Outcomes of Liver Cancer Patients Undergoing Elective Surgery after Recovering from Mild SARS-CoV-2 Omicron Infection: A Retrospective Cohort Study
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
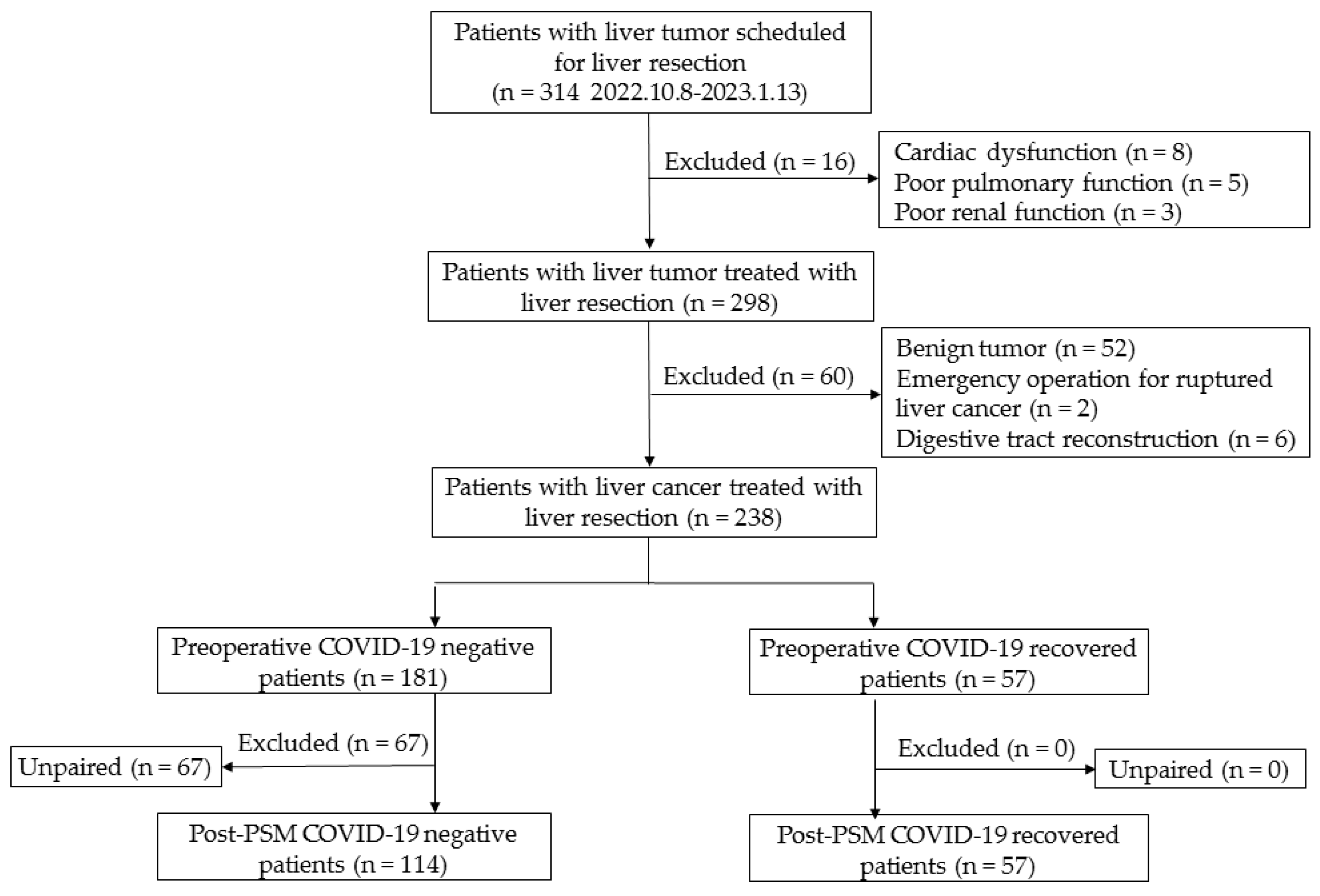
2.2. Participants
2.3. Outcome Measurement
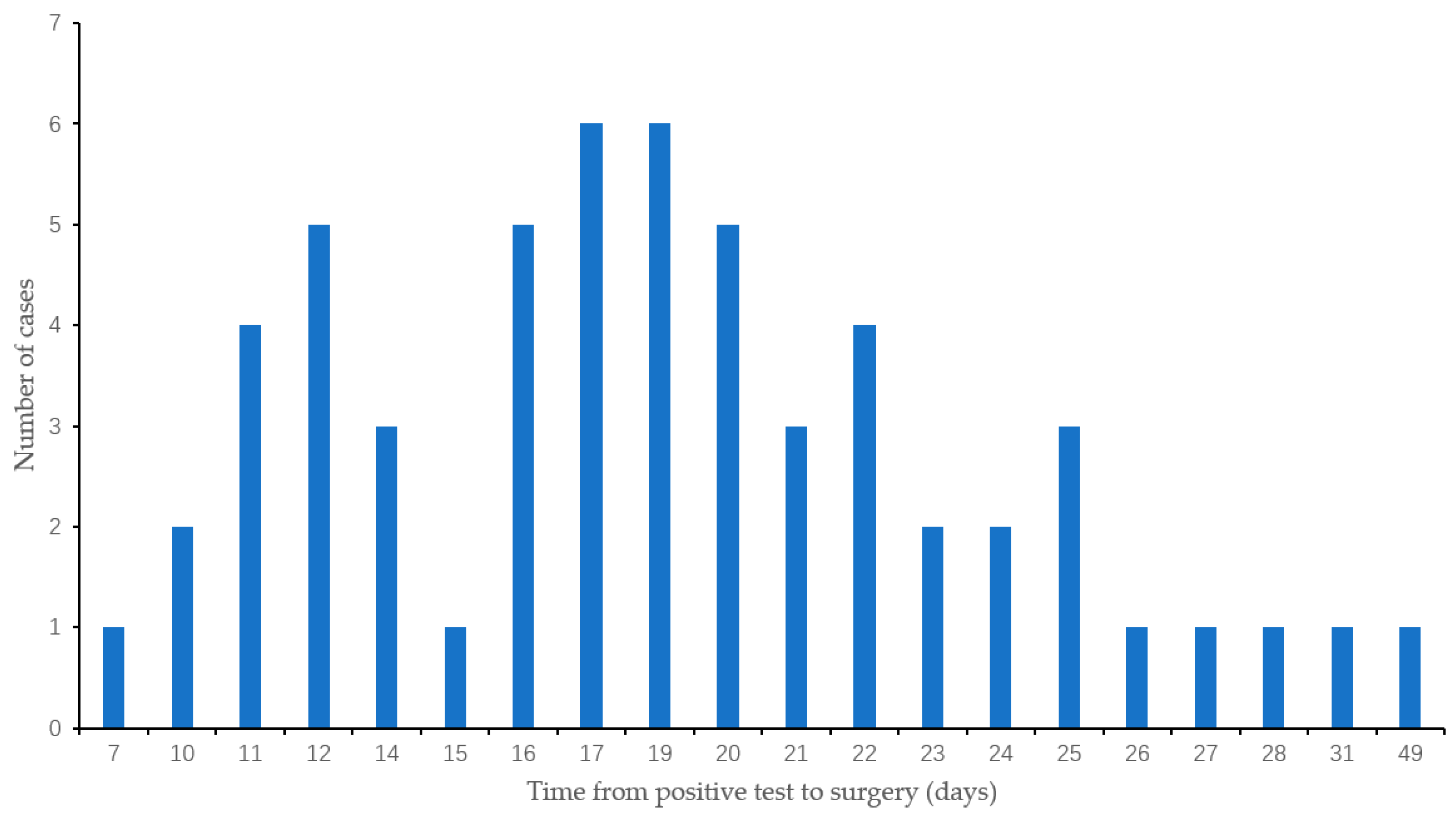
2.4. Diagnosis of COVID-19 Infection
2.5. Data Collection
2.6. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. Overall Outcomes
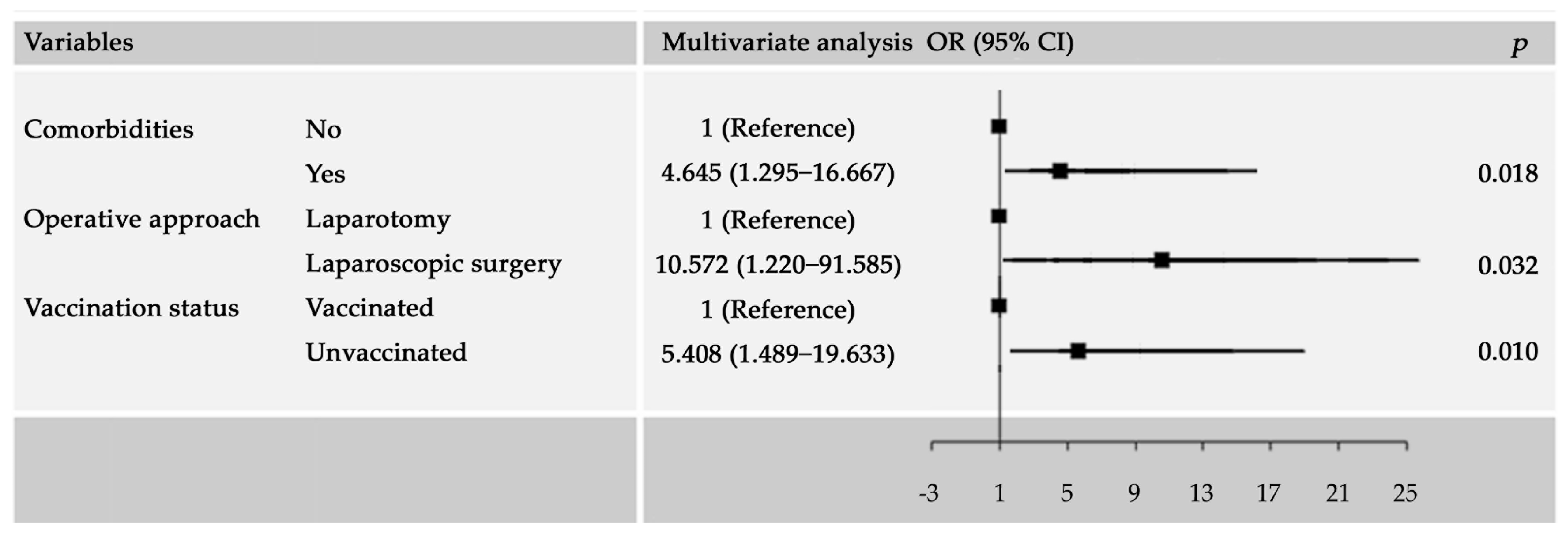
3.3. Factors Associated with Major Complications
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- COVIDSurg Collaborative; GlobalSurg Collaborative; Nepogodiev, D.; Simoes, J.F.; Li, E.; Picciochi, M.; Glasbey, J.C.; Baiocchi, G.; Blanco-Colino, R.; Chaudhry, D.; et al. Timing of surgery following SARS-CoV-2 infection: An international prospective cohort study. Anaesthesia 2021, 76, 748–758. [Google Scholar]
- El-Boghdadly, K.; Cook, T.M.; Goodacre, T.; Kua, J.; Blake, L.; Denmark, S.; McNally, S.; Mercer, N.; Moonesinghe, S.R.; Summerton, D.J. SARS-CoV-2 infection, COVID-19 and timing of elective surgery: A multidisciplinary consensus statement on behalf of the Association of Anaesthetists, the Centre for Peri-operative Care, the Federation of Surgical Specialty Associations, the Royal College. Anaesthesia 2021, 76, 940–946. [Google Scholar] [CrossRef] [PubMed]
- Kougias, P.; Sharath, S.E.; Zamani, N.; Brunicardi, F.C.; Berger, D.H.; Wilson, M.A. Timing of a major operative intervention after a positive COVID-19 test affects postoperative mortality: Results from a nationwide, procedure-matched analysis. Ann. Surg. 2022, 276, 554–561. [Google Scholar] [CrossRef]
- Parmar, A.; Eskander, A.; Sander, B.; Naimark, D.; Irish, J.C.; Chan, K.K.W. Impact of cancer surgery slowdowns on patient survival during the COVID-19 pandemic: A microsimulation modelling study. CMAJ 2022, 194, e408–e414. [Google Scholar] [CrossRef] [PubMed]
- Shiina, S.; Gani, R.A.; Yokosuka, O.; Maruyama, H.; Nagamatsu, H.; Payawal, D.A.; Dokmeci, A.K.; Lesmana, L.A.; Tanwandee, T.; Lau, G.; et al. APASL practical recommendations for the management of hepatocellular carcinoma in the era of COVID-19. Hepatol. Int. 2020, 14, 920–929. [Google Scholar] [CrossRef] [PubMed]
