Pretreatment Tumor Growth Rate and Radiological Response as Predictive Markers of Pathological Response and Survival in Patients with Resectable Lung Cancer Treated by Neoadjuvant Treatment
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Study Design
2.2. Radiological Assessment
2.3. Survival Endpoints
2.4. Pathological Endpoints
2.5. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Prognostic Factors Associated with EFS and OS
3.3. Predictors of Pathological Response after Neoadjuvant Treatment
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Thai, A.A.; Solomon, B.J.; Sequist, L.V.; Gainor, J.F.; Heist, R.S. Lung Cancer. Lancet 2021, 398, 535–554. [Google Scholar] [CrossRef] [PubMed]
- de Koning, H.J.; van der Aalst, C.M.; de Jong, P.A.; Scholten, E.T.; Nackaerts, K.; Heuvelmans, M.A.; Lammers, J.-W.J.; Weenink, C.; Yousaf-Khan, U.; Horeweg, N.; et al. Reduced Lung-Cancer Mortality with Volume CT Screening in a Randomized Trial. N. Engl. J. Med. 2020, 382, 503–513. [Google Scholar] [CrossRef] [PubMed]
- Pastorino, U.; Silva, M.; Sestini, S.; Sabia, F.; Boeri, M.; Cantarutti, A.; Sverzellati, N.; Sozzi, G.; Corrao, G.; Marchianò, A. Prolonged Lung Cancer Screening Reduced 10-Year Mortality in the MILD Trial: New Confirmation of Lung Cancer Screening Efficacy. Ann. Oncol. 2019, 30, 1162–1169. [Google Scholar] [CrossRef]
- Flores, R.; Patel, P.; Alpert, N.; Pyenson, B.; Taioli, E. Association of Stage Shift and Population Mortality Among Patients with Non–Small Cell Lung Cancer. JAMA Netw. Open 2021, 4, e2137508. [Google Scholar] [CrossRef]
