Evolution of Patterns of Care and Outcomes in the Real-Life Setting for Patients with Metastatic GIST Treated in Three French Expert Centers over Three Decades
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
- -
- Patients ≥ 18 years old, with a diagnosis of GIST;
- -
- with expert pathological review performed by members of RREPS (Réseau de Référence En Pathologie des Sarcomes);
- -
- treated in one of the three national coordinating centers from NETSARC (Institut Bergonié, Centre Léon Berard and Institut Gustave Roussy);
- -
- from 1990 to 2018;
- -
- who gave their informed consent to be included in the prospectively maintained FSG database;
- -
- and presented metastatic disease (metastatic at diagnostic or metastatic relapse).
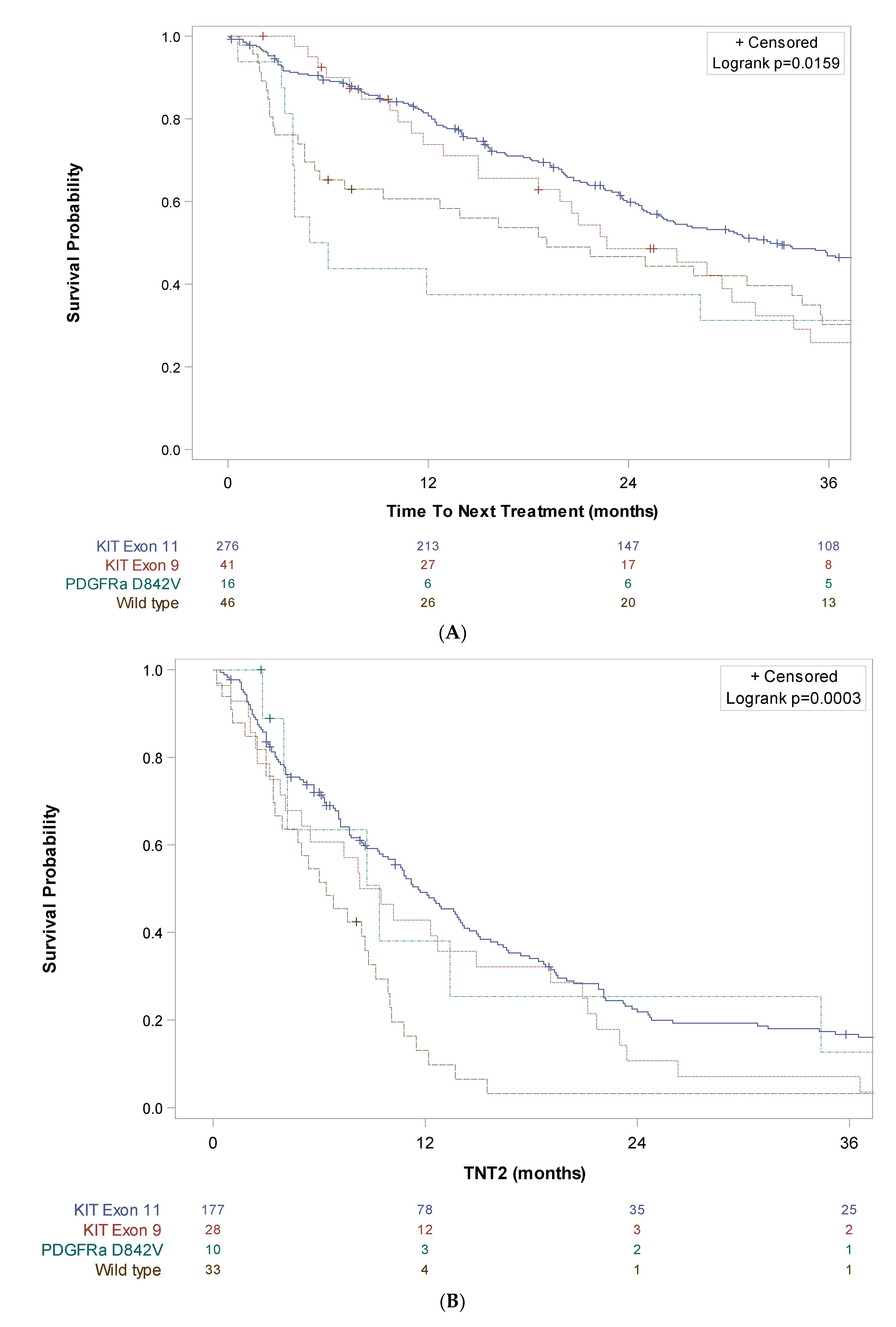
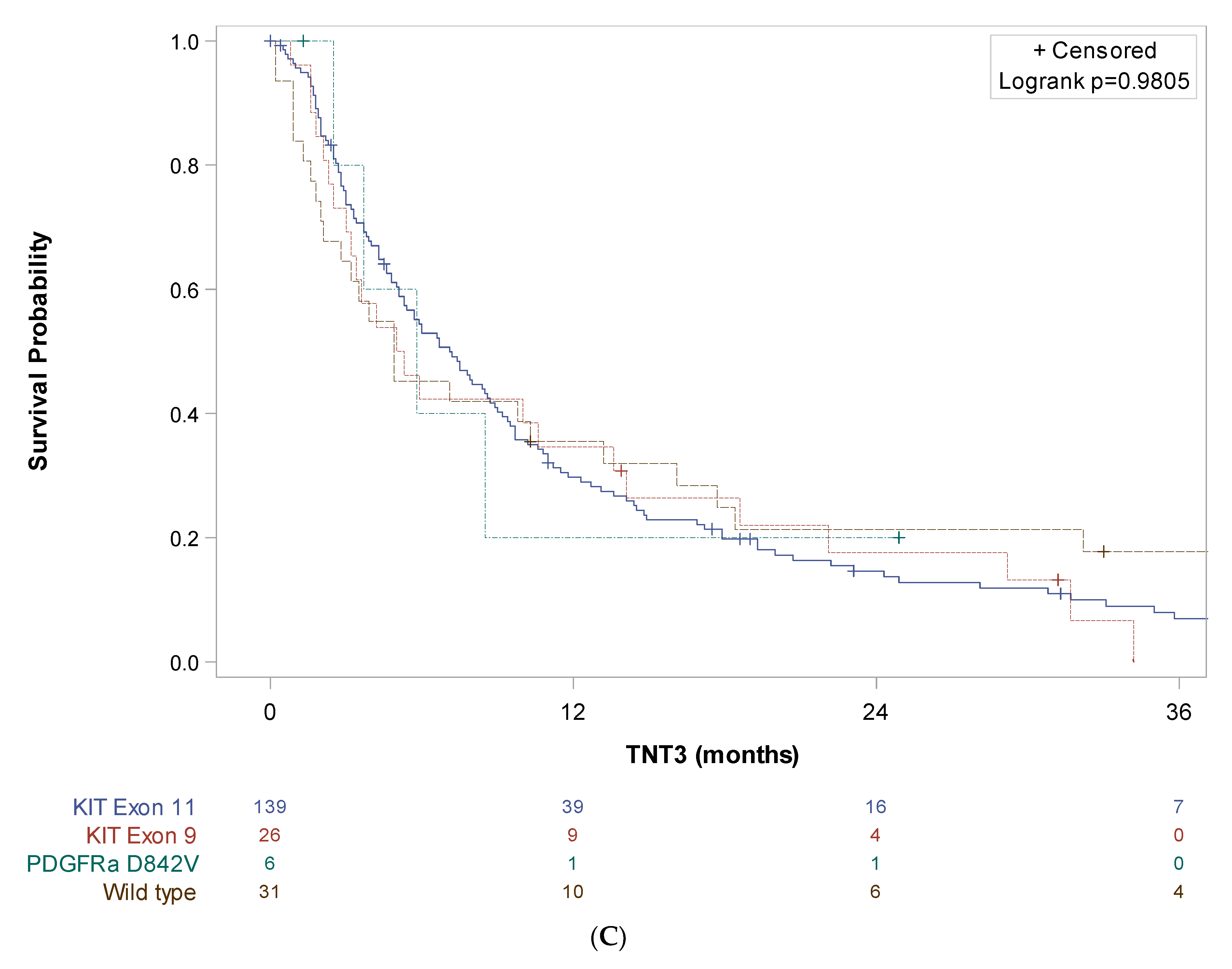
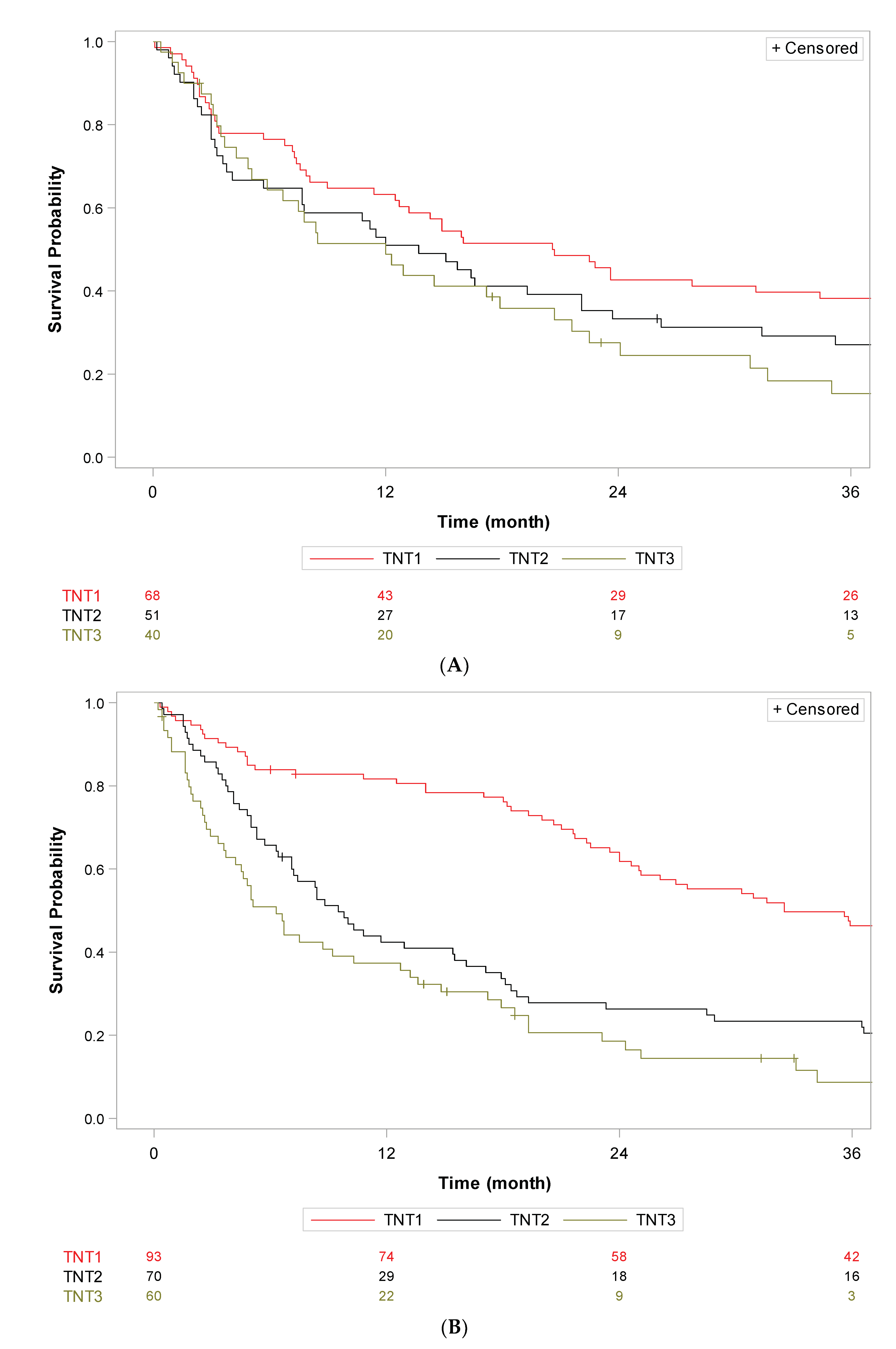
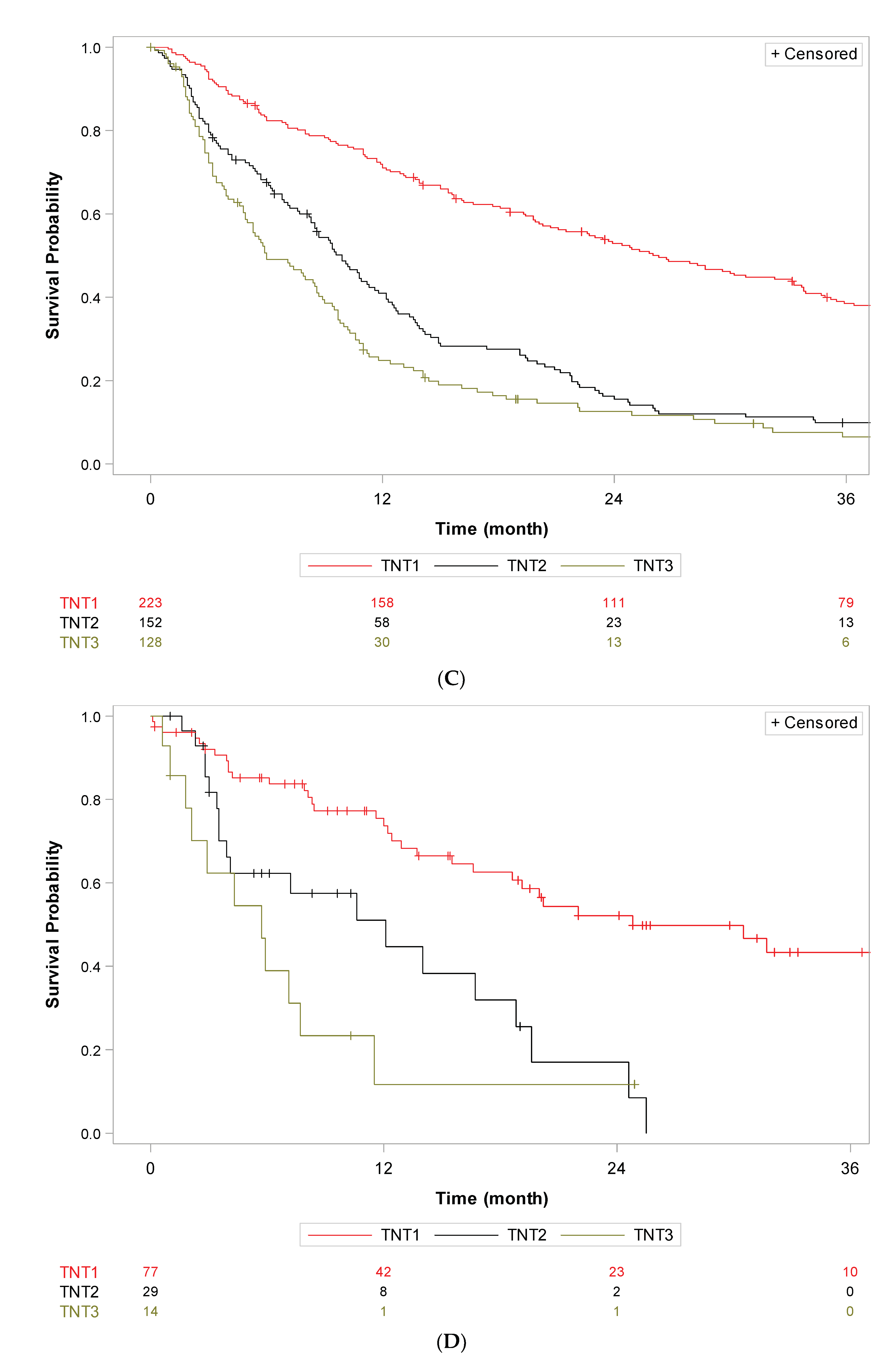
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hirota, S.; Isozaki, K.; Moriyama, Y.; Hashimoto, K.; Nishida, T.; Ishiguro, S.; Kawano, K.; Hanada, M.; Kurata, A.; Takeda, M.; et al. Gain-of-function mutations of c-kit in human gastrointestinal stromal tumors. Science 1998, 279, 577–580. [Google Scholar] [CrossRef] [PubMed]
- Demetri, G.D.; von Mehren, M.; Blanke, C.D.; Van den Abbeele, A.D.; Eisenberg, B.; Roberts, P.J.; Heinrich, M.C.; Tuveson, D.A.; Singer, S.; Janicek, M.; et al. Efficacy and safety of imatinib mesylate in advanced gastrointestinal stromal tumors. N. Engl. J. Med. 2002, 347, 472–480. [Google Scholar] [CrossRef] [PubMed]
- Demetri, G.D.; van Oosterom, A.T.; Garrett, C.R.; Blackstein, M.E.; Shah, M.H.; Verweij, J.; McArthur, G.; Judson, I.R.; Heinrich, M.C.; Morgan, J.A.; et al. Efficacy and safety of sunitinib in patients with advanced gastrointestinal stromal tumour after failure of imatinib: A randomised controlled trial. Lancet 2006, 368, 1329–1338. [Google Scholar] [CrossRef]