- Balakrishnan, A.; Lesurtel, M.; Siriwardena, A.K.; Heinrich, S.; Serrablo, A.; Besselink, M.G.H.; Erkan, M.; Andersson, B.; Polak, W.G.; Laurenzi, A.; et al. Delivery of hepato-pancreato-biliary surgery during the COVID-19 pandemic: An European-African Hepato-Pancreato-Biliary Association (E-AHPBA) cross-sectional survey. HPB 2020, 22, 1128–1134. [Google Scholar] [CrossRef] [PubMed]
- Hamid, S.; da Silva, M.R.A.; Burak, K.W.; Chen, T.; Drenth, J.P.H.; Esmat, G.; Gaspar, R.; LaBrecque, D.; Lee, A.; Macedo, G.; et al. WGO guidance for the care of patients with COVID-19 and liver disease. J. Clin. Gastroenterol. 2021, 55, 1–11. [Google Scholar] [CrossRef]
- Zhao, H.; Zhou, A.; Zhou, J.; Bi, X.; Yan, S.; Jin, J.; Wei, W.; Li, M.; Gong, C.; Chen, Q.; et al. Chinese expert recommendations on management of hepatocellular carcinoma during COVID-19 pandemic: A nationwide multicenter survey. HPB 2022, 24, 342–352. [Google Scholar] [CrossRef]
- Koelle, K.; Martin, M.A.; Antia, R.; Lopman, B.; Dean, N.E. The changing epidemiology of SARS-CoV-2. Science 2022, 375, 1116–1121. [Google Scholar] [CrossRef]
- Kothari, A.N.; DiBrito, S.R.; Lee, J.J.; Caudle, A.S.; Clemens, M.W.; Gottumukkala, V.N.; Katz, M.H.G.; Offodile, A.C.; Uppal, A.; D3CODE Team; et al. Surgical outcomes in cancer patients undergoing elective surgery after recovering from mild-to-moderate SARS-CoV-2 infection. Ann. Surg. Oncol. 2021, 28, 8046–8053. [Google Scholar] [CrossRef]
- Guan, W.J.; Ni, Z.Y.; Hu, Y.; Liang, W.H.; Ou, C.Q.; He, J.X.; Liu, L.; Shan, H.; Lei, C.L.; Hui, D.S.C.; et al. Clinical characteristics of Coronavirus Disease 2019 in China. N. Engl. J. Med. 2020, 382, 1708–1720. [Google Scholar] [CrossRef] [PubMed]
- National Health Commission of China; National Administration of Traditional Chinese Medicine. Diagnosis and Treatment Protocol for COVID-19 (Trial Version 10). Available online: http://www.nhc.gov.cn/xcs/zhengcwj/202301/32de5b2ff9bf4eaa88e75bdf7223a65a.shtml (accessed on 29 June 2023).
- COVIDSurg, Collaborative. Projecting COVID-19 disruption to elective surgery. Lancet 2022, 399, 233–234. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Liu, S.; Lu, F. Impact of National Omicron Outbreak at the end of 2022 on the future outlook of COVID-19 in China. Emerg. Microbes. Infect. 2023, 12, 2191738. [Google Scholar] [CrossRef] [PubMed]
