Fertility Preservation in Children and Adolescents during Oncological Treatment—A Review of Healthcare System Factors and Attitudes of Patients and Their Caregivers
Abstract
Simple Summary
Abstract
1. Introduction
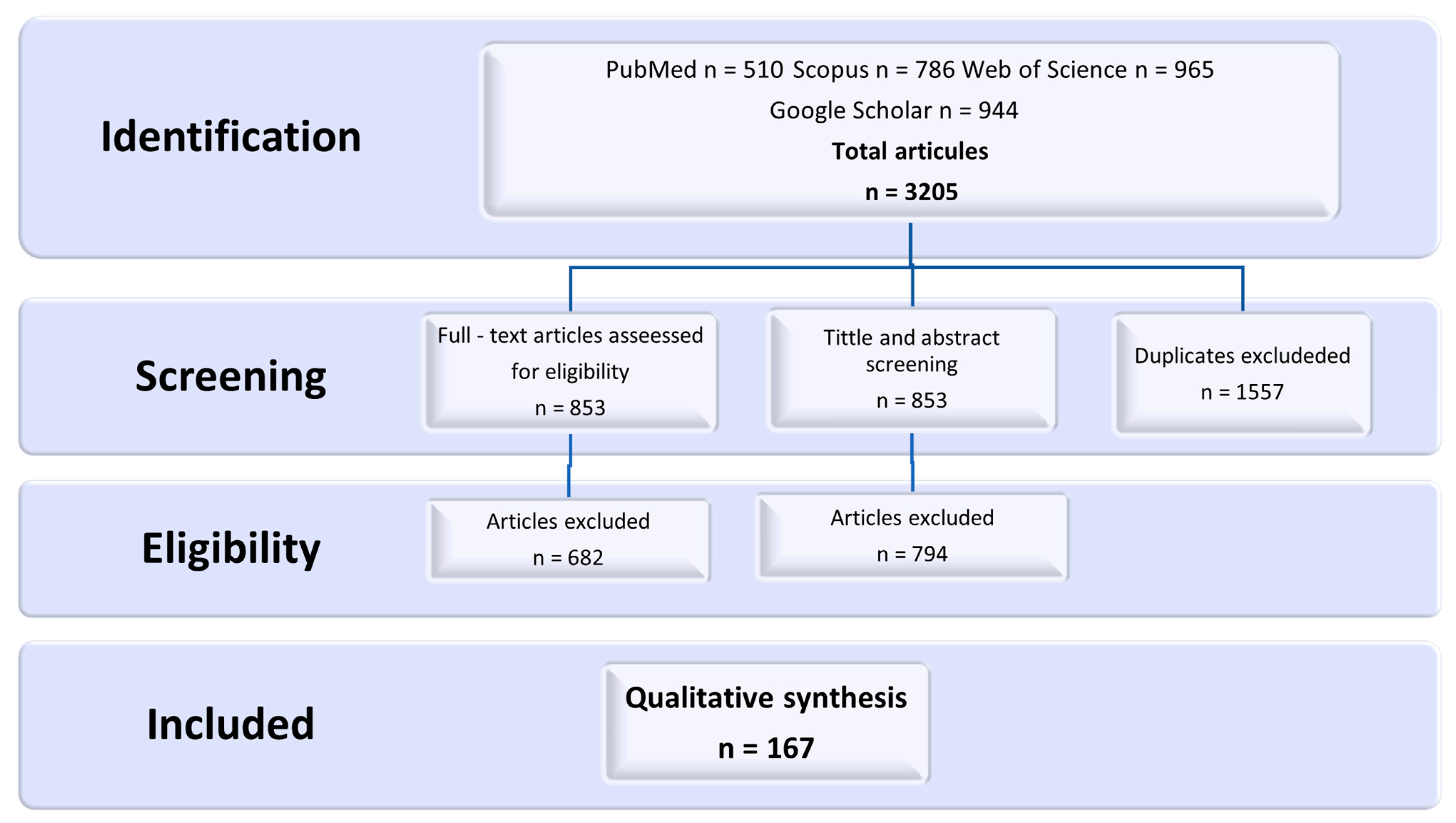
2. Materials and Methods
3. Results
3.1. Fertility Preservation Methods: To Whom, When and How They Should Be Offered
3.2. Oncofertility and Its Funding around the World
3.3. Oncofertility from the Perspective of Healthcare Professionals
3.4. Oncofertility from the Perspective of an Adolescent
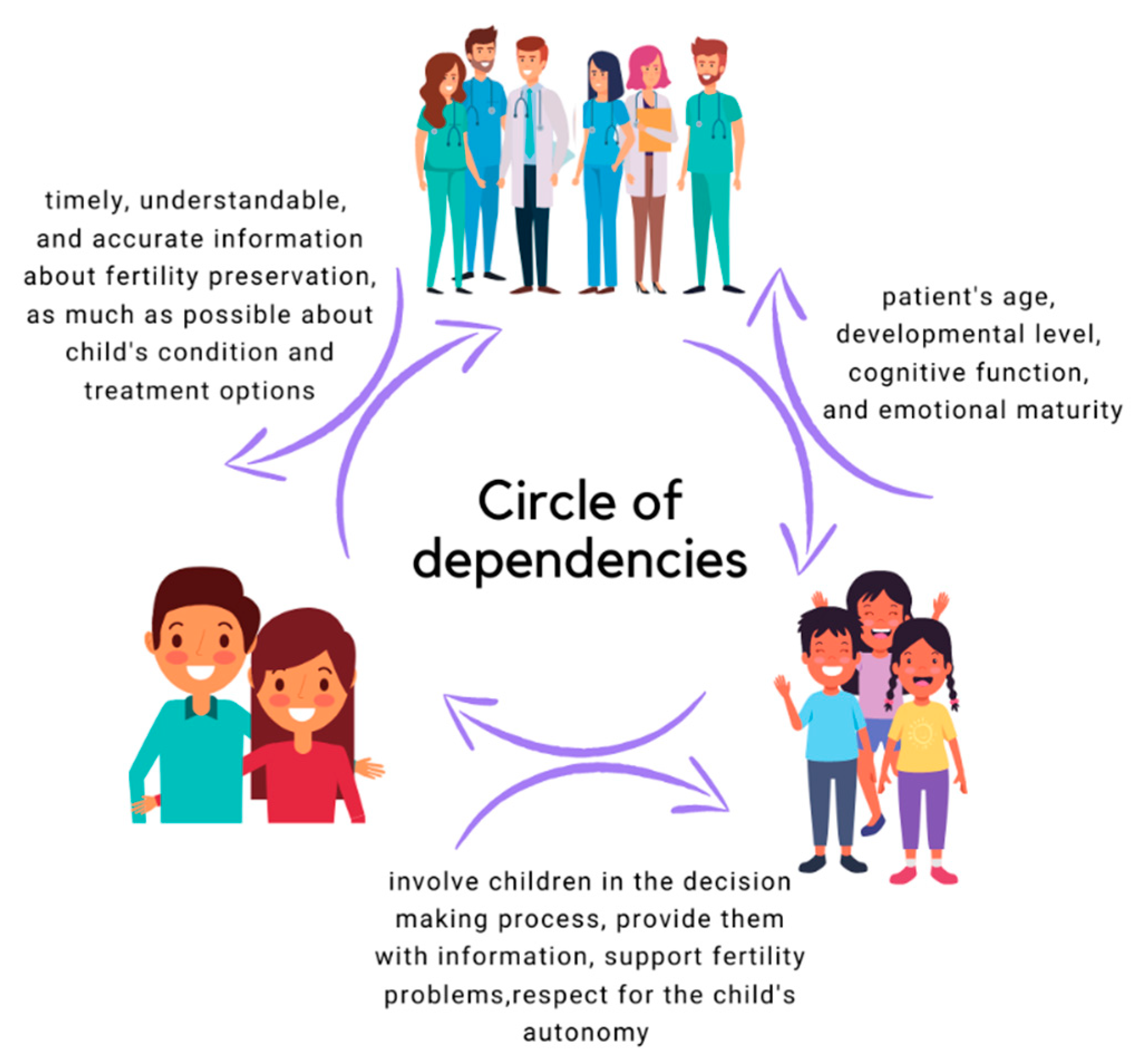
3.5. Oncofertility from the Perspective of Parents
3.6. Legal and Ethical Aspects of Oncofertility
4. Summary
5. Limitations
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Marcotte, E.L.; Domingues, A.M.; Sample, J.M.; Richardson, M.R.; Spector, L.G. Racial and Ethnic Disparities in Pediatric Cancer Incidence among Children and Young Adults in the United States by Single Year of Age. Cancer 2021, 127, 3651–3663. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef]
- Beltrami, A.; Hilliard, A.; Green, A.L. Demographic and Socioeconomic Disparities in Pediatric Cancer in the United States: Current Knowledge, Deepening Understanding, and Expanding Intervention. Cancer Epidemiol. 2022, 76, 102082. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2021. CA Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Johnston, W.T.; Erdmann, F.; Newton, R.; Steliarova-Foucher, E.; Schüz, J.; Roman, E. Childhood Cancer: Estimating Regional and Global Incidence. Cancer Epidemiol. 2021, 71, 101662. [Google Scholar] [CrossRef]
- Miller, K.D.; Fidler-Benaoudia, M.; Keegan, T.H.; Hipp, H.S.; Jemal, A.; Siegel, R.L. Cancer Statistics for Adolescents and Young Adults, 2020. CA Cancer J. Clin. 2020, 70, 443–459. [Google Scholar] [CrossRef]
- Schmidt, R.; Richter, D.; Sender, A.; Geue, K. Motivations for Having Children after Cancer--a Systematic Review of the Literature. Eur. J. Cancer Care 2016, 25, 6–17. [Google Scholar] [CrossRef]
- Ernst, M.; Brähler, E.; Wild, P.S.; Faber, J.; Merzenich, H.; Beutel, M.E. The Desire for Children among Adult Survivors of Childhood Cancer: Psychometric Evaluation of a Cancer-Specific Questionnaire and Relations with Sociodemographic and Psychological Characteristics. Psychooncology 2020, 29, 485–492. [Google Scholar] [CrossRef]
- Edge, B.; Holmes, D.; Makin, G. Sperm Banking in Adolescent Cancer Patients. Arch. Dis. Child. 2006, 91, 149–152. [Google Scholar] [CrossRef]
- Erdmann, F.; Frederiksen, L.E.; Bonaventure, A.; Mader, L.; Hasle, H.; Robison, L.L.; Winther, J.F. Childhood Cancer: Survival, Treatment Modalities, Late Effects and Improvements over Time. Cancer Epidemiol. 2021, 71, 101733. [Google Scholar] [CrossRef]
- Gilleland Marchak, J.; Elchuri, S.V.; Vangile, K.; Wasilewski-Masker, K.; Mertens, A.C.; Meacham, L.R. Perceptions of Infertility Risks Among Female Pediatric Cancer Survivors Following Gonadotoxic Therapy. J. Pediatr. Hematol. Oncol. 2015, 37, 368–372. [Google Scholar] [CrossRef] [PubMed]
- Michalczyk, K.; Cymbaluk-Płoska, A. Fertility Preservation and Long-Term Monitoring of Gonadotoxicity in Girls, Adolescents and Young Adults Undergoing Cancer Treatment. Cancers 2021, 13, 202. [Google Scholar] [CrossRef] [PubMed]
- Delessard, M.; Saulnier, J.; Rives, A.; Dumont, L.; Rondanino, C.; Rives, N. Exposure to Chemotherapy During Childhood or Adulthood and Consequences on Spermatogenesis and Male Fertility. Int. J. Mol. Sci. 2020, 21, 1454. [Google Scholar] [CrossRef] [PubMed]
- Anazodo, A.C.; Choi, S.; Signorelli, C.; Ellis, S.; Johnston, K.; Wakefield, C.E.; Deans, R.; Neville, K.A.; Cohn, R.J. Reproductive Care of Childhood and Adolescent Cancer Survivors: A 12-Year Evaluation. J. Adolesc. Young Adult Oncol. 2021, 10, 131–141. [Google Scholar] [CrossRef]
- Wei, C.; Crowne, E. The Impact of Childhood Cancer and Its Treatment on Puberty and Subsequent Hypothalamic Pituitary and Gonadal Function, in Both Boys and Girls. Best. Pract. Res. Clin. Endocrinol. Metab. 2019, 33, 101291. [Google Scholar] [CrossRef] [PubMed]
- Anazodo, A.; Ataman-Millhouse, L.; Jayasinghe, Y.; Woodruff, T.K. Oncofertility—An Emerging Discipline Rather than a Special Consideration. Pediatr. Blood Cancer 2018, 65, e27297. [Google Scholar] [CrossRef]
- Burns, K.C.; Hoefgen, H.; Strine, A.; Dasgupta, R. Fertility Preservation Options in Pediatric and Adolescent Patients with Cancer. Cancer 2018, 124, 1867–1876. [Google Scholar] [CrossRef]
- Anazodo, A.; Laws, P.; Logan, S.; Saunders, C.; Travaglia, J.; Gerstl, B.; Bradford, N.; Cohn, R.; Birdsall, M.; Barr, R.; et al. How Can We Improve Oncofertility Care for Patients? A Systematic Scoping Review of Current International Practice and Models of Care. Hum. Reprod. Update 2019, 25, 159–179. [Google Scholar] [CrossRef]
- Kim, S.; Kim, S.W.; Han, S.J.; Lee, S.; Park, H.T.; Song, J.Y.; Kim, T. Molecular Mechanism and Prevention Strategy of Chemotherapy- and Radiotherapy-Induced Ovarian Damage. Int. J. Mol. Sci. 2021, 22, 7484. [Google Scholar] [CrossRef]
- Zhang, P.; Mo, L.; Torres, J.; Huang, X. Effects of Cognitive Behavioral Therapy on Psychological Adjustment in Chinese Pediatric Cancer Patients Receiving Chemotherapy: A Randomized Trial. Medicine 2019, 98, e16319. [Google Scholar] [CrossRef]