- Demetri, G.D.; Reichardt, P.; Kang, Y.K.; Blay, J.Y.; Rutkowski, P.; Gelderblom, H.; Hohenberger, P.; Leahy, M.; von Mehren, M.; Joensuu, H.; et al. Efficacy and safety of regorafenib for advanced gastrointestinal stromal tumours after failure of imatinib and sunitinib (GRID): An international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet 2013, 381, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Venkataraman, V.; George, S.; Cote, G.M. Molecular Advances in the Treatment of Advanced Gastrointestinal Stromal Tumor. Oncologist 2023, 28, oyad167. [Google Scholar] [CrossRef]
- Zhang, H.; Jiang, T.; Mu, M.; Zhao, Z.; Yin, X.; Cai, Z.; Zhang, B.; Yin, Y. Radiotherapy in the Management of Gastrointestinal Stromal Tumors: A Systematic Review. Cancers 2022, 14, 3169. [Google Scholar] [CrossRef]
- Lillemoe, H.A.; Brudvik, K.W.; Vauthey, J.N. Treatment Options for Metastatic Gastrointestinal Stromal Tumors to the Liver: A Review. Semin. Liver Dis. 2019, 39, 395–402. [Google Scholar] [CrossRef]
- Patterson, T.; Li, H.; Chai, J.; Debruyns, A.; Simmons, C.; Hart, J.; Pollock, P.; Holloway, C.L.; Truong, P.T.; Feng, X. Locoregional Treatments for Metastatic Gastrointestinal Stromal Tumor in British Columbia: A Retrospective Cohort Study from January 2008 to December 2017. Cancers 2022, 14, 1477. [Google Scholar] [CrossRef]
- Blay, J.Y.; Serrano, C.; Heinrich, M.C.; Zalcberg, J.; Bauer, S.; Gelderblom, H.; Schoffski, P.; Jones, R.L.; Attia, S.; D’Amato, G.; et al. Ripretinib in patients with advanced gastrointestinal stromal tumours (INVICTUS): A double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol. 2020, 21, 923–934. [Google Scholar] [CrossRef]
- Jones, R.L.; Serrano, C.; von Mehren, M.; George, S.; Heinrich, M.C.; Kang, Y.K.; Schoffski, P.; Cassier, P.A.; Mir, O.; Chawla, S.P.; et al. Avapritinib in unresectable or metastatic PDGFRA D842V-mutant gastrointestinal stromal tumours: Long-term efficacy and safety data from the NAVIGATOR phase I trial. Eur. J. Cancer 2021, 145, 132–142. [Google Scholar] [CrossRef]
