Oncotype Dx Score, HER2 Low Expression, and Clinical Outcomes in Early-Stage Breast Cancer: A National Cancer Database Analysis
Abstract
:Simple Summary
Abstract
1. Introduction
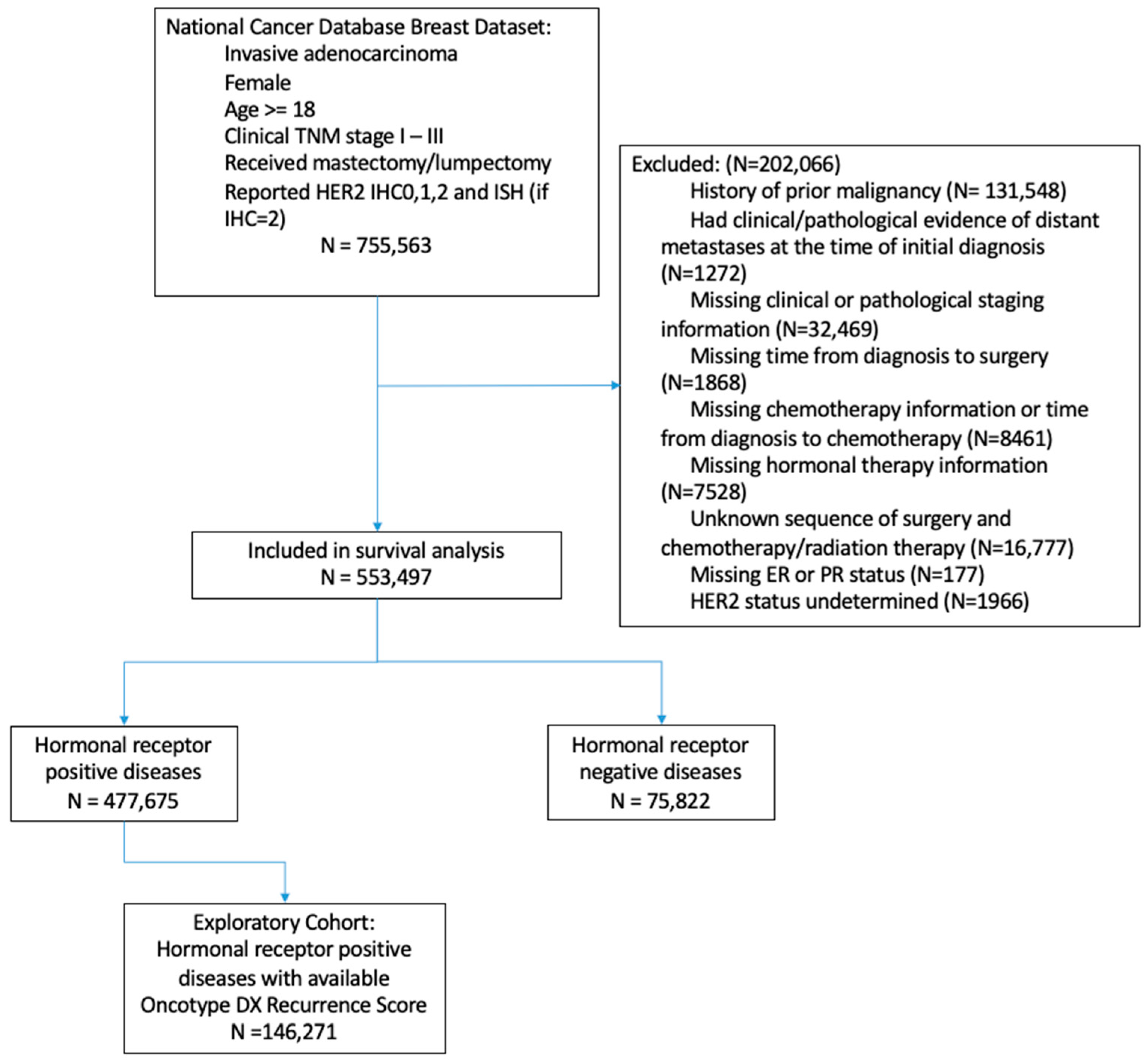
2. Materials and Methods
2.1. Patient Selections
2.2. Statistical Analysis
3. Results
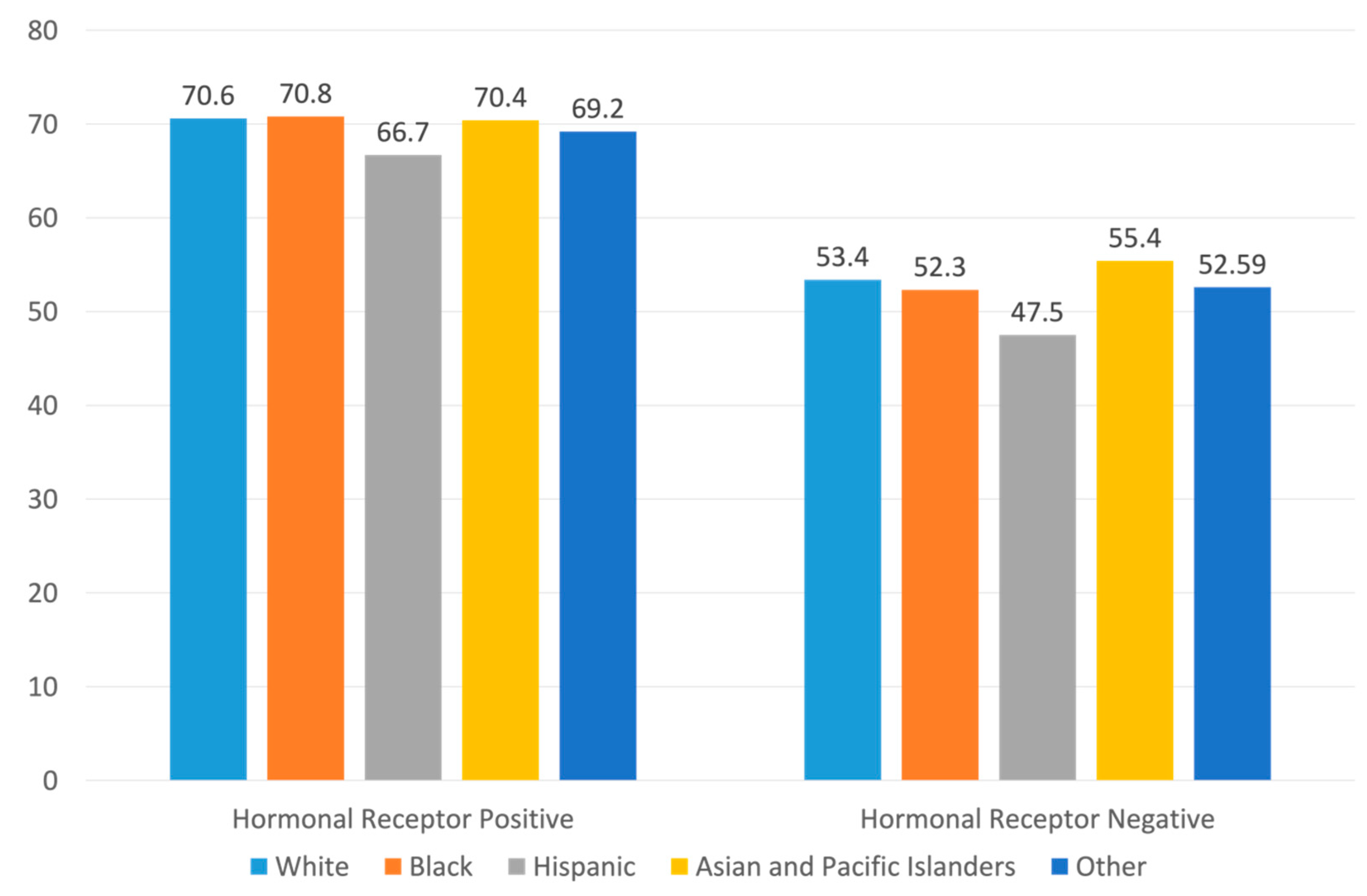
3.1. Patients Characteristics
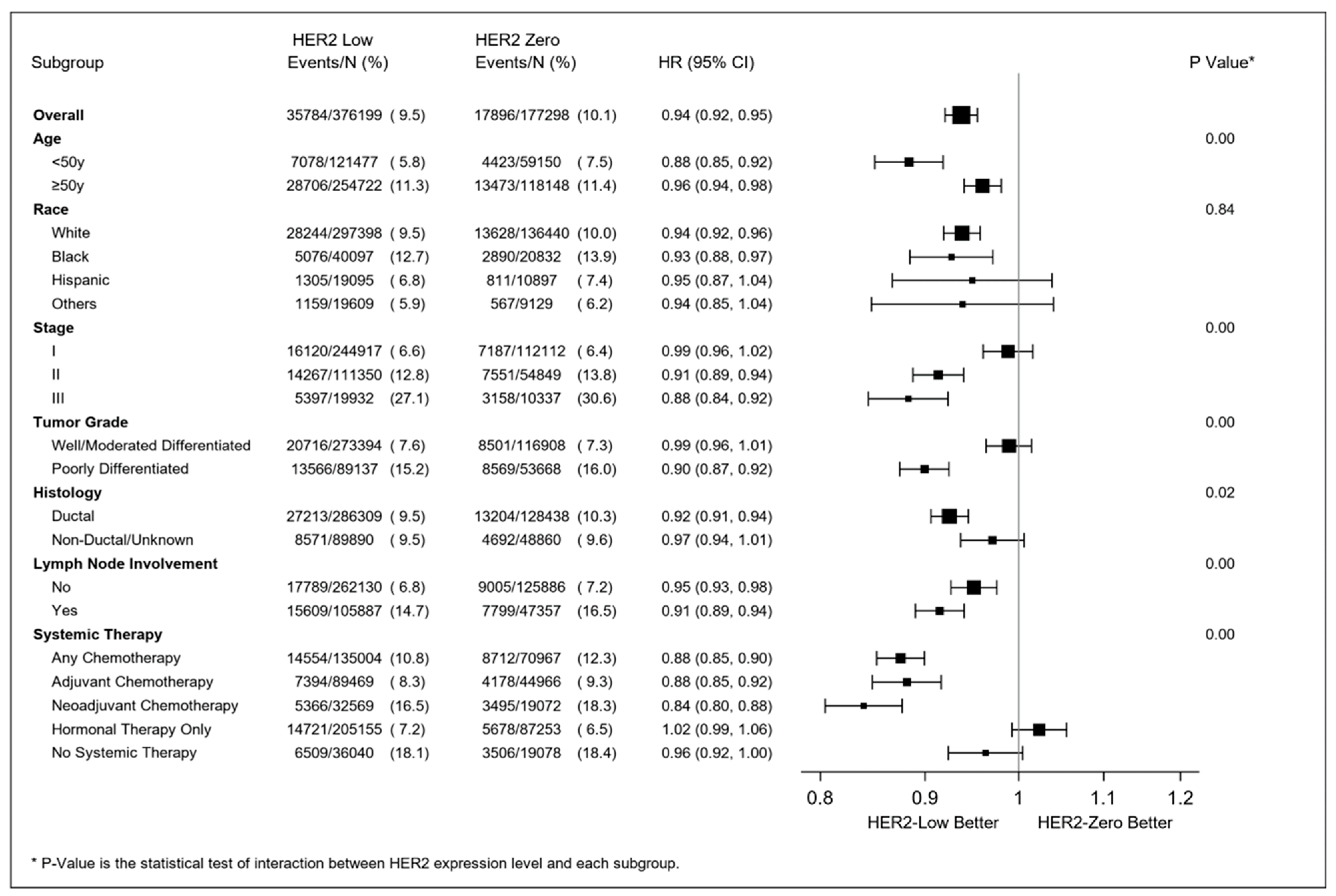
3.2. Kaplan–Meier Estimates
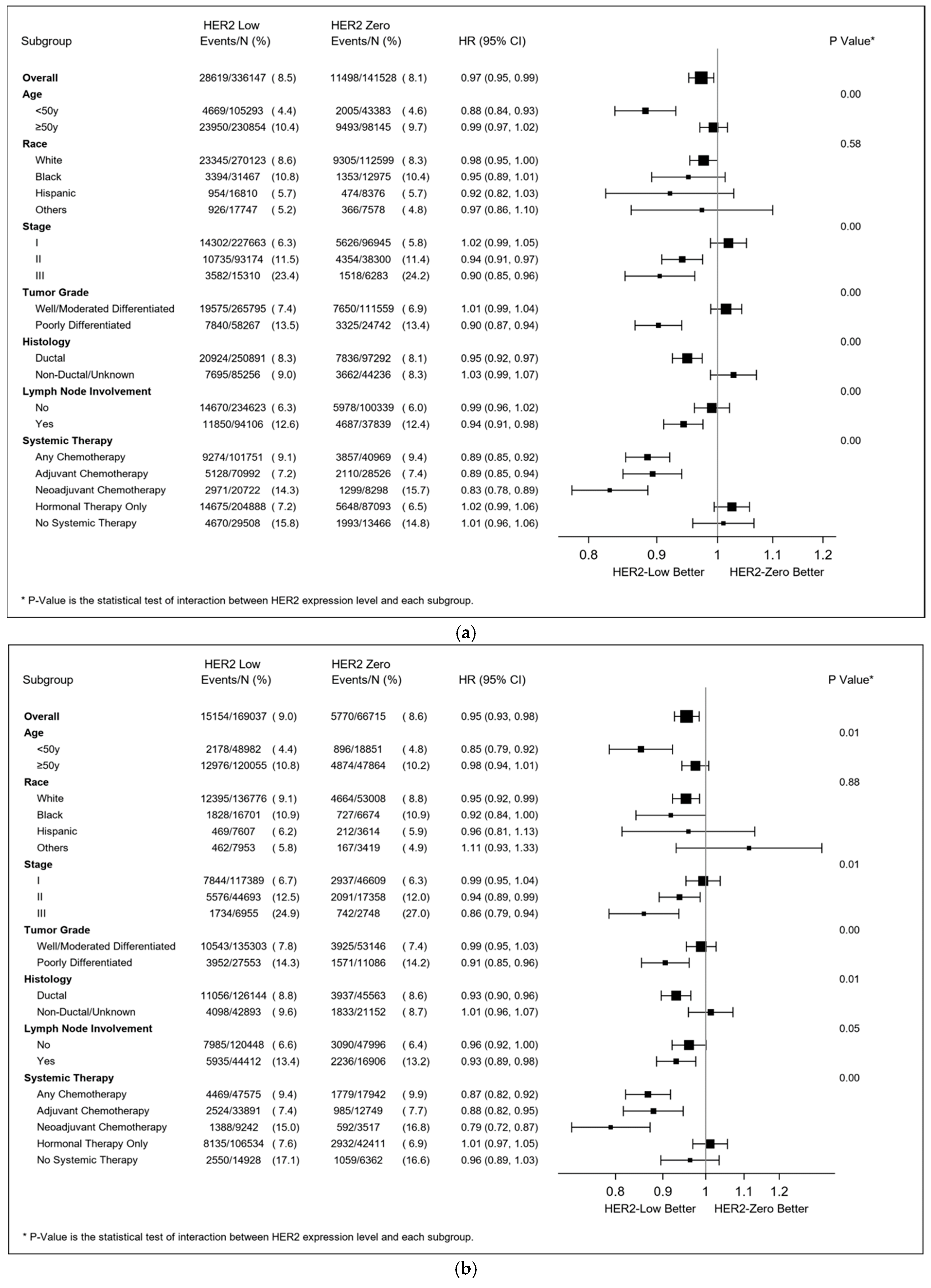
3.3. Response to Neoadjuvant Chemotherapy
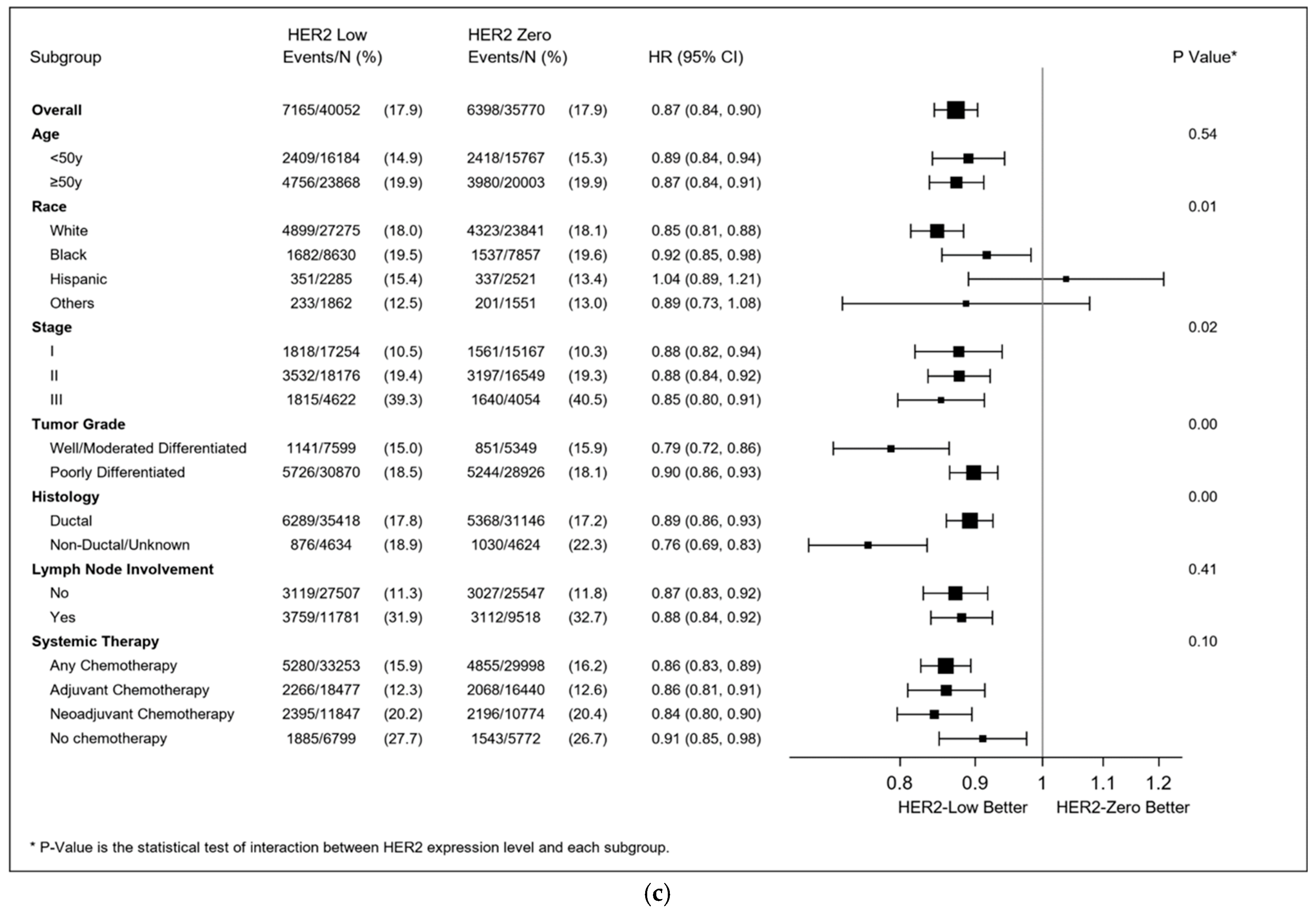
3.4. Multivariable Survival Analyses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hudis, C.A. Trastuzumab—Mechanism of action and use in clinical practice. N. Engl. J. Med. 2007, 357, 39–51. [Google Scholar] [CrossRef] [PubMed]
- Von Minckwitz, G.; Procter, M.; de Azambuja, E.; Zardavas, D.; Benyunes, M.; Viale, G.; Suter, T.; Arahmani, A.; Rouchet, N.; Clark, E.; et al. Adjuvant Pertuzumab and Trastuzumab in Early HER2-Positive Breast Cancer. N. Engl. J. Med. 2017, 377, 122–131. [Google Scholar] [CrossRef] [PubMed]
- Von Minckwitz, G.; Huang, C.S.; Mano, M.S.; Loibl, S.; Mamounas, E.P.; Untch, M.; Wolmark, N.; Rastogi, P.; Schneeweiss, A.; Redondo, A.; et al. Trastuzumab Emtansine for Residual Invasive HER2-Positive Breast Cancer. N. Engl. J. Med. 2019, 380, 617–628. [Google Scholar] [CrossRef] [PubMed]
- Wolff, A.C.; Hammond, M.E.H.; Allison, K.H.; Harvey, B.E.; Mangu, P.B.; Bartlett, J.M.; Bilous, M.; Ellis, I.O.; Fitzgibbons, P.; Hanna, W. Human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline focused update. Arch. Pathol. Lab. Med. 2018, 142, 1364–1382. [Google Scholar] [CrossRef] [PubMed]
- Fehrenbacher, L.; Cecchini, R.S.; Geyer, C.E., Jr.; Rastogi, P.; Costantino, J.P.; Atkins, J.N.; Crown, J.P.; Polikoff, J.; Boileau, J.-F.; Provencher, L. NSABP B-47/NRG oncology phase III randomized trial comparing adjuvant chemotherapy with or without trastuzumab in high-risk invasive breast cancer negative for HER2 by FISH and with IHC 1+ or 2+. J. Clin. Oncol. 2020, 38, 444. [Google Scholar] [CrossRef]
- Tarantino, P.; Hamilton, E.; Tolaney, S.M.; Cortes, J.; Morganti, S.; Ferraro, E.; Marra, A.; Viale, G.; Trapani, D.; Cardoso, F. HER2-low breast cancer: Pathological and clinical landscape. J. Clin. Oncol. 2020, 38, 1951–1962. [Google Scholar] [CrossRef]
