Investigations Using Albumin Binders to Modify the Tissue Distribution Profile of Radiopharmaceuticals Exemplified with Folate Radioconjugates
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis and Preclinical Investigation of Albumin-Binding Radioligands
2.1.1. Synthesis of Albumin-Binding Ligands (DOTA-ALBs)
2.1.2. Preparation and Stability of 177Lu-DOTA-ALBs
2.1.3. Evaluation of the Albumin-Binding Properties of DOTA-ALBs
2.1.4. Blood Clearance Studies of 177Lu-DOTA-ALBs
2.2. Synthesis and Investigation of Albumin-Binding Folate Radioconjugates
2.2.1. Synthesis of Folate Conjugates (RedFols)
2.2.2. Preparation, Stability and Hydrophilic/Lipophilic Properties of 177Lu-RedFols
2.2.3. Determination of Cell Uptake of 177Lu-RedFols
2.2.4. Determination of FR-Binding Affinity of 177Lu-RedFols
2.2.5. Albumin-Binding Properties of 177Lu-RedFols
2.2.6. Biodistribution Studies of 177Lu-RedFols in Tumor-Bearing Mice
2.2.7. SPECT/CT Imaging Studies of 177Lu-RedFols
2.3. License of In Vivo Studies
3. Results
3.1. Synthesis and Preclinical Investigation of Albumin-Binding Radioligands
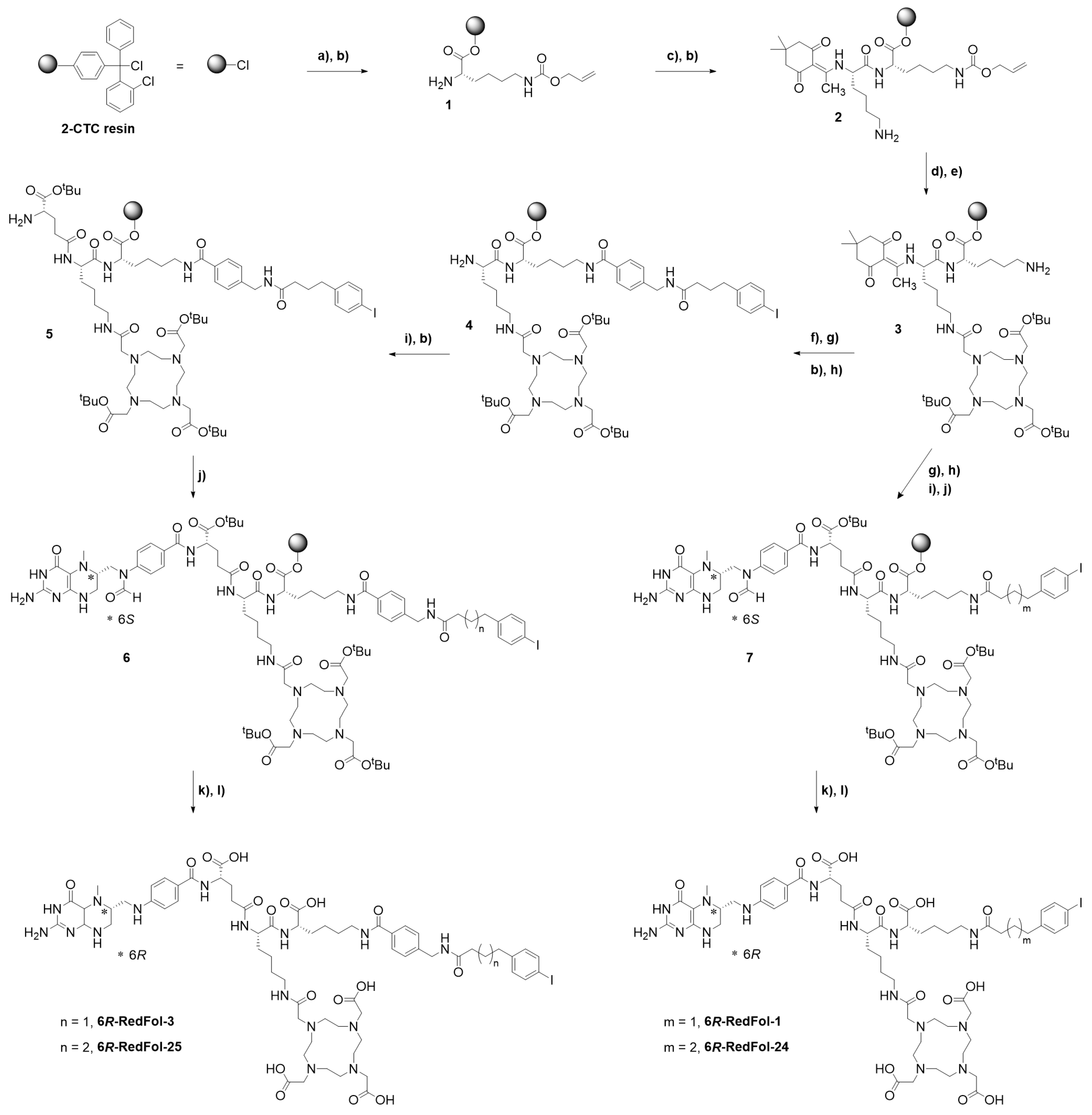
3.1.1. Synthesis of DOTA-ALBs
3.1.2. In Vitro Testing of 177Lu-DOTA-ALBs
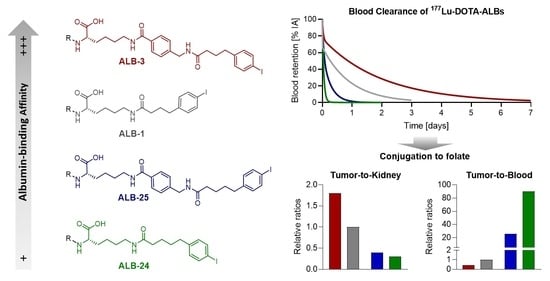
3.1.3. Relative Albumin-Binding Affinities of 177Lu-DOTA-ALBs
3.1.4. Blood Clearance of 177Lu-DOTA-ALBs
3.2. Synthesis and Investigation of Albumin-Binding Folate Radioconjugates
3.2.1. Synthesis of RedFols
3.2.2. Preparation, Stability and Distribution Coefficients of 177Lu-RedFols
3.2.3. FR-Mediated Cell Uptake and FR-Binding Affinity of 177Lu-RedFols
3.2.4. Relative Albumin-Binding Properties of 177Lu-RedFols