- Warton, C.; McDougall, R.J. Fertility Preservation for Transgender Children and Young People in Paediatric Healthcare: A Systematic Review of Ethical Considerations. J. Med. Ethics 2022, 48, 1076–1082. [Google Scholar] [CrossRef] [PubMed]
- Nahata, L.; Dattilo, T.M.; Olsavsky, A.L.; Lipak, K.G.; Whiteside, S.; Yeager, N.D.; Audino, A.; Klosky, J.L.; Rausch, J.; Saraf, A.; et al. Impact of a novel family-centered values clarification tool on adolescent sperm banking attempts at the time of a new cancer diagnosis. J. Assist. Reprod. Genet. 2021, 38, 1561–1569. [Google Scholar] [CrossRef] [PubMed]
- di Mattei, V.E.; Perego, G.; Rancoita, P.M.V.; Taranto, P.; Carnelli, L.; Mangili, G.; Sarais, V.; Bergamini, A.; Candiani, M. Psychological Aspects Associated With Fertility Preservation in Oncology: An Exploratory Study. Front. Psychol. 2020, 11, 3618. [Google Scholar] [CrossRef]
- Stanhiser, J.; Steiner, A.Z. Psychosocial Aspects of Fertility and Assisted Reproductive Technology. Obstet. Gynecol. Clin. N. Am. 2018, 45, 563–574. [Google Scholar] [CrossRef] [PubMed]
- Klosky, J.L.; Simmons, J.L.; Russell, K.M.; Foster, R.H.; Sabbatini, G.M.; Canavera, K.E.; Hodges, J.R.; Schover, L.R.; McDermott, M.J. Fertility as a Priority among At-Risk Adolescent Males Newly Diagnosed with Cancer and Their Parents. Support. Care Cancer 2015, 23, 333–341. [Google Scholar] [CrossRef]
- Tian En, L.; Brougham, M.F.H.; Wallace, W.H.B.; Mitchell, R.T. Impacts of Platinum-Based Chemotherapy on Subsequent Testicular Function and Fertility in Boys with Cancer. Hum. Reprod. Update 2020, 26, 874–885. [Google Scholar] [CrossRef] [PubMed]
- Rives, N.; Courbière, B.; Almont, T.; Kassab, D.; Berger, C.; Grynberg, M.; Papaxanthos, A.; Decanter, C.; Elefant, E.; Dhedin, N.; et al. What should be done in terms of fertility preservation for patients with cancer? The French 2021 guidelines. Eur. J. Cancer 2022, 173, 146–166. [Google Scholar] [CrossRef]
- Kessler, S.; Marzooq, A.; Sood, A.; Beebe, K.; Walsh, A.; Montoya, L.; Price, H. Alopecia in Children Undergoing Chemotherapy, Radiation, and Hematopoietic Stem Cell Transplantation: Scoping Review and Approach to Management. Pediatr. Dermatol. 2022, 39, 354–362. [Google Scholar] [CrossRef]
- Hooke, M.C.; Linder, L.A. Symptoms in Children Receiving Treatment for Cancer—Part I: Fatigue, Sleep Disturbance, and Nausea/Vomiting. J. Pediatr. Oncol. Nurs. 2019, 36, 244–261. [Google Scholar] [CrossRef]
- Gupta, K.; Walton, R.; Kataria, S.P. Chemotherapy-Induced Nausea and Vomiting: Pathogenesis, Recommendations, and New Trends. Cancer Treat. Res. Commun. 2021, 26, 100278. [Google Scholar] [CrossRef]
- Madmoli, M. Evaluation of Chemotherapy Complications in Patients with Cancer: A Systematic Review. Int. J. Res. Stud. Sci. Eng. Technol. 2018, 5, 58–63. [Google Scholar]
- Moravek, M.B.; Appiah, L.C.; Anazodo, A.; Burns, K.C.; Gomez-Lobo, V.; Hoefgen, H.R.; Jaworek Frias, O.; Laronda, M.M.; Levine, J.; Meacham, L.R.; et al. Development of a Pediatric Fertility Preservation Program: A Report From the Pediatric Initiative Network of the Oncofertility Consortium. J. Adolesc. Health 2019, 64, 563–573. [Google Scholar] [CrossRef] [PubMed]
- Tercyak, K.; Mays, D.; Johnson, A.; Murphy, S.; Shad, A.T. Oncofertility and Quality of Life among Adolescent and Young Adult Survivors of Childhood Cancer. J. Clin. Oncol. 2016, 34, 222. [Google Scholar] [CrossRef]
- Johnson, A.C.; Mays, D.; Rehberg, K.; Shad, A.; Tercyak, K.P. Knowledge and Beliefs About Oncofertility and Associations with Quality of Life Among Adolescent and Young Adult Survivors of Pediatric Cancer. J. Adolesc. Young Adult Oncol. 2018, 7, 424–429. [Google Scholar] [CrossRef] [PubMed]
- Pedraza-Fariña, L.G. Constructing Interdisciplinary Collaboration: The Oncofertility Consortium as an Emerging Knowledge Commons. Governing Medical Knowledge Commons. Northwestern Law Econ. Res. Pap. 2016, 16, 259–284. [Google Scholar] [CrossRef]
- Mahey, R.; Kandpal, S.; Gupta, M.; Vanamail, P.; Bhatla, N.; Malhotra, N. Knowledge and Awareness about Fertility Preservation among Female Patients with Cancer: A Cross-Sectional Study. Obstet. Gynecol. Sci. 2020, 63, 480. [Google Scholar] [CrossRef]
- Nekhlyudov, L.; Aziz, N.M.; Lerro, C.; Virgo, K.S. Oncologists’ and Primary Care Physicians’ Awareness of Late and Long-Term Effects of Chemotherapy: Implications for Care of the Growing Population of Survivors. J. Oncol. Pract. 2014, 10, e29–e36. [Google Scholar] [CrossRef]
- Halpern, J.A.; Das, A.; Faw, C.A.; Brannigan, R.E. Oncofertility in Adult and Pediatric Populations: Options and Barriers. Transl. Androl. Urol. 2020, 9, S227–S238. [Google Scholar] [CrossRef]
- Lau, G.A.; Schaeffer, A.J. Pediatric Oncofertility: An Update. Transl. Androl. Urol. 2020, 9, 2416–2421. [Google Scholar] [CrossRef]
- Zalecenia Grupy Roboczej, ds. Zachowania Płodności u Chorych Onkologicznych i Chorych Hematologicznych oraz Innych Chorych Leczonych Terapiami Gonadotoksycznymi “ONCOFERTIILITY” (GROF) Polskiego Towarzystwa Ginekologii Onkologicznej. Available online: https://ptgo.pl/archiwa/rekomendacje/zalecenia-grupy-roboczej-ds-zachowania-plodnosci-u-chorych-onkologicznych-i-chorych-hematologicznych-oraz-innych-chorych-leczonych-terapiami-gonadotoksycznymi-oncofertility-grof-p (accessed on 13 August 2023).