- Raman, V.; Yang, C.-F.J.; Deng, J.Z.; D’Amico, T.A. Surgical Treatment for Early Stage Non-Small Cell Lung Cancer. J. Thorac. Dis. 2018, 10, S898–S904. [Google Scholar] [CrossRef]
- Cruz, C.; Afonso, M.; Oliveiros, B.; Pêgo, A. Recurrence and Risk Factors for Relapse in Patients with Non-Small Cell Lung Cancer Treated by Surgery with Curative Intent. Oncology 2017, 92, 347–352. [Google Scholar] [CrossRef] [PubMed]
- Fedor, D.; Johnson, W.R.; Singhal, S. Local Recurrence Following Lung Cancer Surgery: Incidence, Risk Factors, and Outcomes. Surg. Oncol. 2013, 22, 156–161. [Google Scholar] [CrossRef]
- Kelsey, C.R.; Marks, L.B.; Hollis, D.; Hubbs, J.L.; Ready, N.E.; D’Amico, T.A.; Boyd, J.A. Local Recurrence after Surgery for Early Stage Lung Cancer: An 11-Year Experience with 975 Patients. Cancer 2009, 115, 5218–5227. [Google Scholar] [CrossRef] [PubMed]
- Bria, E.; Gralla, R.J.; Raftopoulos, H.; Cuppone, F.; Milella, M.; Sperduti, I.; Carlini, P.; Terzoli, E.; Cognetti, F.; Giannarelli, D. Magnitude of Benefit of Adjuvant Chemotherapy for Non-Small Cell Lung Cancer: Meta-Analysis of Randomized Clinical Trials. Lung Cancer 2009, 63, 50–57. [Google Scholar] [CrossRef] [PubMed]
- NSCLC Meta-Analyses Collaborative Group. Adjuvant Chemotherapy, with or without Postoperative Radiotherapy, in Operable Non-Small-Cell Lung Cancer: Two Meta-Analyses of Individual Patient Data. Lancet 2010, 375, 1267–1277. [Google Scholar] [CrossRef]
- NSCLC Meta-Analysis Collaborative Group. Preoperative Chemotherapy for Non-Small-Cell Lung Cancer: A Systematic Review and Meta-Analysis of Individual Participant Data. Lancet 2014, 383, 1561–1571. [Google Scholar] [CrossRef]