- Chinese Center for Disease Control and Prevention. China Situation of COVID-19. Available online: https://www.chinacdc.cn/jkzt/crb/zl/szkb_11803/jszl_13141/202304/t20230429_265709.html (accessed on 29 June 2023).
- Quinn, K.L.; Huang, A.; Bell, C.M.; Detsky, A.S.; Lapointe-Shaw, L.; Rosella, L.C.; Urbach, D.R.; Razak, F.; Verma, A.A. Complications following elective major noncardiac surgery among patients with prior SARS-CoV-2 infection. JAMA Netw. Open 2022, 5, e2247341. [Google Scholar] [CrossRef] [PubMed]
- Clancy, P.W.; Knio, Z.O.; Zuo, Z. Positive SARS-CoV-2 detection on intraoperative nasopharyngeal viral testing is not associated with worse outcomes for asymptomatic elective surgical patients. Front. Med. 2022, 9, 1065625. [Google Scholar] [CrossRef] [PubMed]
- El-Boghdadly, K.; Cook, T.M.; Goodacre, T.; Kua, J.; Denmark, S.; Mercer, N.; Moonesinghe, S.R.; Summerton, D.J. Timing of elective surgery and risk assessment after SARS-CoV-2 infection: 2023 update A multidisciplinary consensus statement on behalf of the Association of Anaesthetists, Federation of Surgical Specialty Associations, Royal College of Anaesthetists and Royal College of Surgeons of England. Anaesthesia, 2023; online ahead of print. [Google Scholar] [CrossRef]
- Llanos, A.A.M.; Ashrafi, A.; Ghosh, N.; Tsui, J.; Lin, Y.; Fong, A.J.; Ganesan, S.; Heckman, C.J. Evaluation of inequities in cancer treatment delay or discontinuation following SARS-CoV-2 infection. JAMA Netw. Open 2023, 6, e2251165. [Google Scholar] [CrossRef]
- McKay, S.C.; COVIDSurg, Collaborative. Outcomes of patients undergoing elective liver and pancreas cancer surgery during the SARS-CoV-2 pandemic: An international, multicentre, prospective cohort study. HPB 2022, 24, 1668–1678. [Google Scholar] [CrossRef]
- Doglietto, F.; Vezzoli, M.; Gheza, F.; Lussardi, G.L.; Domenicucci, M.; Vecchiarelli, L.; Zanin, L.; Saraceno, G.; Signorini, L.; Panciani, P.P.; et al. Factors associated with surgical mortality and complications among patients with and without Coronavirus Disease 2019 (COVID-19) in Italy. JAMA Surg. 2020, 155, 691–702. [Google Scholar] [CrossRef]
