic syndrome affects breast cancer risk in postmenopausal women: National Cancer Institute of Naples experience. Cancer Biol. Ther. 2010, 10, 1240–1243. [Google Scholar]
- Khandekar, M.J.; Cohen, P.; Spiegelman, B.M. Molecular mechanisms of cancer development in obesity. Nat. Rev. Cancer 2011, 11, 886–895. [Google Scholar]
- Ligibel, J.A.; Alfano, C.M.; Courneya, K.S.; Demark-Wahnefried, W.; Burger, R.A.; Chlebowski, R.T.; Fabian, C.J.; Gucalp, A.; Hershman, D.L.; Hudson, M.M.; et al. American Society of Clinical Oncology position statement on obesity and cancer. J. Clin. Oncol. 2014, 32, 3568–3574. [Google Scholar]
- Agnoli, C.; Berrino, F.; Abagnato, C.A.; Muti, P.; Panico, S.; Crosignani, P.; Krogh, V. Metabolic syndrome and postmenopausal breast cancer in the ORDET cohort: A nested case-control study. Nutr. Metab. Cardiovasc. Dis. 2010, 20, 41–48. [Google Scholar]
- Chen, G.; Leary, S.; Niu, J.; Perry, R.; Papadaki, A. The Role of the Mediterranean Diet in Breast Cancer Survivorship: A Systematic Review and Meta-Analysis of Observational Studies and Randomised Controlled Trials. Nutrients 2023, 15, 2099. [Google Scholar]
- Shaikh, H.; Bradhurst, P.; Ma, L.X.; Tan, S.Y.; Egger, S.J.; Vardy, J.L. Body weight management in overweight and obese breast cancer survivors. Cochrane Database Syst. Rev. 2020, 12. [Google Scholar] [CrossRef]
- Chan, D.S.M.; Vieira, A.R.; Aune, D.; Bandera, E.V.; Greenwood, D.C.; McTiernan, A.; Navarro Rosenblatt, D.; Thune, I.; Vieira, R.; Norat, T. Body mass index and survival in women with breast cancer-systematic literature review and meta-analysis of 82 follow-up studies. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2014, 25, 1901–1914. [Google Scholar]
- Dandamudi, A.; Tommie, J.; Nommsen-Rivers, L.; Couch, S. Dietary Patterns and Breast Cancer Risk: A Systematic Review. Anticancer Res. 2018, 38, 3209–3222. [Google Scholar] [CrossRef] [PubMed]
- Van den Brandt, P.A.; Schulpen, M. Mediterranean diet adherence and risk of postmenopausal breast cancer: Results of a cohort study and meta-analysis. Int. J. Cancer 2017, 140, 2220–2231. [Google Scholar] [PubMed]
- Weigl, J.; Hauner, H.; Hauner, D. Can Nutrition Lower the Risk of Recurrence in Breast Cancer. Breast Care 2018, 13, 86–91. [Google Scholar] [CrossRef]
- Esposito, K.; Kastorini, C.M.; Panagiotakos, D.B.; Giugliano, D. Mediterranean diet and weight loss: Meta-analysis of randomized controlled trials. Metab. Syndr. Relat. Disord. 2011, 9, 1–12. [Google Scholar] [CrossRef]
- Tosti, V.; Bertozzi, B.; Fontana, L. Health Benefits of the Mediterranean Diet: Metabolic and Molecular Mechanisms. J. Gerontol. A Biol. Sci. Med. Sci. 2018, 73, 318–326. [Google Scholar]
- Caesar, R.; Tremaroli, V.; Kovatcheva-Datchary, P.; Cani, P.D.; Bäckhed, F. Crosstalk between Gut Microbiota and Dietary Lipids Aggravates WAT Inflammation through TLR Signaling. Cell Metab. 2015, 22, 658–668. [Google Scholar] [CrossRef]
