Sleep Duration and Stress Level in the Risk of Gastric Cancer: A Pooled Analysis of Case-Control Studies in the Stomach Cancer Pooling (StoP) Project
Abstract
:Simple Summary
Abstract
1. Introduction
2. Methods
3. Results
3.1. Descriptive Analyses
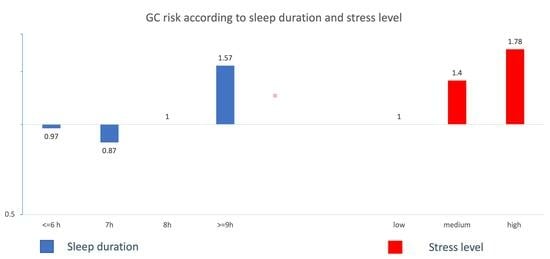
3.2. Analyses of the Association between Sleep Duration and Gastric Cancer
3.3. Analyses of the Association between Stress and Gastric Cancer
3.4. Sensitivity Analyses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Helicobacter and Cancer Collaborative Group. Gastric cancer and Helicobacter pylori: A combined analysis of 12 case control studies nested within prospective cohorts. Gut 2001, 49, 347–353. [Google Scholar] [CrossRef]
- Inoue, M. Public Health Interventions for Gastric Cancer Control. Gastrointest. Endosc. Clin. North Am. 2021, 31, 441–449. [Google Scholar] [CrossRef]
- Lyons, K.; Le, L.C.; Pham, Y.T.H.; Borron, C.; Park, J.Y.; Tran, C.T.; Tran, T.V.; Tran, H.T.T.; Vu, K.T.; Do, C.D.; et al. Gastric cancer: Epidemiology, biology, and prevention: A mini review. Eur. J. Cancer Prev. 2019, 28, 397–412. [Google Scholar] [CrossRef] [PubMed]
- Ferro, A.; Morais, S.; Rota, M.; Pelucchi, C.; Bertuccio, P.; Bonzi, R.; Galeone, C.; Zhang, Z.-F.; Matsuo, K.; Ito, H.; et al. Alcohol intake and gastric cancer: Meta-analyses of published data versus individual participant data pooled analyses (StoP Project). Cancer Epidemiol. 2018, 54, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Collatuzzo, G.; Etemadi, A.; Sotoudeh, M.; Nikmanesh, A.; Poustchi, H.; Khoshnia, M.; Pourshams, A.; Hashemian, M.; Roshandel, G.; Dawsey, S.M.; et al. Meat consumption and risk of esophageal and gastric cancer in the Golestan Cohort Study, Iran. Int. J. Cancer. 2022, 151, 1005–1012. [Google Scholar] [CrossRef]
- Shah, S.C.; Boffetta, P.; Johnson, K.C.; Hu, J.; Palli, D.; Ferraroni, M.; Tsugane, S.; Hamada, G.S.; Hidaka, A.; Zaridze, D.; et al. Occupational exposures and odds of gastric cancer: A StoP project consortium pooled analysis. Int. J. Epidemiol. 2020, 49, 422–434. [Google Scholar] [CrossRef] [PubMed]
- Lv, L.; Liang, X.; Wu, D.; Wang, F.; Zhang, Y.; Cang, H.; Deng, X.; Li, M. Is cardia cancer a special type of gastric cancer? A differential analysis of early cardia cancer and non-cardia cancer. J. Cancer. 2021, 12, 2385–2394. [Google Scholar] [CrossRef]
- Wang, J.E.; Kim, S.E.; Lee, B.E.; Park, S.; Hwang, J.H.; Huang, R.J. The risk of diffuse-type gastric cancer following diagnosis with gastric precancerous lesions: A systematic review and meta-analysis. Cancer Causes Control. 2022, 33, 183–191. [Google Scholar] [CrossRef]
- Sexton, R.E.; Al Hallak, M.N.; Diab, M.; Azmi, A.S. Gastric cancer: A comprehensive review of current and future treatment strategies. Cancer Metastasis Rev. 2020, 39, 1179–1203. [Google Scholar] [CrossRef]