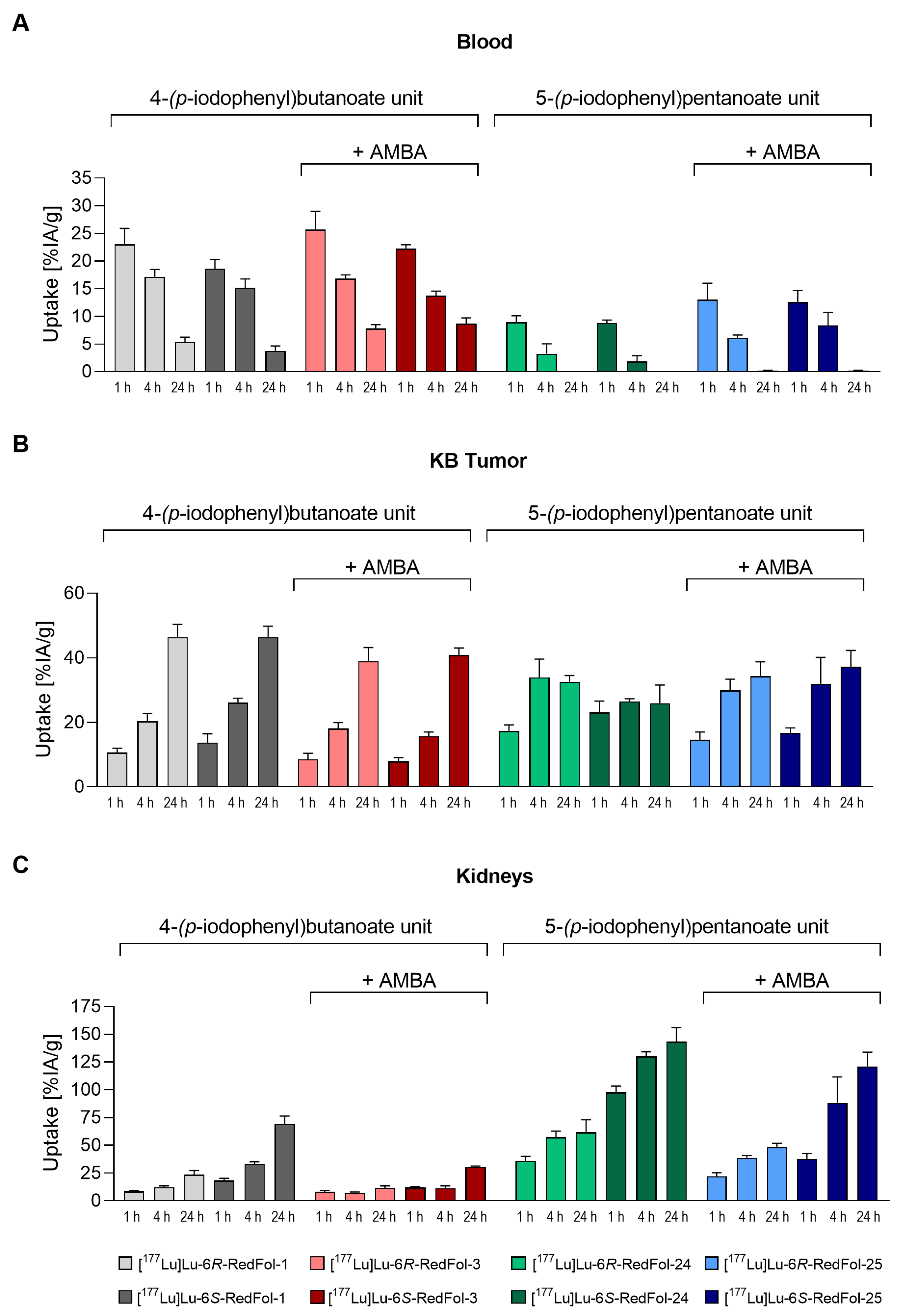
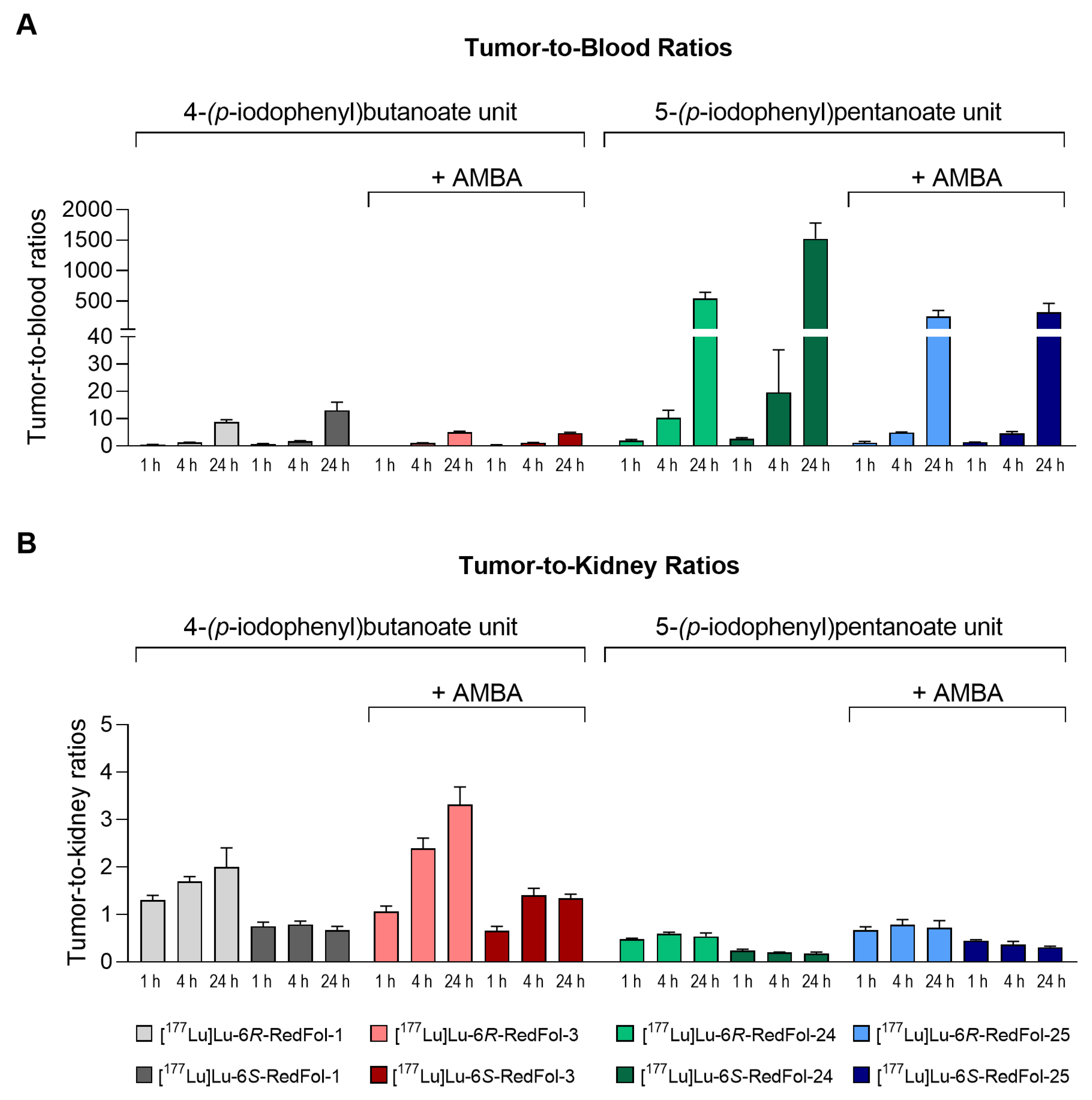
3.2.5. Biodistribution Studies of 177Lu-RedFols
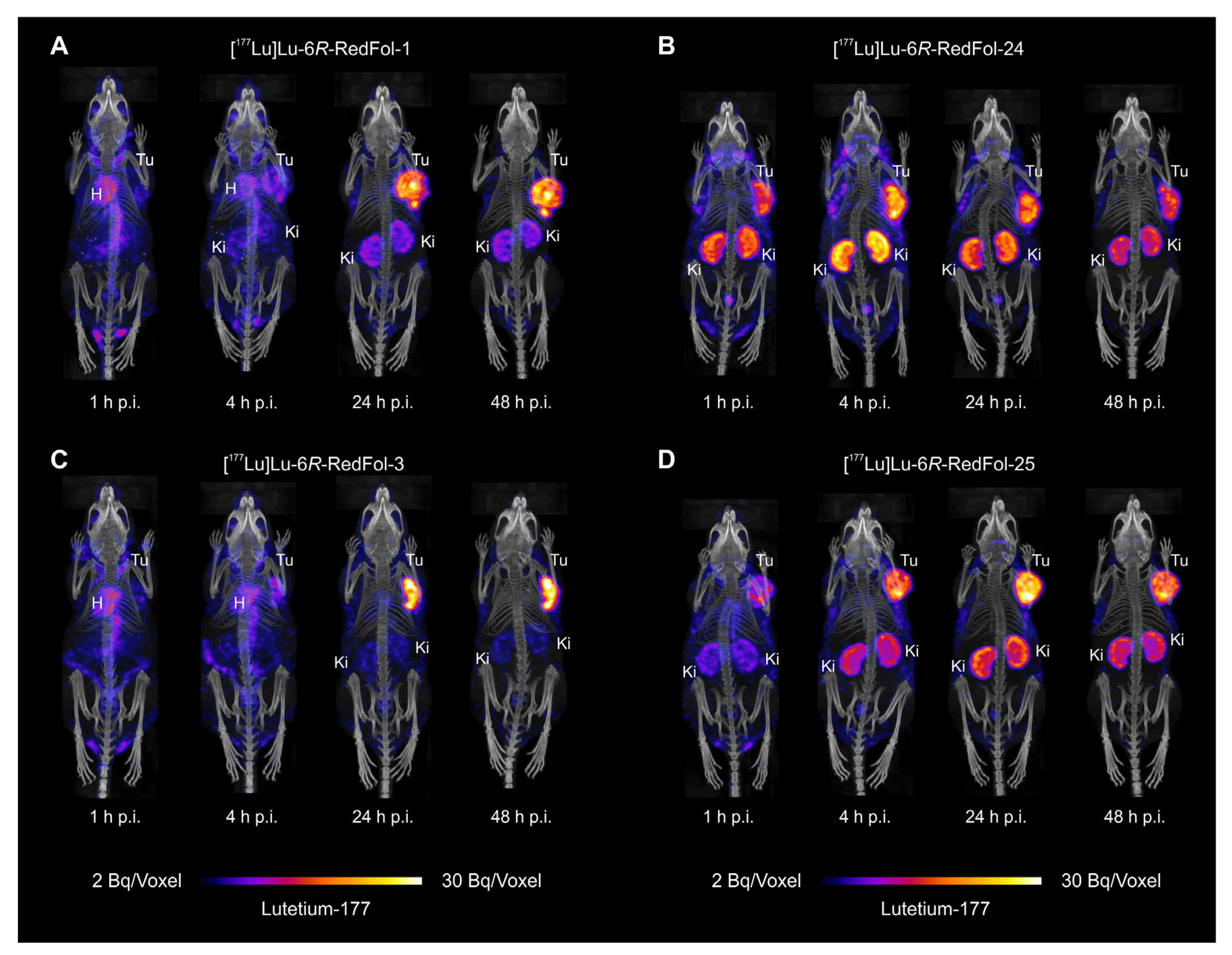
3.2.6. SPECT/CT Imaging Studies of 177Lu-RedFols
4. Discussion
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Parker, N.; Turk, M.J.; Westrick, E.; Lewis, J.D.; Low, P.S.; Leamon, C.P. Folate receptor expression in carcinomas and normal tissues determined by a quantitative radioligand binding assay. Anal. Biochem. 2005, 338, 284–293. [Google Scholar] [CrossRef] [PubMed]
- Low, P.S.; Kularatne, S.A. Folate-targeted therapeutic and imaging agents for cancer. Curr. Opin. Chem. Biol. 2009, 13, 256–262. [Google Scholar] [CrossRef] [PubMed]
- Young, O.; Ngo, N.; Lin, L.; Stanbery, L.; Creeden, J.F.; Hamouda, D.; Nemunaitis, J. Folate receptor as a biomarker and therapeutic target in solid tumors. Curr. Probl. Cancer 2023, 47, 100917. [Google Scholar] [CrossRef] [PubMed]
- Holm, J.; Hansen, S.I.; Hoier-Madsen, M.; Bostad, L. High-affinity folate binding in human choroid plexus. Characterization of radioligand binding, immunoreactivity, molecular heterogeneity and hydrophobic domain of the binding protein. Biochem. J. 1991, 280 Pt 1, 267–271. [Google Scholar] [CrossRef]
- Holm, J.; Hansen, S.I.; Hoier-Madsen, M.; Bostad, L. A high-affinity folate binding protein in proximal tubule cells of human kidney. Kidney Int. 1992, 41, 50–55. [Google Scholar] [CrossRef]
- Birn, H.; Spiegelstein, O.; Christensen, E.I.; Finnell, R.H. Renal tubular reabsorption of folate mediated by folate binding protein 1. J. Am. Soc. Nephrol. JASN 2005, 16, 608–615. [Google Scholar] [CrossRef]
