The Use of 3D Printing Technology in Gynaecological Brachytherapy—A Narrative Review
Abstract
Simple Summary
Abstract
1. Introduction
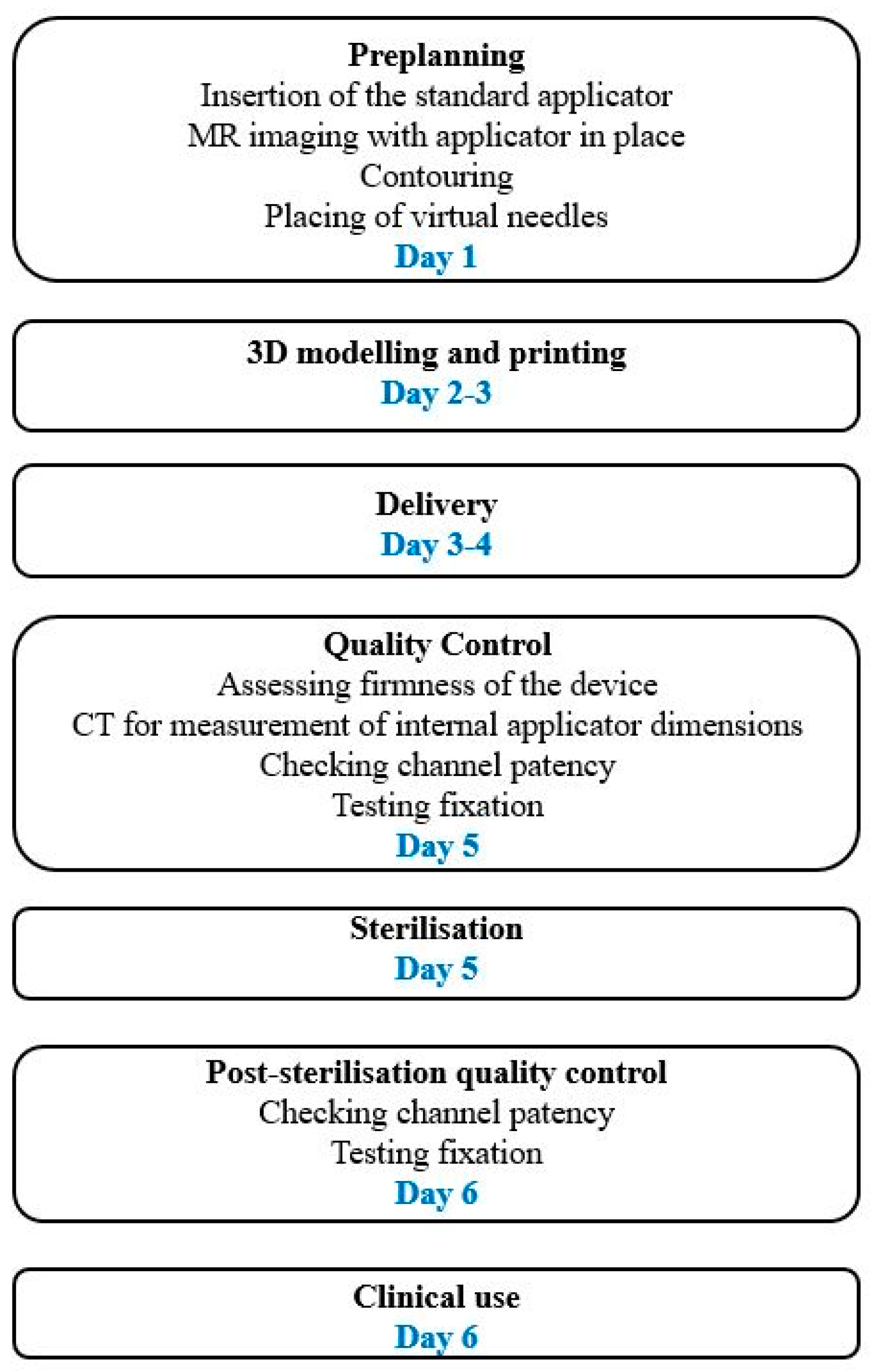
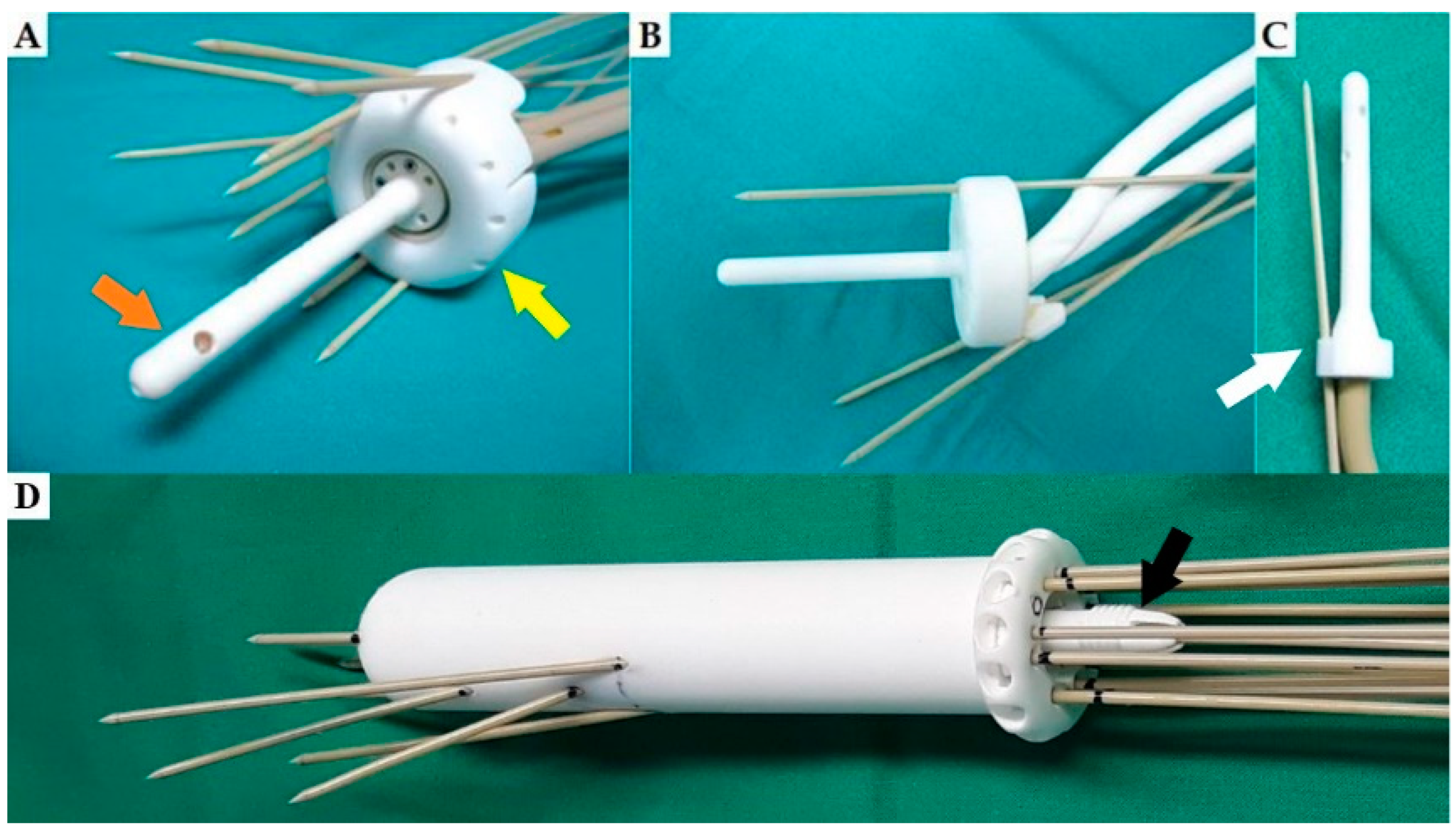
2. The Rationale for Development of 3D-Printed Applicators
3. Three-Dimensional Printing Technology
- Stereolythography (SLA)—the material used is a liquid resin with photoactive mono- and polymers, which gains its final form with photopoylmerisation under UV light and high temperature. Its resolution is high, in the range of 10 μm, the surface is smooth; however, the printing is slow and expensive, and the final product is fragile.