- Koemans, W.; Luijten, J.; van der Kaaij, R.; Grootscholten, C.; Snaebjornsson, P.; Verhoeven, R.; van Sandick, J. The metastatic pattern of intestinal and diffuse type gastric carcinoma—A Dutch national cohort study. Cancer Epidemiol. 2020, 69, 101846. [Google Scholar] [CrossRef] [PubMed]
- Stiekema, J.; Cats, A.; Kuijpers, A.; van Coevorden, F.; Boot, H.; Jansen, E.; Verheij, M.; Ponz, O.B.; Hauptmann, M.; van Sandick, J. Surgical treatment results of intestinal and diffuse type gastric cancer. Implications for a differentiated therapeutic approach? Eur. J. Surg. Oncol. 2013, 39, 686–693. [Google Scholar] [CrossRef]
- Fong, C.; Johnston, E.; Starling, N. Neoadjuvant and Adjuvant Therapy Approaches to Gastric Cancer. Curr. Treat. Options Oncol. 2022, 23, 1247–1268. [Google Scholar] [CrossRef]
- Xin, J.; Wu, Y.; Wang, X.; Li, S.; Chu, H.; Wang, M.; Du, M.; Zhang, Z. A transcriptomic study for identifying cardia- and non-cardia-specific gastric cancer prognostic factors using genetic algorithm-based methods. J. Cell Mol. Med. 2020, 24, 9457–9465. [Google Scholar] [CrossRef] [PubMed]
- Han, Z.; Liu, J.; Zhang, W.; Kong, Q.; Wan, M.; Lin, M.; Lin, B.; Ding, Y.; Duan, M.; Li, Y.; et al. Cardia and non-cardia gastric cancer risk associated with Helicobacter pylori in East Asia and the West: A systematic review, meta-analysis, and estimation of population attributable fraction. Helicobacter 2023, 28, e12950. [Google Scholar] [CrossRef]
- Oue, N.; Sentani, K.; Sakamoto, N.; Uraoka, N.; Yasui, W. Molecular carcinogenesis of gastric cancer: Lauren classification, mucin phenotype expression, and cancer stem cells. Int. J. Clin. Oncol. 2019, 24, 771–778. [Google Scholar] [CrossRef]
- Waldum, H.L.; Fossmark, R. Types of Gastric Carcinomas. Int. J. Mol. Sci. 2018, 19, 4109. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Tan, F.; Wei, L.; Li, X.; Lyu, Z.; Feng, X.; Wen, Y.; Guo, L.; He, J.; Dai, M.; et al. Sleep duration and the risk of cancer: A systematic review and meta-analysis including dose-response relationship. BMC Cancer 2018, 18, 1149. [Google Scholar] [CrossRef]
- Papantoniou, K.; Castaño-Vinyals, G.; Espinosa, A.; Turner, M.C.; Martín-Sánchez, V.; Casabonne, D.; Aragonés, N.; Gómez-Acebo, I.; Ardanaz, E.; Jimenez-Moleon, J.-J.; et al. Sleep duration and napping in relation to colorectal and gastric cancer in the MCC-Spain study. Sci. Rep. 2021, 11, 11822. [Google Scholar] [CrossRef]
- Gu, F.; Xiao, Q.; Chu, L.W.; Yu, K.; Matthews, C.E.; Hsing, A.W.; Caporaso, N.E. Sleep Duration and Cancer in the NIH-AARP Diet and Health Study Cohort. PLoS ONE 2016, 11, e0161561. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, Y.; Li, Q. Mechanisms underlying the effects of stress on tumorigenesis and metastasis (Review). Int. J. Oncol. 2018, 53, 2332–2342. [Google Scholar] [CrossRef]
- Available online: http://www.stop-project.org/ (accessed on 16 August 2023).