- Hilgenbrink, A.R.; Low, P.S. Folate receptor-mediated drug targeting: From therapeutics to diagnostics. J. Pharm. Sci. 2005, 94, 2135–2146. [Google Scholar] [CrossRef]
- Salazar, M.D.; Ratnam, M. The folate receptor: What does it promise in tissue-targeted therapeutics? Cancer Metastasis Rev. 2007, 26, 141–152. [Google Scholar] [CrossRef]
- Müller, C. Folate based radiopharmaceuticals for imaging and therapy of cancer and inflammation. Curr. Pharm. Deisng 2012, 18, 1058–1083. [Google Scholar] [CrossRef]
- Wagner, L.; Kenzhebayeva, B.; Dhaini, B.; Boukhlef, S.; Moussaron, A.; Mordon, S.; Frochot, C.; Collet, C.; Acherar, S. Folate-based radiotracers for nuclear imaging and radionuclide therapy. Coordin Chem. Rev. 2022, 470, 214702. [Google Scholar] [CrossRef]
- Mathias, C.J.; Wang, S.; Waters, D.J.; Turek, J.J.; Low, P.S.; Green, M.A. Indium-111-DTPA-folate as a potential folate-receptor-targeted radiopharmaceutical. J. Nucl. Med. 1998, 39, 1579–1585. [Google Scholar] [PubMed]
- Leamon, C.P.; Parker, M.A.; Vlahov, I.R.; Xu, L.C.; Reddy, J.A.; Vetzel, M.; Douglas, N. Synthesis and biological evaluation of EC20: A new folate-derived, 99mTc-based radiopharmaceutical. Bioconjugate Chem. 2002, 13, 1200–1210. [Google Scholar] [CrossRef] [PubMed]
- Siegel, B.A.; Dehdashti, F.; Mutch, D.G.; Podoloff, D.A.; Wendt, R.; Sutton, G.P.; Burt, R.W.; Ellis, P.R.; Mathias, C.J.; Green, M.A.; et al. Evaluation of 111In-DTPA-folate as a receptor-targeted diagnostic agent for ovarian cancer: Initial clinical results. J. Nucl. Med. 2003, 44, 700–707. [Google Scholar] [PubMed]