- Casali, P.G.; Zalcberg, J.; Le Cesne, A.; Reichardt, P.; Blay, J.Y.; Lindner, L.H.; Judson, I.R.; Schoffski, P.; Leyvraz, S.; Italiano, A.; et al. Ten-Year Progression-Free and Overall Survival in Patients With Unresectable or Metastatic GI Stromal Tumors: Long-Term Analysis of the European Organisation for Research and Treatment of Cancer, Italian Sarcoma Group, and Australasian Gastrointestinal Trials Group Intergroup Phase III Randomized Trial on Imatinib at Two Dose Levels. J. Clin. Oncol. 2017, 35, 1713–1720. [Google Scholar] [CrossRef] [PubMed]
- Bauer, S.; Rutkowski, P.; Hohenberger, P.; Miceli, R.; Fumagalli, E.; Siedlecki, J.A.; Nguyen, B.P.; Kerst, M.; Fiore, M.; Nyckowski, P.; et al. Long-term follow-up of patients with GIST undergoing metastasectomy in the era of imatinib—Analysis of prognostic factors (EORTC-STBSG collaborative study). Eur. J. Surg. Oncol. 2014, 40, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.; Ryu, M.H.; Lee, Y.; Park, Y.S.; Kim, K.H.; Kim, J.H.; Park, Y.; Lee, S.M.; Kim, C.W.; Kim, B.S.; et al. Role of Resection Following Focal Progression with Standard Doses of Imatinib in Patients with Advanced Gastrointestinal Stromal Tumors: Results of Propensity Score Analyses. Oncologist 2019, 24, e1443–e1449. [Google Scholar] [CrossRef] [PubMed]
- Casali, P.G.; Blay, J.Y.; Abecassis, N.; Bajpai, J.; Bauer, S.; Biagini, R.; Bielack, S.; Bonvalot, S.; Boukovinas, I.; Bovee, J.; et al. Gastrointestinal stromal tumours: ESMO-EURACAN-GENTURIS Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2022, 33, 20–33. [Google Scholar] [CrossRef]
- Schrage, Y.; Hartgrink, H.; Smith, M.; Fiore, M.; Rutkowski, P.; Tzanis, D.; Messiou, C.; Servois, V.; Bonvalot, S.; van der Hage, J. Surgical management of metastatic gastrointestinal stromal tumour. Eur. J. Surg. Oncol. 2018, 44, 1295–1300. [Google Scholar] [CrossRef]
- Jones, R.L.; McCall, J.; Adam, A.; O’Donnell, D.; Ashley, S.; Al-Muderis, O.; Thway, K.; Fisher, C.; Judson, I.R. Radiofrequency ablation is a feasible therapeutic option in the multi modality management of sarcoma. Eur. J. Surg. Oncol. 2010, 36, 477–482. [Google Scholar] [CrossRef]
- Chudley, L.; McCann, K.; Mander, A.; Tjelle, T.; Campos-Perez, J.; Godeseth, R.; Creak, A.; Dobbyn, J.; Johnson, B.; Bass, P.; et al. DNA fusion-gene vaccination in patients with prostate cancer induces high-frequency CD8(+) T-cell responses and increases PSA doubling time. Cancer Immunol. Immunother. 2012, 61, 2161–2170. [Google Scholar] [CrossRef]