- Pelucchi, C.; Lunet, N.; Boccia, S.; Zhang, Z.F.; Praud, D.; Boffetta, P.; Levi, F.; Matsuo, K.; Ito, H.; Hu, J.; et al. The stomach cancer pooling (StoP) project: Study design and presentation. Eur. J. Cancer Prev. 2015, 24, 16–23. [Google Scholar] [CrossRef]
- Zhang, Z.-F.; Kurtz, R.C.; Klimstra, D.S.; Yu, G.-P.; Sun, M.; Harlap, S.; Marshall, J.R. Helicobacter pylori infection on the risk of stomach cancer and chronic atrophic gastritis. Cancer Detect. Prev. 1999, 23, 357–367. [Google Scholar] [CrossRef]
- Santibañez, M.; Alguacil, J.; de la Hera, M.G.; Navarrete-Muñoz, E.M.; Llorca, J.; Aragonés, N.; Kauppinen, T.; Vioque, J.; PANESOES Study Group. Occupational exposures and risk of stomach cancer by histological type. Occup. Environ. Med. 2012, 69, 268–275. [Google Scholar] [CrossRef]
- Nishimoto, I.N.; Hamada, G.S.; Kowalski, L.P.; Rodrigues, J.G.; Iriya, K.; Sasazuki, S.; Hanaoka, T.; Tsugane, S.; São Paulo—Japan Cancer Project Gastric Cancer Study Group. Risk factors for stomach cancer in Brazil (I): A case-control study among non-Japanese Brazilians in São Paulo. Jpn. J. Clin. Oncol. 2002, 32, 277–283. [Google Scholar] [CrossRef]
- Hamada, G.S.; Kowalski, L.P.; Nishimoto, I.N.; Rodrigues, J.J.G.; Iriya, K.; Sasazuki, S.; Hanaoka, T.; Tsugane, S.; São Paulo—Japan Cancer Project Gastric Cancer Study Group. Risk factors for stomach cancer in Brazil (II): A case-control study among Japanese Brazilians in São Paulo. Jpn. J. Clin. Oncol. 2002, 32, 284–290. [Google Scholar] [CrossRef] [PubMed]
- Hirshkowitz, M.; Whiton, K.; Albert, S.M.; Alessi, C.; Bruni, O.; DonCarlos, L.; Hazen, N.; Herman, J.; Adams Hillard, P.J.; Katz, E.S.; et al. National Sleep Foundation’s updated sleep duration recommendations: Final report. Sleep Health 2015, 1, 233–243. [Google Scholar] [CrossRef]
- StataCorp. Stata Statistical Software: Release 16, StataCorp LLC: College Station, TX, USA, 2019.
- Liu, Y.; Wheaton, A.G.; Chapman, D.P.; Cunningham, T.J.; Lu, H.; Croft, J.B. Prevalence of Healthy Sleep Duration among Adults--United States, 2014. MMWR Morb. Mortal Wkly. Rep. 2016, 65, 137–141. [Google Scholar] [CrossRef]
- Schmid, S.M.; Hallschmid, M.; Schultes, B. The metabolic burden of sleep loss. Lancet Diabetes Endocrinol. 2015, 3, 52–62. [Google Scholar] [CrossRef] [PubMed]
- Heyde, I.; Oster, H. Differentiating external zeitgeber impact on peripheral circadian clock resetting. Sci. Rep. 2019, 9, 20114. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Croft, J.B.; Wheaton, A.G.; Perry, G.S.; Chapman, D.P.; Strine, T.W.; McKnight-Eily, L.R.; Presley-Cantrell, L. Association between perceived insufficient sleep, frequent mental distress, obesity and chronic diseases among US adults, 2009 behavioral risk factor surveillance system. BMC Public Health 2013, 13, 84. [Google Scholar] [CrossRef] [PubMed]