- Selective laser sintering (SLS) or powder bed fusion (PBF)—the materials used are powders, which can be plastic, ceramic, metal or glass and are fused into solid form using a laser beam. Similar to SLA, its resolution is high, in the range of 80–250 μm, but the process is slow and costly.
- Fused deposition modelling (FDM)—the materials used are continuous fibre-reinforced polymers and filaments of thermoplastic polymers, which are heated to a semi-liquid form and ejected through the nozzle layer by layer. The method is simple, fast and cheap, with a resolution of 50–200 μm, and its major limitations are the lack of more thermoplastic materials to choose from, rough surface and mechanical fragility of the final product.
- Laminated object manufacturing (LOM)—it is used in different materials including metal, paper and polymer composites. Its advantages are low cost and a variety of materials to choose from, while its major drawbacks are poor surface quality and unsuitability for finely detailed shapes due to low dimensional accuracy.
- Inkjet printing (IP)—the material mostly used is ceramic in a form of particle dispersion, which is ejected from the printer nozzle and deposited on the surface. This method is fast, but the resolution is coarse and adhesion between layers is poor.
- Direct energy deposition (DED)—mostly metal materials in the form of powder or a wire are fused together using focused thermal energy. DED produces devices of excellent mechanical properties, and the time and costs are low; however, surface quality is poor and resolution is low at 250 μm, which renders printing of fine details hard.
4. Clinical Evidence
5. Discussion
6. Conclusions and Future Directions
Author Contributions
Funding
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: Globocan Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Kamrava, M.; Leung, E.; Bachand, F.; Beriwal, S.; Chargari, C.; D’Souza, D.; Erickson, B.; Fokdal, L.; Han, K.; Harkenrider, M.; et al. GEC-ESTRO (ACROP)–ABS–CBG Consensus Brachytherapy Target Definition Guidelines for Recurrent Endometrial and Cervical Tumors in the Vagina. Int. J. Radiat. Oncol. 2023, 115, 654–663. [Google Scholar] [CrossRef]
- Cibula, D.; Raspollini, M.R.; Planchamp, F.; Centeno, C.; Chargari, C.; Felix, A.; Fischerová, D.; Jahnn-Kuch, D.; Joly, F.; Kohler, C.; et al. ESGO/ESTRO/ESP Guidelines for the management of patients with cervical cancer–Update 2023. Radiother. Oncol. 2023, 184, 109682. [Google Scholar] [CrossRef]
- Concin, N.; Matias-Guiu, X.; Vergote, I.; Cibula, D.; Mirza, M.R.; Marnitz, S.; Ledermann, J.; Bosse, T.; Chargari, C.; Fagotti, A.; et al. ESGO/ESTRO/ESP guidelines for the management of patients with endometrial carcinoma. Radiother. Oncol. 2021, 154, 327–353. [Google Scholar] [CrossRef] [PubMed]
- Nesvacil, N.; Tanderup, K.; Lindegaard, J.C.; Pötter, R.; Kirisits, C. Can reduction of uncertainties in cervix cancer brachytherapy potentially improve clinical outcome? Radiother. Oncol. 2016, 120, 390–396. [Google Scholar] [CrossRef] [PubMed]
- Roberts, S.A.; Hendry, J.H. A realistic closed-form radiobiological model of clinical tumor-control data incorporating intertumor heterogeneity. Int. J. Radiat. Oncol. 1998, 41, 689–699. [Google Scholar] [CrossRef]
- Huang, Z.; Mayr, N.A.; Gao, M.; Lo, S.S.; Wang, J.Z.; Jia, G.; Yuh, W.T. Onset Time of Tumor Repopulation for Cervical Cancer: First Evidence From Clinical Data. Int. J. Radiat. Oncol. 2012, 84, 478–484. [Google Scholar] [CrossRef]
- Tanderup, K.; Fokdal, L.U.; Sturdza, A.; Haie-Meder, C.; Mazeron, R.; van Limbergen, E.; Jürgenliemk-Schulz, I.; Petric, P.; Hoskin, P.; Dörr, W.; et al. Effect of tumor dose, volume and overall treatment time on local control after radiochemotherapy including MRI guided brachytherapy of locally advanced cervical cancer. Radiother. Oncol. 2016, 120, 441–446. [Google Scholar] [CrossRef]
- Tanderup, K.; Nesvacil, N.; Kirchheiner, K.; Serban, M.; Spampinato, S.; Jensen, N.B.K.; Schmid, M.; Smet, S.; Westerveld, H.; Ecker, S.; et al. Evidence-Based Dose Planning Aims and Dose Prescription in Image-Guided Brachytherapy Combined with Radiochemotherapy in Locally Advanced Cervical Cancer. Semin. Radiat. Oncol. 2020, 30, 311–327. [Google Scholar] [CrossRef]
- Schmid, M.P.; Lindegaard, J.C.; Mahantshetty, U.; Tanderup, K.; Haie-Meder, C.; Fokdal, L.U.; Sturdza, A.; Hoskin, P.; Segedin, B.; Bruheim, K.; et al. Risk Factors for Local Failure Following Chemoradiation and Magnetic Resonance Image–Guided Brachytherapy in Locally Advanced Cervical Cancer: Results from the EMBRACE-I Study. J. Clin. Oncol. 2023, 41, 1933–1942. [Google Scholar] [CrossRef]
- Tanderup, K.; Eifel, P.J.; Yashar, C.M.; Pötter, R.; Grigsby, P.W. Curative Radiation Therapy for Locally Advanced Cervical Cancer: Brachytherapy Is NOT Optional. Int. J. Radiat. Oncol. 2014, 88, 537–539. [Google Scholar] [CrossRef]
- Chakrabarti, B.; Pal, S.K.; Sepai, H.M.; Basu-Roy, S.; Kar, S.K.; Lahiri, A.; Das, S.; Bala, A. Clinical and dosimetric consequences of imperfect applicator insertion in cervical cancer brachytherapy. J. Contemp. Brachyther. 2018, 10, 321–336. [Google Scholar] [CrossRef]