- Liang, C.; Li, L.; Fraser, C.D.; Ko, A.; Corzo, D.; Enger, C.; Patt, D. The treatment patterns, efficacy, and safety of nab ((R))-paclitaxel for the treatment of metastatic breast cancer in the United States: Results from health insurance claims analysis. BMC Cancer 2015, 15, 1019. [Google Scholar] [CrossRef]
- Dudeck, O.; Zeile, M.; Reichardt, P.; Pink, D. Comparison of RECIST and Choi criteria for computed tomographic response evaluation in patients with advanced gastrointestinal stromal tumor treated with sunitinib. Ann. Oncol. 2011, 22, 1828–1833. [Google Scholar] [CrossRef]
- Schramm, N.; Englhart, E.; Schlemmer, M.; Hittinger, M.; Ubleis, C.; Becker, C.R.; Reiser, M.F.; Berger, F. Tumor response and clinical outcome in metastatic gastrointestinal stromal tumors under sunitinib therapy: Comparison of RECIST, Choi and volumetric criteria. Eur. J. Radiol. 2013, 82, 951–958. [Google Scholar] [CrossRef]
- Shinagare, A.B.; Jagannathan, J.P.; Kurra, V.; Urban, T.; Manola, J.; Choy, E.; Demetri, G.D.; George, S.; Ramaiya, N.H. Comparison of performance of various tumour response criteria in assessment of regorafenib activity in advanced gastrointestinal stromal tumours after failure of imatinib and sunitinib. Eur. J. Cancer 2014, 50, 981–986. [Google Scholar] [CrossRef] [PubMed]
- Cassier, P.A.; Fumagalli, E.; Rutkowski, P.; Schoffski, P.; Van Glabbeke, M.; Debiec-Rychter, M.; Emile, J.F.; Duffaud, F.; Martin-Broto, J.; Landi, B.; et al. Outcome of patients with platelet-derived growth factor receptor alpha-mutated gastrointestinal stromal tumors in the tyrosine kinase inhibitor era. Clin. Cancer Res. 2012, 18, 4458–4464. [Google Scholar] [CrossRef] [PubMed]
- Heinrich, M.C.; Maki, R.G.; Corless, C.L.; Antonescu, C.R.; Harlow, A.; Griffith, D.; Town, A.; McKinley, A.; Ou, W.B.; Fletcher, J.A.; et al. Primary and secondary kinase genotypes correlate with the biological and clinical activity of sunitinib in imatinib-resistant gastrointestinal stromal tumor. J. Clin. Oncol. 2008, 26, 5352–5359. [Google Scholar] [CrossRef] [PubMed]