- Diéras, V.; Deluche, E.; Lusque, A.; Pistilli, B.; Bachelot, T.; Pierga, J.-Y.; Viret, F.; Levy, C.; Salabert, L.; Du, F.L. Abstract PD8-02: Trastuzumab deruxtecan (T-DXd) for advanced breast cancer patients (ABC), regardless HER2 status: A phase II study with biomarkers analysis (DAISY). Cancer Res. 2022, 82, PD8-02. [Google Scholar] [CrossRef]
- Modi, S.; Park, H.; Murthy, R.K.; Iwata, H.; Tamura, K.; Tsurutani, J.; Moreno-Aspitia, A.; Doi, T.; Sagara, Y.; Redfern, C. Antitumor activity and safety of trastuzumab deruxtecan in patients with HER2-low–expressing advanced breast cancer: Results from a phase Ib study. J. Clin. Oncol. 2020, 38, 1887. [Google Scholar] [CrossRef]
- Nakada, T.; Sugihara, K.; Jikoh, T.; Abe, Y.; Agatsuma, T. The latest research and development into the antibody–drug conjugate, [fam-] trastuzumab deruxtecan (DS-8201a), for HER2 cancer therapy. Chem. Pharm. Bull. 2019, 67, 173–185. [Google Scholar] [CrossRef]
- Saura, C.; Thistlethwaite, F.; Banerji, U.; Lord, S.; Moreno, V.; MacPherson, I.; Boni, V.; Rolfo, C.D.; de Vries, E.G.; Van Herpen, C.M. A phase I expansion cohorts study of SYD985 in heavily pretreated patients with HER2-positive or HER2-low metastatic breast cancer. J. Clin. Oncol. 2018, 36, 1014. [Google Scholar] [CrossRef]
- Modi, S.; Jacot, W.; Yamashita, T.; Sohn, J.; Vidal, M.; Tokunaga, E.; Tsurutani, J.; Ueno, N.T.; Prat, A.; Chae, Y.S.; et al. Trastuzumab Deruxtecan in Previously Treated HER2-Low Advanced Breast Cancer. N. Engl. J. Med. 2022, 387, 9–20. [Google Scholar] [CrossRef] [PubMed]
- Roy, A.M.; Kumarasamy, V.M.; Dhakal, A.; O’Regan, R.; Gandhi, S. A review of treatment options in HER2-low breast cancer and proposed treatment sequencing algorithm. Cancer 2023, 129, 2773–2788. [Google Scholar] [CrossRef]
- Bao, K.K.; Sutanto, L.; Shirley, S.; Cheung, K.M.; Chan, J.C. The association of ERBB2-low expression with the efficacy of cyclin-dependent kinase 4/6 inhibitor in hormone receptor–positive, ERBB2-negative metastatic breast cancer. JAMA Netw. Open 2021, 4, e2133132. [Google Scholar] [CrossRef] [PubMed]
- Dehghani, M.; Keshavarz, P.; Talei, A.; Akrami, M.; Tahmasebi, S.; Safaie, A.; Ghanbari, M. The effects of low HER2/neu expression on the clinicopathological characteristics of triple-negative breast cancer patients. Asian Pac. J. Cancer Prev. 2020, 21, 3027. [Google Scholar] [CrossRef]