- Forde, P.M.; Spicer, J.; Lu, S.; Provencio, M.; Mitsudomi, T.; Awad, M.M.; Felip, E.; Broderick, S.R.; Brahmer, J.R.; Swanson, S.J.; et al. Neoadjuvant Nivolumab plus Chemotherapy in Resectable Lung Cancer. N. Engl. J. Med. 2022, 386, 1973–1985. [Google Scholar] [CrossRef] [PubMed]
- Provencio, M.; Serna-Blasco, R.; Nadal, E.; Insa, A.; García-Campelo, M.R.; Casal Rubio, J.; Dómine, M.; Majem, M.; Rodríguez-Abreu, D.; Martínez-Martí, A.; et al. Overall Survival and Biomarker Analysis of Neoadjuvant Nivolumab Plus Chemotherapy in Operable Stage IIIA Non–Small-Cell Lung Cancer (NADIM Phase II Trial). J. Clin. Oncol. 2022, 40, 2924–2933. [Google Scholar] [CrossRef]
- Ferté, C.; Fernandez, M.; Hollebecque, A.; Koscielny, S.; Levy, A.; Massard, C.; Balheda, R.; Bot, B.; Gomez-Roca, C.; Dromain, C.; et al. Tumor Growth Rate Is an Early Indicator of Antitumor Drug Activity in Phase I Clinical Trials. Clin. Cancer Res. 2014, 20, 246–252. [Google Scholar] [CrossRef] [PubMed]
- Lamarca, A.; Ronot, M.; Moalla, S.; Crona, J.; Opalinska, M.; Lopez Lopez, C.; Pezzutti, D.; Najran, P.; Carvhalo, L.; Bezerra, R.O.F.; et al. Tumor Growth Rate as a Validated Early Radiological Biomarker Able to Reflect Treatment-Induced Changes in Neuroendocrine Tumors: The GREPONET-2 Study. Clin. Cancer Res. 2019, 25, 6692–6699. [Google Scholar] [CrossRef] [PubMed]
- Ramtohul, T.; Cohen, A.; Rodrigues, M.; Piperno-Neumann, S.; Cabel, L.; Cassoux, N.; Lumbroso-Le Rouic, L.; Malaise, D.; Gardrat, S.; Pierron, G.; et al. Tumor Growth Rate Improves Tumor Assessment and First-Line Systemic Treatment Decision-Making for Immunotherapy in Patients with Liver Metastatic Uveal Melanoma. Br. J. Cancer 2022, 127, 258–267. [Google Scholar] [CrossRef] [PubMed]