- Heinrich, M.C.; Owzar, K.; Corless, C.L.; Hollis, D.; Borden, E.C.; Fletcher, C.D.; Ryan, C.W.; von Mehren, M.; Blanke, C.D.; Rankin, C.; et al. Correlation of kinase genotype and clinical outcome in the North American Intergroup Phase III Trial of imatinib mesylate for treatment of advanced gastrointestinal stromal tumor: CALGB 150105 Study by Cancer and Leukemia Group B and Southwest Oncology Group. J. Clin. Oncol. 2008, 26, 5360–5367. [Google Scholar] [CrossRef]
- Khosroyani, H.M.; Klug, L.R.; Heinrich, M.C. TKI Treatment Sequencing in Advanced Gastrointestinal Stromal Tumors. Drugs 2023, 83, 55–73. [Google Scholar] [CrossRef]
- Saesen, R.; Van Hemelrijck, M.; Bogaerts, J.; Booth, C.M.; Cornelissen, J.J.; Dekker, A.; Eisenhauer, E.A.; Freitas, A.; Gronchi, A.; Hernan, M.A.; et al. Defining the role of real-world data in cancer clinical research: The position of the European Organisation for Research and Treatment of Cancer. Eur. J. Cancer 2023, 186, 52–61. [Google Scholar] [CrossRef]
- Visvanathan, K.; Levit, L.A.; Raghavan, D.; Hudis, C.A.; Wong, S.; Dueck, A.; Lyman, G.H. Untapped Potential of Observational Research to Inform Clinical Decision Making: American Society of Clinical Oncology Research Statement. J. Clin. Oncol. 2017, 35, 1845–1854. [Google Scholar] [CrossRef]
- Toulmonde, M.; Blay, J.Y.; Bouche, O.; Mir, O.; Penel, N.; Isambert, N.; Duffaud, F.; Bompas, E.; Esnaud, T.; Boidot, R.; et al. Activity and Safety of Palbociclib in Patients with Advanced Gastrointestinal Stromal Tumors Refractory to Imatinib and Sunitinib: A Biomarker-driven Phase II Study. Clin. Cancer Res. 2019, 25, 4611–4615. [Google Scholar] [CrossRef]
- Lagarde, P.; Perot, G.; Kauffmann, A.; Brulard, C.; Dapremont, V.; Hostein, I.; Neuville, A.; Wozniak, A.; Sciot, R.; Schoffski, P.; et al. Mitotic checkpoints and chromosome instability are strong predictors of clinical outcome in gastrointestinal stromal tumors. Clin. Cancer Res. 2012, 18, 826–838. [Google Scholar] [CrossRef]