- Mogavero, M.P.; DelRosso, L.M.; Fanfulla, F.; Bruni, O.; Ferri, R. Sleep disorders and cancer: State of the art and future perspectives. Sleep Med. Rev. 2021, 56, 101409. [Google Scholar] [CrossRef] [PubMed]
- Blask, D.E. Melatonin, sleep disturbance and cancer risk. Sleep Med. Rev. 2009, 13, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Goldstein-Piekarski, A.N.; Greer, S.M.; Saletin, J.M.; Harvey, A.G.; Williams, L.M.; Walker, M.P. Sex, Sleep Deprivation, and the Anxious Brain. J. Cogn. Neurosci. 2018, 30, 565–578. [Google Scholar] [CrossRef]
- Blackwelder, A.; Hoskins, M.; Huber, L. Effect of Inadequate Sleep on Frequent Mental Distress. Prev. Chronic Dis. 2021, 18, E61. [Google Scholar] [CrossRef]
- Clevers, E.; Lutin, E.; Cornelis, J.; Van Oudenhove, L. Gastrointestinal symptoms in office workers are predicted by psychological distress and short sleep duration. J. Psychosom. Res. 2020, 138, 110230. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, Y.; Arakawa, T.; Fass, R. Gastroesophageal reflux disease and sleep disturbances. J. Gastroenterol. 2012, 47, 760–769. [Google Scholar] [CrossRef]
- Orr, W.C.; Fass, R.; Sundaram, S.S.; Scheimann, A.O. The effect of sleep on gastrointestinal functioning in common digestive diseases. Lancet Gastroenterol. Hepatol. 2020, 5, 616–624. [Google Scholar] [CrossRef]
- Besedovsky, L.; Lange, T.; Born, J. Sleep and immune function. Pflugers Arch. 2012, 463, 121–137. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.H.; Lyu, Z.Y.; Wang, G.; Feng, X.S.; Xie, S.H.; Chen, S.H.; Yin, J.; Ren, J.S.; Mi, Z.H.; Wang, S.; et al. Relationship of sleep duration and annual changes in sleep duration with the incidence of gastrointestinal cancers: A prospective cohort study. Chin. Med. J. 2021, 134, 2976–2984. [Google Scholar] [CrossRef]
- Fang, H.F.; Miao, N.F.; Chen, C.D.; Sithole, T.; Chung, M.H. Risk of Cancer in Patients with Insomnia, Parasomnia, and Obstructive Sleep Apnea: A Nationwide Nested Case-Control Study. J. Cancer. 2015, 6, 1140–1147. [Google Scholar] [CrossRef]
- Chaput, J.P.; Dutil, C.; Sampasa-Kanyinga, H. Sleeping hours: What is the ideal number and how does age impact this? Nat. Sci. Sleep 2018, 10, 421–430. [Google Scholar] [CrossRef] [PubMed]
- Smiley, A.; King, D.; Bidulescu, A. The Association between Sleep Duration and Metabolic Syndrome: The NHANES 2013/2014. Nutrients 2019, 11, 2582. [Google Scholar] [CrossRef] [PubMed]
- Winer, J.R.; Deters, K.D.; Kennedy, G.; Jin, M.; Goldstein-Piekarski, A.; Poston, K.L.; Mormino, E.C. Association of Short and Long Sleep Duration With Amyloid-β Burden and Cognition in Aging. JAMA Neurol. 2021, 78, 1187–1196. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Vitiello, M.V.; Gooneratne, N.S. Sleep in Normal Aging. Sleep Med. Clin. 2018, 13, 1–11. [Google Scholar] [CrossRef]