- Fisher, R.E.; Siegel, B.A.; Edell, S.L.; Oyesiku, N.M.; Morgenstern, D.E.; Messmann, R.A.; Amato, R.J. Exploratory study of 99mTc-EC20 imaging for identifying patients with folate receptor-positive solid tumors. J. Nucl. Med. 2008, 49, 899–906. [Google Scholar] [CrossRef]
- Lau, J.; Jacobson, O.; Niu, G.; Lin, K.S.; Benard, F.; Chen, X. Bench to bedside: Albumin binders for improved cancer radioligand therapies. Bioconjugate Chem. 2019, 30, 487–502. [Google Scholar] [CrossRef]
- Brandt, M.; Cardinale, J.; Giammei, C.; Guarrochena, X.; Happl, B.; Jouini, N.; Mindt, T.L. Mini-review: Targeted radiopharmaceuticals incorporating reversible, low molecular weight albumin binders. Nucl. Med. Biol. 2019, 70, 46–52. [Google Scholar] [CrossRef]
- Sand, K.M.K.; Bern, M.; Nilsen, J.; Noordzij, H.T.; Sandlie, I.; Andersen, J.T. Unraveling the Interaction between FcRn and albumin: Ppportunities for design of albumin-based therapeutics. Front. Immunol. 2015, 5, 682. [Google Scholar] [CrossRef] [PubMed]
- Merlot, A.M.; Kalinowski, D.S.; Richardson, D.R. Unraveling the mysteries of serum albumin—More than just a serum protein. Front. Physiol. 2014, 5, 299. [Google Scholar] [CrossRef]
- Dumelin, C.E.; Trüssel, S.; Buller, F.; Trachsel, E.; Bootz, F.; Zhang, Y.; Mannocci, L.; Beck, S.C.; Drumea-Mirancea, M.; Seeliger, M.W.; et al. A portable albumin binder from a DNA-encoded chemical library. Angew. Chem. Int. Ed. Engl. 2008, 47, 3196–3201. [Google Scholar] [CrossRef]
- Müller, C.; Struthers, H.; Winiger, C.; Zhernosekov, K.; Schibli, R. DOTA conjugate with an albumin-binding entity enables the first folic acid-targeted 177Lu-radionuclide tumor therapy in mice. J. Nucl. Med. 2013, 54, 124–131. [Google Scholar] [CrossRef]