- Mulder, R.L.; Font-Gonzalez, A.; Green, D.M.; Loeffen, E.A.H.; Hudson, M.M.; Loonen, J.; Yu, R.; Ginsberg, J.P.; Mitchell, R.T.; Byrne, J.; et al. PanCareLIFE Consortium. Fertility preservation for male patients with childhood, adolescent, and young adult cancer: Recommendations from the PanCareLIFE Consortium and the International Late Effects of Childhood Cancer Guideline Harmonization Group. Lancet Oncol. 2021, 22, e57–e67. [Google Scholar] [CrossRef]
- Balduzzi, A.; Dalle, J.H.; Jahnukainen, K.; von Wolff, M.; Lucchini, G.; Ifversen, M.; Macklon, K.T.; Poirot, C.; Diesch, T.; Jarisch, A.; et al. Fertility preservation issues in pediatric hematopoietic stem cell transplantation: Practical approaches from the consensus of the Pediatric Diseases Working Party of the EBMT and the International BFM Study Group. Bone Marrow Transpl. 2017, 52, 1406–1415. [Google Scholar] [CrossRef] [PubMed]
- Goossens, E.; Jahnukainen, K.; Mitchell, R.; van Pelt, A.; Pennings, G.; Rives, N.; Poels, J.; Wyns, C.; Lane, S.; Rodriguez-Wallberg, K.; et al. Fertility Preservation in Boys: Recent Developments and New Insights. Hum. Reprod. Open. 2020, 2020, hoaa016. [Google Scholar] [CrossRef] [PubMed]
- Bîcă, O.; Sârbu, I.; Ciongradi, C.I. Pediatric and Adolescent Oncofertility in Male Patients—From Alpha to Omega. Genes 2021, 12, 701. [Google Scholar] [CrossRef] [PubMed]
- Moussaoui, D.; Surbone, A.; Adam, C.; Diesch-Furlanetto, T.; Girardin, C.; Bénard, J.; Vidal, I.; Bernard, F.; Busiah, K.; Bouthors, T.; et al. Testicular tissue cryopreservation for fertility preservation in prepubertal and adolescent boys: A 6 year experience from a Swiss multi-center network. Front. Pediatr. 2022, 10, 909000. [Google Scholar] [CrossRef]
- Joshi, V.B.; Behl, S.; Pittock, S.T.; Arndt, C.A.S.; Zhao, Y.; Khan, Z.; Granberg, C.F.; Chattha, A. Establishment of a Pediatric Ovarian and Testicular Cryopreservation Program for Malignant and Non-Malignant Conditions: The Mayo Clinic Experience. J. Pediatr. Adolesc. Gynecol. 2021, 34, 673–680. [Google Scholar] [CrossRef]
- Ginsberg, J.P.; Li, Y.; Carlson, C.A.; Gracia, C.R.; Hobbie, W.L.; Miller, V.A.; Mulhall, J.; Shnorhavorian, M.; Brinster, R.L.; Kolon, T.F. Testicular tissue cryopreservation in prepubertal male children: An analysis of parental decision-making. Pediatr. Blood Cancer. 2014, 61, 1673–1678. [Google Scholar] [CrossRef]
- Hermann, B.P.; Sukhwani, M.; Winkler, F.; Pascarella, J.N.; Peters, K.A.; Sheng, Y.; Valli, H.; Rodriguez, M.; Ezzelarab, M.; Dargo, G.; et al. Spermatogonial Stem Cell Transplantation into Rhesus Testes Regenerates Spermatogenesis Producing Functional Sperm. Cell Stem Cell 2012, 11, 715–726. [Google Scholar] [CrossRef]
- Ntemou, E.; Kadam, P.; Van Laere, S.; Van Saen, D.; Vicini, E.; Goossens, E. Effect of Recombinant Human Vascular Endothelial Growth Factor on Testis Tissue Xenotransplants from Prepubertal Boys: A Three-Case Study. Reprod. Biomed. Online 2019, 39, 119–133. [Google Scholar] [CrossRef]
- Poels, J.; Abou-Ghannam, G.; Decamps, A.; Leyman, M.; Rieux, A.D.; Wyns, C. Transplantation of Testicular Tissue in Alginate Hydrogel Loaded with VEGF Nanoparticles Improves Spermatogonial Recovery. J. Control. Release 2016, 234, 79–89. [Google Scholar] [CrossRef]
- Sadri-Ardekani, H.; Akhondi, M.A.; Van Der Veen, F.; Repping, S.; Van Pelt, A.M.M. In Vitro Propagation of Human Prepubertal Spermatogonial Stem Cells. JAMA 2011, 305, 2416–2418. [Google Scholar] [CrossRef]
- Shetty, G.; Mitchell, J.M.; Lam, T.N.A.; Wu, Z.; Zhang, J.; Hill, L.; Tailor, R.C.; Peters, K.A.; Penedo, M.C.; Orwig, K.E.; et al. Donor Spermatogenesis in de Novo Formed Seminiferous Tubules from Transplanted Testicular Cells in Rhesus Monkey Testis. Hum. Reprod. 2018, 33, 2249–2255. [Google Scholar] [CrossRef] [PubMed]
- De Michele, F.; Poels, J.; Vermeulen, M.; Ambroise, J.; Gruson, D.; Guiot, Y.; Wyns, C. Haploid Germ Cells Generated in Organotypic Culture of Testicular Tissue From Prepubertal Boys. Front. Physiol. 2018, 9, 1413. [Google Scholar] [CrossRef] [PubMed]
- Sato, T.; Katagiri, K.; Gohbara, A.; Inoue, K.; Ogonuki, N.; Ogura, A.; Kubota, Y.; Ogawa, T. In Vitro Production of Functional Sperm in Cultured Neonatal Mouse Testes. Nature 2011, 471, 504–508. [Google Scholar] [CrossRef] [PubMed]
- Del-Pozo-Lérida, S.; Salvador, C.; Martínez-Soler, F.; Tortosa, A.; Perucho, M.; Giménez-Bonafé, P. Preservation of Fertility in Patients with Cancer (Review). Oncol. Rep. 2019, 41, 2607–2614. [Google Scholar] [CrossRef] [PubMed]
- Creux, H.; Monnier, P.; Son, W.Y.; Buckett, W. Thirteen Years’ Experience in Fertility Preservation for Cancer Patients after in Vitro Fertilization and in Vitro Maturation Treatments. J. Assist. Reprod. Genet. 2018, 35, 583–592. [Google Scholar] [CrossRef]
- Telfer, E.E.; Andersen, C.Y. In vitro growth and maturation of primordial follicles and immature oocytes. Fertil. Steril. 2021, 115, 1116–1125. [Google Scholar] [CrossRef] [PubMed]
- Karavani, G.; Schachter-Safrai, N.; Revel, A.; Mordechai-Daniel, T.; Bauman, D.; Imbar, T. In Vitro Maturation Rates in Young Premenarche Patients. Fertil. Steril. 2019, 112, 315–322. [Google Scholar] [CrossRef]
- Loren, A.W.; Mangu, P.B.; Beck, L.N.; Brennan, L.; Magdalinski, A.J.; Partridge, A.H.; Quinn, G.; Wallace, W.H.; Oktay, K. American Society of Clinical Oncology. Fertility preservation for patients with cancer: American Society of Clinical Oncology clinical practice guideline update. J. Clin. Oncol. 2013, 31, 2500–2510. [Google Scholar] [CrossRef]
- Harada, M.; Osuga, Y. Fertility Preservation for Female Cancer Patients. Int. J. Clin. Oncol. 2019, 24, 28–33. [Google Scholar] [CrossRef]
- Blumenfeld, Z. Fertility Preservation Using GnRH Agonists: Rationale, Possible Mechanisms, and Explanation of Controversy. Clin. Med. Insights Reprod. Health 2019, 13, 1179558119870163. [Google Scholar] [CrossRef]
- ESHRE Guideline Group on Female Fertility Preservation; Anderson, R.A.; Amant, F.; Braat, D.; D’Angelo, A.; Chuva de Sousa Lopes, S.M.; Demeestere, I.; Dwek, S.; Frith, L.; Lambertini, M.; et al. ESHRE guideline: Female fertility preservation. Hum. Reprod. Open. 2020, 2020, hoaa052. [Google Scholar] [CrossRef]
- ESHRE Guideline Female Fertility Preservation. Available online: https://www.eshre.eu/Guidelines-and-Legal/Guidelines/Female-fertility-preservation (accessed on 13 August 2023).