- Kissel, M.; Fournier-Bidoz, N.; Henry, O.; Bockel, S.; Kumar, T.; Espenel, S.; Chargari, C. Venezia applicator with oblique needles improves clinical target volume coverage in distal parametrial tumor residue compared to parallel needles only. J. Contemp. Brachytherapy 2021, 13, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Zhang, Y.; Cui, M.; Miao, J.; Gao, L.; Hu, J.; Tian, D.; You, J. Dosimetry comparison between a 3D printed minimally invasive guidance template and free implantation in the brachytherapy treatment of postoperative recurrent cervical carcinoma. Cancer Manag. Res. 2019, 11, 5013–5018. [Google Scholar] [CrossRef]
- Fortin, I.; Tanderup, K.; Haie-Meder, C.; Lindegaard, J.C.; Mahantshetty, U.; Segedin, B.; Jürgenliemk-Schulz, I.M.; Hoskin, P.; Kirisits, C.; Pötter, R. Image Guided Brachytherapy in Cervical Cancer: A Comparison between Intracavitary and Combined Intracavitary/Interstitial Brachytherapy in Regard to Doses to HR CTV, OARs and Late Morbidity—Early Results from the Embrace Study in 999 Patients. Brachytherapy 2016, 15, S21. [Google Scholar] [CrossRef]
- Tanderup, K.; Nielsen, S.K.; Nyvang, G.-B.; Pedersen, E.M.; Røhl, L.; Aagaard, T.; Fokdal, L.; Lindegaard, J.C. From point A to the sculpted pear: MR image guidance significantly improves tumour dose and sparing of organs at risk in brachytherapy of cervical cancer. Radiother. Oncol. 2010, 94, 173–180. [Google Scholar] [CrossRef]
- Fokdal, L.; Tanderup, K.; Hokland, S.B.; Røhl, L.; Pedersen, E.M.; Nielsen, S.K.; Paludan, M.; Lindegaard, J.C. Clinical feasibility of combined intracavitary/interstitial brachytherapy in locally advanced cervical cancer employing MRI with a tandem/ring applicator in situ and virtual preplanning of the interstitial component. Radiother. Oncol. 2013, 107, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Chargari, C.; Tanderup, K.; Planchamp, F.; Chiva, L.; Humphrey, P.; Sturdza, A.; Tan, L.T.; van der Steen-Banasik, E.; Zapardiel, I.; Nout, R.A.; et al. ESGO/ESTRO quality indicators for radiation therapy of cervical cancer. Int. J. Gynecol. Cancer 2023, 33, 862–875. [Google Scholar] [CrossRef] [PubMed]
- Gerbaulet, A.; Pötter, R.; Mazeron, J.-J.; Meertens, H.; Van Limbergen, E. The GEC ESTRO Handbook of Brachytherapy, 1st ed.; ESTRO: Shibuya, Tokyo, 2002. [Google Scholar]
- Logar, H.B.Z.; Hudej, R.; Šegedin, B. Development and assessment of 3D-printed individual applicators in gynecological MRI-guided brachytherapy. J. Contemp. Brachyther. 2019, 11, 128–136. [Google Scholar] [CrossRef]
- Lindegaard, J.C.; Madsen, M.L.; Traberg, A.; Meisner, B.; Nielsen, S.K.; Tanderup, K.; Spejlborg, H.; Fokdal, L.U.; Nørrevang, O. Individualised 3D printed vaginal template for MRI guided brachytherapy in locally advanced cervical cancer. Radiother. Oncol. 2016, 118, 173–175. [Google Scholar] [CrossRef]
- Xu, Y.; Lin, S.; Hamilton, R.; Watchman, C.; Dougherty, S. Improved Dose Distribution with 3D Printed Vaginal Cylinder Applicator for VariSource HDR Afterloader. Int. J. Radiat. Oncol. 2018, 102, e480–e481. [Google Scholar] [CrossRef]
- Sekii, S.; Tsujino, K.; Kosaka, K.; Yamaguchi, S.; Kubota, H.; Matsumoto, Y.; Ota, Y.; Sasaki, R.; Soejima, T. Inversely designed, 3D-printed personalized template-guided interstitial brachytherapy for vaginal tumors. J. Contemp. Brachyther. 2018, 10, 470–477. [Google Scholar] [CrossRef] [PubMed]
- Wiebe, E.; Easton, H.; Thomas, G.; Barbera, L.; D’Alimonte, L.; Ravi, A. Customized vaginal vault brachytherapy with computed tomography imaging-derived applicator prototyping. Brachytherapy 2015, 14, 380–384. [Google Scholar] [CrossRef]
- Xenofontos, P.; Zamani, R.; Akrami, M. The application of 3D printing in preoperative planning for transcatheter aortic valve replacement: A systematic review. Biomed. Eng. Online 2022, 21, 59. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.Y.; Skewes, J.; Desselle, M.; Wong, C.; Woodruff, M.A.; Dasgupta, P.; Rukin, N.J. Current applications of three-dimensional printing in urology. BJU Int. 2019, 125, 17–27. [Google Scholar] [CrossRef]
- Jonathan, S.; Babazadeh, S.M.; Mackay, N. Three-dimensional printing in orthopaedic surgery: Review of current and future applications. ANZ J. Surgeryournal. Surg. 2016, 86, 648–653. [Google Scholar]
- Tack, P.; Victor, J.; Gemmel, P.; Annemans, L. 3D-printing techniques in a medical setting: A systematic literature review. Biomed. Eng. Online 2016, 15, 115. [Google Scholar] [CrossRef]
- Hong, C.J.; Giannopoulos, A.A.; Hong, B.Y.; Witterick, I.J.; Irish, J.C.; Lee, J.; Vescan, A.; Mitsouras, D.; Dang, W.; Campisi, P.; et al. Clinical applications of three-dimensional printing in otolaryngology–head and neck surgery: A systematic review. Laryngoscope 2019, 129, 2045–2052. [Google Scholar] [CrossRef]