- Eggemann, H.; Ignatov, T.; Burger, E.; Kantelhardt, E.J.; Fettke, F.; Thomssen, C.; Costa, S.D.; Ignatov, A. Moderate HER2 expression as a prognostic factor in hormone receptor positive breast cancer. Endocr. Relat. Cancer 2015, 22, 725–733. [Google Scholar] [CrossRef]
- Schettini, F.; Chic, N.; Brasó-Maristany, F.; Paré, L.; Pascual, T.; Conte, B.; Martínez-Sáez, O.; Adamo, B.; Vidal, M.; Barnadas, E. Clinical, pathological, and PAM50 gene expression features of HER2-low breast cancer. npj Breast Cancer 2021, 7, 1. [Google Scholar] [CrossRef]
- Li, Y.; Abudureheiyimu, N.; Mo, H.; Guan, X.; Lin, S.; Wang, Z.; Chen, Y.; Chen, S.; Li, Q.; Cai, R. In real life, low-level HER2 expression may be associated with better outcome in HER2 negative breast cancer: A study of the National Cancer Center, China. Front. Oncol. 2022, 11, 774577. [Google Scholar] [CrossRef]
- Won, H.S.; Ahn, J.; Kim, Y.; Kim, J.S.; Song, J.Y.; Kim, H.K.; Lee, J.; Park, H.K.; Kim, Y.S. Clinical significance of HER2-low expression in early breast cancer: A nationwide study from the Korean Breast Cancer Society. Breast Cancer Res. 2022, 24, 22. [Google Scholar] [CrossRef]
- Shang, J.; Sun, X.; Xu, Z.; Cai, L.; Liu, C.; Wu, S.; Liu, Y. Evolution and clinical significance of HER2-low status after neoadjuvant therapy for breast cancer. Front. Oncol. 2023, 13, 1086480. [Google Scholar] [CrossRef]
- Miglietta, F.; Griguolo, G.; Bottosso, M.; Giarratano, T.; Lo Mele, M.; Fassan, M.; Cacciatore, M.; Genovesi, E.; De Bartolo, D.; Vernaci, G.; et al. HER2-low-positive breast cancer: Evolution from primary tumor to residual disease after neoadjuvant treatment. npj Breast Cancer 2022, 8, 66. [Google Scholar] [CrossRef]
- Tarantino, P.; Jin, Q.; Tayob, N.; Jeselsohn, R.M.; Schnitt, S.J.; Vincuilla, J.; Parker, T.; Tyekucheva, S.; Li, T.; Lin, N.U.; et al. Prognostic and Biologic Significance of ERBB2-Low Expression in Early-Stage Breast Cancer. JAMA Oncol. 2022, 8, 1177–1183. [Google Scholar] [CrossRef] [PubMed]
- Sparano, J.A.; Gray, R.J.; Makower, D.F.; Pritchard, K.I.; Albain, K.S.; Hayes, D.F.; Geyer, C.E., Jr.; Dees, E.C.; Goetz, M.P.; Olson, J.A., Jr.; et al. Adjuvant Chemotherapy Guided by a 21-Gene Expression Assay in Breast Cancer. N. Engl. J. Med. 2018, 379, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Puglisi, F.; Gerratana, L.; Lambertini, M.; Ceppi, M.; Boni, L.; Montemurro, F.; Russo, S.; Bighin, C.; De Laurentiis, M.; Giuliano, M.; et al. Composite risk and benefit from adjuvant dose-dense chemotherapy in hormone receptor-positive breast cancer. npj Breast Cancer 2021, 7, 82. [Google Scholar] [CrossRef]