- Siwowska, K.; Haller, S.; Bortoli, F.; Benesova, M.; Groehn, V.; Bernhardt, P.; Schibli, R.; Müller, C. Preclinical comparison of albumin-binding radiofolates: Impact of linker entities on the in vitro and in vivo properties. Mol. Pharm. 2017, 14, 523–532. [Google Scholar] [CrossRef] [PubMed]
- Haller, S.; Reber, J.; Brandt, S.; Bernhardt, P.; Groehn, V.; Schibli, R.; Müller, C. Folate receptor-targeted radionuclide therapy: Preclinical investigation of anti-tumor effects and potential radionephropathy. Nucl. Med. Biol. 2015, 42, 770–779. [Google Scholar] [CrossRef] [PubMed]
- Benesova, M.; Guzik, P.; Deberle, L.M.; Busslinger, S.D.; Landolt, T.; Schibli, R.; Müller, C. Design and evaluation of novel albumin-binding folate radioconjugates: Systematic approach of varying the linker entities. Mol. Pharm. 2022, 19, 963–973. [Google Scholar] [CrossRef] [PubMed]
- Scaglione, F.; Panzavolta, G. Folate, folic acid and 5-methyltetrahydrofolate are not the same thing. Xenobiotica 2014, 44, 480–488. [Google Scholar] [CrossRef] [PubMed]
- Boss, S.D.; Müller, C.; Siwowska, K.; Schmid, R.M.; Groehn, V.; Schibli, R.; Ametamey, S.M. Diastereomerically pure 6R- and 6S-3′-aza-2′-18F-fluoro-5-methyltetrahydrofolates show unprecedentedly high uptake in folate receptor-positive KB tumors. J. Nucl. Med. 2019, 60, 135–141. [Google Scholar] [CrossRef]
- Guzik, P.; Fang, H.Y.; Deberle, L.M.; Benesova, M.; Cohrs, S.; Boss, S.D.; Ametamey, S.M.; Schibli, R.; Müller, C. Identification of a PET radiotracer for imaging of the folate receptor-alpha: A potential tool to select patients for targeted tumor therapy. J. Nucl. Med. 2021, 62, 1475–1481. [Google Scholar] [CrossRef]