- Jensen, A.K.; Macklon, K.T.; Fedder, J.; Ernst, E.; Humaidan, P.; Andersen, C.Y. 86 Successful Births and 9 Ongoing Pregnancies Worldwide in Women Transplanted with Frozen-Thawed Ovarian Tissue: Focus on Birth and Perinatal Outcome in 40 of These Children. J. Assist. Reprod. Genet. 2017, 34, 325–336. [Google Scholar] [CrossRef] [PubMed]
- Donnez, J.; Dolmans, M.M.; Diaz, C.; Pellicer, A. Ovarian Cortex Transplantation: Time to Move on from Experimental Studies to Open Clinical Application. Fertil. Steril. 2015, 104, 1097–1098. [Google Scholar] [CrossRef] [PubMed]
- Affdal, A.O.; Ravitsky, V. Chapitre 9. Oncofertilité et jeunes filles prépubères: Un « droit à un avenir ouvert » ? [Chapitre 9. Oncofertility and prepubescent girls: A ‘right to an open future’?]. J. Int. Bioethique Ethique Sci. 2019, 30, 159–177. [Google Scholar] [CrossRef] [PubMed]
- Wallace, W.H.B.; Anderson, R.A.; Irvine, D.S. Fertility Preservation for Young Patients with Cancer: Who Is at Risk and What Can Be Offered? Lancet Oncol. 2005, 6, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Dolmans, M.M.; von Wolff, M.; Poirot, C.; Diaz-Garcia, C.; Cacciottola, L.; Boissel, N.; Liebenthron, J.; Pellicer, A.; Donnez, J.; Andersen, C.Y. Transplantation of Cryopreserved Ovarian Tissue in a Series of 285 Women: A Review of Five Leading European Centers. Fertil. Steril. 2021, 115, 1102–1115. [Google Scholar] [CrossRef]
- Bastings, L.; Beerendonk, C.C.M.; Westphal, J.R.; Massuger, L.F.A.G.; Kaal, S.E.J.; van Leeuwen, F.E.; Braat, D.D.M.; Peek, R. Autotransplantation of Cryopreserved Ovarian Tissue in Cancer Survivors and the Risk of Reintroducing Malignancy: A Systematic Review. Hum. Reprod. Update 2013, 19, 483–506. [Google Scholar] [CrossRef]
- Matthews, S.J.; Picton, H.; Ernst, E.; Andersen, C.Y. Successful Pregnancy in a Woman Previously Suffering from β-Thalassemia Following Transplantation of Ovarian Tissue Cryopreserved before Puberty. Minerva Ginecol. 2018, 70, 432–435. [Google Scholar] [CrossRef]
- Sönmezer, M.; Türküolu, I.; Cokun, U.; Oktay, K. Random-Start Controlled Ovarian Hyperstimulation for Emergency Fertility Preservation in Letrozole Cycles. Fertil. Steril. 2011, 95, 2125.e9–2125.e11. [Google Scholar] [CrossRef]
- Yu, L.; Wellenstein, W.; Streich-Tilles, T.; Breech, L. Laparoscopic Oophoropexy with Combined Fixation to Uterosacral Ligament and Pelvic Side Wall in a Prepubertal Girl with Recurrent Ovarian Torsion. J. Pediatr. Adolesc. Gynecol. 2022, 35, 241. [Google Scholar] [CrossRef]
- Published Recommendations from the International Late Effects of Childhood Cancer Guideline Harmonization Group. 2022. Available online: https://www.ighg.org/guidelines/topics/male-gonadotoxicity/recommendations/ (accessed on 15 August 2023).
- Salama, M.; Isachenko, V.; Isachenko, E.; Rahimi, G.; Mallmann, P. Updates in Preserving Reproductive Potential of Prepubertal Girls with Cancer: Systematic Review. Crit. Rev. Oncol. Hematol. 2016, 103, 10–21. [Google Scholar] [CrossRef] [PubMed]
- Tavana, S.; Valojerdi, M.R.; Azarnia, M.; Shahverdi, A. Restoration of Ovarian Tissue Function and Estrous Cycle in Rat after Autotransplantation Using Hyaluronic Acid Hydrogel Scaffold Containing VEGF and BFGF. Growth Factors 2016, 34, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Nahata, L.; Woodruff, T.K.; Quinn, G.P.; Meacham, L.R.; Chen, D.; Appiah, L.C.; Finlayson, C.; Orwig, K.E.; Laronda, M.M.; Rowell, E.E.; et al. Ovarian Tissue Cryopreservation as Standard of Care: What Does This Mean for Pediatric Populations? J. Assist. Reprod. Genet. 2020, 37, 1323–1326. [Google Scholar] [CrossRef] [PubMed]
- Pampanini, V.; Wagner, M.; Asadi-Azarbaijani, B.; Oskam, I.C.; Sheikhi, M.; Sjödin, M.O.D.; Lindberg, J.; Hovatta, O.; Sahlin, L.; Bjorvang, R.D.; et al. Reply: Impact of First-Line Cancer Treatment on Follicle Quality in Cryopreserved Ovarian Samples. Hum. Reprod. 2020, 35, 1249. [Google Scholar] [CrossRef]
- Pampanini, V.; Wagner, M.; Asadi-Azarbaijani, B.; Oskam, I.C.; Sheikhi, M.; Sjödin, M.O.D.; Lindberg, J.; Hovatta, O.; Sahlin, L.; Björvang, R.D.; et al. Impact of First-Line Cancer Treatment on the Follicle Quality in Cryopreserved Ovarian Samples from Girls and Young Women. Hum. Reprod. 2019, 34, 1674–1685. [Google Scholar] [CrossRef]
- Dolmans, M.M.; Falcone, T.; Patrizio, P. Importance of patient selection to analyze in vitro fertilization outcome with transplanted cryopreserved ovarian tissue. Fertil. Steril. 2020, 114, 279–280. [Google Scholar] [CrossRef]
- Demeestere, I.; Simon, P.; Dedeken, L.; Moffa, F.; Tsépélidis, S.; Brachet, C.; Delbaere, A.; Devreker, F.; Ferster, A. Live birth after autograft of ovarian tissue cryopreserved during childhood. Hum. Reprod. 2015, 30, 2107–2109. [Google Scholar] [CrossRef]
- Fraison, E.; Huberlant, S.; Labrune, E.; Cavalieri, M.; Montagut, M.; Brugnon, F.; Courbiere, B. Live birth rate after female fertility preservation for cancer or haematopoietic stem cell transplantation: A systematic review and meta-analysis of the three main techniques; embryo, oocyte and ovarian tissue cryopreservation. Hum. Reprod. 2023, 38, 489–502. [Google Scholar] [CrossRef]
- Yamashiro, C.; Sasaki, K.; Yabuta, Y.; Kojima, Y.; Nakamura, T.; Okamoto, I.; Yokobayashi, S.; Murase, Y.; Ishikura, Y.; Shirane, K.; et al. Generation of Human Oogonia from Induced Pluripotent Stem Cells in Vitro. Science 2018, 362, 356–360. [Google Scholar] [CrossRef]
- Brungardt, J.G.; Burns, K.C.; Dasgupta, R. Fertility Preservation in Children and Young Adults with Cancer. Curr. Opin. Pediatr. 2022, 34, 48–52. [Google Scholar] [CrossRef]
- Ellis, S.J.; Wakefield, C.E.; McLoone, J.K.; Robertson, E.G.; Cohn, R.J. Fertility Concerns among Child and Adolescent Cancer Survivors and Their Parents: A Qualitative Analysis. J. Psychosoc. Oncol. 2016, 34, 347–362. [Google Scholar] [CrossRef] [PubMed]
- Adam, C.; Deffert, C.; Leyvraz, C.; Primi, M.P.; Simon, J.P.; Beck Popovic, M.; Bénard, J.; Bouthors, T.; Girardin, C.; Streuli, I.; et al. Use and Effectiveness of Sperm Cryopreservation for Adolescents and Young Adults: A 37-Year Bicentric Experience. J. Adolesc. Young Adult Oncol. 2021, 10, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Gat, I.; Toren, A.; Hourvitz, A.; Raviv, G.; Band, G.; Baum, M.; Lerner-Geva, L.; Inbar, R.; Madgar, I. Sperm Preservation by Electroejaculation in Adolescent Cancer Patients. Pediatr. Blood Cancer 2014, 61, 286–290. [Google Scholar] [CrossRef]
- Otani, T. Clinical Review of Ejaculatory Dysfunction. Reprod. Med. Biol. 2019, 18, 331–343. [Google Scholar] [CrossRef] [PubMed]
- Ruan, X.; Cheng, J.; Du, J.; Jin, F.; Gu, M.; Li, Y.; Ju, R.; Wu, Y.; Wang, H.; Yang, W.; et al. Analysis of Fertility Preservation by Ovarian Tissue Cryopreservation in Pediatric Children in China. Front. Endocrinol. (Lausanne). 2022, 13, 930786. [Google Scholar] [CrossRef] [PubMed]
- Gellert, S.E.; Pors, S.E.; Kristensen, S.G.; Bay-Bjørn, A.M.; Ernst, E.; Yding Andersen, C. Transplantation of frozen-thawed ovarian tissue: An update on worldwide activity published in peer-reviewed papers and on the Danish cohort. J. Assist. Reprod. Genet. 2018, 35, 561–570. [Google Scholar] [CrossRef] [PubMed]
- Pors, S.E.; Ramløse, M.; Nikiforov, D.; Lundsgaard, K.; Cheng, J.; Yding Andersen, C.; Kristensen, S.G. Initial Steps in Reconstruction of the Human Ovary: Survival of Pre-Antral Stage Follicles in a Decellularized Human Ovarian Scaffold. Hum. Reprod. 2019, 34, 1523–1535. [Google Scholar] [CrossRef]
- Chiti, M.C.; Dolmans, M.M.; Orellana, R.; Soares, M.; Paulini, F.; Donnez, J.; Amorim, C.A. Influence of Follicle Stage on Artificial Ovary Outcome Using Fibrin as a Matrix. Hum. Reprod. 2016, 31, 427–435. [Google Scholar] [CrossRef]
- Oktay, K.; Harvey, B.E.; Partridge, A.H.; Quinn, G.P.; Reinecke, J.; Taylor, H.S.; Hamish Wallace, W.; Wang, E.T.; Loren, A.W. Fertility Preservation in Patients With Cancer: ASCO Clinical Practice Guideline Update. J. Clin. Oncol. 2018, 36, 1994–2001. [Google Scholar] [CrossRef]
- Meacham, L.R.; Burns, K.; Orwig, K.E.; Levine, J. Standardizing Risk Assessment for Treatment-Related Gonadal Insufficiency and Infertility in Childhood Adolescent and Young Adult Cancer: The Pediatric Initiative Network Risk Stratification System. J. Adolesc. Young Adult Oncol. 2020, 9, 662–666. [Google Scholar] [CrossRef]
- Bourlon, M.T.; Anazodo, A.; Woodruff, T.K.; Segelov, E. Oncofertility as a Universal Right and a Global Oncology Priority. JCO Glob. Oncol. 2020, 6, 314–316. [Google Scholar] [CrossRef] [PubMed]
- Campo-Engelstein, L. Common Ethical Issues in Oncofertility. Textb. Oncofertility Res. Pract. 2019, 355–362. [Google Scholar] [CrossRef]
- Oncofertility Consensus Document Compiled by Members of the CCLG Late Effects Group. 2019. Available online: https://www.cclg.org.uk/write/MediaUploads/Member%20area/Treatment%20guidelines/CCLG_Oncofertility_Final_Oct2019.pdf (accessed on 15 August 2023).