- Savina, M.; Le Cesne, A.; Blay, J.Y.; Ray-Coquard, I.; Mir, O.; Toulmonde, M.; Cousin, S.; Terrier, P.; Ranchere-Vince, D.; Meeus, P.; et al. Patterns of care and outcomes of patients with METAstatic soft tissue SARComa in a real-life setting: The METASARC observational study. BMC Med. 2017, 15, 78. [Google Scholar] [CrossRef]
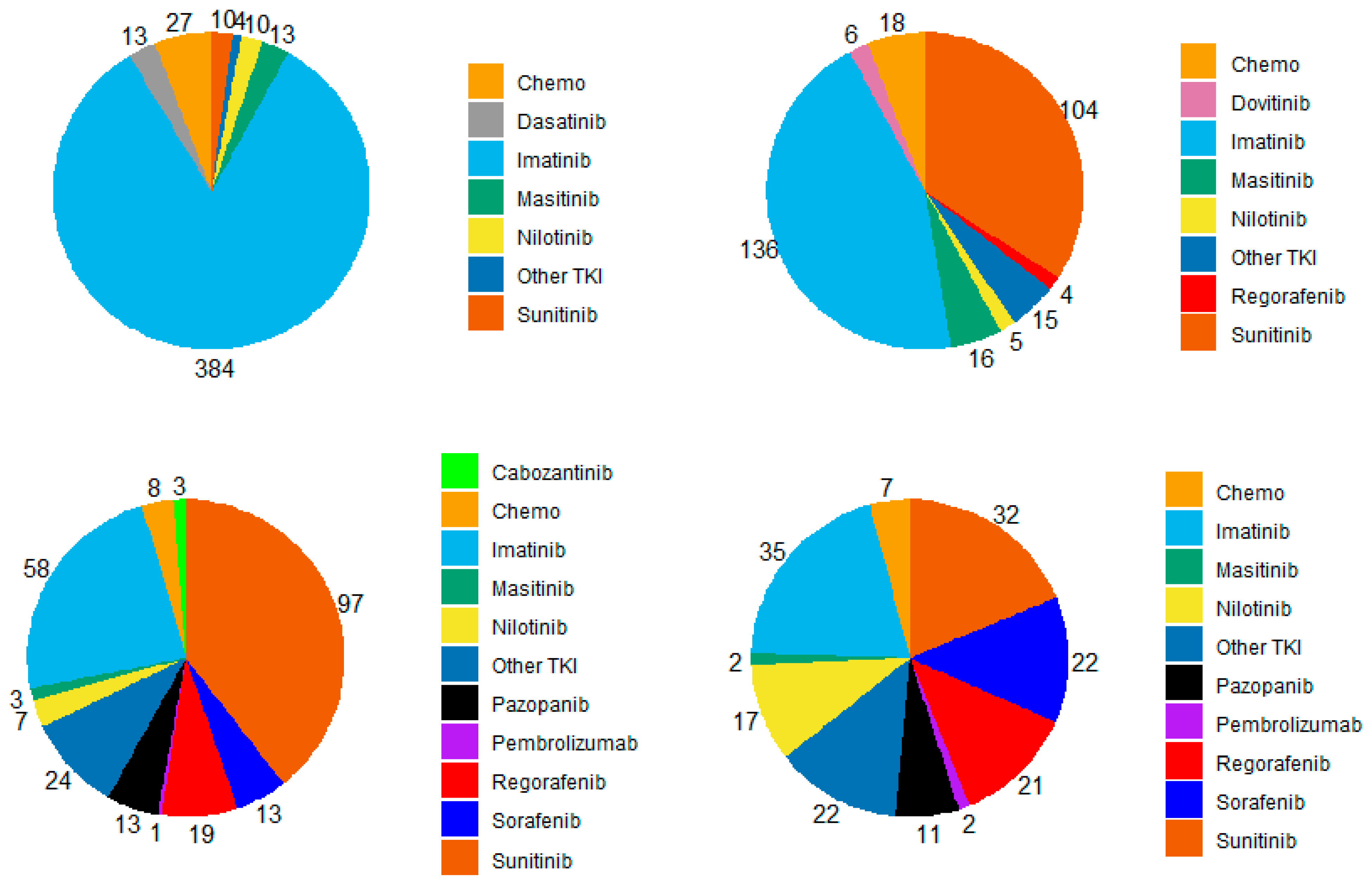
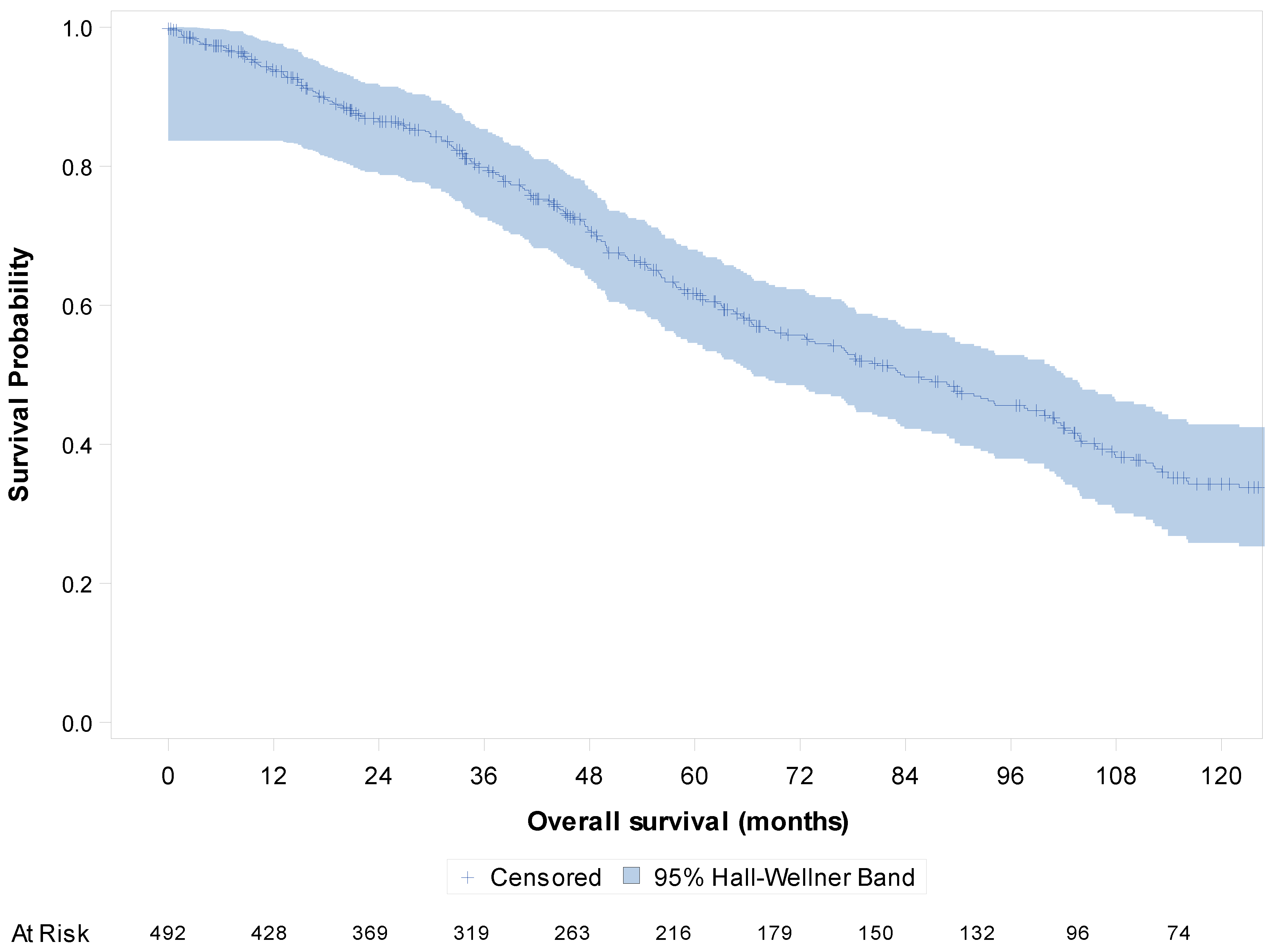
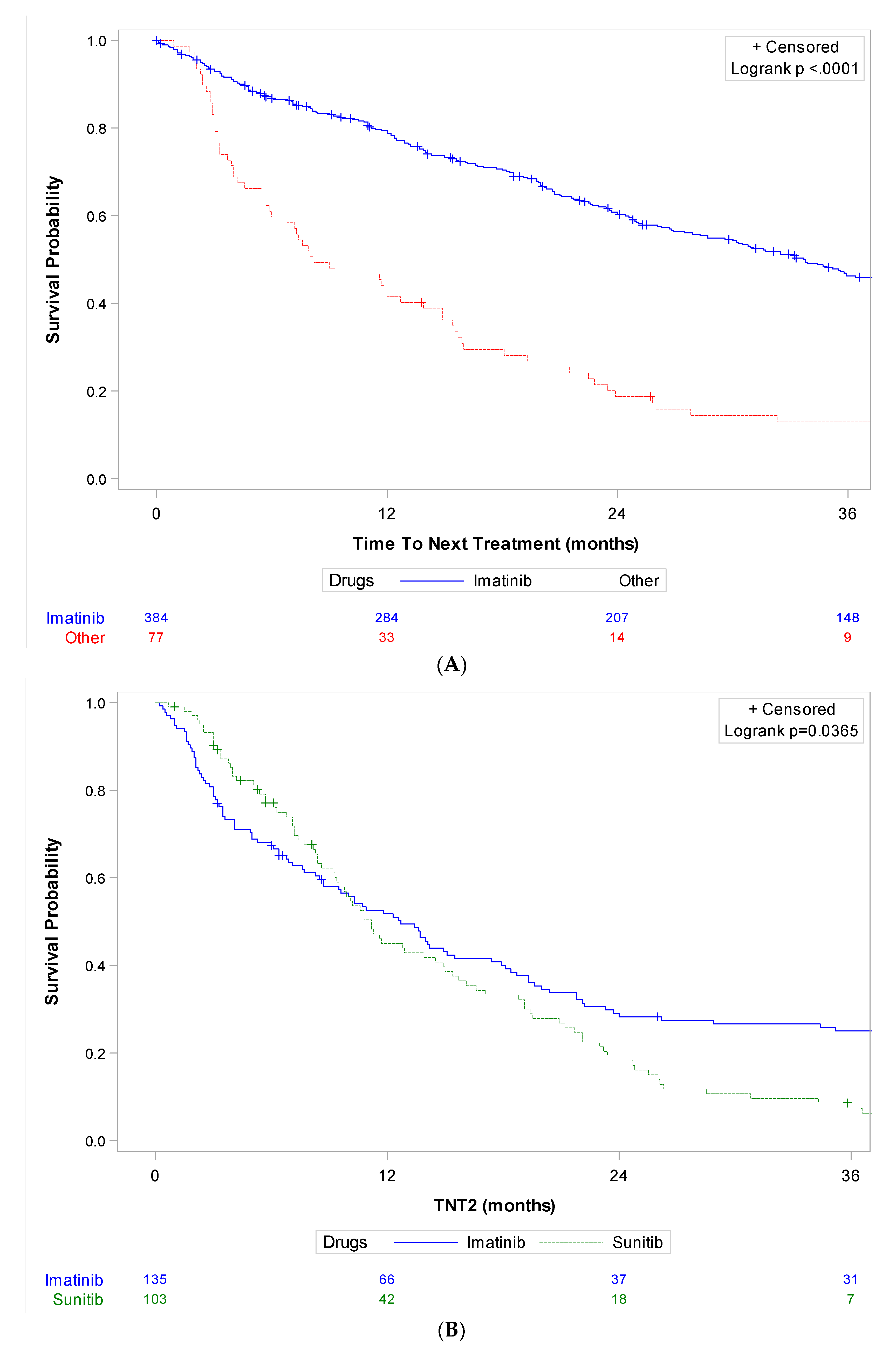
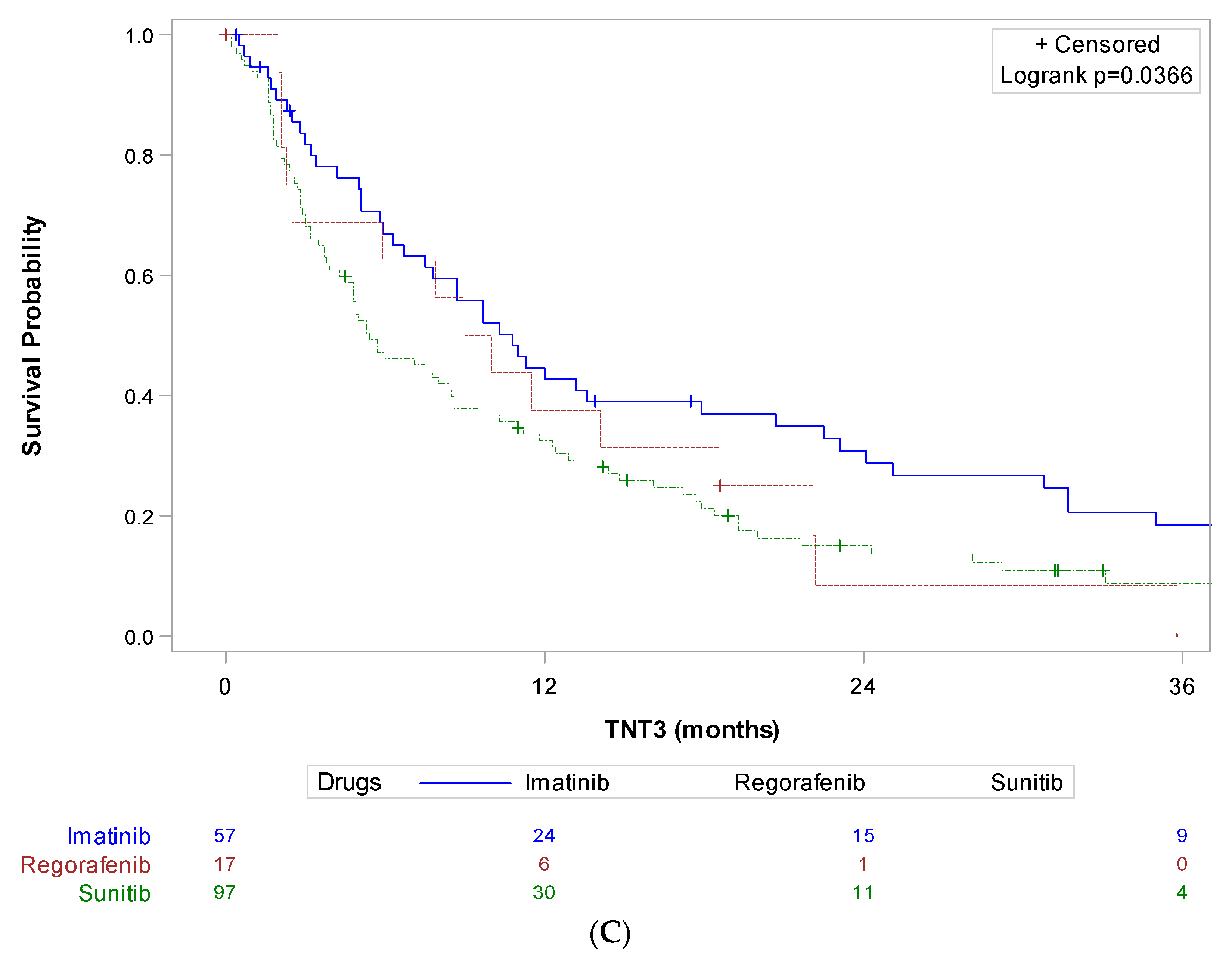
| All Patients (n = 1038) | Study Patients (n = 492) | |||
|---|---|---|---|---|
| n | % | n | % | |
| Sex | 547 | 52.7 | 292 | 59.3 |
| Male | ||||
| Female | 491 | 47.3 | 200 | 40.7 |
| Median age at diagnosis, years (min-max) | 61 | (19–93) | 59 | (19–93) |
| Significant previous history | ||||
| No | 850 | 81.9 | 424 | 86.2 |
| Previous cancer | 106 | 10.2 | 36 | 7.3 |
| NF1 | 26 | 2.5 | 8 | 1.6 |
| Other | 21 | 2 | 7 | 1.4 |
| Unknown | 19 | 1.8 | 6 | 1.2 |
| Tumor site | ||||
| Stomach | 527 | 50.8 | 204 | 41.5 |
| Small intestine | 327 | 31.5 | 192 | 39.0 |
| Duodenum | 60 | 5.8 | 26 | 5.3 |
| Rectum | 55 | 5.3 | 24 | 4.9 |
| Peritoneum | 30 | 2.9 | 19 | 3.9 |
| Colon | 24 | 2.3 | 17 | 3.5 |
| Esophagus | 15 | 1.4 | 10 | 2.0 |
| Median tumor size, mm (min-max) | 80 | (3–450) | 100 | (18–400) |
| Median mitotic index/50HPF (min–max) | 5 | (0–350) | 10 | (0–350) |
| Miettinen AFIP scoring | ||||
| High risk | 501 | 48.3 | 340 | 69.1 |
| Intermediate risk | 186 | 17.9 | 69 | 14.0 |
| Low risk | 137 | 13.2 | 18 | 3.7 |
| Very low risk | 124 | 11.9 | 5 | 1.0 |
| NA | 90 | 8.7 | 60 | 12.2 |
| Mutational status | ||||
| KIT Exon 11 | 543 | 52.3 | 287 | 58.3 |
| KIT Exon 9 | 75 | 7.2 | 42 | 8.5 |
| PDGFRa Exon 18 D842V | 61 | 5.9 | 20 | 4.1 |
| Wild type | 115 | 11.1 | 52 | 10.6 |
| Other * | 77 | 7.4 | 26 | 5.3 |
| NA | 167 | 16.1 | 65 | 13.2 |
| Metastasis at diagnosis | ||||
| No | 774 | 74.6 | 233 | 47.4 |
| Yes ** | 260 | 25.0 | 259 | 52.6 |
| NA | 4 | 0.4 | ||
| Status At Expert Center Referral | ||||
| First event | 871 | 83.9 | 349 | 70.9 |