- Winkler, M.R.; Telke, S.; Ahonen, E.Q.; Crane, M.M.; Mason, S.M.; Neumark-Sztainer, D. Constrained choices: Combined influences of work, social circumstances, and social location on time-dependent health behaviors. SSM Popul. Health. 2020, 11, 100562. [Google Scholar] [CrossRef]
- Porkka-Heiskanen, T.; Kalinchuk, A.V. Adenosine, energy metabolism and sleep homeostasis. Sleep Med. Rev. 2011, 15, 123–135. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, A.; Inoue, H.; Kuwano, T. Low energy intake and dietary quality are associated with low objective sleep quality in young Japanese women. Nutr. Res. 2020, 80, 44–54. [Google Scholar] [CrossRef]
- Wilunda, C.; Abe, S.K.; Svensson, T.; Sawada, N.; Tsugane, S.; Wada, K.; Nagata, C.; Kimura, T.; Tamakoshi, A.; Sugawara, Y.; et al. Sleep duration and risk of cancer incidence and mortality: A pooled analysis of six population-based cohorts in Japan. Int. J. Cancer. 2022, 151, 1068–1080. [Google Scholar] [CrossRef]
- Harkness, J.A.; Richter, M.B.; Panayi, G.S.; Van de Pette, K.; Unger, A.; Pownall, R.; Geddawi, M. Circadian variation in disease activity in rheumatoid arthritis. Br. Med. J. 1982, 284, 551–554. [Google Scholar] [CrossRef]
- Titova, O.E.; Michaëlsson, K.; Vithayathil, M.; Mason, A.M.; Kar, S.; Burgess, S.; Larsson, S.C. Sleep duration and risk of overall and 22 site-specific cancers: A Mendelian randomization study. Int. J. Cancer. 2021, 148, 914–920. [Google Scholar] [CrossRef]
- Berisha, A.; Shutkind, K.; Borniger, J.C. Sleep Disruption and Cancer: Chicken or the Egg? Front. Neurosci. 2022, 16, 856235. [Google Scholar] [CrossRef] [PubMed]
- Keskin, G. Approach to stress endocrine response: Somatization in the context of gastroenterological symptoms: A systematic review. Afr. Health Sci. 2019, 19, 2537–2545. [Google Scholar] [CrossRef]
- Rohleder, N. Stress and inflammation—The need to address the gap in the transition between acute and chronic stress effects. Psychoneuroendocrinology 2019, 105, 164–171. [Google Scholar] [CrossRef] [PubMed]
- Sandi, C.; Haller, J. Stress and the social brain: Behavioural effects and neurobiological mechanisms. Nat. Rev. Neurosci. 2015, 16, 290–304. [Google Scholar] [CrossRef]
- Powell, N.D.; Tarr, A.J.; Sheridan, J.F. Psychosocial stress and inflammation in cancer. Brain Behav. Immun. 2013, 30, S41–S47. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | Cases | Controls |
|---|---|---|
| N (%) | N (%) | |
| Total | 1293 (100.0) | 4439 (100.0) |
| Sex | ||
| Male | 871 (25.7) | 2518 (74.3) |
| Female | 422 (18.0) | 1921 (82.0) |
| Age (years) | ||
| <55 | 286 (22.2) | 1000 (77.8) |
| 55–59 | 160 (25.0) | 480 (75.0) |
| 60–64 | 163 (19.6) | 667 (80.4) |
| 65–69 | 237 (23.7) | 809 (77.3) |
| ≥70 | 447 (23.2) | 1483 (76.8) |