- Deberle, L.M.; Benesova, M.; Becker, A.E.; Ratz, M.; Guzik, P.; Schibli, R.; Müller, C. Novel synthetic strategies enable the efficient development of folate conjugates for cancer radiotheranostics. Bioconjugate Chem. 2021, 32, 1617–1628. [Google Scholar] [CrossRef]
- Guzik, P.; Benesova, M.; Ratz, M.; Monne Rodriguez, J.M.; Deberle, L.M.; Schibli, R.; Müller, C. Preclinical evaluation of 5-methyltetrahydrofolate-based radioconjugates-new perspectives for folate receptor-targeted radionuclide therapy. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 972–983. [Google Scholar] [CrossRef]
- Kelly, J.M.; Amor-Coarasa, A.; Nikolopoulou, A.; Wustemann, T.; Barelli, P.; Kim, D.; Williams, C., Jr.; Zheng, X.; Bi, C.; Hu, B.; et al. Dual-target binding ligands with modulated pharmacokinetics for endoradiotherapy of prostate cancer. J. Nucl. Med. 2017, 58, 1442–1449. [Google Scholar] [CrossRef]
- Müller, C.; Farkas, R.; Borgna, F.; Schmid, R.M.; Benesova, M.; Schibli, R. Synthesis, radiolabeling, and characterization of plasma protein-binding ligands: Potential tools for modulation of the pharmacokinetic properties of (radio)pharmaceuticals. Bioconjugate Chem. 2017, 28, 2372–2383. [Google Scholar] [CrossRef]
- Umbricht, C.A.; Benesova, M.; Schibli, R.; Müller, C. Preclinical development of novel PSMA-targeting radioligands: Modulation of albumin-binding properties to improve prostate cancer therapy. Mol. Pharm. 2018, 15, 2297–2306. [Google Scholar] [CrossRef] [PubMed]
- Deberle, L.M.; Tschan, V.J.; Borgna, F.; Sozzi-Guo, F.; Bernhardt, P.; Schibli, R.; Müller, C. Albumin-binding PSMA radioligands: Impact of minimal structural changes on the tissue distribution profile. Molecules 2020, 25, 2542. [Google Scholar] [CrossRef] [PubMed]