- Yasmin, E.; Balachandren, N.; Davies, M.C.; Jones, G.L.; Lane, S.; Mathur, R.; Webber, L.; Anderson, R.A. Fertility Preservation for Medical Reasons in Girls and Women: British Fertility Society Policy and Practice Guideline. Hum. Fertil. 2018, 21, 3–26. [Google Scholar] [CrossRef]
- Newton, H.L.; Picton, H.M.; Friend, A.J.; Hayden, C.M.; Brougham, M.; Cox, R.; Grandage, V.; Kwok-Williams, M.; Lane, S.; Mitchell, R.T.; et al. Inconsistencies in Fertility Preservation for Young People with Cancer in the UK. Arch. Dis. Child. 2022, 107, 265–270. [Google Scholar] [CrossRef]
- NHS. Thames Valley Priorities Committee Commissioning Policy Statement. Procedure That Requires Prior Approval Thames Valley Priorities Committee Commissioning Policy Statement Policy Statement 11g: Assisted Reproduction Services for Infertile Couples. Available online: https://fundingrequests.scwcsu.nhs.uk/wp-content/uploads/2021/06/011k-TVPC11g-Assisted-reproduction-services-v3.pdf (accessed on 15 August 2023).
- Hussein, A.A.; Tran, N.D.; Smith, J.F. Fertility preservation for boys and adolescents facing sterilizing medical therapy. Transl. Androl. Urol. 2014, 3, 382–390. [Google Scholar] [CrossRef]
- Oxford University Hospital. NHS Foundation Trust Testicular Tissue Cryopreservation: Parent/Carer. Available online: https://www.ouh.nhs.uk/patient-guide/leaflets/files/13065Ptesticular.pdf (accessed on 13 August 2023).
- Nilsson, J.; Jervaeus, A.; Lampic, C.; Eriksson, L.E.; Widmark, C.; Armuand, G.M.; Malmros, J.; Marshall Heyman, M.; Wettergren, L. Will I be able to have a baby?’Results from online focus group discussions with childhood cancer survivors in Sweden. Hum. Reprod. 2014, 29, 2704–2711. [Google Scholar] [CrossRef]
- Rodriguez-Wallberg, K.A.; Borgström, B.; Petersen, C.; Thurin-Kjellberg, A.; Mörse, H.; Giwercman, A.; Jarfelt, M. National Guidelines and Multilingual Age-Adapted Patient Brochures and Videos as Decision Aids for Fertility Preservation (FP) of Children and Teenagers with Cancer-A Multidisciplinary Effort to Improve Children’s Information and Access to FP in Sweden. Acta Obstet. Gynecol. Scand. 2019, 98, 679–680. [Google Scholar] [CrossRef]
- Regionala Cancercentrum i Samverkan. Available online: https://cancercentrum.se/samverkan/ (accessed on 11 November 2022).
- Nationell Vävnadsdokumentation Åtgärder För Att Bevara Reproduktions-Förmågan Hos Unga. Available online: https://vavnad.se/atgarder-for-att-bevara-av-reproduktionsformaga-hos-unga/ (accessed on 11 November 2022).
- Rodriguez-Wallberg, K.A.; Tanbo, T.; Tinkanen, H.; Thurin-Kjellberg, A.; Nedstrand, E.; Kitlinski, M.L.; Macklon, K.T.; Ernst, E.; Fedder, J.; Tiitinen, A.; et al. Ovarian Tissue Cryopreservation and Transplantation among Alternatives for Fertility Preservation in the Nordic Countries—Compilation of 20 Years of Multicenter Experience. Acta Obstet. Gynecol. Scand. 2016, 95, 1015–1026. [Google Scholar] [CrossRef]
- Affdal, A.O.; Grynberg, M.; Hessissen, L.; Ravitsky, V. Impact of Legislation and Public Funding on Oncofertility: A Survey of Canadian, French and Moroccan Pediatric Hematologists/Oncologists. BMC Med. Ethics 2020, 21, 25. [Google Scholar] [CrossRef]
- Summary—Plan Cancer 2014–2019—Ref: PLANK3SYNTANG14. Available online: https://www.e-cancer.fr/Expertises-et-publications/Catalogue-des-publications/Summary-Plan-cancer-2014-2019 (accessed on 11 November 2022).
- Courbiere, B.; le Roux, E.; Mathieu, E.D.; Torre, A.; Patrat, C.; Poncelet, C.; Montagut, J.; Gremeau, A.-S.; Creux, H.; Peigné, M.; et al. Oocyte Vitrification for Fertility Preservation in Women with Benign Gynecologic Disease: French Clinical Practice Guidelines Developed by a Modified Delphi Consensus Process Clinical Medicine Oocyte Vitrification for Fertility Preservation in Women with Benign Gynecologic Disease: French Clinical Practice Guidelines Developed by a Modified Delphi Consensus Process and on Behalf of the PreFerBe Expert Panel. J. Clin. Med. 2021, 10, 3810. [Google Scholar] [CrossRef]
- LOI N° 2004-800 Du 6 Août 2004 Relative à La Bioéthique—Légifrance. Available online: https://www.legifrance.gouv.fr/jorf/id/JORFTEXT000000441469 (accessed on 11 November 2022).
- Référentiel Préservation de la Fertilité Enfant (Fille, Garçon) et Adolescente. Available online: https://www.aphp.fr/contenu/referentiel-preservation-de-la-fertilite-enfant-fille-garcon-et-adolescente (accessed on 11 November 2022).
- Fertility Network|NHS Funding FAQ|Fertility Network. Available online: https://fertilitynetworkuk.org/access-support/nhs-funding/funding-faqs/ (accessed on 11 November 2022).
- Committee of the American Society for Reproductive Medicine, E. Fertility Preservation and Reproduction in Patients Facing Gonadotoxic Therapies: An Ethics Committee Opinion. Fertil. Steril. 2018, 110, 380–386. [Google Scholar] [CrossRef]
- Patient and Survivor Care|ASCO. Available online: https://old-prod.asco.org/practice-patients/guidelines/patient-and-survivor-care?fbclid=IwAR0TAV2K9Sq95d6wsrbUy1E3au_YrTFCReMA6yI4auxc8fWj5D0gfrWLU5g (accessed on 11 November 2022).
- Alberto, R.; Francesca, S.; Stefano, C.; Marta, S.; Bernadette, E.; Gianluca, G.; Chiara, B. Oncofertility: Meeting the Fertility Goals of Adolescents and Young Adults with Cancer. Cancer J. 2018, 24, 328. [Google Scholar] [CrossRef]
- Mgboji, G.E.; Cordeiro Mitchell, C.N.; Bedrick, B.S.; Vaidya, D.; Tao, X.; Liu, Y.; Maher, J.Y.; Christianson, M.S. Predictive Factors for Fertility Preservation in Pediatric and Adolescent Girls with Planned Gonadotoxic Treatment. J. Assist. Reprod. Genet. 2021, 38, 2713–2721. [Google Scholar] [CrossRef] [PubMed]
- Ceballo, R.; Graham, E.T.; Hart, J. Silent and Infertile: An Intersectional Analysis of the Experiences of Socioeconomically Diverse African American Women With Infertility. Psychol. Women Q. 2015, 39, 497–511. [Google Scholar] [CrossRef]
- Kyweluk, M.A.; Reinecke, J.; Chen, D. Fertility Preservation Legislation in the United States: Potential Implications for Transgender Individuals. LGBT Health 2019, 6, 331. [Google Scholar] [CrossRef] [PubMed]
- State Laws & Legislation|Alliance for Fertility Preservation. Available online: https://www.allianceforfertilitypreservation.org/state-legislation/ (accessed on 11 November 2022).
- COSA:AYA Cancer Fertility Preservation/Options for Treatment/Testicular Biopsy—Cancer Guidelines Wiki. Available online: https://www.cancer.org.au/clinical-guidelines/cancer-fertility-preservation/options-for-treatment/testicular-biopsy (accessed on 6 July 2023).
- COSA:AYA Cancer Fertility Preservation/Options for Treatment/Sperm cryopreservation—Cancer Guidelines Wiki. Available online: https://www.cancer.org.au/clinical-guidelines/cancer-fertility-preservation/options-for-treatment/sperm-cryopreservation (accessed on 6 July 2023).
- COSA:AYA Cancer Fertility Preservation/Options for Treatment/Ovarian Tissue Cryopreservation —Cancer Guidelines Wiki. Available online: https://www.cancer.org.au/clinical-guidelines/cancer-fertility-preservation/options-for-treatment/ovarian-tissue (accessed on 6 July 2023).
- COSA:AYA Cancer Fertility Preservation/Options for Treatment/Embryo Cryopreservation—Cancer Guidelines Wiki. Available online: https://www.cancer.org.au/clinical-guidelines/cancer-fertility-preservation/options-for-treatment/embryo-cryopreservation (accessed on 6 July 2023).
- COSA:AYA Cancer Fertility Preservation/Options for Treatment/Ovarian Suppression with GnRH Analogues—Cancer Guidelines Wiki. Available online: https://www.cancer.org.au/clinical-guidelines/cancer-fertility-preservation/options-for-treatment/ovarian-suppression (accessed on 6 July 2023).
- Cancer Council Australia. A Guide for People with Cancer, Their Families and Friends. 2022. Available online: https://www.cancercouncil.com.au/wp-content/uploads/2022/12/Living-with-Advanced-Cancer_2022.pdf (accessed on 13 November 2022).