| Sleep duration (hours) | ||
| <6 h | 356 (26.3) | 1354 (73.7) |
| 7 h | 250 (20.8) | 1131 (79.2) |
| 8 h | 476 (18.1) | 1332 (81.9) |
| ≥9 h | 192 (32.3) | 402 (67.7) |
| Stress level | ||
| Low | 274 (59.4) | 400 (40.7) |
| Intermediate | 244 (53.3) | 278 (46.7) |
| High | 325 (47.8) | 298 (52.2) |
| Cigarette smoking | ||
| Never | 549 (21.4) | 2018 (78.6) |
| Former | 409 (21.5) | 1491 (78.5) |
| Current | 324 (27.1) | 871 (72.3) |
| Alcohol drinking † | ||
| Never | 476 (30.7) | 1074 (69.3) |
| Low | 312 (16.8) | 1548 (83.2) |
| Intermediate | 247 (21.4) | 905 (78.6) |
| High | 143 (37.2) | 241 (62.8) |
| Socioeconomic status | ||
| Low | 867 (26.7) | 2381 (73.3) |
| Intermediate | 293 (19.2) | 1235 (80.8) |
| High | 131 (13.8) | 818 (86.2) |
| Salt intake | ||
| Low | 599 (26.5) | 1664 (73.5) |
| Intermediate | 216 (15.9) | 1145 (84.1) |
| High | 363 (24.5) | 1116 (75.5) |
| Vegetables and fruit intake | ||
| Low | 310 (22.0) | 1102 (78.0) |
| Intermediate | 320 (21.8) | 1147 (78.2) |
| High | 548 (26.5) | 1524 (73.6) |
| Study population | NA | |
| Hospitalized | 891 (21.1%) | |
| Nonhospitalized | 13,328 (79.9%) | |
| Anatomical site of GC | NA | |
| Cardia | 215 | |
| Noncardia | 919 | |
| Histological type of GC | NA | |
| Intestinal | 619 | |
| Diffuse | 353 |
| Sleep Duration | Stress Level | ||||||
|---|---|---|---|---|---|---|---|
| ≤6 h | 7 h | 8 h | ≥9 h | Low | Intermediate | High | |
| Sex | |||||||
| Male | 56.7% | 59.4% | 60.0% | 62.5% | 73.9% | 64.9% | 55.7% |
| Female | 43.3% | 40.6% | 40.0% | 37.5% | 26.1% | 35.1% | 43.3% |
| Age | |||||||
| <55 | 21.6% | 23.5% | 28.3% | 11.6% | 18.3% | 28.7% | 32.4% |
| 55–59 | 11.1% | 11.9% | 12.7% | 6.73% | 9.94% | 16.1% | 14.6% |
| 60–64 | 14.4% | 14.4% | 16.2% | 11.6% | 14.8% | 16.1% | 16.0% |
| 65–69 | 19.5% | 18.4% | 16.4% | 19.0% | 20.9% | 19.4% | 18.0% |
| ≥70 | 33.4% | 31.9% | 26.4% | 51.0% | 35.1% | 19.7% | 19.0% |
| Cigarette smoking | |||||||
| Never | 44.9% | 42.1% | 47.0% | 50.8% | 43.1% | 50.7% | 48.8% |
| Former | 34.7% | 35.7% | 30.2% | 32.6% | 33.1% | 28.0% | 25.8% |
| Current | 20.4% | 22.2% | 22.8% | 16.6% | 23.8% | 21.3% | 25.5% |
| Alcohol drinking | |||||||
| Never | 33.3% | 23.4% | 33.8% | 35.9% | 50.5% | 60.4% | 48.7% |
| Low | 39.8% | 44.3% | 34.0% | 27.7% | 20.6% | 16.2% | 24.2% |
| Intermediate | 21.0% | 24.5% | 23.5% | 26.6% | 18.3% | 14.8% | 17.5% |
| High | 5.90% | 7.80% | 8.70% | 9.87% | 10.6% | 8.65% | 9.60% |
| Socioeconomic status | |||||||
| Low | 55.7% | 46.3% | 58.0% | 73.4% | 61.4% | 62.8% | 62.8% |
| Intermediate | 27.7% | 29.9% | 26.1% | 20.2% | 26.4% | 27.6% | 24.2% |
| High | 16.6% | 23.8% | 15.8% | 6.40% | 12.2% | 9.6% | 13.0% |
| Vegetables and fruit intake | |||||||
| Low | 27.8% | 31.2% | 27.6% | 26.1% | 19.7% | 18.8% | 24.8% |