- Licata, L.; Viale, G.; Giuliano, M.; Curigliano, G.; Chavez-MacGregor, M.; Foldi, J.; Oke, O.; Collins, J.; Del Mastro, L.; Puglisi, F.; et al. Oncotype DX results increase concordance in adjuvant chemotherapy recommendations for early-stage breast cancer. npj Breast Cancer 2023, 9, 51. [Google Scholar] [CrossRef]
- Garutti, M.; Griguolo, G.; Botticelli, A.; Buzzatti, G.; De Angelis, C.; Gerratana, L.; Molinelli, C.; Adamo, V.; Bianchini, G.; Biganzoli, L.; et al. Definition of High-Risk Early Hormone-Positive HER2-Negative Breast Cancer: A Consensus Review. Cancers 2022, 14, 1898. [Google Scholar] [CrossRef]
- Peiffer, D.S.; Zhao, F.; Chen, N.; Hahn, O.M.; Nanda, R.; Olopade, O.I.; Huo, D.; Howard, F.M. Clinicopathologic Characteristics and Prognosis of ERBB2-Low Breast Cancer Among Patients in the National Cancer Database. JAMA Oncol. 2023, 9, 500–510. [Google Scholar] [CrossRef]
- Rusthoven, C.G.; Rabinovitch, R.A.; Jones, B.L.; Koshy, M.; Amini, A.; Yeh, N.; Jackson, M.W.; Fisher, C.M. The impact of postmastectomy and regional nodal radiation after neoadjuvant chemotherapy for clinically lymph node-positive breast cancer: A National Cancer Database (NCDB) analysis. Ann. Oncol. 2016, 27, 818–827. [Google Scholar] [CrossRef]
- Zeidman, M.; Alberty-Oller, J.J.; Ru, M.; Pisapati, K.V.; Moshier, E.; Ahn, S.; Mazumdar, M.; Port, E.; Schmidt, H. Use of neoadjuvant versus adjuvant chemotherapy for hormone receptor-positive breast cancer: A National Cancer Database (NCDB) study. Breast Cancer Res. Treat. 2020, 184, 203–212. [Google Scholar] [CrossRef]
- Fayanju, O.M.; Ren, Y.; Thomas, S.M.; Greenup, R.A.; Plichta, J.K.; Rosenberger, L.H.; Tamirisa, N.; Force, J.; Boughey, J.C.; Hyslop, T.; et al. The Clinical Significance of Breast-only and Node-only Pathologic Complete Response (pCR) After Neoadjuvant Chemotherapy (NACT): A Review of 20,000 Breast Cancer Patients in the National Cancer Data Base (NCDB). Ann. Surg. 2018, 268, 591–601. [Google Scholar] [CrossRef]