- Kelly, J.M.; Amor-Coarasa, A.; Ponnala, S.; Nikolopoulou, A.; Williams, C., Jr.; DiMagno, S.G.; Babich, J.W. Albumin-binding PSMA ligands: Implications for expanding the therapeutic window. J. Nucl. Med. 2019, 60, 656–663. [Google Scholar] [CrossRef] [PubMed]
- Brandt, F.; Ullrich, M.; Laube, M.; Kopka, K.; Bachmann, M.; Loser, R.; Pietzsch, J.; Pietzsch, H.J.; van den Hoff, J.; Wodtke, R. “Clickable” albumin binders for modulating the tumor uptake of targeted radiopharmaceuticals. J. Med. Chem. 2022, 65, 710–733. [Google Scholar] [CrossRef]
- Colclough, N.; Ruston, L.; Wood, J.M.; MacFaul, P.A. Species differences in drug plasma protein binding. MedChemComm 2014, 5, 963–967. [Google Scholar] [CrossRef]
- Ketrat, S.; Japrung, D.; Pongprayoon, P. Exploring how structural and dynamic properties of bovine and canine serum albumins differ from human serum albumin. J. Mol. Graph. Model. 2020, 98, 107601. [Google Scholar] [CrossRef]



| 177Lu-DOTA-ALBs | Half-Life Phase a (% Phase a) | Half-Life Phase b (% Phase b) | Time to Reach 50% IA | Blood AUC0–7days 1 | Rel. Blood AUC0–7days 2 |
|---|---|---|---|---|---|
| [h] | [h] | [h] | [% IA× h] | ||
| 1 | 0.006 (33%) | 16 (67%) | ~8 | 1500 | 1.0 |
| 3 | 0.5 (23%) | 34 (77%) | ~24 | 3617 | 2.4 |
| 24 | 0.8 (99%) | 8.9 (1%) | ~0.8 | 119 | 0.08 |
| 25 | 0.7 (41%) | 4.5 (59%) | ~2 | 425 | 0.3 |
| 177Lu-RedFols | logD Values 1 | KD Value 2 | Total Cell Uptake 3 | Cell-Internalized Fraction 3 |
|---|---|---|---|---|
| [nM] | [%] | [%] | ||
| 1 (6R) | −3.9 ± 0.2 4 | 2.6 (2.0–3.5) 5 | 49 ± 11 | 12 ± 2 |
| 1 (6S) | −3.7 ± 0.1 4 | 1.7 (1.0–2.4) 5 | 62 ± 6 | 18 ± 1 |
| 3 (6R) | −3.2 ± 0.1 | 4.7 (3.4–6.4) | 39 ± 14 | 19 ± 8 |