| Intermediate | 28.2% | 32.0% | 29.6% | 28.8% | 20.6% | 20.0% | 26.4% |
| High | 44.0% | 36.8% | 42.8% | 45.1% | 59.6% | 61.2% | 48.8% |
| Anatomical site of GC | |||||||
| Cardia | 19.8% | 19.7% | 17.9% | 19.0% | 21.6% | 12.0% | 16.2% |
| Noncardia | 80.2% | 80.3% | 82.1% | 81.0% | 78.4% | 88.0% | 87.8% |
| Histological type of GC | |||||||
| Intestinal | 61.8% | 60.8% | 66.0% | 64.4% | 70.1% | 60.0% | 62.5% |
| Diffuse | 38.2% | 39.2% | 34.0% | 35.6% | 29.9% | 40.0% | 37.5% |
| OR1 (95% CI) | OR2 (95% CI) | |
|---|---|---|
| Sleep duration (hours) | ||
| ≤6 h | 0.97 (0.80–1.18) | 0.99 (0.77–1.28) |
| 7 h | 0.86 (0.70–1.05) | 1.03 (0.79–1.35) |
| 8 h | Ref | Ref |
| ≥9 h | 1.57 (1.23–2.00) | 1.60 (1.14–2.23) |
| Stress level | ||
| Low | Ref | Ref |
| Intermediate | 1.40 (1.10–1.81) | 1.44 (1.12–1.85) |
| High | 1.77 (1.39–2.26) | 1.82 (1.42–2.32) |
| Ordinal * | 1.33 (1.18–1.50) | 1.35 (1.19–1.52) |
| Anatomical Subsite | Histological Type | |||
|---|---|---|---|---|
| Cardia | Noncardia | Diffuse | Intestinal | |
| OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | |
| Sleep duration (hours) | ||||
| ≤6 h | 0.93 (0.61–1.41) N = 63 | 0.96 (0.77–1.19) N = 256 | 0.99 (0.73–1.35) N = 99 | 1.00 (0.78–1.28) N = 160 |
| 7 h | 0.80 (0.51–1.24) N = 43 | 0.86 (0.68–1.09) N = 175 | 0.95 (0.68–1.33) N = 71 | 0.84 (0.64–1.10) N = 110 |
| 8 h | Ref N = 72 | Ref N = 331 | Ref N = 126 | Ref N = 245 |
| ≥9 h | 1.63 (0.97–2.72) N = 34 | 1.59 (1.22–2.08) N = 145 | 1.65 (1.14–2.40) N = 52 | 1.24 (0.91–1.67) N = 94 |
| Stress level | ||||
| Low | Ref N = 53 | Ref N = 192 | Ref N = 66 | Ref N = 155 |
| Intermediate | 0.92 (0.52–1.62) N = 26 | 1.45 (1.10–1.90) N = 190 | 1.49(1.03–2.15) N = 82 | 1.22 (0.91–1.63) N = 123 |
| High | 0.79 (0.46–1.36) N = 39 | 1.62 (1.24–2.13) N = 201 | 1.77 (1.24–2.53) N = 100 | 1.52 (1.15–2.00) N = 167 |
| Ordinal * | 0.89 (0.68–1.17) | 1.28 (1.12–1.46) | 1.32 (1.11–1.58) | 1.23 (1.07–1.42) |
| Sex | Age | Cigarette Smoking | Socioeconomic Status | ||||||
|---|---|---|---|---|---|---|---|---|---|
| OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | ||||||
| Male | Female | <65 years old | ≥65 years old | Never | Former | Current | Low | High | |
| Sleep duration (hours) | |||||||||
| ≤6 h | 1.00 (0.80–1.27) N = 319 | 0.88 (0.62–1.23) N = 157 | 0.87 (0.66–1.15) N = 234 | 1.06 (0.81–1.40) N = 242 | 0.97 (0.73–1.30) N = 210 | 0.74 (0.52–1.05) N = 140 | 1.26 (0.83–1.93) N = 125 | 1.11 (0.86–1.43) N = 233 | 0.59 (0.32–1.08) N = 35 |
| 7 h | 0.84 (0.66–1.08) N = 237 | 0.84 (0.58–1.22) N = 119 | 0.75 (0.56–1.01) N = 181 | 1.00 (0.75–1.35) N = 175 | 0.82 (0.59–1.13) N = 149 | 0.66 (0.46–0.96) N = 118 | 1.18 (0.78–1.79) N = 82 | 0.87 (0.67–1.15) N = 151 | 0.73 (0.41–1.30) N = 41 |
| 8 h | Ref N = 180 | Ref N = 70 | Ref N = 126 | Ref N = 124 | Ref N = 88 | Ref N = 87 | Ref N = 74 | Ref N = 323 | Ref N = 44 |