- Osorio, B.; Yegya-Raman, N.; Kim, S.; Simone II, C.B.; Theodorou Ross, C.; Deek, M.P.; Gaines, D.; Zou, W.; Lin, L.; Malhotra, J.; et al. Clinical Significance of Pretreatment Tumor Growth Rate for Locally Advanced Non-Small Cell Lung Cancer. Ann. Transl. Med. 2019, 7, 95. [Google Scholar] [CrossRef]
- Champiat, S.; Dercle, L.; Ammari, S.; Massard, C.; Hollebecque, A.; Postel-Vinay, S.; Chaput, N.; Eggermont, A.; Marabelle, A.; Soria, J.-C.; et al. Hyperprogressive Disease Is a New Pattern of Progression in Cancer Patients Treated by Anti-PD-1/PD-L1. Clin. Cancer Res. 2017, 23, 1920–1928. [Google Scholar] [CrossRef]
- Uprety, D.; Mandrekar, S.J.; Wigle, D.; Roden, A.C.; Adjei, A.A. Neoadjuvant Immunotherapy for NSCLC: Current Concepts and Future Approaches. J. Thorac. Oncol. 2020, 15, 1281–1297. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Zhang, X.; Li, H.; Chen, T.; Chen, C.; Zhou, Y.; Lin, Z.; Du, W.; Fang, W.; Yang, Y.; et al. Pre-Treatment Tumor Growth Rate Predicts Clinical Outcomes of Patients With Advanced Non-Small Cell Lung Cancer Undergoing Anti-PD-1/PD-L1 Therapy. Front. Oncol. 2021, 10, 621329. [Google Scholar] [CrossRef]
- Wagner, N.B.; Lenders, M.M.; Kühl, K.; Reinhardt, L.; André, F.; Dudda, M.; Ring, N.; Ebel, C.; Stäger, R.; Zellweger, C.; et al. Pretreatment Metastatic Growth Rate Determines Clinical Outcome of Advanced Melanoma Patients Treated with Anti-PD-1 Antibodies: A Multicenter Cohort Study. J. Immunother. Cancer 2021, 9, e002350. [Google Scholar] [CrossRef] [PubMed]