- Von Minckwitz, G.; Untch, M.; Blohmer, J.U.; Costa, S.D.; Eidtmann, H.; Fasching, P.A.; Gerber, B.; Eiermann, W.; Hilfrich, J.; Huober, J.; et al. Definition and impact of pathologic complete response on prognosis after neoadjuvant chemotherapy in various intrinsic breast cancer subtypes. J. Clin. Oncol. 2012, 30, 1796–1804. [Google Scholar] [CrossRef]
- Denkert, C.; Seither, F.; Schneeweiss, A.; Link, T.; Blohmer, J.U.; Just, M.; Wimberger, P.; Forberger, A.; Tesch, H.; Jackisch, C.; et al. Clinical and molecular characteristics of HER2-low-positive breast cancer: Pooled analysis of individual patient data from four prospective, neoadjuvant clinical trials. Lancet Oncol. 2021, 22, 1151–1161. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Katerji, H.; Turner, B.M.; Audeh, W.; Hicks, D.G. HER2-low breast cancers: Incidence, HER2 staining patterns, clinicopathologic features, MammaPrint and BluePrint genomic profiles. Mod. Pathol. 2022, 35, 1045–1054. [Google Scholar] [CrossRef] [PubMed]
- Catalfamo, K.; Attwood, K.; Kapoor, A.; Jatwani, K.; Roy, A.M. Racial disparity in the clinical outcomes of HER2-low and HER2-zero early-stage breast cancer. J. Clin. Oncol. 2023, 41, e12602. [Google Scholar] [CrossRef]
- Chang, J.; Ormerod, M.; Powles, T.; Allred, D.; Ashley, S.; Dowsett, M. Apoptosis and proliferation as predictors of chemotherapy response in patients with breast carcinoma. Cancer Interdiscip. Int. J. Am. Cancer Soc. 2000, 89, 2145–2152. [Google Scholar] [CrossRef]
- Amadori, D.; Volpi, A.; Maltoni, R.; Nanni, O.; Amaducci, L.; Amadori, A.; Giunchi, D.C.; Vio, A.; Saragoni, A.; Silvestrini, R. Cell proliferation as a predictor of response to chemotherapy in metastatic breast cancer: A prospective study. Breast Cancer Res. Treat. 1997, 43, 7–14. [Google Scholar] [CrossRef]
- Mutai, R.; Barkan, T.; Moore, A.; Sarfaty, M.; Shochat, T.; Yerushalmi, R.; Stemmer, S.M.; Goldvaser, H. Prognostic impact of HER2-low expression in hormone receptor positive early breast cancer. Breast 2021, 60, 62–69. [Google Scholar] [CrossRef]
- Jiang, C.; Perimbeti, S.; Deng, L.; Shapiro, C.L.; Gandhi, S. Clinical outcomes of de novo metastatic HER2-low breast cancer: A National Cancer Database Analysis. npj Breast Cancer 2022, 8, 135. [Google Scholar] [CrossRef]
- American College of Surgeons. National Cancer Database. Available online: https://www.facs.org/quality-programs/cancer/ncdb (accessed on 21 March 2022).