| Metastatic Relapse/Progression | 155 | 14.9 | 143 | 29.1 |
| NA | 12 | 1.2 | ||
| A | ||||
|---|---|---|---|---|
| Clinical and Molecular Factors | p-Value (khi-2) | Hazard Ratio | HR Lower Conf. Limit | HR Upper Conf. Limit |
| Age at diagnosis | ||||
| Over 60 yo vs. under 60 yo | 0.022 | 1.38 | 1.05 | 1.81 |
| Mitotic Index | <0.001 | 1.01 | 1.00 | 1.01 |
| Mutational Status | 0.019 | |||
| PDGFRa D842V vs. KIT Exon 11 | 0.597 | 1.25 | 0.54 | 2.89 |
| KIT Exon 9 vs. KIT Exon 11 | 0.011 | 1.80 | 1.14 | 2.85 |
| Other vs. KIT Exon 11 | 0.507 | 1.26 | 0.64 | 2.50 |
| Wild type vs. KIT Exon 11 | 0.002 | 1.90 | 1.26 | 2.86 |
| NA vs. KIT Exon 11 | 0.910 | 1.03 | 0.66 | 1.60 |
| Surgery of primary tumor | ||||
| Yes vs. No | 0.001 | 0.48 | 0.31 | 0.76 |
| LR treatment of metastatic sites | ||||
| Yes vs. No | <0.001 | 0.60 | 0.46 | 0.79 |
| B | ||||
| Clinical and molecular factors | p-Value (chi-2) | Hazard Ratio | HR Lower Conf. Limit | HR Upper Conf. Limit |
| Tumor size | 0.004 | 1.00 | 1.00 | 1.01 |
| Mitotic Index | 0.015 | 1.01 | 1.00 | 1.01 |
| Mutational Status | 0.010 | |||
| PDGFRa D842V vs. KIT Exon 11 | 0.075 | 0.16 | 0.02 | 1.21 |
| KIT Exon 9 vs. KIT Exon 11 | <0.001 | 2.76 | 1.57 | 4.86 |
| Other vs. KIT Exon 11 | 0.951 | 0.96 | 0.23 | 3.95 |
| Wild type vs. KIT Exon 11 | 0.003 | 2.77 | 1.43 | 5.35 |
| NA vs. KIT Exon 11 | 0.732 | 1.12 | 0.59 | 2.14 |
| Surgery of primary tumor | ||||
| Yes vs. No | <0.001 | 0.34 | 0.21 | 0.57 |
| Clinical and Molecular Factors | p-Value (khi-2) | Hazard Ratio | HR Lower Conf. Limit | HR Upper Conf. Limit |
|---|---|---|---|---|
| Age at diagnosis | ||||
| Over 60 yo vs. under 60 yo | 0.037 | 1.28 | 1.01 | 1.61 |
| AFIP Miettinen classification | 0.006 | |||
| Intermediate risk vs. High risk | 0.115 | 0.76 | 0.54 | 1.07 |
| Low/very low risk vs. High risk | <0.001 | 0.30 | 0.15 | 0.61 |
| NA/Missing vs. High risk | 0.971 | |||
| Mutational Status | <0.001 | |||
| PDGFRa D842V vs. KIT Exon 11 | 0.004 | 2.25 | 1.29 | 3.93 |
| KIT Exon 9 vs. KIT Exon 11 | 0.018 | 1.61 | 1.08 | 2.39 |
| Other vs. KIT Exon 11 | 0.305 | 1.33 | 0.77 | 2.31 |