- Matsumura, S.; Kato, T.; Kujime, Y.; Kitakaze, H.; Nakano, K.; Hongo, S.; Yoshioka, I.; Okumi, M.; Nonomura, N.; Takada, S. Pre-Treatment Metastatic Growth Rate Is Associated with Clinical Outcome in Patients with Metastatic Renal Cell Carcinoma Treated with Nivolumab. BMC Urol. 2023, 23, 107. [Google Scholar] [CrossRef]
- Zhang, L.; Wu, L.; Chen, Q.; Zhang, B.; Liu, J.; Liu, S.; Mo, X.; Li, M.; Chen, Z.; Chen, L.; et al. Predicting Hyperprogressive Disease in Patients with Advanced Hepatocellular Carcinoma Treated with Anti-Programmed Cell Death 1 Therapy. EClinicalMedicine 2021, 31, 100673. [Google Scholar] [CrossRef]
- Peng, Y.; Mei, W.; Ma, K.; Zeng, C. Circulating Tumor DNA and Minimal Residual Disease (MRD) in Solid Tumors: Current Horizons and Future Perspectives. Front. Oncol. 2021, 11, 763790. [Google Scholar] [CrossRef] [PubMed]
- Pitt, J.M.; Marabelle, A.; Eggermont, A.; Soria, J.-C.; Kroemer, G.; Zitvogel, L. Targeting the Tumor Microenvironment: Removing Obstruction to Anticancer Immune Responses and Immunotherapy. Ann. Oncol. 2016, 27, 1482–1492. [Google Scholar] [CrossRef]
- Altorki, N.K.; Markowitz, G.J.; Gao, D.; Port, J.L.; Saxena, A.; Stiles, B.; McGraw, T.; Mittal, V. The Lung Microenvironment: An Important Regulator of Tumour Growth and Metastasis. Nat. Rev. Cancer 2019, 19, 9–31. [Google Scholar] [CrossRef] [PubMed]
- Rothschild, S.I.; Zippelius, A.; Eboulet, E.I.; Savic Prince, S.; Betticher, D.; Bettini, A.; Früh, M.; Joerger, M.; Lardinois, D.; Gelpke, H.; et al. SAKK 16/14: Durvalumab in Addition to Neoadjuvant Chemotherapy in Patients with Stage IIIA(N2) Non–Small-Cell Lung Cancer—A Multicenter Single-Arm Phase II Trial. J. Clin. Oncol. 2021, 39, 2872–2880. [Google Scholar] [CrossRef]