- Meyer-Szary, J.; Luis, M.S.; Mikulski, S.; Patel, A.; Schulz, F.; Tretiakow, D.; Fercho, J.; Jaguszewska, K.; Frankiewicz, M.; Pawłowska, E.; et al. The Role of 3D Printing in Planning Complex Medical Procedures and Training of Medical Professionals—Cross-Sectional Multispecialty Review. Int. J. Environ. Res. Public Health 2022, 19, 3331. [Google Scholar] [CrossRef]
- Magklara, E.; Angelis, S.; Solia, E.; Apostolopoulos, A.P.; Tsakotos, G.; Vlasis, K.; Katsimantas, A.; Filippou, D.K. Three-Dimensional (3D) Printing in Orthopedics Education. J. Autom. Inf. Sci. 2020, 30, 255–258. [Google Scholar] [CrossRef]
- Kut, C.; Kao, T.; Morcos, M.; Kim, Y.; Boctor, E.; Viswanathan, A.N. 3D-printed Magnetic Resonance (MR)-based gynecological phantom for image-guided brachytherapy training. Brachytherapy 2022, 21, 799–805. [Google Scholar] [CrossRef] [PubMed]
- Jones, E.-L.; Baldion, A.T.; Thomas, C.; Burrows, T.; Byrne, N.; Newton, V.; Aldridge, S. Introduction of novel 3D-printed superficial applicators for high-dose-rate skin brachytherapy. Brachytherapy 2017, 16, 409–414. [Google Scholar] [CrossRef]
- Bielęda, G.; Chicheł, A.; Boehlke, M.; Zwierzchowski, G.; Chyrek, A.; Burchardt, W.; Stefaniak, P.; Wiśniewska, N.; Czereba, K.; Malicki, J. 3D printing of individual skin brachytherapy applicator: Design, manufacturing, and early clinical results. J. Contemp. Brachyther. 2022, 14, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Conejo, I.M.; Cegarra, O.P.; Arnalot, P.F.; Castillejo, A.R.; de Dios, N.R.; Latiesas, X.S.; Vallverdú, R.M.P.; Jordana, J.Q.; Cepria, E.F.-V.; Muñoz, V.A.; et al. Custom 3D-printed applicators for high dose-rate brachytherapy in skin cancer. Brachytherapy 2021, 20, 1257–1264. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.-W.; Zhang, J.-G.; Zheng, L.; Liu, S.-M.; Yu, G.-Y. Accuracy evaluation of a 3D-printed individual template for needle guidance in head and neck brachytherapy. J. Radiat. Res. 2016, 57, 662–667. [Google Scholar] [CrossRef]
- Scholten, A.N.; van Putten, W.L.; Beerman, H.; Smit, V.T.; Koper, P.C.; Lybeert, M.L.; Jobsen, J.J.; Wárlám-Rodenhuis, C.C.; De Winter, K.A.; Lutgens, L.C.; et al. Postoperative radiotherapy for Stage 1 endometrial carcinoma: Long-term outcome of the randomized PORTEC trial with central pathology review. Int. J. Radiat. Oncol. 2005, 63, 834–838. [Google Scholar] [CrossRef]
- Subashi, E.; Jacobs, C.; Hood, R.; Kirsch, D.G.; Craciunescu, O. A design process for a 3D printed patient-specific applicator for HDR brachytherapy of the orbit. 3D Print. Med. 2020, 6, 4–8. [Google Scholar] [CrossRef]
- Zwierzchowski, G.; Bielęda, G.; Szymbor, A.; Boehlke, M. Personalized Superficial HDR Brachytherapy—Dosimetric Verification of Dose Distribution with Lead Shielding of Critical Organs in the Head and Neck Region. J. Pers. Med. 2022, 12, 1432. [Google Scholar] [CrossRef]
- Harris, V.A.; Staffurth, J.; Naismith, O.; Esmail, A.; Gulliford, S.; Khoo, V.; Lewis, R.; Littler, J.; McNair, H.; Sadoyze, A.; et al. Consensus Guidelines and Contouring Atlas for Pelvic Node Delineation in Prostate and Pelvic Node Intensity Modulated Radiation Therapy. Int. J. Radiat. Oncol. 2015, 92, 874–883. [Google Scholar] [CrossRef]
- Tao, H.; Xiaodan, Y.; Ying, X.; Zhendong, Z.; Ying, Y.; Ning, W. Therapeutic value of 3-D printing template-assisted 125I-seed implantation in the treatment of malignant liver tumors. OncoTargets Ther. 2017, 10, 3277–3283. [Google Scholar] [CrossRef]
- Aristei, C.; Lancellotta, V.; Piergentini, M.; Costantini, G.; Saldi, S.; Chierchini, S.; Cavalli, A.; Di Renzo, L.; Fiorucci, O.; Guasticchi, M.; et al. Individualized 3D-printed templates for high-dose-rate interstitial multicathether brachytherapy in patients with breast cancer. Brachytherapy 2019, 18, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Lu, J.; Chen, K.-M.; Wu, Z.-Y.; Wang, Q.-B.; Liu, J.-J.; Gong, J.; Chen, Z.-J.; Ding, X.-Y.; Wang, Z.-M. Preliminary application of 3D-printed coplanar template for iodine-125 seed implantation therapy in patients with advanced pancreatic cancer. World J. Gastroenterol. 2018, 24, 5280–5287. [Google Scholar] [CrossRef] [PubMed]
- Ji, Z.; Sun, H.; Jiang, Y.; Chen, Y.; Guo, F.; Fan, J.; Wang, J. Analysis on the accuracy of CT-guided radioactive I-125 seed implantation with 3D printing template assistance in the treatment of thoracic malignant tumors. J. Radiat. Res. 2021, 62, 910–917. [Google Scholar] [CrossRef] [PubMed]
- Chen, E.; Zhang, Y.; Zhang, H.; Jia, C.; Liang, Y.; Wang, J. Dosimetry study of three-dimensional print template for 125I implantation therapy. Radiat. Oncol. 2021, 16, 115. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Shen, Z.; Qu, A.; Jiang, P.; Jiang, Y.; Wang, J. A comparative study of dosimetric parameters of 3D-printed non-coplanar template-assisted CT-guided iodine-125 seed implantation brachytherapy in patients with inguinal lymph node metastatic carcinomas. J. Contemp. Brachyther. 2022, 14, 452–461. [Google Scholar] [CrossRef]
- D’Alimonte, L.; Ravi, A.; Helou, J.; Morrison, D.; Mendez, L.C.; Easton, H.; Morton, G. Optimized penile surface mold brachytherapy using latest stereolithography techniques: A single-institution experience. Brachytherapy 2019, 18, 348–352. [Google Scholar] [CrossRef]