- Jach, R.; Spaczynski, R.; Kurzawa, R.; Radwan, M.; Rzepka, J.; Swornik, M.; Pabian, W. Updating the recommendations of the Working Group for the Preservation of Fertility in Oncological and Hematological Patients and Other Patients Treating Gonadier Therapies “ONCOFERTILITY” (GROF) of the Polish Society of Oncological Gynecology regarding cryopreserves and autologous transplant. Ginekol. Pol. 2021, 92, 668–672. [Google Scholar] [CrossRef]
- Madeleine van der Perk, M.E.; van der Kooi, A.L.L.F.; van de Wetering, M.D.; IJgosse, I.M.; van Dulmen-Den Broeder, E.; Broer, S.L.; Klijn, A.J.; Versluys, A.B.; Arends, B.; Oude Ophuis, R.J.A.; et al. Oncofertility Care for Newly Diagnosed Girls with Cancer in a National Pediatric Oncology Setting, the First Full Year Experience from the Princess Máxima Center, the PEARL Study. PLoS ONE 2021, 16, e0246344. [Google Scholar] [CrossRef]
- Osmani, A.H.; Haider, G.; Ali, S.; Ali, F.; Irfan, M.; e Fatima, D. Knowledge and Perceptions about Cancer Treatment-Associated Infertility among Young Patients at a Tertiary Care Hospital in Pakistan. Asian Pac. J. Cancer Prev. 2017, 18, 3261–3265. [Google Scholar] [CrossRef]
- Sigman, M. Introduction: Cancer Treatment and Male Fertility: Effects of Therapy and Current and Future Management Options. Fertil. Steril. 2013, 100, 1179. [Google Scholar] [CrossRef]
- Wright, C.I.; Coad, J.; Morgan, S.; Stark, D.; Cable, M. “Just in Case”: The Fertility Information Needs of Teenagers and Young Adults with Cancer. Eur. J. Cancer Care 2014, 23, 189–198. [Google Scholar] [CrossRef]
- Nagel, K.; Neal, M. Discussions Regarding Sperm Banking with Adolescent and Young Adult Males Who Have Cancer. J. Pediatr. Oncol. Nurs. 2008, 25, 102–106. [Google Scholar] [CrossRef]
- Loren, A.W.; Brazauskas, R.; Chow, E.J.; Gilleece, M.; Halter, J.; Jacobsohn, D.A.; Joshi, S.; Pidala, J.; Quinn, G.P.; Wang, Z.; et al. Physician Perceptions and Practice Patterns Regarding Fertility Preservation in Hematopoietic Cell Transplant Recipients. Bone Marrow Transpl. 2013, 48, 1091–1097. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Wallberg, K.A.; Oktay, K. Fertility Preservation Medicine: Options for Young Adults and Children with Cancer. J. Pediatr. Hematol. Oncol. 2010, 32, 390–396. [Google Scholar] [CrossRef]
- Coccia, P.F.; Pappo, A.S.; Altman, J.; Bhatia, S.; Borinstein, S.C.; Flynn, J.; Frazier, A.L.; George, S.; Goldsby, R.; Hayashi, R.; et al. Adolescent and Young Adult Oncology, Version 2.2014. J. Natl. Compr. Canc Netw. 2014, 12, 21–32. [Google Scholar] [CrossRef]
- Rodriguez-Wallberg, K.A.; Anastacio, A.; Vonheim, E.; Deen, S.; Malmros, J.; Borgström, B. Fertility Preservation for Young Adults, Adolescents, and Children with Cancer. Ups. J. Med. Sci. 2020, 125, 112–120. [Google Scholar] [CrossRef] [PubMed]
- Schover, L.R.; Brey, K.; Lichtin, A.; Lipshultz, L.I.; Jeha, S. Oncologists’ Attitudes and Practices Regarding Banking Sperm before Cancer Treatment. J. Clin. Oncol. 2002, 20, 1890–1897. [Google Scholar] [CrossRef] [PubMed]
- Thompson, K.; Dyson, G.; Holland, L.; Joubert, L. An Exploratory Study of Oncology Specialists’ Understanding of the Preferences of Young People Living with Cancer. Soc. Work. Health Care 2013, 52, 166–190. [Google Scholar] [CrossRef]
- Benedict, C.; Nieh, J.L.; Hahn, A.L.; McCready, A.; Diefenbach, M.; Ford, J.S. “Looking at future cancer survivors, give them a roadmap”: Addressing fertility and family-building topics in post-treatment cancer survivorship care. Support. Care Cancer 2021, 29, 2203–2213. [Google Scholar] [CrossRef]
- Ginsberg, J.P.; Ogle, S.K.; Tuchman, L.K.; Carlson, C.A.; Reilly, M.M.; Hobbie, W.L.; Rourke, M.; Zhao, H.; Meadows, A.T. Sperm Banking for Adolescent and Young Adult Cancer Patients: Sperm Quality, Patient, and Parent Perspectives. Pediatr. Blood Cancer 2008, 50, 594–598. [Google Scholar] [CrossRef]
- de Vries, M.C.; Bresters, D.; Engberts, D.P.; Wit, J.M.; van Leeuwen, E. Attitudes of Physicians and Parents towards Discussing Infertility Risks and Semen Cryopreservation with Male Adolescents Diagnosed with Cancer. Pediatr. Blood Cancer 2009, 53, 386–391. [Google Scholar] [CrossRef]
- Fallat, M.E.; Hutter, J. Preservation of Fertility in Pediatric and Adolescent Patients with Cancer. Pediatrics 2008, 121, e1461–e1469. [Google Scholar] [CrossRef] [PubMed]
- Oosterhuis, B.E.; Goodwin, T.; Kiernan, M.; Hudson, M.M.; Dahl, G.v. Concerns about Infertility Risks among Pediatric Oncology Patients and Their Parents. Pediatr. Blood Cancer 2008, 50, 85–89. [Google Scholar] [CrossRef] [PubMed]
- Gracia, C.R.; Chang, J.; Kondapalli, L.; Prewitt, M.; Carlson, C.A.; Mattei, P.; Jeffers, S.; Ginsberg, J.P. Ovarian Tissue Cryopreservation for Fertility Preservation in Cancer Patients: Successful Establishment and Feasibility of a Multidisciplinary Collaboration. J. Assist. Reprod. Genet. 2012, 29, 495–502. [Google Scholar] [CrossRef] [PubMed]
- Anderson, R.A.; Weddell, A.; Spoudeas, H.A.; Douglas, C.; Shalet, S.M.; Levitt, G.; Wallace, W.H.B. Do Doctors Discuss Fertility Issues before They Treat Young Patients with Cancer? Hum. Reprod. 2008, 23, 2246–2251. [Google Scholar] [CrossRef] [PubMed]
- Crawshaw, M.A.; Sloper, P. ‘Swimming against the Tide’—The Influence of Fertility Matters on the Transition to Adulthood or Survivorship Following Adolescent Cancer. Eur. J. Cancer Care 2010, 19, 610–620. [Google Scholar] [CrossRef] [PubMed]
- Coccia, P.F.; Altman, J.; Bhatia, S.; Borinstein, S.C.; Flynn, J.; George, S.; Goldsby, R.; Hayashi, R.; Huang, M.S.; Johnson, R.H.; et al. Adolescent and young adult oncology. J. Natl. Compr. Canc Netw. 2012, 10, 1112–1150. [Google Scholar] [CrossRef]
- Murphy, D.; Orgel, E.; Termuhlen, A.; Shannon, S.; Warren, K.; Quinn, G.P. Why healthcare providers should focus on the fertility of AYA cancer survivors: It’s not too late! Front. Oncol. 2013, 7, 248. [Google Scholar] [CrossRef]
- Geue, K.; Richter, D.; Schmidt, R.; Sender, A.; Siedentopf, F.; Brähler, E.; Stöbel-Richter, Y. The desire for children and fertility issues among young German cancer survivors. J. Adolesc. Health 2014, 54, 527–535. [Google Scholar] [CrossRef]
- Wilkes, S.; Coulson, S.; Crosland, A.; Rubin, G.; Stewart, J. Experience of Fertility Preservation among Younger People Diagnosed with Cancer. Hum. Fertil. 2010, 13, 151–158. [Google Scholar] [CrossRef]
- Quinn, G.P.; Vadaparampil, S.T. Fertility Preservation and Adolescent/Young Adult Cancer Patients: Physician Communication Challenges. J. Adolesc. Health 2009, 44, 394–400. [Google Scholar] [CrossRef]
- Barbour, R.S.; Porter, M.A.; Peddie, V.L.; Bhattacharya, S. Counselling in the Context of Fertility and Cancer: Some Sociological Insights. Hum. Fertil. 2013, 16, 54–58. [Google Scholar] [CrossRef] [PubMed]
- Klosky, J.L.; Lehmann, V.; Flynn, J.S.; Su, Y.; Zhang, H.; Russell, K.M.; Schenck, L.A.M.; Schover, L.R. Patient Factors Associated with Sperm Cryopreservation among At-Risk Adolescents Newly Diagnosed with Cancer. Cancer 2018, 124, 3567. [Google Scholar] [CrossRef] [PubMed]
- Perez, G.K.; Salsman, J.M.; Fladeboe, K.; Kirchhoff, A.C.; Park, E.R.; Rosenberg, A.R. Taboo Topics in Adolescent and Young Adult Oncology: Strategies for Managing Challenging but Important Conversations Central to Adolescent and Young Adult Cancer Survivorship. Am. Soc. Clin. Oncol. Educ. Book 2020, 40, e171–e185. [Google Scholar] [CrossRef]
- Vadaparampil, S.; Quinn, G.; King, L.; Wilson, C.; Nieder, M. Barriers to Fertility Preservation among Pediatric Oncologists. Patient Educ. Couns. 2008, 72, 402–410. [Google Scholar] [CrossRef] [PubMed]
- Quinn, G.P.; Vadaparampil, S.T.; King, L.; Miree, C.A.; Wilson, C.; Raj, O.; Watson, J.; Lopez, A.; Albrecht, T.L. Impact of Physicians’ Personal Discomfort and Patient Prognosis on Discussion of Fertility Preservation with Young Cancer Patients. Patient Educ. Couns. 2009, 77, 338–343. [Google Scholar] [CrossRef]
- King, L.; Quinn, G.P.; Vadaparampil, S.T.; Gwede, C.K.; Miree, C.A.; Wilson, C.; Clayton, H.; Perrin, K. Oncology Nurses’ Perceptions of Barriers to Discussion of Fertility Preservation with Patients with Cancer. Clin. J. Oncol. Nurs. 2008, 12, 467–476. [Google Scholar] [CrossRef]
- Vindrola-Padros, C.; Dyer, K.E.; Cyrus, J.; Lubker, I.M. Healthcare professionals’ views on discussing fertility preservation with young cancer patients: A mixed method systematic review of the literature. Psychooncology 2017, 26, 4–14. [Google Scholar] [CrossRef]
- Sandheinrich, T.; Wondmeneh, S.B.; Mohrmann, C.; Gettinger, K.; Henry, J.; Hayashi, R.J. Knowledge and Perceptions of Infertility in Female Cancer Survivors and Their Parents. Support. Care Cancer 2018, 26, 2433–2439. [Google Scholar] [CrossRef]