| ≥9 h | 1.51 (1.11–2.03) N = 124 | 1.74 (1.15–2.64) N = 68 | 1.37 (0.91–2.07) N = 62 | 1.70 (1.25–2.32) N = 130 | 1.46 (1.02–2.07) N = 92 | 1.34 (0.86–2.08) N = 59 | 1.89 (1.09–3.28) N = 39 | 1.58 (1.18–2.11) N = 145 | 1.37 (0.49–3.84) N = 10 |
| Stress level | |||||||||
| Low | Ref N = 209 | Ref N = 65 | Ref N = 108 | Ref N = 166 | Ref N = 113 | Ref N = 92 | Ref N = 67 | Ref | Ref |
| Intermediate | 1.75 (1.28–2.37) N = 175 | 0.97 (0.61–1.53) N = 69 | 1.41 (1.00–1.99) N = 142 | 1.39 (0.96–2.03) N = 102 | 1.13 (0.79–1.63) N = 107 | 1.69 (1.05–2.72) N = 77 | 1.84 (1.06–3.18) N = 57 | 1.70 (1.23–2.35) N = 169 | 0.54 (0.23–1.27) N = 39 |
| High | 1.86 (1.37–2.53) N = 193 | 1.62 (1.07–2.46) N = 132 | 1.75 (1.26–2.44) N = 193 | 1.94 (1.34–2.82) N = 103 | 1.38 (0.97–1.97) N = 140 | 1.75 (1.10–2.80) N = 83 | 3.32 (1.98–5.56) N = 100 | 2.32 (1.70–3.18) N = 268 | 0.43 (0.20–0.96) N = 17 |
| Ordinal * | 1.38 (1.19–1.61) | 1.30 (1.05–1.60) | 1.32 (1.12–1.56) | 1.39 (1.16–1.68) | 1.18 (0.99–1.40) | 1.34 (1.06–1.69) | 1.82 (1.41–2.36) | 1.53 (1.30–1.79) N = 220 | 0.65 (0.44–0.97) N = 28 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Collatuzzo, G.; Pelucchi, C.; Negri, E.; Kogevinas, M.; Huerta, J.M.; Vioque, J.; de la Hera, M.G.; Tsugane, S.; Shigueaki Hamada, G.; Hidaka, A.; et al. Sleep Duration and Stress Level in the Risk of Gastric Cancer: A Pooled Analysis of Case-Control Studies in the Stomach Cancer Pooling (StoP) Project. Cancers 2023, 15, 4319. https://doi.org/10.3390/cancers15174319
Collatuzzo G, Pelucchi C, Negri E, Kogevinas M, Huerta JM, Vioque J, de la Hera MG, Tsugane S, Shigueaki Hamada G, Hidaka A, et al. Sleep Duration and Stress Level in the Risk of Gastric Cancer: A Pooled Analysis of Case-Control Studies in the Stomach Cancer Pooling (StoP) Project. Cancers. 2023; 15(17):4319. https://doi.org/10.3390/cancers15174319
Chicago/Turabian StyleCollatuzzo, Giulia, Claudio Pelucchi, Eva Negri, Manolis Kogevinas, José María Huerta, Jesus Vioque, Manoli García de la Hera, Shoichiro Tsugane, Gerson Shigueaki Hamada, Akihisa Hidaka, and et al. 2023. "Sleep Duration and Stress Level in the Risk of Gastric Cancer: A Pooled Analysis of Case-Control Studies in the Stomach Cancer Pooling (StoP) Project" Cancers 15, no. 17: 4319. https://doi.org/10.3390/cancers15174319
APA StyleCollatuzzo, G., Pelucchi, C., Negri, E., Kogevinas, M., Huerta, J. M., Vioque, J., de la Hera, M. G., Tsugane, S., Shigueaki Hamada, G., Hidaka, A., Zhang, Z.-F., Camargo, M. C., Curado, M. P., Lunet, N., La Vecchia, C., & Boffetta, P. (2023). Sleep Duration and Stress Level in the Risk of Gastric Cancer: A Pooled Analysis of Case-Control Studies in the Stomach Cancer Pooling (StoP) Project. Cancers, 15(17), 4319. https://doi.org/10.3390/cancers15174319