- American College of Surgery. PUF Data Dictionary. Available online: https://www.facs.org/quality-programs/cancer/ncdb/puf (accessed on 21 March 2022).
- Fernandez, A.I.; Liu, M.; Bellizzi, A.; Brock, J.; Fadare, O.; Hanley, K.; Harigopal, M.; Jorns, J.M.; Kuba, M.G.; Ly, A.; et al. Examination of Low ERBB2 Protein Expression in Breast Cancer Tissue. JAMA Oncol. 2022, 8, 607–610. [Google Scholar] [CrossRef]
- Wolff, A.C.; Hammond, M.E.; Hicks, D.G.; Dowsett, M.; McShane, L.M.; Allison, K.H.; Allred, D.C.; Bartlett, J.M.; Bilous, M.; Fitzgibbons, P.; et al. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. J. Clin. Oncol. 2013, 31, 3997–4013. [Google Scholar] [CrossRef]
- Gupta, R.K.; Roy, A.M.; Gupta, A.; Takabe, K.; Dhakal, A.; Opyrchal, M.; Kalinski, P.; Gandhi, S. Systemic Therapy De-Escalation in Early-Stage Triple-Negative Breast Cancer: Dawn of a New Era? Cancers 2022, 14, 1856. [Google Scholar] [CrossRef]
- Akrida, I.; Mulita, F. The clinical significance of HER2 expression in DCIS. Med. Oncol. 2022, 40, 16. [Google Scholar] [CrossRef] [PubMed]



| Variable | Level | N | Overall N = 553,497 | Her2-Low N = 376,199 | Her2-Zero N = 177,298 | p-Value |
|---|---|---|---|---|---|---|
| Age | 553,497 | 60.6 ± 12.6 | 60.7 ± 12.6 | 60.4 ± 12.7 | <0.001 | |
| Race | 1. White | 553,497 | 433,838 (78.4%) | 297,398 (79.1%) | 136,440 (77.0%) | <0.001 |
| 2. Black | 60,929 (11.0%) | 40,097 (10.7%) | 20,832 (11.7%) | |||
| 3. Hispanic | 29,992 (5.4%) | 19,095 (5.1%) | 10,897 (6.1%) | |||
| 4. Asian and Pacific Islanders | 20,555 (3.7%) | 14,123 (3.8%) | 6432 (3.6%) | |||
| 5. Other or unknown | 8183 (1.5%) | 5486 (1.5%) | 2697 (1.5%) | |||
| Insurance | 1. Private | 55,3497 | 294,343 (53.2%) | 199,250 (53.0%) | 95,093 (53.6%) | <0.001 |
| 2. Public insurance | 245,077 (44.3%) | 167,671 (44.6%) | 77,406 (43.7%) | |||
| 3. Uninsured | 8952 (1.6%) | 5889 (1.6%) | 3063 (1.7%) | |||
| 4. Unknown | 5125 (0.9%) | 3389 (0.9%) | 1736 (1.0%) | |||
| Household Income | 1. <$40,227 | 553,497 | 70,895 (12.8%) | 47,742 (12.7%) | 23,153 (13.1%) | <0.001 |
| 2. $40,227–$50,353 | 94,587 (17.1%) | 64,914 (17.3%) | 29,673 (16.7%) | |||
| 3. $50,354–$63,332 | 111,351 (20.1%) | 75,856 (20.2%) | 35,495 (20.0%) | |||
| 4. ≥$63,333 | 200,876 (36.3%) | 133,969 (35.6%) | 66,907 (37.7%) | |||
| 5. Unknown | 75,788 (13.7%) | 53,718 (14.3%) | 22,070 (12.4%) | |||
| Treatment Setting | 1. Community cancer program | 553,497 | 36,308 (6.6%) | 25,402 (6.8%) | 10,906 (6.2%) | <0.001 |
| 2. Comprehensive community cancer program | 219,471 (39.7%) | 153,811 (40.9%) | 65,660 (37.0%) | |||
| 3. Academic comprehensive cancer program | 159,912 (28.9%) | 102,545 (27.3%) | 57,367 (32.4%) | |||
| 4. Integrated network cancer program | 112,286 (20.3%) | 77,899 (20.7%) | 34,387 (19.4%) | |||
| 5. Unknown | 25,520 (4.6%) | 16,542 (4.4%) | 8978 (5.1%) | |||
| Treatment Location | 1. Metro | 553,497 | 470,060 (84.9%) | 317,798 (84.5%) | 152,262 (85.9%) | <0.001 |
| 2. Urban | 62,460 (11.3%) | 44,003 (11.7%) | 18,457 (10.4%) | |||
| 3. Rural | 8055 (1.5%) | 5826 (1.5%) | 2229 (1.3%) | |||
| 4. Unknown | 12,922 (2.3%) | 8572 (2.3%) | 4350 (2.5%) | |||
| Histology | 1. Ductal adenocarcinoma | 553,497 | 414,747 (74.9%) | 286,309 (76.1%) | 128,438 (72.4%) | <0.001 |
| 2. Lobular adenocarcinoma | 58,463 (10.6%) | 38,155 (10.1%) | 20,308 (11.5%) | |||
| 3. Mixed or unknown histology | 80,287 (14.5%) | 51,735 (13.8%) | 28,552 (16.1%) | |||
| Tumor Grade | 1. Well differentiated | 553,497 | 142,090 (25.7%) | 99,817 (26.5%) | 42,273 (23.8%) | <0.001 |
| 2. Moderately differentiated | 248,212 (44.8%) | 173,577 (46.1%) | 74,635 (42.1%) | |||
| 3. Poorly differentiated/undifferentiated | 142,805 (25.8%) | 89,137 (23.7%) | 53,668 (30.3%) | |||
| 4. Unknown | 20,390 (3.7%) | 13,668 (3.6%) | 6722 (3.8%) | |||
| Clinical Stage | Stage I | 553,497 | 357,029 (64.5%) | 244,917 (65.1%) | 112,112 (63.2%) | <0.001 |
| Stage II | 166,199 (30.0%) | 111,350 (29.6%) | 54,849 (30.9%) | |||
| Stage III | 30,269 (5.5%) | 19,932 (5.3%) | 10,337 (5.8%) | |||
| Lymph Node Involvement at Diagnosis | 1. No lymph node | 553,497 | 388,016 (70.1%) | 262,130 (69.7%) | 125,886 (71.0%) | <0.001 |