- de Ziegler, D.; Streuli, I.; Vasilopoulos, I.; Decanter, C.; This, P.; Chapron, C. Cancer and Fecundity Issues Mandate a Multidisciplinary Approach. Fertil. Steril. 2010, 93, 691–696. [Google Scholar] [CrossRef]
- Vadaparampil, S.T.; Clayton, H.; Quinn, G.P.; King, L.M.; Nieder, M.; Wilson, C. Pediatric Oncology Nurses’ Attitudes Related to Discussing Fertility Preservation with Pediatric Cancer Patients and Their Families. J. Pediatr. Oncol. Nurs. 2007, 24, 255–263. [Google Scholar] [CrossRef]
- Saraf, A.J.; Stanek, J.; Audino, A.; DaJusta, D.; Hansen-Moore, J.; McCracken, K.; Whiteside, S.; Yeager, N.; Nahata, L. Examining Predictors and Outcomes of Fertility Consults among Children, Adolescents, and Young Adults with Cancer. Pediatr. Blood Cancer 2018, 65, e27409. [Google Scholar] [CrossRef]
- Schover, L.R.; van der Kaaij, M.; van Dorst, E.; Creutzberg, C.; Huyghe, E.; Kiserud, C.E. Sexual Dysfunction and Infertility as Late Effects of Cancer Treatment. EJC Suppl. 2014, 12, 41. [Google Scholar] [CrossRef]
- Lehmann, V.; Nahata, L.; Ferrante, A.C.; Hansen-Moore, J.A.; Yeager, N.D.; Klosky, J.L.; Gerhardt, C.A. Fertility-Related Perceptions and Impact on Romantic Relationships Among Adult Survivors of Childhood Cancer. J. Adolesc. Young Adult Oncol. 2018, 7, 409–414. [Google Scholar] [CrossRef] [PubMed]
- Borgmann-Staudt, A.; Kunstreich, M.; Schilling, R.; Balcerek, M.; Dirksen, U.; Cario, H.; Kepakova, K.; Klco-Brosius, S.; Korte, E.; Kruseova, J.; et al. Fertility Knowledge and Associated Empowerment Following an Educational Intervention for Adolescent Cancer Patients. Psychooncology 2019, 28, 2218–2225. [Google Scholar] [CrossRef] [PubMed]
- Schneider, T.; Clasen, N.H.Z.; Van Der Perk, M.E.M.; Neggers, S.J.C.M.M.; Bos, A.M.E.; Van Den Heuvel-Eibrink, M.M. Experiences of Female Childhood Cancer Patients and Survivors Regarding Information and Counselling on Gonadotoxicity Risk and Fertility Preservation at Diagnosis: A Systematic Review. Cancers 2023, 15, 1946. [Google Scholar] [CrossRef]
- Peddie, V.L.; Porter, M.A.; Barbour, R.; Culligan, D.; MacDonald, G.; King, D.; Horn, J.; Bhattacharya, S. Factors Affecting Decision Making about Fertility Preservation after Cancer Diagnosis: A Qualitative Study. BJOG 2012, 119, 1049–1057. [Google Scholar] [CrossRef]
- Skaczkowski, G.; White, V.; Thompson, K.; Bibby, H.; Coory, M.; Orme, L.M.; Conyers, R.; Phillips, M.B.; Osborn, M.; Harrup, R.; et al. Factors Influencing the Provision of Fertility Counseling and Impact on Quality of Life in Adolescents and Young Adults with Cancer. J. Psychosoc. Oncol. 2018, 36, 484–502. [Google Scholar] [CrossRef]
- Heritage, S.R.; Feast, A.; Mourad, M.; Smith, L.; Hatcher, H.; Critoph, D.J. Documentation of Oncofertility Communication in Adolescents and Young Adults with Cancer: A Retrospective Analysis. J. Adolesc. Young Adult Oncol. 2022, 11, 275–283. [Google Scholar] [CrossRef]
- Crawshaw, M.A.; Glaser, A.W.; Hale, J.P.; Sloper, P. Male and Female Experiences of Having Fertility Matters Raised alongside a Cancer Diagnosis during the Teenage and Young Adult Years. Eur. J. Cancer Care 2009, 18, 381–390. [Google Scholar] [CrossRef]
- Hershberger, P.E.; Finnegan, L.; Altfeld, S.; Lake, S.; Hirshfeld-Cytron, J. Toward theoretical understanding of the fertility preservation decision-making process: Examining information processing among young women with cancer. Res. Theory Nurs. Pract. 2013, 27, 257–275. [Google Scholar] [CrossRef]
- Jones, G.L.; Hughes, J.; Mahmoodi, N.; Greenfield, D.; Brauten-Smith, G.; Skull, J.; Gath, J.; Yeomanson, D.; Baskind, E.; Snowden, J.A.; et al. Observational Study of the Development and Evaluation of a Fertility Preservation Patient Decision Aid for Teenage and Adult Women Diagnosed with Cancer: The Cancer, Fertility and Me Research Protocol. BMJ Open 2017, 7, e013219. [Google Scholar] [CrossRef]
- Goossens, J.; Delbaere, I.; van Lancker, A.; Beeckman, D.; Verhaeghe, S.; van Hecke, A. Cancer Patients’ and Professional Caregivers’ Needs, Preferences and Factors Associated with Receiving and Providing Fertility-Related Information: A Mixed-Methods Systematic Review. Int. J. Nurs. Stud. 2014, 51, 300–319. [Google Scholar] [CrossRef]
- Wyns, C.; Collienne, C.; Shenfield, F.; Robert, A.; Laurent, P.; Roegiers, L.; Brichard, B. Fertility Preservation in the Male Pediatric Population: Factors Influencing the Decision of Parents and Children. Hum. Reprod. 2015, 30, 2022–2030. [Google Scholar] [CrossRef]
- Tschudin, S.; Bitzer, J. Psychological Aspects of Fertility Preservation in Men and Women Affected by Cancer and Other Life-Threatening Diseases. Hum. Reprod. Update 2009, 15, 587–597. [Google Scholar] [CrossRef]
- Quinn, G.P.; Vadaparampil, S.T. More Research, More Responsibility: The Expansion of Duty to Warn in Cancer Patients Considering Fertility Preservation. Am. J. Obstet. Gynecol. 2013, 209, 98–102. [Google Scholar] [CrossRef]
- Nieman, C.L.; Kinahan, K.E.; Yount, S.E.; Rosenbloom, S.K.; Yost, K.J.; Hahn, E.A.; Volpe, T.; Dilley, K.J.; Zoloth, L.; Woodruff, T.J. Fertility preservation and adolescent cancer patients: Lessons from adult survivors of childhood cancer and their parents. Cancer Treat. Res. 2007, 138, 201–217. [Google Scholar] [CrossRef]
- Quinn, G.P.; Knapp, C.; Murphy, D.; Sawczyn, K.; Sender, L. Congruence of reproductive concerns among adolescents with cancer and parents: Pilot testing an adapted instrument. Pediatrics 2012, 129, e930–e936. [Google Scholar] [CrossRef] [PubMed]
- Lipstein, E.A.; Brinkman, W.B.; Britto, M.T. What Is Known about Parents’ Treatment Decisions? A Narrative Review of Pediatric Decision Making. Med. Decis. Mak. 2012, 32, 246–258. [Google Scholar] [CrossRef] [PubMed]
- Mack, J.W.; Fasciano, K.M.; Block, S.D. Adolescent and Young Adult Cancer Patients’ Experiences With Treatment Decision-Making. Pediatrics 2019, 143, e20182800. [Google Scholar] [CrossRef]
- Allingham, C.; Gillam, L.; McCarthy, M.; Zacharin, M.; Jayasuriya, S.; Heloury, Y.; Orme, L.; Sullivan, M.; Peate, M.; Jayasinghe, Y. Fertility Preservation in Children and Adolescents With Cancer: Pilot of a Decision Aid for Parents of Children and Adolescents With Cancer. JMIR Pediatr. Parent. 2018, 1, e10463. [Google Scholar] [CrossRef] [PubMed]
- Khalife, D.; Kutteh, W.; Tarhini, H.; Khalil, A.; Beyrouthy, C.; Ghazeeri, G. Parental Attitudes Toward Fertility Preservation in Female Adolescent Cancer Patients in Lebanon. J. Pediatr. Adolesc. Gynecol. 2019, 32, 525–529. [Google Scholar] [CrossRef] [PubMed]
- Kazak, A.E.; Boeving, C.A.; Alderfer, M.A.; Hwang, W.T.; Reilly, A. Posttraumatic Stress Symptoms during Treatment in Parents of Children with Cancer. J. Clin. Oncol. 2005, 23, 7405–7410. [Google Scholar] [CrossRef] [PubMed]
- Korte, E.; Schilling, R.; Balcerek, M.; Byrne, J.; DIrksen, U.; Herrmann, G.; Kepak, T.; Klco-Brosius, S.; Kruseova, J.; Kunstreich, M.; et al. Fertility-Related Wishes and Concerns of Adolescent Cancer Patients and Their Parents. J. Adolesc. Young Adult Oncol. 2020, 9, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Barlevy, D.; Wangmo, T.; Ash, S.; Elger, B.S.; Ravitsky, V. Oncofertility Decision Making: Findings from Israeli Adolescents and Parents. J. Adolesc. Young Adult Oncol. 2019, 8, 74–83. [Google Scholar] [CrossRef] [PubMed]
- Stinson, J.N.; Sung, L.; Gupta, A.; White, M.E.; Jibb, L.A.; Dettmer, E.; Baker, N. Disease Self-Management Needs of Adolescents with Cancer: Perspectives of Adolescents with Cancer and Their Parents and Healthcare Providers. J. Cancer Surviv. 2012, 6, 278–286. [Google Scholar] [CrossRef]
- Klosky, J.L.; Flynn, J.S.; Lehmann, V.; Russell, K.M.; Wang, F.; Hardin, R.N.; Eddinger, J.R.; Zhang, H.; Schenck, L.A.M.; Schover, L.R. Parental Influences on Sperm Banking Attempts among Adolescent Males Newly Diagnosed with Cancer. Fertil. Steril. 2017, 108, 1043–1049. [Google Scholar] [CrossRef]
- Weaver, M.S.; Baker, J.N.; Gattuso, J.S.; Gibson, D.V.; Sykes, A.D.; Hinds, P.S. Adolescents’ Preferences for Treatment Decisional Involvement during Their Cancer. Cancer 2015, 121, 4416–4424. [Google Scholar] [CrossRef]
- Lavery, S.A.; Islam, R.; Hunt, J.; Carby, A.; Anderson, R.A. The Medical and Ethical Challenges of Fertility Preservation in Teenage Girls: A Case Series of Sickle Cell Anaemia Patients Prior to Bone Marrow Transplant. Hum. Reprod. 2016, 31, 1501–1507. [Google Scholar] [CrossRef]
- Recommendations for Reproductive Fertility Preservation for Female CAYA Cancer Patients by International Guideline Harmonization Group. 2020. Available online: https://www.ighg.org/wp-content/uploads/2021/03/IGHG-Female-fertility-preservation-recommendations_2020.pdf (accessed on 8 August 2023).