| 3 (6S) | −3.2 ± 0.1 | 2.8 (1.9–4.2) | 44 ± 11 | 23 ± 8 |
| 24 (6R) | −3.4 ± 0.2 | 4.7 (3.0–7.1) | 40 ± 6 | 15 ± 1 |
| 24 (6S) | −3.1 ± 0.1 | 4.3 (3.0–6.2) | 58 ± 13 | 22 ± 4 |
| 25 (6R) | −3.0 ± 0.1 | 3.6 (2.8–4.6) | 29 ± 8 | 17 ± 3 |
| 25 (6S) | −3.0 ± 0.1 | 2.2 (1.5–3.3) | 55 ± 5 | 31 ± 3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Busslinger, S.D.; Becker, A.E.; Vaccarin, C.; Deberle, L.M.; Renz, M.-L.; Groehn, V.; Schibli, R.; Müller, C. Investigations Using Albumin Binders to Modify the Tissue Distribution Profile of Radiopharmaceuticals Exemplified with Folate Radioconjugates. Cancers 2023, 15, 4259. https://doi.org/10.3390/cancers15174259
Busslinger SD, Becker AE, Vaccarin C, Deberle LM, Renz M-L, Groehn V, Schibli R, Müller C. Investigations Using Albumin Binders to Modify the Tissue Distribution Profile of Radiopharmaceuticals Exemplified with Folate Radioconjugates. Cancers. 2023; 15(17):4259. https://doi.org/10.3390/cancers15174259
Chicago/Turabian StyleBusslinger, Sarah D., Anna E. Becker, Christian Vaccarin, Luisa M. Deberle, Marie-Luise Renz, Viola Groehn, Roger Schibli, and Cristina Müller. 2023. "Investigations Using Albumin Binders to Modify the Tissue Distribution Profile of Radiopharmaceuticals Exemplified with Folate Radioconjugates" Cancers 15, no. 17: 4259. https://doi.org/10.3390/cancers15174259
APA StyleBusslinger, S. D., Becker, A. E., Vaccarin, C., Deberle, L. M., Renz, M.-L., Groehn, V., Schibli, R., & Müller, C. (2023). Investigations Using Albumin Binders to Modify the Tissue Distribution Profile of Radiopharmaceuticals Exemplified with Folate Radioconjugates. Cancers, 15(17), 4259. https://doi.org/10.3390/cancers15174259