- Yang, J.; Tan, Q.; Fu, Q.; Zhou, Y.; Hu, Y.; Tang, S.; Zhou, Y.; Zhang, J.; Qiu, J.; Lv, Q. Gastrointestinal microbiome and breast cancer: Correlations, mechanisms and potential clinical implications. Breast Cancer 2017, 24, 220–228. [Google Scholar]
- Caputo, M.; Pigni, S.; Antoniotti, V.; Agosti, E.; Caramaschi, A.; Antonioli, A.; Aimaretti, G.; Manfredi, M.; Bona, E.; Prodam, F. Targeting microbiota in dietary obesity management: A systematic review on randomized control trials in adults. Crit. Rev. Food Sci. Nutr. 2022, 8, 1–33. [Google Scholar]
- Miller, B.M.L.; Brennan, L. Measuring and reporting attrition from obesity treatment programs: A call to action! Obes. Res. Clin. Pract. 2015, 9, 187–202. [Google Scholar] [CrossRef] [PubMed]
- Leung, A.W.Y.; Chan, R.S.M.; Sea, M.M.M.; Woo, J. An Overview of Factors Associated with Adherence to Lifestyle Modification Programs for Weight Management in Adults. Int. J. Environ. Res. Public Health 2017, 14, 922. [Google Scholar] [CrossRef] [PubMed]
- Serra-Majem, L.; Tomaino, L.; Dernini, S.; Berry, E.M.; Lairon, D.; de la Cruz, J.N.; Bach-Faig, A.; Donini, L.M.; Medina, F.X.; Belahsen, R.; et al. Updating the Mediterranean Diet Pyramid towards Sustainability: Focus on Environmental Concerns. Int. J. Environ. Res. Public Health 2020, 17, 8758. [Google Scholar] [CrossRef] [PubMed]
- Finocchiaro, C.; Ossola, M.; Monge, T.; Fadda, M.; Brossa, L.; Caudera, V.; De Francesco, A. Effect of specific educational program on dietary change and weight loss in breast-cancer survivors. Clin. Nutr. 2016, 35, 864–870. [Google Scholar] [CrossRef]
- Grosso, G.; Bella, F.; Godos, J.; Sciacca, S.; Del Rio, D.; Ray, S.; Galvano, F.; Giovannucci, E.L. Possible role of diet in cancer: Systematic review and multiple meta-analyses of dietary patterns, lifestyle factors, and cancer risk. Nutr. Rev. 2017, 75, 405–419. [Google Scholar] [CrossRef]
- James, F.R.; Wootton, S.; Jackson, A.; Wiseman, M.; Copson, E.R.; Cutress, R.I. Obesity in breast cancer—What is the risk factor? Eur. J. Cancer 2015, 51, 705–720. [Google Scholar] [CrossRef]
- Ecker, B.L.; Lee, J.Y.; Sterner, C.J.; Solomon, A.C.; Pant, D.K.; Shen, F.; Peraza, J.; Vaught, L.; Mahendra, S.; Belka, G.K.; et al. Impact of obesity on breast cancer recurrence and minimal residual disease. Breast Cancer Res. 2019, 21, 1–16. [Google Scholar] [CrossRef]
- Campbell, K.L.; Van Patten, C.L.; Neil, S.E.; Kirkham, A.A.; Gotay, C.C.; Gelmon, K.A.; McKenzie, D.C. Feasibility of a lifestyle intervention on body weight and serum biomarkers in breast cancer survivors with overweight and obesity. J. Acad. Nutr. Diet. 2012, 112, 559–567. [Google Scholar] [CrossRef]