| 2. 1–3 lymph nodes | 113,966 (20.6%) | 78,894 (21.0%) | 35,072 (19.8%) | |||
| 3. 4+ lymph nodes | 39,278 (7.1%) | 26,993 (7.2%) | 12,285 (6.9%) | |||
| 4. Unknown | 12,237 (2.2%) | 8182 (2.2%) | 4055 (2.3%) | |||
| Hormonal Receptor Status | 1. Yes | 553,497 | 477,675 (86.3%) | 336,147 (89.4%) | 141,528 (79.8%) | <0.001 |
| 2. No | 75,822 (13.7%) | 40,052 (10.6%) | 35,770 (20.2%) | |||
| Surgical Treatment | 1. Lumpectomy or partial mastectomy | 553,497 | 349,935 (63.2%) | 236,913 (63.0%) | 113,022 (63.7%) | <0.001 |
| 2. Total mastectomy | 203,562 (36.8%) | 139,286 (37.0%) | 64,276 (36.3%) | |||
| Adjuvant Radiation | 1. Yes | 553,497 | 368,206 (66.5%) | 250,112 (66.5%) | 118,094 (66.6%) | 0.363 |
| 2. No | 185,291 (33.5%) | 126,087 (33.5%) | 59,204 (33.4%) | |||
| Chemotherapy | 1. Yes | 553,497 | 205,971 (37.2%) | 135,004 (35.9%) | 70,967 (40.0%) | <0.001 |
| 2. No | 347,526 (62.8%) | 241,195 (64.1%) | 106,331 (60.0%) | |||
| Neoadjuvant Chemotherapy | 1. Yes | 553,497 | 51,641 (9.3%) | 32,569 (8.7%) | 19,072 (10.8%) | <0.001 |
| 2. No | 501,856 (90.7%) | 343,630 (91.3%) | 158,226 (89.2%) | |||
| Adjuvant Chemotherapy | 1. Yes | 553,497 | 134,435 (24.3%) | 89,469 (23.8%) | 44,966 (25.4%) | <0.001 |
| 2. No | 419,062 (75.7%) | 286,730 (76.2%) | 132,332 (74.6%) | |||
| Hormone Treatment | 1. Yes | 553,497 | 426,986 (77.1%) | 301,202 (80.1%) | 125,784 (70.9%) | <0.001 |
| 2. No | 126,511 (22.9%) | 74,997 (19.9%) | 51,514 (29.1%) | |||
| Comorbidity Score | 0 | 553,497 | 459,789 (83.1%) | 312,377 (83.0%) | 147,412 (83.1%) | 0.022 |
| 1 | 72,834 (13.2%) | 49,709 (13.2%) | 23,125 (13.0%) | |||
| 2 | 14,976 (2.7%) | 10,197 (2.7%) | 4779 (2.7%) | |||
| ≥3 | 5898 (1.1%) | 3916 (1.0%) | 1982 (1.1%) | |||
| Year of Diagnosis | 2010 | 553,497 | 48,104 (8.7%) | 33,414 (8.9%) | 14,690 (8.3%) | <0.001 |
| 2011 | 55,715 (10.1%) | 38,793 (10.3%) | 16,922 (9.5%) | |||
| 2012 | 60,516 (10.9%) | 43,645 (11.6%) | 16,871 (9.5%) | |||
| 2013 | 68,550 (12.4%) | 47,071 (12.5%) | 21,479 (12.1%) | |||
| 2014 | 72,624 (13.1%) | 50,208 (13.3%) | 22,416 (12.6%) | |||
| 2015 | 78,184 (14.1%) | 53,022 (14.1%) | 25,162 (14.2%) | |||
| 2016 | 83,765 (15.1%) | 55,478 (14.7%) | 28,287 (16.0%) | |||
| 2017 | 86,039 (15.5%) | 54,568 (14.5%) | 31,471 (17.8%) |
| Variable | Level | N | Overall N = 146,271 | Her2-Low N = 104,517 | Her2-Zero N = 41,754 | p-Value |
|---|---|---|---|---|---|---|
| Oncotype Dx Recurrence Score | 0–15 | 146,271 | 73,250 (50.1%) | 52,114 (49.9%) | 21,136 (50.6%) | 0.008 |
| 16–25 | 53,649 (36.7%) | 38,592 (36.9%) | 15,057 (36.1%) | |||
| 26–100 | 19,372 (13.2%) | 13,811 (13.2%) | 5561 (13.3%) | |||
| Age | 146,271 | 58.8 ± 10.4 | 58.7 ± 10.5 | 58.9 ± 10.4 | 0.004 | |
| Race | 1. White | 146,271 | 120,210 (82.2%) | 86,244 (82.5%) | 33,966 (81.3%) | <0.001 |
| 2. Black | 11,471 (7.8%) | 8257 (7.9%) | 3214 (7.7%) | |||
| 3. Hispanic | 6726 (4.6%) | 4491 (4.3%) | 2235 (5.4%) | |||
| 4. Asian and Pacific Islanders | 5687 (3.9%) | 4011 (3.8%) | 1676 (4.0%) | |||
| 5. Other or unknown | 2177 (1.5%) | 1514 (1.4%) | 663 (1.6%) | |||
| Insurance | 1. Private | 146,271 | 89,946 (61.5%) | 64,140 (61.4%) | 25,806 (61.8%) | 0.148 |
| 2. Public insurance | 53,452 (36.5%) | 38,352 (36.7%) | 15,100 (36.2%) | |||
| 3. Uninsured | 1627 (1.1%) | 1136 (1.1%) | 491 (1.2%) | |||
| 4. Unknown | 1246 (0.9%) | 889 (0.9%) | 357 (0.9%) | |||
| Household Income | 1. <$40,227 | 146,271 | 15,136 (10.3%) | 10,839 (10.4%) | 4297 (10.3%) | <0.001 |
| 2. $40,227–$50,353 | 22,687 (15.5%) | 16,528 (15.8%) | 6159 (14.8%) | |||
| 3. $50,354–$63,332 | 29,330 (20.1%) | 21,036 (20.1%) | 8294 (19.9%) | |||
| 4. ≥$63,333 | 57,928 (39.6%) | 40,237 (38.5%) | 17,691 (42.4%) | |||
| 5. Unknown | 21,190 (14.5%) | 15,877 (15.2%) | 5313 (12.7%) | |||
| Treatment Setting | 1. Community cancer program | 146,271 | 8246 (5.6%) | 6072 (5.8%) | 2174 (5.2%) | <0.001 |
| 2. Comprehensive community cancer program | 57,187 (39.1%) | 42,054 (40.2%) | 15,133 (36.2%) | |||
| 3. Academic comprehensive cancer program | 46,672 (31.9%) | 31,274 (29.9%) | 15,398 (36.9%) | |||
| 4. Integrated network cancer program | 29,872 (20.4%) | 21,975 (21.0%) | 7897 (18.9%) | |||
| 5. Unknown | 4294 (2.9%) | 3142 (3.0%) | 1152 (2.8%) | |||