- Lancellotta, V.; Pagano, S.; Tagliaferri, L.; Piergentini, M.; Ricci, A.; Montecchiani, S.; Saldi, S.; Chierchini, S.; Cianetti, S.; Valentini, V.; et al. Individual 3-dimensional printed mold for treating hard palate carcinoma with brachytherapy: A clinical report. J. Prosthet. Dent. 2019, 121, 690–693. [Google Scholar] [CrossRef]
- Petric, P.; Hudej, R.; Al-Hammadi, N.; Segedin, B. Virtual modelling of novel applicator prototypes for cervical cancer brachytherapy. Radiol. Oncol. 2016, 50, 433–441. [Google Scholar] [CrossRef][Green Version]
- Mourya, A.; Aggarwal, L.M.; Choudhary, S. Evolution of Brachytherapy Applicators for the Treatment of Cervical Cancer. J. Med. Phys. 2021, 46, 231–243. [Google Scholar] [CrossRef]
- Mahantshetty, U.; Sturdza, A.; Ch, P.N.; Berger, D.; Fortin, I.; Motisi, L.; Schmid, M.P.; Aravindakshan, D.; Ghadi, Y.; Swamidas, J.V.; et al. Vienna-II ring applicator for distal parametrial/pelvic wall disease in cervical cancer brachytherapy: An experience from two institutions: Clinical feasibility and outcome. Radiother. Oncol. 2019, 141, 123–129. [Google Scholar] [CrossRef]
- Marar, M.; Niedermayr, T.; Kidd, E.A. Developing Next-Generation 3-Dimensional Printing for Cervical Cancer Hybrid Brachytherapy: A Guided Interstitial Technique Enabling Improved Flexibility, Dosimetry, and Efficiency. Int. J. Radiat. Oncol. 2023. [Google Scholar] [CrossRef] [PubMed]
- Chopra, S. SP-0373 Tumour biology to guide brachytherapy delivery and combination therapies. Radiother. Oncol. 2023, 182, S279. [Google Scholar] [CrossRef]
- Fokdal, L.; Sturdza, A.; Mazeron, R.; Haie-Meder, C.; Tan, L.T.; Gillham, C.; Šegedin, B.; Jürgenliemk-Schultz, I.; Kirisits, C.; Hoskin, P.; et al. Image guided adaptive brachytherapy with combined intracavitary and interstitial technique improves the therapeutic ratio in locally advanced cervical cancer: Analysis from the retroEMBRACE study. Radiother. Oncol. 2016, 120, 434–440. [Google Scholar] [CrossRef] [PubMed]
- Viswanathan, A.N.; Moughan, J.; Small, W.; Levenback, C.; Iyer, R.; Hymes, S.; Dicker, A.P.; Miller, B.; Erickson, B.; Gaffney, D.K. The Quality of Cervical Cancer Brachytherapy Implantation and the Impact on Local Recurrence and Disease-Free Survival in RTOG Prospective Trials 0116 and 0128. Int. J. Gynecol. Cancer 2012, 22, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Corn, B.W.; Hanks, G.E.; Schultheiss, T.E.; Hunt, M.A.; Lee, W.; Coia, L.R. Conformal treatment of prostate cancer with improved targeting: Superior prostate-specific antigen response compared to standard treatment. Int. J. Radiat. Oncol. 1995, 32, 325–330. [Google Scholar] [CrossRef] [PubMed]
- Qiu, B.; Jiang, Y.; Ji, Z.; Sun, H.; Fan, J.; Li, W.; Shao, Y.; Jiang, P.; Wang, J. The Accuracy of Individualized 3D-Printing Template-Assisted I125 Radioactive Seed Implantation for Recurrent/Metastatic Head and Neck Cancer. Front. Oncol. 2021, 11, 664996. [Google Scholar] [CrossRef]
- Wang, B.; Qiu, B.; Wu, L.; Liu, Y.; Zhang, J.; Wang, R.; Zhang, K.; Wang, J. Efficacy and safety of 3D printing coplanar template-assisted iodine-125 seed implantation as palliative treatment for inoperable pancreatic cancer. J. Contemp. Brachyther. 2022, 14, 140–147. [Google Scholar] [CrossRef]
- Logar, H.; Hudej, R.; Kobav, M. 86 3D-printed multi-channel vaginal applicator for brachytherapy in gynecological cancer. Int. J. Gynecol. Cancer 2020, 30, 102–103. [Google Scholar]
- Yan, J.; Qin, X.; Zhang, F.; Hou, X.; Yu, L.; Qiu, J. Comparing multichannel cylinder and 3D-printed applicators for vaginal cuff brachytherapy with preliminary exploration of post-hysterectomy vaginal morphology. J. Contemp. Brachyther. 2021, 13, 641–648. [Google Scholar] [CrossRef]
- Sethi, R.; Cunha, A.; Mellis, K.; Siauw, T.; Diederich, C.; Pouliot, J.; Hsu, I.-C. Clinical applications of custom-made vaginal cylinders constructed using three-dimensional printing technology. J. Contemp. Brachyther. 2016, 3, 208–214. [Google Scholar] [CrossRef]
- Ngo, T.D.; Kashani, A.; Imbalzano, G.; Nguyen, K.T.Q.; Hui, D. Additive Manufacturing (3D Printing): A Review of Materials, Methods, Applications and Challenges. Compos. Part B Eng. 2018, 143, 172–196. [Google Scholar] [CrossRef]
- Salmi, M. Additive Manufacturing Processes in Medical Applications. Materials 2021, 14, 191. [Google Scholar] [CrossRef] [PubMed]
- Javaid, M.; Haleem, A. Current status and challenges of Additive manufacturing in orthopaedics: An overview. J. Clin. Orthop. Trauma 2019, 10, 380–386. [Google Scholar] [CrossRef] [PubMed]
- Shopova, D.; Yaneva, A.; Bakova, D.; Mihaylova, A.; Kasnakova, P.; Hristozova, M.; Sbirkov, Y.; Sarafian, V.; Semerdzhieva, M. (Bio)printing in Personalized Medicine—Opportunities and Potential Benefits. Bioengineering 2023, 10, 287. [Google Scholar] [CrossRef]
- Tian, Y.; Chen, C.; Xu, X.; Wang, J.; Hou, X.; Li, K.; Lu, X.; Shi, H.; Lee, E.-S.; Jiang, H.B. A Review of 3D Printing in Dentistry: Technologies, Affecting Factors, and Applications. Scanning 2021, 2021, 9950131. [Google Scholar] [CrossRef]