- Rock, C.L.; Flatt, S.W.; Byers, T.E.; Colditz, G.A.; Demark-Wahnefried, W.; Ganz, P.A.; Wolin, K.Y.; Elias, A.; Krontiras, H.; Liu, J.; et al. Results of the Exercise and Nutrition to Enhance Recovery and Good Health for You (ENERGY) Trial: A Behavioral Weight Loss Intervention in Overweight or Obese Breast Cancer Survivors. J. Clin. Oncol. 2015, 33, 3169–3176. [Google Scholar] [CrossRef]
- Wong, A.L.; Seng, K.Y.; Ong, E.M.; Wang, L.Z.; Oscar, H.; Cordero, M.T.; Copones, R.; Fan, L.; Tan, S.H.; Goh, B.C.; et al. Body fat composition impacts the hematologic toxicities and pharmacokinetics of doxorubicin in Asian breast cancer patients. Breast Cancer Res. Treat. 2014, 144, 143–152. [Google Scholar] [CrossRef]
- Gurunathan, U.; Myles, P.S. Limitations of body mass index as an obesity measure of perioperative risk. Br. J. Anaesth. 2016, 116, 319–321. [Google Scholar] [CrossRef] [PubMed]
- Gill, R.S.; Karmali, S.; Hadi, G.; Al-Adra, D.P.; Shi, X.; Birch, D.W. Predictors of attrition in a multidisciplinary adult weight management clinic. Can. J. Surg. 2012, 55, 239–243. [Google Scholar] [CrossRef] [PubMed]
- Dalle Grave, R.; Calugi, S.; Marchesini, G. The influence of cognitive factors in the treatment of obesity: Lessons from the QUOVADIS study. Behav. Res. Ther. 2014, 63, 157–161. [Google Scholar] [CrossRef] [PubMed]
- Dalle Grave, R.; Calugi, S.; Compare, A.; El Ghoch, M.; Petroni, M.L.; Tomasi, F.; Mazzali, G.; Marchesini, G. Weight Loss Expectations and Attrition in Treatment-Seeking Obese Women. Obes. Facts 2015, 8, 311–318. [Google Scholar] [CrossRef]
- Zuniga, K.E.; Parma, D.L.; Muñoz, E.; Spaniol, M.; Wargovich, M.; Ramirez, A.G. Dietary intervention among breast cancer survivors increased adherence to a Mediterranean-style, anti-inflammatory dietary pattern: The Rx for Better Breast Health Randomized Controlled Trial. Breast Cancer Res. Treat. 2019, 173, 145–154. [Google Scholar] [CrossRef] [PubMed]
- Wurz, A.; St-Aubin, A.; Brunet, J. Breast cancer survivors’ barriers and motives for participating in a group-based physical activity program offered in the community. Support. Care Cancer 2015, 23, 2407–2416. [Google Scholar] [CrossRef] [PubMed]
- Ortner Hadžiabdić, M.; Mucalo, I.; Hrabač, P.; Matić, T.; Rahelić, D.; Božikov, V. Factors predictive of drop-out and weight loss success in weight management of obese patients. J. Hum. Nutr. Diet. 2015, 28 (Suppl. 2), 24–32. [Google Scholar] [CrossRef]
- Moroshko, I.; Brennan, L.; O’Brien, P. Predictors of dropout in weight loss interventions: A systematic review of the literature. Obes. Rev. 2011, 12, 912–934. [Google Scholar] [CrossRef]
- Rothberg, A.E.; McEwen, L.N.; Kraftson, A.T.; Ajluni, N.; Fowler, C.E.; Miller, N.M.; Zurales, K.R.; Herman, W.H. Factors associated with participant retention in a clinical, intensive, behavioral weight management program. BMC Obes. 2015, 2, 1–7. [Google Scholar] [CrossRef]
| Baseline Characteristics | N (%) |