- Jonker, P.K.C.; van der Plas, W.Y.; Steinkamp, P.J.; Poelstra, R.; Emous, M.; van der Meij, W.; Thunnissen, F.; Bierman, W.F.W.; Struys, M.M.R.F.; de Reuver, P.R.; et al. Perioperative SARS-CoV-2 infections increase mortality, pulmonary complications, and thromboembolic events: A Dutch, multicenter, matched-cohort clinical study. Surgery 2021, 169, 264–274. [Google Scholar] [CrossRef]
- Deng, J.Z.; Chan, J.S.; Potter, A.L.; Chen, Y.W.; Sandhu, H.S.; Panda, N.; Chang, D.C.; Yang, C.J. The risk of postoperative complications after major elective surgery in active or resolved COVID-19 in the United States. Ann. Surg. 2022, 275, 242–246. [Google Scholar] [CrossRef]
- Lieberman, N.; Racine, A.; Nair, S.; Semczuk, P.; Azimaraghi, O.; Freda, J.; Eikermann, M.; Wongtangman, K. Should asymptomatic patients testing positive for SARS-CoV-2 wait for elective surgical procedures? Br. J. Anaesth. 2022, 128, e311–e314. [Google Scholar] [CrossRef] [PubMed]
- Ranganathan, P.; Salunke, B.; Wajekar, A.; Siddique, A.; Daruwalla, K.; Chawathey, S.; Niyogi, D.; Nayak, P.; Divatia, J. Outcomes of elective cancer surgery in COVID-19 survivors: An observational study. J. Surg. Oncol. 2023, 127, 11–17. [Google Scholar] [CrossRef]
- Garnier, M.; Constantin, J.M.; Cinotti, R.; Daoui, C.; Margetis, D.; Destruhaut, G.; Cirenei, C.; Noll, E.; Quesnel, C.; Lecinq, A.; et al. Association of preoperative COVID-19 and postoperative respiratory morbidity during the Omicron epidemic wave: The DROMIS-22 multicentre prospective observational cohort study. EClinicalMedicine 2023, 58, 101881. [Google Scholar] [CrossRef] [PubMed]
- Codner, J.A.; Archer, R.H.; Lynde, G.C.; Sharma, J. Timing is everything: Surgical outcomes for SARS-CoV-2 positive patients. World J. Surg. 2023, 47, 437–444. [Google Scholar] [CrossRef] [PubMed]
- Maslo, C.; Friedland, R.; Toubkin, M.; Laubscher, A.; Akaloo, T.; Kama, B. Characteristics and outcomes of hospitalized patients in South Africa during the COVID-19 Omicron wave compared with previous waves. JAMA 2022, 327, 583–584. [Google Scholar] [CrossRef] [PubMed]
- Pinato, D.J.; Aguilar-Company, J.; Ferrante, D.; Hanbury, G.; Bower, M.; Salazar, R.; Mirallas, O.; Sureda, A.; Plaja, A.; Cucurull, M.; et al. Outcomes of the SARS-CoV-2 omicron (B.1.1.529) variant outbreak among vaccinated and unvaccinated patients with cancer in Europe: Results from the retrospective, multicentre, OnCovid registry study. Lancet Oncol. 2022, 23, 865–875. [Google Scholar] [CrossRef]