- Ballard, D.H.; Tappa, K.; Boyer, C.J.; Jammalamadaka, U.; Hemmanur, K.; Weisman, J.A.; Alexander, J.S.; Mills, D.K.; Woodard, P.K. Antibiotics in 3D-printed implants, instruments and materials: Benefits, challenges and future directions. J. 3D Print. Med. 2019, 3, 83–93. [Google Scholar] [CrossRef] [PubMed]
- Choonara, Y.E.; du Toit, L.C.; Kumar, P.; Kondiah, P.P.D.; Pillay, V. 3D-printing and the effect on medical costs: A new era? Expert Rev. Pharmacoecon. Outcomes Res. 2016, 16, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Alssabbagh, M.; Tajuddin, A.A.; Abdulmanap, M.; Zainon, R. Evaluation of 3D printing materials for fabrication of a novel multi-functional 3D thyroid phantom for medical dosimetry and image quality. Radiat. Phys. Chem. 2017, 135, 106–112. [Google Scholar] [CrossRef]
- Ricotti, R.; Ciardo, D.; Pansini, F.; Bazani, A.; Comi, S.; Spoto, R.; Noris, S.; Cattani, F.; Baroni, G.; Orecchia, R.; et al. Dosimetric characterization of 3D printed bolus at different infill percentage for external photon beam radiotherapy. Phys. Medica 2017, 39, 25–32. [Google Scholar] [CrossRef]
- Rooney, M.K.; Rosenberg, D.M.; Braunstein, S.; Cunha, A.; Damato, A.L.; Ehler, E.; Pawlicki, T.; Robar, J.; Tatebe, K.; Golden, D.W. Three-dimensional printing in radiation oncology: A systematic review of the literature. J. Appl. Clin. Med Phys. 2020, 21, 15–26. [Google Scholar] [CrossRef]
- Tino, R.B.; Leary, M.; Yeo, A.U.; Kyriakou, E.; Kron, T.; Brandt, M. Additive manufacturing in radiation oncology: A review of clinical practice, emerging trends and research opportunities. Int. J. Extreme Manuf. 2020, 2, 012003. [Google Scholar] [CrossRef]
- Yan, Q.; Dong, H.; Su, J.; Han, J.; Song, B.; Wei, Q.; Shi, Y. A Review of 3D Printing Technology for Medical Applications. Engineering 2018, 4, 729–742. [Google Scholar] [CrossRef]
- Cunha, J.A.M.; Mellis, K.; Sethi, R.; Siauw, T.; Sudhyadhom, A.; Garg, A.; Goldberg, K.; Hsu, I.-C.; Pouliot, J. Evaluation of PC-ISO for customized, 3D printed, gynecologic 192Ir HDR brachytherapy applicators. J. Appl. Clin. Med. Phys. 2015, 16, 246–253. [Google Scholar] [CrossRef] [PubMed]
- Sohn, J.J.; Polizzi, M.; Richeson, D.; Gholami, S.; Das, I.J.; Song, W.Y. A Novel Workflow with a Customizable 3D Printed Vaginal Template and a Direction Modulated Brachytherapy (DMBT) Tandem Applicator for Adaptive Interstitial Brachytherapy of the Cervix. J. Clin. Med. 2022, 11, 6989. [Google Scholar] [CrossRef] [PubMed]
- Semeniuk, O.; Cherpak, A.; Robar, J. Design and evaluation of 3D printable patient-specific applicators for gynecologic HDR brachytherapy. Med. Phys. 2021, 48, 4053–4063. [Google Scholar] [CrossRef]
- Laan, R.C.; Nout, R.A.; Dankelman, J.; van de Berg, N.J. MRI-driven design of customised 3D printed gynaecological brachytherapy applicators with curved needle channels. 3D Print. Med. 2019, 5, 8. [Google Scholar] [CrossRef]
- Wang, J.; Kang, W.; Zhang, H.; Liang, Y.; Chen, E.; Zhao, J.; Gao, Z. Comparison of three-dimensional-printed template-guided and traditional implantation of 125I seeds for gynecological tumors: A dosimetric and efficacy study. J. Cancer Res. Ther. 2021, 17, 688–694. [Google Scholar] [CrossRef]
- Marar, M.; Simiele, E.; Niedermayr, T.; Kidd, E.A. Applying 3D-Printed Templates in High-Dose-Rate Brachytherapy for Cervix Cancer: Simplified Needle Insertion for Optimized Dosimetry. Int. J. Radiat. Oncol. 2022, 114, 111–119. [Google Scholar] [CrossRef]
- Kudla, M.; Bachand, F.; Moore, J.; Batchelar, D. Patient-specific cylinder templates for hybrid interstitial vaginal brachytherapy: Feasibility of automated 3-D design, 3D printing, and dosimetric outlook. Brachytherapy 2023, 22, 468–476. [Google Scholar] [CrossRef]
- Serban, M.; Fokdal, L.; Nielsen, S.K.; Hokland, S.B.; Hansen, A.T.; Spejlborg, H.; Rylander, S.; Petric, P.; Lindegaard, J.C.; Tanderup, K. Characterization of combined intracavitary/interstitial brachytherapy including oblique needles in locally advanced cervix cancer. Brachytherapy 2021, 20, 796–806. [Google Scholar] [CrossRef]
- Jiang, W.; Jiang, P.; Wei, S.; Jiang, Y.; Ji, Z.; Sun, H.; Fan, J.; Li, W.; Shao, Y.; Wang, J. The accuracy and safety of CT-guided iodine-125 seed implantation assisted by 3D non-coplanar template for retroperitoneal recurrent carcinoma. World J. Surg. Oncol. 2020, 18, 307. [Google Scholar] [CrossRef]
- Jiang, P.; Qu, A.; Wei, S.; Sun, H.; Zhang, X.; Li, X.; Wang, J. The Preliminary Results of 3-Dimensional Printed Individual Template Assisted 192Ir High-Dose Rate Interstitial Brachytherapy for Central Recurrent Gynecologic Cancer. Technol. Cancer Res. Treat. 2021, 32, e15. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.; Zhang, F.; Hou, X.; Yu, L.; Yu, L.; Yan, J.; Qiu, J. Efficacy and safety of a 3D-printed applicator for vaginal brachytherapy in patients with central pelvic-recurrent cervical cancer after primary hysterectomy. Brachytherapy 2022, 21, 193–201. [Google Scholar] [CrossRef]