|---|---|
| Age | |
| Mean + SD | 53.9 ± 10.4 |
| Median (IQR) | 53 (15) |
| Educational Level (missing = 19) | |
| Middle school or lower | 66 (40.5%) |
| High school or technical institutes | 73 (44.8%) |
| Degree (any) | 24 (14.7%) |
| Menopausal state (missing = 17) | |
| Yes | 84 (50.9%) |
| No | 81 (49.1%) |
| Breastfeeding history (missing = 17) | |
| Yes | 94 (57%) |
| No | 71 (43%) |
| Smoking history (missing = 17) | |
| Yes | 65 (39.4%) |
| No | 100 (60.6%) |
| Habitual alcohol consumption (missing = 18) | |
| Yes | 66 (40.2%) |
| No | 98 (59.8%) |
| Years since first breast cancer diagnosis (missing = 13) | |
| From 0 to 4 | 125 (74%) |
| 5 or more | 44 (26%) |
| Breast cancer medications (missing = 18) | |
| Aromatase inhibitors | 62 (37.8%) |
| SERMs | 24 (14.6%) |
| Anti-HER2 | 32 (19.5%) |
| GnRH agonists No therapy Combination of two drugs | 4 (2.4%) 20 (12.2%) 22 (13.4%) |
| -Aromatase inhibitors + Anti-HER2 | 6 (3.7%) |
| -Aromatase inhibitors + SERMs | 13 (7.9%) |
| -Aromatase inhibitors + GnRH agonists | 1 (0.6%) |
| -SERMs + Anti-HER2 | 1 (0.6%) |
| -SERMs + GnRH agonists | 1 (0.6%) |
| NW | OW | OB | p-Value | |
|---|---|---|---|---|
| Patients (%) | 20 (11%) | 59 (32.4%) | 103 (56.6%) | |
| Age (y) | 52 (15) | 51 (10) | 53 (17) | Ns |
| BW(Kg) | 61.9 (7.2) | 71.2 (7.8) | 87.7 (15.8) | <0.001 1 |
| BMI (Kg/m2) | 23.7 (1.1) | 27.6 (2.4) | 34.1 (5.5) | <0.001 1 |
| Waist (cm) | 88.8 ± 6.4 | 98.5 ± 6.6 | 112.9 ± 9.8 | <0.001 1 |
| PA (°) | 5.1 ± 0.47 | 5.2 ± 0.4 | 5.2 ± 0.5 | Ns |
| FM% | 29.8 ± 3 | 34.4 ± 4.5 | 43.3 ± 4.6 | <0.001 2 |
| ECW % | 50.4 ± 2.6 | 49.6 ± 2.4 | 49.7 ± 2.8 | Ns |
| Glucose (mg/dL) | 85 (11.5) | 88(13.5) | 96 (17) | <0.001 3 |
| Insulin (μUI/mL) | 8.7 ± 6.2 | 11.7 ± 11.6 | 15.5 ± 9.2 | Ns |
| HOMA index | 1.79 ± 1.26 | 2.59 ± 2.5 | 4.0 ± 3.0 | <0.05 4 |
| Tot Chol (mg/dL) | 204.8 ± 39.4 | 206.4 ± 35.6 | 207.9 ± 40.4 | Ns |
| HDL (mg/dL) | 55 (18) | 56.5 (17.5) | 50.5 (20) | Ns |
| LDL (mg/dL) | 122.8 ± 31.7 | 124.0 ± 28.6 | 125.6 ± 39.3 | Ns |
| Triglycerides (mg/dL) | 91 (69.2) | 106 (87) | 130.5 (96) | Ns |
| SBP (mmHg) | 120 ± 8.9 | 117.9 ± 15.7 | 129.1 ± 15.3 | <0.01 5 |
| DBP (mmHg) | 76.2 ± 6.9 | 72.9 ± 9.4 | 80.3 ± 11.9 | <0.05 5 |
| NW | OW | OB | ||||
|---|---|---|---|---|---|---|
| T1 | T2 | T1 | T2 | T1 | T2 | |
| BW (Kg) | 61.9 (3.0) | 58.8 (3.7) °° | 71.0 (5.4) | 66.5 (7.4) °°° | 85.2 (16.8) | 79.0 (16.0) °°° |
| BMI (Kg/m2) | 24.0 (1.4) | 23.0 (1.6) °° | 27.4 (2.6) | 26.6 (2.1) °°° | 33.5 (4.8) | 31.8 (5.7) °°° |
| Waist (cm) | 87.6 ± 9.1 | 84.6 ± 7.2 * | 97.9 ± 5.7 | 94.2 ± 7.6 *** | 111.8 ± 8.4 | 106.9 ± 9.2 *** |
| FM % (% of BW) | 28.7 ± 0.5 | 28.4 ± 1.3 | 34.1 ± 7.1 | 33.2 ± 7.2 | 42.7 ± 4.4 | 39.4 ± 6.1 * |
| ECW % (% of TBW) | 51.1 ± 4.0 | 52.2 ± 2.2 | 49.5 ± 2.5 | 49.2 ± 1.3 | 48.8 ± 3.3 | 48.2 ± 5.8 |