- Qi, X.; Wang, J.; Zhang, Q.; Ai, J.; Liu, C.; Li, Q.; Gu, Y.; Lv, J.; Huang, Y.; Liu, Y.; et al. Safety and immunogenicity of COVID-19 vaccination in patients with hepatocellular carcinoma (CHESS-NMCID 2101): A multicenter prospective study. J. Med. Virol. 2022, 94, 5553–5559. [Google Scholar] [CrossRef]
- Liu, F.; Feng, X.; Du, J.; Ruan, M.; Liu, H. Serologic status and safety of inactivated Covid-19 vaccine for hepatocellular carcinoma patients with cirrhosis after curative liver resection. Cancer Commun. 2023, 43, 409–412. [Google Scholar] [CrossRef]
| Variables | Pre-Matching | Post-Matching | ||||
|---|---|---|---|---|---|---|
| COVID-19 Negative (n = 181) | COVID-19 Recovered (n = 57) | p | COVID-19 Negative (n = 114) | COVID-19 Recovered (n = 57) | p | |
| Age, years (SD) | 59.2 ± 11.2 | 56.6 ± 9.2 | 0.115 | 57.4 ± 11.8 | 56.6 ± 9.2 | 0.670 |
| Sex | 0.690 | 0.905 | ||||
| Male | 135 (74.6%) | 41 (71.9%) | 81 (71.1%) | 41 (71.9%) | ||
| Female | 46 (25.4%) | 16 (28.1%) | 33 (28.9%) | 16 (28.1%) | ||
| BMI, kg/m2 (SD) | 24.4 ± 3.2 | 24.6 ± 3.4 | 0.642 | 24.1 ± 3.1 | 24.6 ± 3.4 | 0.332 |
| Smoking status | 0.122 | 0.100 | ||||
| Never | 142 (78.5%) | 50 (87.7%) | 88 (77.2%) | 50 (87.7%) | ||
| Current | 39 (21.5%) | 7 (12.3%) | 26 (22.8%) | 7 (12.3%) | ||
| Comorbidities | 0.185 | 0.129 | ||||
| Pulmonary | 6 (3.3%) | 4 (7.0%) | 2 (1.8%) | 4 (7.0%) | ||
| Cardiovascular | 9 (5.0%) | 0 (0.0%) | 4 (3.5%) | 0 (0.0%) | ||
| Hypertension | 54 (29.8%) | 8 (14.0%) | 27 (23.7%) | 8 (14.0%) | ||
| Diabetes | 25 (13.8%) | 8 (14.0%) | 11 (9.6%) | 8 (14.0%) | ||
| Other | 12 (6.6%) | 2 (3.5%) | 9 (7.9%) | 2 (3.5%) | ||
| Multiple | 27 (14.9%) | 6 (10.5%) | 14 (12.3%) | 6 (10.5%) | ||
| ECOG performance score | 0.430 | 0.769 | ||||
| 0 | 109 (60.2%) | 39 (68.4%) | 76 (66.7%) | 39 (68.4%) | ||
| 1 | 70 (38.7%) | 18 (31.6%) | 37 (32.5%) | 18 (31.6%) | ||
| 2 | 2 (1.1%) | 0 (0.0%) | 1 (0.9%) | 0 (0.0%) | ||
| Child–Pugh class | 0.386 | 0.156 | ||||
| A | 180 (99.4%) | 56 (98.2%) | 114 (100.0%) | 56 (98.2%) | ||
| B | 1 (0.6%) | 1 (1.8%) | 0 (0.0%) | 1 (1.8%) | ||
| Chest X-ray | ||||||
| Normal | 107 (59.1%) | 23 (40.4%) | 0.013 * | 73 (64.0%) | 23 (40.4%) | 0.003 * |