- Liao, Y.; Tatebe, K.; Barry, P.; Wang, D.; Turian, J. A novel use of 3D-printed template in vaginal HDR brachytherapy. Brachytherapy 2022, 21, 238–243. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Zhang, D.; Jiang, S.; Zhou, L.; Zhou, Z.; Wang, W. Individualized and inverse optimized needle configuration for combined intracavitary-interstitial brachytherapy in locally advanced cervical cancer. J. Cancer Res. Ther. 2019, 15, 1589–1596. [Google Scholar] [CrossRef]
- Liu, Y.; Jiang, P.; Zhang, H.; Wang, J. Safety and efficacy of 3D-printed templates assisted CT-guided radioactive iodine-125 seed implantation for the treatment of recurrent cervical carcinoma after external beam radiotherapy. J. Gynecol. Oncol. 2021, 32, e15. [Google Scholar] [CrossRef]
- Fowler, T.L.; Buyyounouski, M.K.; Jenkins, C.H.; Fahimian, B.P. Clinical Implementation of 3D Printing for Brachytherapy: Techniques and Emerging Applications. Brachytherapy 2016, 15, S166. [Google Scholar] [CrossRef]
- Barnes, E.A.; Tsao, M.N.; Taggar, A.S.; Ravi, A.; Paudel, M.R. Optimizing surface mould brachytherapy for treatment of nasal basal cell carcinoma using customized applicators. Brachytherapy 2023. [Google Scholar] [CrossRef]
- Otani, Y.; Sumida, I.; Nose, T.; Shimamoto, S.; Okubo, H.; Ogawa, K. High-dose rate intracavitary brachytherapy pretreatment dwell position verification using a transparent applicator. J. Appl. Clin. Med. Phys. 2018, 19, 428–434. [Google Scholar] [CrossRef] [PubMed]
- Biltekin, F.; Akyol, H.F.; Gültekin, M.; Yildiz, F. Brachytherapy Applicator: Feasibility Study. J. Contemp. Brachyther. 2020, 12, 17–26. [Google Scholar] [CrossRef]
- Lin, S.-M.; Ku, H.-Y.; Chang, T.-C.; Liu, T.-W.; Hong, J.-H. The prognostic impact of overall treatment time on disease outcome in uterine cervical cancer patients treated primarily with concomitant chemoradiotherapy: A nationwide Taiwanese cohort study. Oncotarget 2017, 8, 85203–85213. [Google Scholar] [CrossRef] [PubMed]
- Bielęda, G.; Marach, A.; Boehlke, M.; Zwierzchowski, G.; Malicki, J. 3D-printed surface applicators for brachytherapy: A phantom study. J. Contemp. Brachyther. 2021, 13, 549–562. [Google Scholar] [CrossRef]
- Skinner, L.B.; Niedermayr, T.; Prionas, N.; Perl, J.; Fahimian, B.; Kidd, E.A. Intensity modulated Ir-192 brachytherapy using high-Z 3D printed applicators. Phys. Med. Biol. 2020, 65, 155018. [Google Scholar] [CrossRef] [PubMed]
- Sohn, J.J.; Polizzi, M.; Kang, S.-W.; Ko, W.-H.; Cho, Y.-H.; Eom, K.-Y.; Chung, J.-B. Intensity Modulated High Dose Rate (HDR) Brachytherapy Using Patient Specific 3D Metal Printed Applicators: Proof of Concept. Front. Oncol. 2022, 12, 829529. [Google Scholar] [CrossRef]
- Tun, S.Y.Y.; Madanian, S.; Mirza, F. Internet of things (IoT) applications for elderly care: A reflective review. Aging Clin. Exp. Res. 2021, 33, 855–867. [Google Scholar] [CrossRef]
- Al-Kahtani, M.S.; Khan, F.; Taekeun, W. Application of Internet of Things and Sensors in Healthcare. Sensors 2022, 22, 5738. [Google Scholar] [CrossRef]
- Sadoughi, F.; Behmanesh, A.; Sayfouri, N. Internet of things in medicine: A systematic mapping study. J. Biomed. Inform. 2020, 103, 103383. [Google Scholar] [CrossRef]
- Mulita, F.; Verras, G.-I.; Anagnostopoulos, C.-N.; Kotis, K. A Smarter Health through the Internet of Surgical Things. Sensors 2022, 22, 4577. [Google Scholar] [CrossRef]
- Muhsen, I.N.; Rasheed, O.W.; Habib, E.A.; Alsaad, R.K.; Maghrabi, M.K.; Rahman, A.; Sicker, D.; Wood, W.A.; Beg, M.S.; Sung, A.D.; et al. Current status and future perspectives on the Internet of Things in oncology. Hematol. Stem Cell Ther. 2021, 16, 102–109. [Google Scholar] [CrossRef]
- Heshmat, M.; Shehata, A.R.S. A framework about using internet of things for smart cancer treatment process. Proc. Int. Conf. Ind. Eng. Oper. Manag. 2018, 2018, 1206–1211. [Google Scholar]
- de Queiroz, D.A.; da Costa, C.A.; de Queiroz, E.A.I.F.; da Silveira, E.F.; Righi, R.d.R. Internet of Things in active cancer Treatment: A systematic review. J. Biomed. Inform. 2021, 118, 103814. [Google Scholar] [CrossRef] [PubMed]
- Thaker, N.G.; De, B.; Shah, C.; Manda, S.; Royce, T.J.; Beriwal, S. Practical Applications of the Internet of Things in Radiation Oncology. Appl. Radiat. Oncol. 2022. [Google Scholar] [CrossRef]
- Taunk, N.K.; Shah, N.K.; Hubley, E.; Anamalayil, S.; Trotter, J.W.; Li, T. Virtual reality-based simulation improves gynecologic brachytherapy proficiency, engagement, and trainee self-confidence. Brachytherapy 2021, 20, 695–700. [Google Scholar] [CrossRef] [PubMed]
| Author, Publication Year | Type of Study/No. of Pts | Patient Selection | Type of 3D-Printed Applicator | Results |
|---|---|---|---|---|
| Yuan et al., 2019 [14] | Randomised/21 pts | Recurrent cervical cancer | VC with oblique needles, compared to freehand | Higher D90 for CTVHR, lower D2cc all OARs; Fewer needles with 3D cylinder |
| Yan et al., 2021 [60] | Prospective/48 pts | Postoperative endometrial cancer | MVC, compared to commercial cylinder | Higher D90 for CTV, more homogeneous dose, fewer air pockets |