| PA (°) | 5.0 ± 0.7 | 4.8 ± 0.4 | 5.2 ± 0.4 | 5.3 ± 0.2 | 5.4 ± 0.6 | 5.6 ± 1.3 |
| Glucose (mg/dL) | 82.0 (7.0) | 83.0 (8.0) | 88.5 (14.7) | 90.0 (17.7) | 96.0 (17.0) | 97.0 (20.5) |
| Tot Chol (mg/dL) | 222.0 ± 28.3 | 206.5 ± 6.4 | 212.9 ± 38.3 | 207.2 ± 35.7 | 207.5 ± 39.6 | 200.5 ± 31.4 |
| HDL (mg/dL) | 54.0 (5.7) | 55.0 (4.8) | 57.0 (19.0) | 53.0 (24.0) | 50.5 (18.5) | 52.5 (13.7) ° |
| Triglycerides (mg/dL) | 77.0 (132.2) | 78.5 (68.4) | 97.0 (86.0) | 95.0 (110.0) | 136.5 (94.0) | 113.5 (73.5) ° |
| T1 | T2 | T3 | |
|---|---|---|---|
| Time | Baseline | 6 months | 12 months |
| N (% of the first sample) | 182 | 101 (55.5) | 61 (33.9) |
| NW (% of total) | 19 (10.4) | 14 (14.5) | 9 (24.6) |
| OW (% of total) | 57 (31.3) | 38 (37.6) | 28 (45.9) |
| OB (% of total) | 102 (57.3) | 49 (47.5) | 24 (39.3) |
| Class I–II–II obesity (% of OB) | 50.0–30.4–19.6 | 60.8–21.4–17.8 | 58.3–25.1–16.6 |
| N | Completers 101 | Dropouts 77 | p-Value |
|---|---|---|---|
| Age (y) | 53.0 (15.0) | 52.0 (15.0) | 0.528 |
| Educational level | 1 = 40 2 = 41 3 = 13 | 1 = 25 2 = 29 3 = 11 | Χ2 = 0.410 p = 0.815 |
| Menopausal state | No = 42 Yes = 53 | No = 36 Yes = 30 | Χ2 = 1.665 p = 0.197 |
| Breastfeeding history | No = 47 Yes = 48 | No = 23 Yes = 43 | Χ2 = 3.390 p = 0.066 |
| Smoking history | No = 58 Yes = 37 | No = 39 Yes = 27 | Χ2 = 0.063 p = 0.802 |
| Habitual alcohol consumption | No = 58 Yes = 36 | No = 36 Yes = 30 | Χ2 = 0.819 p = 0.395 |
| Years since first BC diagnosis | From 0 to 4 = 72 5 or more = 25 | From 0 to 4 = 50 5 or more = 18 | Χ2 = 0.010 p = 0.920 |
| BC medications | Anti-HER2 = 18 Aromatase inhib = 32 SERM = 15 GnRh agonist = 3 Combinations = 14 No therapy = 12 | Anti-HER2 = 14 Aromatase inhib = 29 SERM = 9 GnRh agonist = 1 Combinations = 7 No therapy = 6 | Χ2 = 5.229 p = 0.515 |
| Body weight (Kg) | 78.2 (17.8) | 78.3 (18.6) | 0.801 |
| BMI (Kg/m2) | 31.4 (6.6) | 30.6 (9.5) | 0.450 |
| Waist (cm) | 106.0 ± 11.5 | 105.5 ± 13.3 | 0.784 |
| PA (°) | 5.25 ± 0.46 | 5.18 ± 0.50 | 0.548 |
| FM % | 38.4 ± 7.5 | 36.8 ± 6.4 | 0.378 |
| ECW % | 49.6 ± 2.5 | 50.0 ± 2.7 | 0.608 |
| Glucose (mg/dL) | 90.0 (18.7) | 92.0 (14.0) | 0.551 |
| Insulin (μUI/mL) | 13.5 ± 10.3 | 13.4 ± 7.9 | 0.943 |
| HOMA index | 3.28 ± 2.66 | 3.23 ± 2.14 | 0.934 |
| Tot Chol (mg/dL) | 205.3 ± 35.9 | 211.7 ± 43.4 | 0.337 |
| HDL (mg/dL) | 52.5 (17.5) | 52.5 (17.5) | 0.639 |
| LDL (mg/dL) | 122.2 ± 32.4 | 132.2 ± 39.0 | 0.217 |
| Triglycerides (mg/dL) | 122.0 (100.0) | 123.0 (88.5) | 0.863 |
| SBP (mmHg) | 125.5 ± 14.2 | 124.6 ± 18.7 | 0.805 |
| DBP (mmHg) | 77.7 ± 10.9 | 78.4 ± 12.0 | 0.781 |
| N | Completers 61 | Dropouts 117 | p-Value |
| Age (y) | 55.0 (15.0) | 51.0 (15.0) | <0.05 |
| Educational level | 1 = 28 2 = 22 3 = 7 | 1 = 37 2 = 48 3 = 17 | Χ2 = 2.537 p = 0.281 |
| Menopausal state | No = 20 Yes = 38 | No = 58 Yes = 45 | Χ2 = 7.079 p < 0.01 |