| Wild type vs. KIT Exon 11 | <0.001 | 1.95 | 1.36 | 2.81 |
| NA vs. KIT Exon 11 | 0.008 | 1.70 | 1.15 | 2.50 |
| Surgery of primary tumor | ||||
| Yes vs. No | 0.005 | 0.56 | 0.38 | 0.84 |
| Participated in a clin. trial in 1st line | ||||
| Yes vs. No | 0.008 | 0.73 | 0.58 | 0.92 |
| LR treatment of metastatic sites | ||||
| Yes vs. No | 0.015 | 0.75 | 0.59 | 0.95 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Toulmonde, M.; Dinart, D.; Brahmi, M.; Verret, B.; Jean-Denis, M.; Ducimetière, F.; Desolneux, G.; Méeus, P.; Palussière, J.; Buy, X.; et al. Evolution of Patterns of Care and Outcomes in the Real-Life Setting for Patients with Metastatic GIST Treated in Three French Expert Centers over Three Decades. Cancers 2023, 15, 4306. https://doi.org/10.3390/cancers15174306
Toulmonde M, Dinart D, Brahmi M, Verret B, Jean-Denis M, Ducimetière F, Desolneux G, Méeus P, Palussière J, Buy X, et al. Evolution of Patterns of Care and Outcomes in the Real-Life Setting for Patients with Metastatic GIST Treated in Three French Expert Centers over Three Decades. Cancers. 2023; 15(17):4306. https://doi.org/10.3390/cancers15174306
Chicago/Turabian StyleToulmonde, Maud, Derek Dinart, Mehdi Brahmi, Benjamin Verret, Myriam Jean-Denis, Françoise Ducimetière, Gregoire Desolneux, Pierre Méeus, Jean Palussière, Xavier Buy, and et al. 2023. "Evolution of Patterns of Care and Outcomes in the Real-Life Setting for Patients with Metastatic GIST Treated in Three French Expert Centers over Three Decades" Cancers 15, no. 17: 4306. https://doi.org/10.3390/cancers15174306
APA StyleToulmonde, M., Dinart, D., Brahmi, M., Verret, B., Jean-Denis, M., Ducimetière, F., Desolneux, G., Méeus, P., Palussière, J., Buy, X., Bouhamama, A., Gillon, P., Dufresne, A., Hénon, C., Le Loarer, F., Karanian, M., Ngo, C., Mathoulin-Pélissier, S., Bellera, C., ... Italiano, A. (2023). Evolution of Patterns of Care and Outcomes in the Real-Life Setting for Patients with Metastatic GIST Treated in Three French Expert Centers over Three Decades. Cancers, 15(17), 4306. https://doi.org/10.3390/cancers15174306