- Recommendations for Reproductive Fertility Preservation for Male CAYA Cancer Patients by International Guideline Harmonization Group. 2020. Available online: https://www.ighg.org/wp-content/uploads/2021/03/IGHG-Male-fertility-preservation-recommendations_2020.pdf (accessed on 8 August 2023).
- Recommendations Regarding Ongoing Communication of Treatment-Related Infertility Risk and Fertility Preservation in Patients with CAYA Cancer by International Guideline Harmonization Group. 2020. Available online: https://www.ighg.org/wp-content/uploads/2021/03/IGHG-Communication-and-ethical-considerations-fertility-preservation-recommendations_2020.pdf (accessed on 8 August 2023).
- National Institute for Health and Care Excellence. Fertility Problems: Assessment and Treatment Clinical Guideline. Available online: https://www.nice.org.uk/guidance/cg156 (accessed on 11 November 2022).
- Fertility Preservation in Patients Undergoing Gonadotoxic Therapy or Gonadectomy: A Committee Opinion. Fertil. Steril. 2013, 100, 1214–1223. [CrossRef]
- Rowell, E.E.; Lautz, T.B.; Lai, K.; Weidler, E.M.; Johnson, E.K.; Finlayson, C.; van Leeuwen, K. The Ethics of Offering Fertility Preservation to Pediatric Patients: A Case-Based Discussion of Barriers for Clinicians to Consider. Semin. Pediatr. Surg. 2021, 30, 151095. [Google Scholar] [CrossRef]
- Mulder, R.L.; Font-Gonzalez, A.; van Dulmen-den Broeder, E.; Quinn, G.P.; Ginsberg, J.P.; Loeffen, E.A.H.; Hudson, M.M.; Burns, K.C.; van Santen, H.M.; Berger, C.; et al. PanCareLIFE Consortium (2021). Communication and ethical considerations for fertility preservation for patients with childhood, adolescent, and young adult cancer: Recommendations from the PanCareLIFE Consortium and the International Late Effects of Childhood Cancer Guideline Harmonization Group. Lancet Oncol. 2021, 22, e68–e80. [Google Scholar] [CrossRef] [PubMed]
- Mertes, H. Let’s not forget that many prepubertal girls do have other options besides ovarian tissue cryopreservation. Hum. Reprod. 2015, 30, 2011–2013. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Dolmans, M.M.; Luyckx, V.; Donnez, J.; Andersen, C.Y.; Greve, T. Risk of Transferring Malignant Cells with Transplanted Frozen-Thawed Ovarian Tissue. Fertil. Steril. 2013, 99, 1514–1522. [Google Scholar] [CrossRef] [PubMed]
- Article L1211-2—Code de La Santé Publique—Légifrance. Available online: https://www.legifrance.gouv.fr/codes/article_lc/LEGIARTI000043895792 (accessed on 11 November 2022).
- General Medical Council. 0–18 Years: Guidance for All Doctors. Available online: https://www.gmc-uk.org/ethical-guidance/ethical-guidance-for-doctors/0-18-years (accessed on 23 January 2023).
- National Health Service. Sperm Banking before Cancer Treatment. Macmillan Cancer Support. Available online: https://www.nhs.uk/ipgmedia/national/Macmillan%20Cancer%20Support/Assets/Spermbankingbeforecancertreatment-798 MCS4pages.pdf (accessed on 11 November 2022).
- HFEA. Code of Practice 9th Edition. Available online: https://portal.hfea.gov.uk/media/it1n3vpo/2022-07-01-code-of-practice-2021.pdf (accessed on 23 January 2023).
- Human Fertilisation and Embryology Act. 2008. Available online: https://www.legislation.gov.uk/ukpga/2008/22/contents (accessed on 23 January 2023).
- Health and Care Act 2022. Available online: https://www.legislation.gov.uk/ukpga/2022/31/contents/enacted (accessed on 23 January 2023).
- Gamete (Egg, Sperm) and Embryo Storage Limits: Response to Consultation—GOV.UK. Available online: https://www.gov.uk/government/consultations/egg-sperm-and-embryo-storage-limits/outcome/gamete-egg-sperm-and-embryo-storage-limits-response-to-consultation (accessed on 11 November 2022).
- Written Statements—Written Questions, Answers and Statements—UK Parliament. Available online: https://questions-statements.parliament.uk/written-statements/detail/2021-09-06/hlws252 (accessed on 11 November 2022).
- The Human Fertilisation and Embryology (Statutory Storage Period for Embryos and Gametes) Regulations 2009. Available online: https://www.legislation.gov.uk/uksi/2009/1582/contents/made (accessed on 23 January 2023).
- The Human Fertilisation and Embryology (Statutory Storage Period for Embryos and Gametes) (Coronavirus) Regulations 2020. Available online: https://www.legislation.gov.uk/uksi/2020/566/contents/made (accessed on 23 January 2023).
- Legislation.Gov.Uk. Available online: https://www.legislation.gov.uk/primary+secondary?title=gametes%20and%20embryos (accessed on 11 November 2022).
| Method | Puberty | Legislation | References |
|---|---|---|---|
| Female Patients | [41,42,43,44,45,46] | ||
| Embryo cryopreservation | Postpubertal | Established | |
| Mature oocyte cryopreservation | Postpubertal | Established | |
| In vitro maturation | Prepubertal Postpubertal | Experimental | |
| Ovarian tissue cryopreservation | Prepubertal Postpubertal | Experimental | |
| GnRH analogs | Postpubertal | Experimental | |
| Male Patients | [42,43,44,45] | ||
| Embryo cryopreservation | Postpubertal | Established | |
| Sperm cryopreservation | Postpubertal | Established | |
| Testicular tissue cryopreservation | Prepubertal Postpubertal | Experimental | |
| Country | Method | Funding | Additional Information |
|---|---|---|---|
| UK | Cryopreservation of embryos and oocytes. Cryopreservation of ovarian tissue and testicular tissue in underage patients. | Public financing from the NHS, CCGs, LCGs, and foundations. | The cryopreservation of embryos is a widely accepted method for sexually mature girls. Funding often comes from public funds of the NHS, CCGs, LCGs, and charitable initiatives. |
| Sweden | Cryopreservation of embryos, oocytes, ovarian tissue, and testicular tissue. | Charitable funds and grants. | Sweden has an extensively developed fertility care program for patients undergoing oncological treatment. All Swedish university hospitals have established fertility care programs. The cryopreservation of ovarian tissue is conducted in centers approved by the Ethics Review Board as part of scientific research. |
| France | Cryopreservation of ovarian and testicular tissue for underage patients. | Healthcare funding, a standard for adolescent patients. | France has introduced the obligation to provide oncofertility through the current National Cancer Plan and the Bioethics Law. Funding includes tissue collection, future transplants, and in vitro fertilization. France has implemented specific legislation requiring oncofertility provision for patients at risk of fertility loss. |
| USA | Cryopreservation of gametes and embryos is a standard for adult patients. Experimental methods are only applied in research. | Patient’s insurance and private funds. The 2018 law introduced the obligation for insurance coverage for oncofertility services. | The use of GnRH analogs and in vitro maturation is still experimental. Various states in the USA have different regulations concerning insurance coverage for oncofertility. |
| Australia | Cryopreservation of gametes and embryos is the standard for adolescent patients. | Limited funding is dependent on the location and type of insurance. | The cryopreservation of ovarian tissue is considered an experimental method, and testicular tissue is cryopreserved only within research approved by the Ethics Review Committee. |
| Country | Legal and Ethical Aspects | Year of Publication of the Source Materials |
|---|---|---|
| Sweden |
| 2023 |
| France |
| 2004, 2022 and 2023 |
| United Kingdom |
| 2013 and 2023 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pawłowski, P.; Ziętara, K.J.; Michalczyk, J.; Fryze, M.; Buchacz, A.; Zaucha-Prażmo, A.; Zawitkowska, J.; Torres, A.; Samardakiewicz, M. Fertility Preservation in Children and Adolescents during Oncological Treatment—A Review of Healthcare System Factors and Attitudes of Patients and Their Caregivers. Cancers 2023, 15, 4393. https://doi.org/10.3390/cancers15174393
Pawłowski P, Ziętara KJ, Michalczyk J, Fryze M, Buchacz A, Zaucha-Prażmo A, Zawitkowska J, Torres A, Samardakiewicz M. Fertility Preservation in Children and Adolescents during Oncological Treatment—A Review of Healthcare System Factors and Attitudes of Patients and Their Caregivers. Cancers. 2023; 15(17):4393. https://doi.org/10.3390/cancers15174393
Chicago/Turabian StylePawłowski, Piotr, Karolina Joanna Ziętara, Justyna Michalczyk, Magdalena Fryze, Anna Buchacz, Agnieszka Zaucha-Prażmo, Joanna Zawitkowska, Anna Torres, and Marzena Samardakiewicz. 2023. "Fertility Preservation in Children and Adolescents during Oncological Treatment—A Review of Healthcare System Factors and Attitudes of Patients and Their Caregivers" Cancers 15, no. 17: 4393. https://doi.org/10.3390/cancers15174393
APA StylePawłowski, P., Ziętara, K. J., Michalczyk, J., Fryze, M., Buchacz, A., Zaucha-Prażmo, A., Zawitkowska, J., Torres, A., & Samardakiewicz, M. (2023). Fertility Preservation in Children and Adolescents during Oncological Treatment—A Review of Healthcare System Factors and Attitudes of Patients and Their Caregivers. Cancers, 15(17), 4393. https://doi.org/10.3390/cancers15174393