| Abnormal | 31 (17.1%) | 13 (22.8%) | 0.335 | 15 (13.2%) | 13 (22.8%) | 0.108 |
| Not performed | 43 (23.8%) | 21 (36.8%) | 0.052 | 26 (22.8%) | 21 (36.8%) | 0.053 |
| Thorax CT | ||||||
| Normal | 3 (1.7%) | 3 (5.3%) | 0.130 | 2 (1.8%) | 3 (5.3%) | 0.199 |
| Pulmonary infiltration | 8 (4.4%) | 10 (17.5%) | 0.001 * | 7 (6.1%) | 10 (17.5%) | 0.019 * |
| Other abnormal | 20 (11.0%) | 17 (29.8%) | <0.001 * | 9 (7.9%) | 17 (29.8%) | <0.001 * |
| Not performed | 150 (82.9%) | 27 (47.4%) | <0.001 * | 96 (84.2%) | 27 (47.4%) | <0.001 * |
| ECG | ||||||
| Normal | 80 (44.2%) | 39 (68.4%) | 0.001 * | 50 (43.9%) | 39 (68.4%) | 0.002 * |
| ST-T change | 37 (20.4%) | 9 (15.8%) | 0.438 | 24 (21.1%) | 9 (15.8%) | 0.411 |
| Other abnormal | 61 (33.7%) | 9 (15.8%) | 0.010 * | 39 (34.2%) | 9 (15.8%) | 0.011 * |
| Not performed | 3 (1.7%) | 0 (0.0%) | 0.328 | 1 (0.9%) | 0 (0.0%) | 0.478 |
| Vaccination status | 0.185 | 0.137 | ||||
| Full | 145 (80.1%) | 40 (70.2%) | 95 (83.3%) | 40 (70.2%) | ||
| Partial | 3 (1.7%) | 2 (3.5%) | 2 (1.8%) | 2 (3.5%) | ||
| Unvaccinated | 32 (18.2%) | 15 (26.3%) | 17 (14.9%) | 15 (26.3%) | ||
| ASA grade | 0.360 | 0.973 | ||||
| 1 | 105 (58.0%) | 39 (68.4%) | 76 (66.7%) | 39 (68.4%) | ||
| 2 | 73 (40.3%) | 17 (29.8%) | 36 (31.6%) | 17 (29.8%) | ||
| 3 | 3 (1.7%) | 1 (1.8%) | 2 (1.8%) | 1 (1.8%) | ||
| Operative approach | 0.413 | 0.350 | ||||
| Laparotomy | 123 (68.0%) | 42 (73.7%) | 76 (66.7%) | 42 (73.7%) | ||
| Laparoscopic surgery | 58 (32.0%) | 15 (26.3%) | 38 (33.3%) | 15 (26.3%) | ||
| Extent of resection | 0.101 | 0.096 | ||||
| Minor hepatectomy | 105 (58.0%) | 40 (70.2%) | 65 (57.0%) | 40 (70.2%) | ||
| Major hepatectomy | 76 (42.0%) | 17 (29.8%) | 49 (43.0%) | 17 (29.8%) | ||
| Tumor type | 0.270 | 0.221 | ||||
| Hepatocellular carcinoma | 109 (60.2%) | 41 (71.9%) | 67 (58.8%) | 41 (71.9%) | ||
| Intrahepatic CC | 61 (33.7%) | 14 (24.6%) | 43 (37.7%) | 14 (24.6%) | ||
| Other | 11 (6.1%) | 2 (3.5%) | 4 (3.5%) | 2 (3.5%) | ||
| TNM stage | 0.291 | 0.449 | ||||
| I | 124 (68.5%) | 32 (56.1%) | 77 (67.5%) | 32 (56.1%) | ||
| II | 30 (16.6%) | 11 (19.3%) | 19 (16.7%) | 11 (19.3%) | ||
| III | 24 (13.3%) | 13 (22.8%) | 16 (5.3%) | 13 (22.8%) | ||
| IV | 3 (1.7%) | 1 (1.8%) | 2 (1.8%) | 1 (1.8%) | ||