| Jiang et al., 2020 [83] | Prospective/32 pts | Central recurrences | MVC with oblique needles | Good reproducibility of preplanned needle positions, technique feasible |
| Logar et al., 2019 [20] | Prospective/9 pts | Primary and recurrent gyn tumours | Depending on tumour type (see text), compared to standard applicator | V100, D98, D90 and D100 for GTV and CTVHR higher compared to standard applicator |
| Kudla et al., 2023 [80] | Retrospective/10 pts | Primary and recurrent vaginal tumours | MVC with oblique needles, compared to transperineal implant | Shorter needle path with 3D applicator; Similar DVH parameters |
| Marar et al., 2022 [79] | Retrospective/70 pts | Cervical cancer | TARGIT add-on for T&O, compared to T&O | V100, D90, D98 for CTVHR higher with TARGIT, longer insertion time |
| Marar et al., 2023 [52] | Retrorospective/41 pts | Cervical cancer | TARGIT FX add-on for T&O, compared to TARGIT | V100, D90, D98 for CTVHR higher with TARGIT-FX Insertion time 30% shorter |
| Kang et al., 2021 [78] | Retrospective/28 pts | Gynaecological tumours | Template for seed insertion, compared to freehand | Better reproducibility of preplanned seed geometry |
| Sekii et al., 2018 [23] | Case report/2 pts | Vaginal tumours | MVC with oblique needles | Presenting workflow, reporting DVH parameters |
| Sethi et al., 2016 [61] | Case report/3 pts | Gynaecological tumours | Customised MVC | Favourable DVH parameters for target and OARs |
| Laan et al., 2019 [77] | Case report/2 pts | Recurrent gyn tumours | Personalised needle template | Presenting workflow and applicator modelling No DVH data |
| Lindegaard et al., 2016 [21] | Case report/1 pt | Cervical cancer | Tandem and 3D-printed ring-like template | Presenting workflow, applicator modelling and DVH parameters |
| Wiebe et al., 2015 [24] | Case report/1 pt | Postoperative endometrial cancer | Customised MVC, compared to standard single-chanel VC | V100, D90, D98 for CTV higher, V200 lower 13.2% better coverage |
| Sohn et al., 2022 [75] | Retrospective/5 pts | Cervical cancer | 3D vaginal template + T&O, compared to T&O + freehand needles | Better optimality, target coverage and OAR sparing |
| Qin et al., 2022 [84] | Prospective/9 pts | Recurrent cervical cancer | MVC with needles, compared to commercial single-channel VC | Planning aims achieved in all 3D print plans, but failed in 3 VC plans |
| Serban et al., 2021 [81] | Retrospective/20 pts | Cervical cancer | 3D vaginal template + T&R + freehand needles | CTVHR D90 93 Gy, D2cc bladder/rectum/sigmoid/bowel 78/65/59/61 Gy |
| Liao et al., 2022 [85] | Prospective/6 pts | Postoperative endometrial cancer | Template for VC fixation | Better reproducibility of VC position, less difference in D2cc btw fractions, non-significant |
| Zhang et al., 2019 [86] | Case report/3 pts | Cervical cancer | Customised IC/IS applicator, compared to standard applicator, inverse planning | Higher D90 for CTVHR, better OAR sparing compared to standard applicator |
| Liu et al., 2021 [87] | Retrospective/103 pts | Recurrent cervical cancer post-EBRT | Template for non-coplanar 125I seeds implantation | Safe, effective, minimally invasive, 1 > G2 acute, 2 > G2 late adverse events |
| Strengths | Limitations |
|---|---|
| Fast production of customised and complex forms | Additional steps needed (application, imaging, preplanning, modelling, QA/QC) |
| Allow complex geometry, oblique angles, non-coplanar needle distribution | New skills required, additional education |
| Shorter applications—less time in OR | Accuracy of 3D printers |
| Better position accuracy, favourable geometry | Materials not tested for repeated sterilisation |
| Better reproducibility, consistent placement | Limited possibility for post-sterilisation QA/QC |
| Higher dose to target volume—better local control | Material biocompatibility issues |
| Reducing dose to OARs | No guidelines for applicator manufacture, commissioning and QA/QC |
| Reducing patient discomfort | Applicator fixation issues |
| Better fit to patient’s anatomy | Potentially prolonged OTT |
| Possibility of shielded applicators | Potentially increased costs |
| Lack of good quality prospective clinical data |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Segedin, B.; Kobav, M.; Zobec Logar, H.B. The Use of 3D Printing Technology in Gynaecological Brachytherapy—A Narrative Review. Cancers 2023, 15, 4165. https://doi.org/10.3390/cancers15164165
Segedin B, Kobav M, Zobec Logar HB. The Use of 3D Printing Technology in Gynaecological Brachytherapy—A Narrative Review. Cancers. 2023; 15(16):4165. https://doi.org/10.3390/cancers15164165
Chicago/Turabian StyleSegedin, Barbara, Manja Kobav, and Helena Barbara Zobec Logar. 2023. "The Use of 3D Printing Technology in Gynaecological Brachytherapy—A Narrative Review" Cancers 15, no. 16: 4165. https://doi.org/10.3390/cancers15164165
APA StyleSegedin, B., Kobav, M., & Zobec Logar, H. B. (2023). The Use of 3D Printing Technology in Gynaecological Brachytherapy—A Narrative Review. Cancers, 15(16), 4165. https://doi.org/10.3390/cancers15164165