| Breastfeeding history | No = 27 Yes = 31 | No = 43 Yes = 60 | Χ2 = 0.348 p = 0.555 |
| Smoking history | No = 36 Yes = 22 | No = 61 Yes = 42 | Χ2 = 0.125 p = 0.723 |
| Habitual alcohol consumption | No = 38 Yes = 19 | No = 56 Yes = 47 | Χ2 = 2.290 p = 0.130 |
| Years since first BC diagnosis | From 0 to 4 = 42 5 or more = 17 | From 0 to 4 = 80 5 or more = 26 | Χ2 = 0.361 p = 0.548 |
| BC medications | Anti-HER2 = 7 Aromatase inhib = 20 SERM = 8 GnRh agonist = 2 Combinations = 12 No therapy = 8 | Anti-HER2 = 25 Aromatase inhib = 41 SERM = 16 GnRh agonist = 2 Combinations = 9 No therapy = 10 | Χ2 = 9.555 p = 0.145 |
| Body weight (Kg) | 77.5 (18.9) | 78.8 (17.8) | 0.822 |
| BMI (Kg/m2) | 30.8 (6.7) | 30.8 (8.3) | 0.578 |
| Waist (cm) | 106.1 ± 11.9 | 105.6 ± 12.6 | 0.773 |
| PA (°) | 5.14 ± 0.42 | 5.23 ± 0.50 | 0.530 |
| FM% | 34.9 ± 7.1 | 38.3 ± 6.7 | 0.113 |
| ECW % | 50.2 ± 2.5 | 49.7 ± 2.6 | 0.528 |
| Glucose (mg/dL) | 91.0 (19.8) | 90.0 (16.0) | 0.539 |
| Insulin (μUI/mL) | 13.9 ± 11.2 | 13.1 ± 8.4 | 0.666 |
| HOMA index | 3.35 ± 2.61 | 3.20 ± 2.47 | 0.769 |
| Tot Chol (mg/dL) | 205.0 ± 37.8 | 209.5 ± 39.6 | 0.493 |
| HDL (mg/dL) | 49.0 (15.5) | 55.0 (16.2) | <0.05 |
| LDL (mg/dL) | 120.9 ± 31.7 | 130.0 ± 37.5 | 0.242 |
| Triglycerides (mg/dL) | 133.0 (105.0) | 117.0 (82.0) | 0.129 |
| SBP (mmHg) | 122.3 ± 13.2 | 126.8 ± 17.1 | 0.194 |
| DBP (mmHg) | 74.4 ± 11.3 | 79.9 ± 10.9 | <0.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cava, E.; Spadaccini, D.; Aimaretti, G.; Marzullo, P.; Cavigiolo, B.; Farinelli, D.; Gennari, A.; Saggia, C.; Carbonelli, M.G.; Riso, S.; et al. Weight Management Effectiveness and Predictors of Dropout in Breast Cancer Survivors: A Retrospective Study. Cancers 2023, 15, 4401. https://doi.org/10.3390/cancers15174401
Cava E, Spadaccini D, Aimaretti G, Marzullo P, Cavigiolo B, Farinelli D, Gennari A, Saggia C, Carbonelli MG, Riso S, et al. Weight Management Effectiveness and Predictors of Dropout in Breast Cancer Survivors: A Retrospective Study. Cancers. 2023; 15(17):4401. https://doi.org/10.3390/cancers15174401
Chicago/Turabian StyleCava, Edda, Daniele Spadaccini, Gianluca Aimaretti, Paolo Marzullo, Beatrice Cavigiolo, Deborah Farinelli, Alessandra Gennari, Chiara Saggia, Maria Grazia Carbonelli, Sergio Riso, and et al. 2023. "Weight Management Effectiveness and Predictors of Dropout in Breast Cancer Survivors: A Retrospective Study" Cancers 15, no. 17: 4401. https://doi.org/10.3390/cancers15174401
APA StyleCava, E., Spadaccini, D., Aimaretti, G., Marzullo, P., Cavigiolo, B., Farinelli, D., Gennari, A., Saggia, C., Carbonelli, M. G., Riso, S., & Prodam, F. (2023). Weight Management Effectiveness and Predictors of Dropout in Breast Cancer Survivors: A Retrospective Study. Cancers, 15(17), 4401. https://doi.org/10.3390/cancers15174401