| Variables | Pre-Matching | Post-Matching | ||||
|---|---|---|---|---|---|---|
| COVID-19 Negative (n = 181) | COVID-19 Recovered (n = 57) | p | COVID-19 Negative (n = 181) | COVID-19 Recovered (n = 57) | p | |
| 30-day mortality | 4 (2.2%) | 0 (0.0%) | 0.258 | - | - | |
| Pulmonary complication | ||||||
| Pneumonia | 2 (1.1%) | 1 (1.8%) | 0.702 | 1 (0.9%) | 1 (1.8%) | 0.615 |
| Pulmonary embolism | 1 (0.6%) | 0 (0.0%) | - | - | ||
| Pleural effusion | 6 (3.3%) | 1 (1.8%) | 0.574 | 4 (3.5%) | 1 (1.8%) | 0.521 |
| Cardiac complication | ||||||
| Elevated troponin (IQR) | 0.047 | 0.008 | 0.505 | 0.025 | 0.008 | 0.089 |
| (0.001–1.9) | (0.002–0.023) | (0.001–0.143) | (0.002–0.023) | |||
| Missing | 106 (58.6%) | 43 (75.4%) | 0.022 * | 71 (62.3%) | 43 (75.4%) | 0.085 |
| Pre-BNP (IQR) | 109.0 (12–409) | 73.9 (21–311) | 0.096 | 103.8 (15–354) | 73.9 (21–311) | 0.157 |
| Missing | 84 (46.4%) | 37 (64.9%) | 0.574 | 59 (51.8%) | 37 (64.9%) | 0.102 |
| Atrial fibrillation | 1 (0.6%) | 0 (0.0%) | 0.574 | - | - | |
| Cardiac failure | 1 (0.6%) | 0 (0.0%) | 0.425 | 1 (0.9%) | 0 (0.0%) | 0.478 |
| Postoperative bleeding | 2 (1.1%) | 0 (0.0%) | 0.205 | 1 (0.9%) | 0 (0.0%) | 0.478 |
| Liver failure | 5 (2.8%) | 0 (0.0%) | 0.153 | 3 (2.6%) | 0 (0.0%) | 0.217 |
| Bile leak | 17 (9.4%) | 2 (3.5%) | 0.765 | 12 (10.5%) | 2 (3.5%) | 0.115 |
| Abdominal effusion | 8 (4.4%) | 2 (3.5%) | 0.574 | 5 (4.4%) | 2 (3.5%) | 0.785 |
| Surgical site infection | 7 (3.9%) | 1 (1.8%) | 0.440 | 6 (5.3%) | 1 (1.8%) | 0.275 |
| Unplanned ICU admission | 2 (1.1%) | 0 (0.0%) | 0.425 | 1 (0.9%) | 0 (0.0%) | 0.478 |
| Postoperative nasal cannula use | 6 (3.3%) | 0 (0.0%) | 0.164 | 2 (1.8%) | 0 (0.0%) | 0.314 |
| Postoperative ECG monitored | 9 (5.0%) | 0 (0.0%) | 0.086 | 4 (3.5%) | 0 (0.0%) | 0.152 |
| Reoperation | 2 (1.1%) | 0 (0.0%) | 0.425 | 1 (0.9%) | 0 (0.0%) | 0.478 |
| Hospital length of stay, days (SD) | 10.1 ± 5.4 | 8.3 ± 2.2 | 0.011 * | 10.2 ± 5.9 | 8.3 ± 2.2 | 0.016 * |
| 30-day readmission | 4 (2.2%) | 0 (0.0%) | 0.258 | 4 (3.5%) | 0 (0.0%) | 0.152 |
| Variables | Univariable OR (95% CI) | Multivariable OR (95% CI) |
|---|---|---|
| COVID-19 | ||
| Negative | Reference | |
| Recovered | 0.146 (0.013–1.654) | |
| BMI | ||
| <24 | Reference | |
| ≥24 | 0.087 (0.011–0.693) | |
| Comorbidities | ||