| Treatment Location | 1. Metro | 146,271 | 124,162 (84.9%) | 88,356 (84.5%) | 35,806 (85.8%) | <0.001 |
| 2. Urban | 16,165 (11.1%) | 12,019 (11.5%) | 4146 (9.9%) | |||
| 3. Rural | 2076 (1.4%) | 1558 (1.5%) | 518 (1.2%) | |||
| 4. Unknown | 3868 (2.6%) | 2584 (2.5%) | 1284 (3.1%) | |||
| Histology | 1. Ductal adenocarcinoma | 146,271 | 107,552 (73.5%) | 78,570 (75.2%) | 28,982 (69.4%) | <0.001 |
| 2. Lobular adenocarcinoma | 18,336 (12.5%) | 12,042 (11.5%) | 6294 (15.1%) | |||
| 3. Mixed or unknown histology | 20,383 (13.9%) | 13,905 (13.3%) | 6478 (15.5%) | |||
| Tumor Grade | 1. Well differentiated | 146,271 | 41,082 (28.1%) | 29,755 (28.5%) | 11,327 (27.1%) | <0.001 |
| 2. Moderately differentiated | 79,377 (54.3%) | 56,355 (53.9%) | 23,022 (55.1%) | |||
| 3. Poorly differentiated/undifferentiated | 21,597 (14.8%) | 15,359 (14.7%) | 6238 (14.9%) | |||
| 4. Unknown | 4215 (2.9%) | 3048 (2.9%) | 1167 (2.8%) | |||
| Clinical Stage | Stage I | 146,271 | 111,871 (76.5%) | 80,023 (76.6%) | 31,848 (76.3%) | 0.282 |
| Stage II | 33,795 (23.1%) | 24,075 (23.0%) | 9720 (23.3%) | |||
| Stage III | 605 (0.4%) | 419 (0.4%) | 186 (0.4%) | |||
| Lymph Node Involvement | 1. No lymph node | 146,271 | 120,058 (82.1%) | 85,804 (82.1%) | 34,254 (82.0%) | 0.622 |
| 2. 1–3 lymph nodes | 24,643 (16.8%) | 17,612 (16.9%) | 7031 (16.8%) | |||
| 3. 4+ lymph nodes | 912 (0.6%) | 634 (0.6%) | 278 (0.7%) | |||
| 4. Unknown | 658 (0.4%) | 467 (0.4%) | 191 (0.5%) | |||
| Hormonal Receptor Status | 1. Yes | 146,271 | 146,271 (100.0%) | 104,517 (100.0%) | 41,754 (100.0%) | - |
| Surgical Treatment | 1. Lumpectomy or partial mastectomy | 146,271 | 102,047 (69.8%) | 72,410 (69.3%) | 29,637 (71.0%) | <0.001 |
| 2. Total mastectomy | 44,224 (30.2%) | 32,107 (30.7%) | 12,117 (29.0%) | |||
| Adjuvant Radiation | 1. Yes | 146,271 | 102,105 (69.8%) | 72,605 (69.5%) | 29,500 (70.7%) | <0.001 |
| 2. No | 44,166 (30.2%) | 31,912 (30.5%) | 12,254 (29.3%) | |||
| Adjuvant Chemotherapy | 1. Yes | 146,271 | 28,879 (19.7%) | 20,726 (19.8%) | 8153 (19.5%) | 0.187 |
| 2. No | 117,392 (80.3%) | 83,791 (80.2%) | 33,601 (80.5%) | |||
| Hormone Treatment | 1. Yes | 146,271 | 144,993 (99.1%) | 103,637 (99.2%) | 41,356 (99.0%) | 0.039 |
| 2. No | 1278 (0.9%) | 880 (0.8%) | 398 (1.0%) | |||
| Comorbidity Score | 0 | 146,271 | 123,996 (84.8%) | 88,494 (84.7%) | 35,502 (85.0%) | 0.113 |
| 1 | 17,937 (12.3%) | 12,927 (12.4%) | 5010 (12.0%) | |||
| 2 | 3245 (2.2%) | 2335 (2.2%) | 910 (2.2%) | |||
| ≥3 | 1093 (0.7%) | 761 (0.7%) | 332 (0.8%) | |||
| Year of Diagnosis | 2010 | 146,271 | 8747 (6.0%) | 6360 (6.1%) | 2387 (5.7%) | <0.001 |
| 2011 | 11,556 (7.9%) | 8475 (8.1%) | 3081 (7.4%) | |||
| 2012 | 13,880 (9.5%) | 10,541 (10.1%) | 3339 (8.0%) | |||
| 2013 | 17,042 (11.7%) | 12,449 (11.9%) | 4593 (11.0%) | |||
| 2014 | 19,750 (13.5%) | 14,303 (13.7%) | 5447 (13.0%) | |||
| 2015 | 22,835 (15.6%) | 16,392 (15.7%) | 6443 (15.4%) | |||
| 2016 | 25,392 (17.4%) | 17,748 (17.0%) | 7644 (18.3%) | |||
| 2017 | 27,069 (18.5%) | 18,249 (17.5%) | 8820 (21.1%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roy, A.M.; Jiang, C.; Perimbeti, S.; Deng, L.; Shapiro, C.L.; Gandhi, S. Oncotype Dx Score, HER2 Low Expression, and Clinical Outcomes in Early-Stage Breast Cancer: A National Cancer Database Analysis. Cancers 2023, 15, 4264. https://doi.org/10.3390/cancers15174264
Roy AM, Jiang C, Perimbeti S, Deng L, Shapiro CL, Gandhi S. Oncotype Dx Score, HER2 Low Expression, and Clinical Outcomes in Early-Stage Breast Cancer: A National Cancer Database Analysis. Cancers. 2023; 15(17):4264. https://doi.org/10.3390/cancers15174264
Chicago/Turabian StyleRoy, Arya Mariam, Changchuan Jiang, Stuthi Perimbeti, Lei Deng, Charles L. Shapiro, and Shipra Gandhi. 2023. "Oncotype Dx Score, HER2 Low Expression, and Clinical Outcomes in Early-Stage Breast Cancer: A National Cancer Database Analysis" Cancers 15, no. 17: 4264. https://doi.org/10.3390/cancers15174264
APA StyleRoy, A. M., Jiang, C., Perimbeti, S., Deng, L., Shapiro, C. L., & Gandhi, S. (2023). Oncotype Dx Score, HER2 Low Expression, and Clinical Outcomes in Early-Stage Breast Cancer: A National Cancer Database Analysis. Cancers, 15(17), 4264. https://doi.org/10.3390/cancers15174264