| No | Reference | Reference |
| Yes | 9.912 (1.613–60.928) | 4.645 (1.295–16.667) |
| Smoking Status | ||
| Never | Reference | |
| Current | 2.265 (0.382–13.441) | |
| Chest X-ray or CT | ||
| Normal | Reference | |
| Abnormal | 0.000 (0.000–0.000) | |
| ECG | ||
| Normal | Reference | |
| Abnormal | 0.986 (0.194–5.011) | |
| Child–Pugh class | ||
| A | Reference | |
| B | 0.004 (0.000–1.299) | |
| Extent of resection | ||
| Minor hepatectomy | Reference | |
| Major hepatectomy | 1.698 (0.543–5.306) | |
| Operative approach | ||
| Laparotomy | Reference | Reference |
| Laparoscopic surgery | 6.408 (0.812–50.582) | 10.572 (1.220–91.585) |
| Tumor type | ||
| Hepatocellular carcinoma | Reference | |
| Other | 3.924 (0.743–20.732) | |
| TNM stage | ||
| I-II | Reference | |
| III-IV | 0.825 (0.128–5.330) | |
| Vaccination status | ||
| Vaccinated | Reference | Reference |
| Unvaccinated | 46.054 (4.615–459.543) | 5.408 (1.489–19.633) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Ma, J.; Wu, Y.; Zhang, S.; Li, X.; Xia, Y.; Yan, Z.; Liu, J.; Shen, F.; Zhang, X. Outcomes of Liver Cancer Patients Undergoing Elective Surgery after Recovering from Mild SARS-CoV-2 Omicron Infection: A Retrospective Cohort Study. Cancers 2023, 15, 4254. https://doi.org/10.3390/cancers15174254
Wang Y, Ma J, Wu Y, Zhang S, Li X, Xia Y, Yan Z, Liu J, Shen F, Zhang X. Outcomes of Liver Cancer Patients Undergoing Elective Surgery after Recovering from Mild SARS-CoV-2 Omicron Infection: A Retrospective Cohort Study. Cancers. 2023; 15(17):4254. https://doi.org/10.3390/cancers15174254
Chicago/Turabian StyleWang, Yizhou, Junyong Ma, Yali Wu, Shichao Zhang, Xifeng Li, Yong Xia, Zhenlin Yan, Jian Liu, Feng Shen, and Xiaofeng Zhang. 2023. "Outcomes of Liver Cancer Patients Undergoing Elective Surgery after Recovering from Mild SARS-CoV-2 Omicron Infection: A Retrospective Cohort Study" Cancers 15, no. 17: 4254. https://doi.org/10.3390/cancers15174254
APA StyleWang, Y., Ma, J., Wu, Y., Zhang, S., Li, X., Xia, Y., Yan, Z., Liu, J., Shen, F., & Zhang, X. (2023). Outcomes of Liver Cancer Patients Undergoing Elective Surgery after Recovering from Mild SARS-CoV-2 Omicron Infection: A Retrospective Cohort Study. Cancers, 15(17), 4254. https://doi.org/10.3390/cancers15174254