- Provencio, M.; Nadal, E.; González-Larriba, J.L.; Martínez-Martí, A.; Bernabé, R.; Bosch-Barrera, J.; Casal-Rubio, J.; Calvo, V.; Insa, A.; Ponce, S.; et al. Perioperative Nivolumab and Chemotherapy in Stage III Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2023, 389, 504–513. [Google Scholar] [CrossRef] [PubMed]
- Hellmann, M.D.; Chaft, J.E.; William, W.N.; Rusch, V.; Pisters, K.M.W.; Kalhor, N.; Pataer, A.; Travis, W.D.; Swisher, S.G.; Kris, M.G. Pathological Response after Neoadjuvant Chemotherapy in Resectable Non-Small-Cell Lung Cancers: Proposal for the Use of Major Pathological Response as a Surrogate Endpoint. Lancet Oncol. 2014, 15, e42–e50. [Google Scholar] [CrossRef]
- Rosner, S.; Liu, C.; Forde, P.M.; Hu, C. Association of Pathologic Complete Response and Long-Term Survival Outcomes Among Patients Treated with Neoadjuvant Chemotherapy or Chemoradiotherapy for NSCLC: A Meta-Analysis. JTO Clin. Res. Rep. 2022, 3, 100384. [Google Scholar] [CrossRef]
- Pataer, A.; Weissferdt, A.; Vaporciyan, A.A.; Correa, A.M.; Sepesi, B.; Wistuba, I.I.; Heymach, J.V.; Cascone, T.; Swisher, S.G. Evaluation of Pathologic Response in Lymph Nodes of Patients with Lung Cancer Receiving Neoadjuvant Chemotherapy. J. Thorac. Oncol. 2021, 16, 1289–1297. [Google Scholar] [CrossRef] [PubMed]
- Huynh, C.; Walsh, L.A.; Spicer, J.D. Surgery after Neoadjuvant Immunotherapy in Patients with Resectable Non-Small Cell Lung Cancer. Transl. Lung Cancer Res. 2021, 10, 563–580. [Google Scholar] [CrossRef] [PubMed]
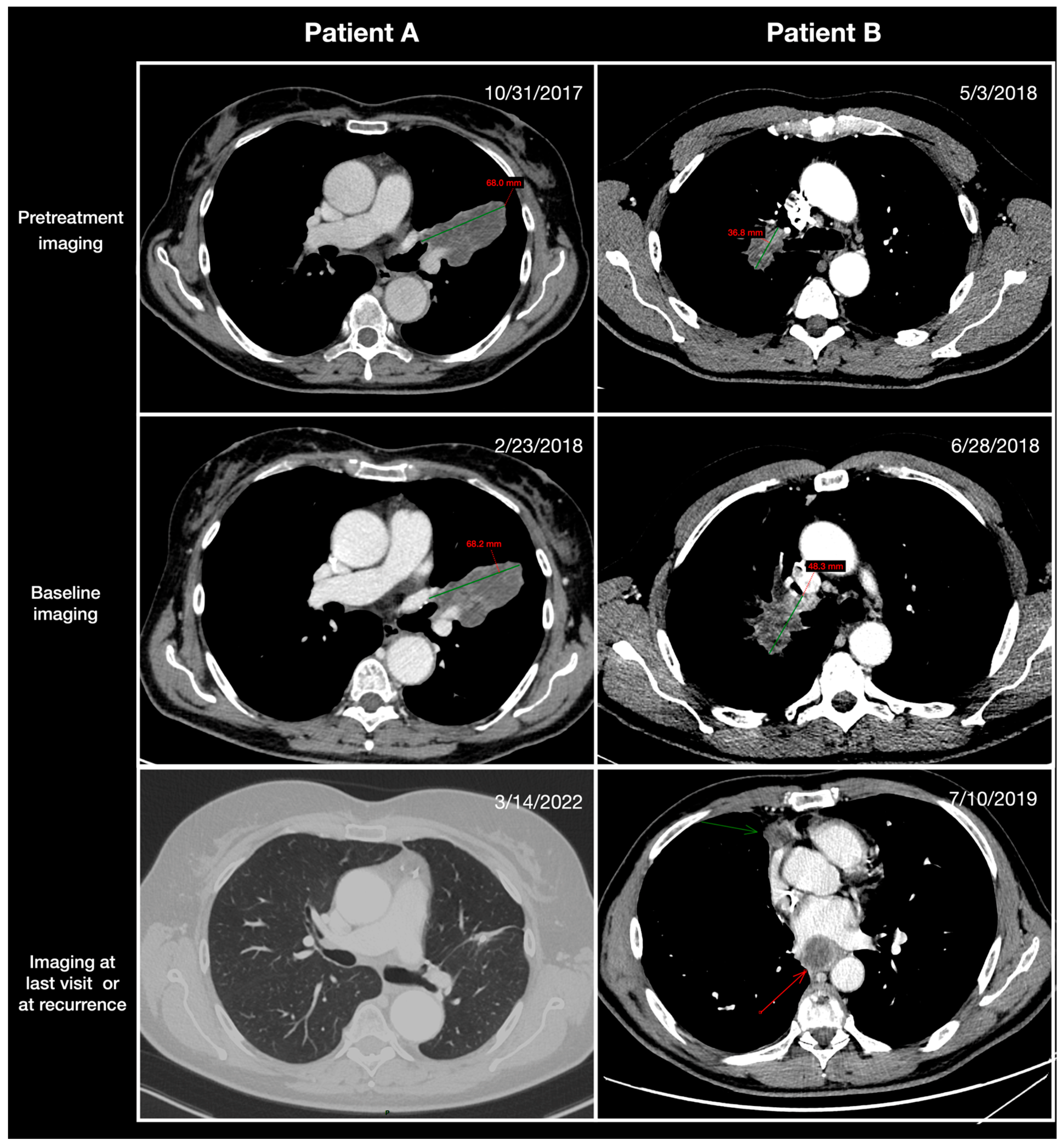
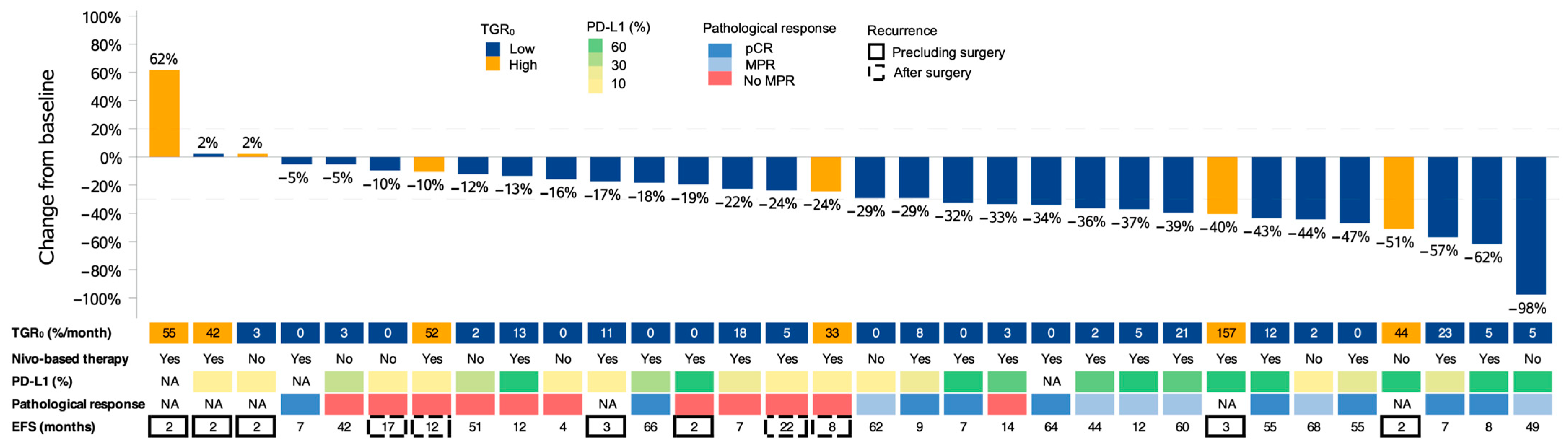
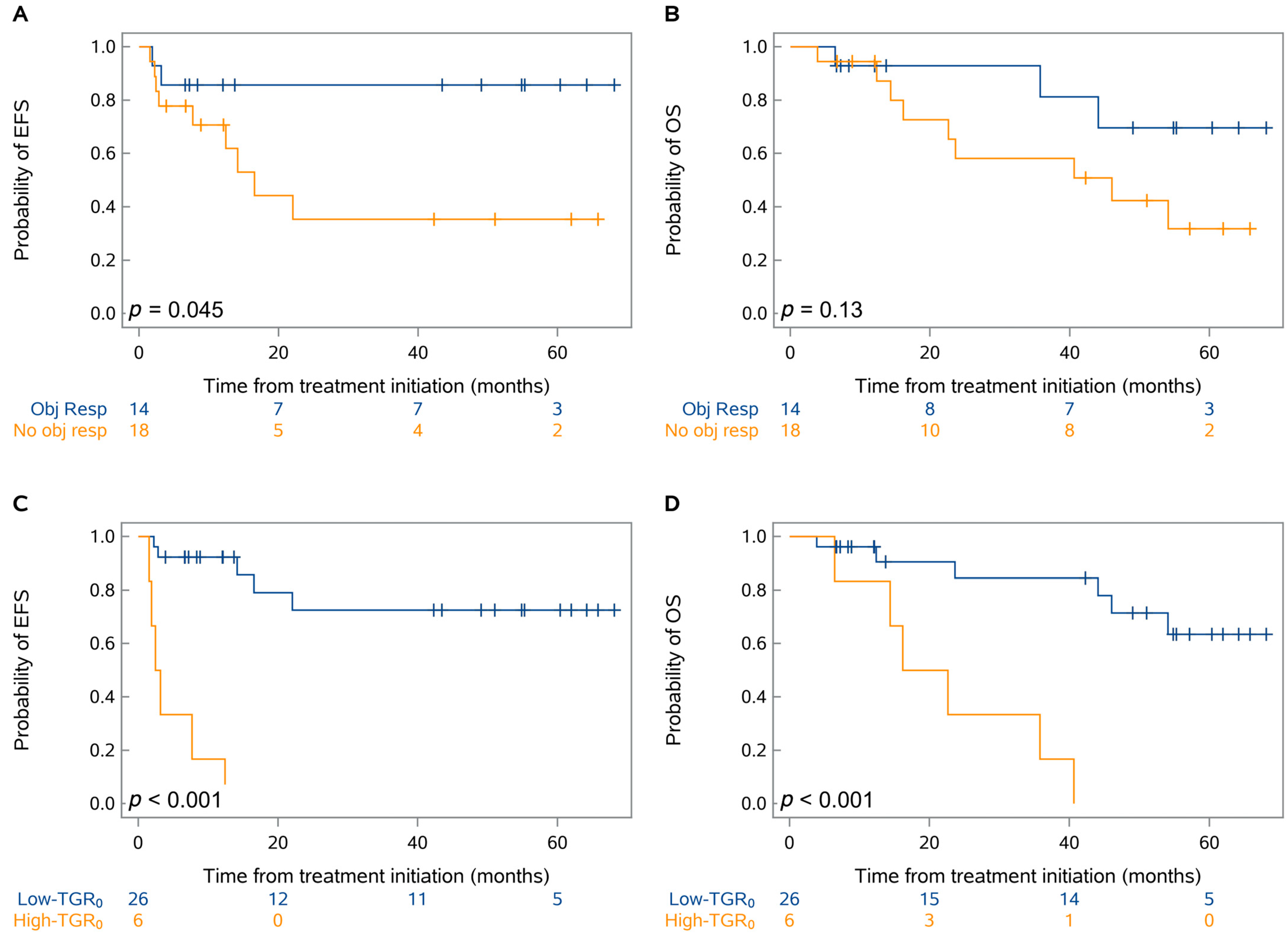
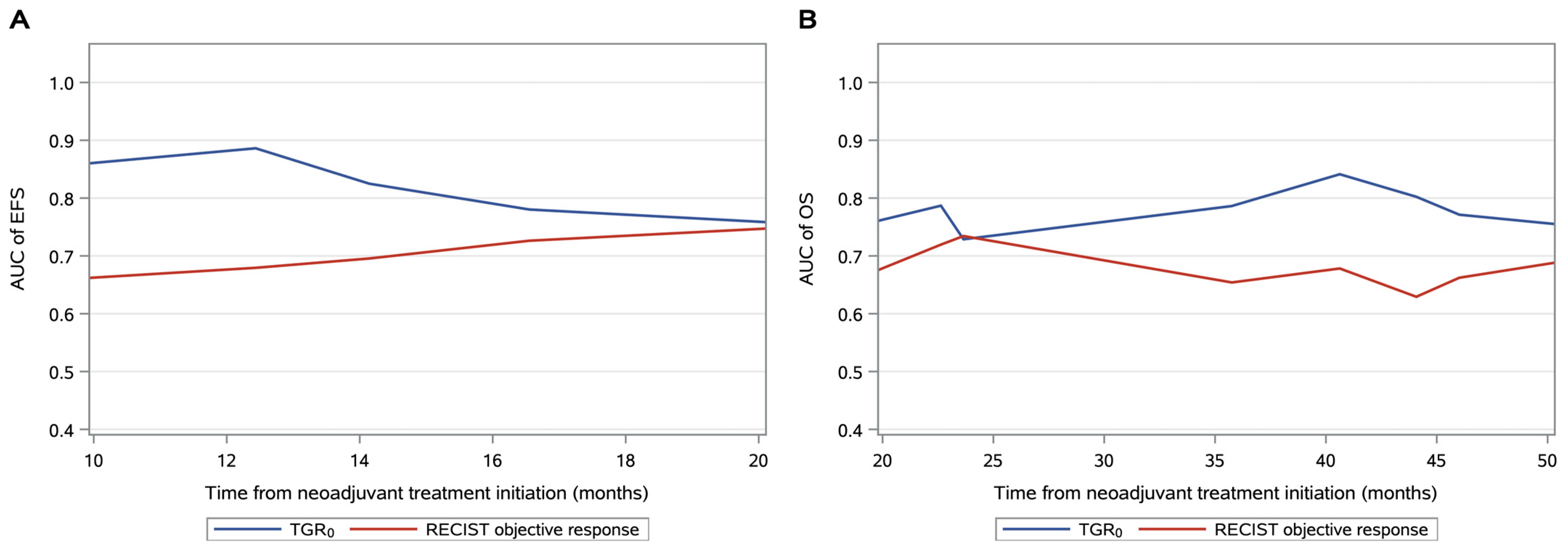

| Variables | Whole Cohort | Pretreatment TGR0 | p-Value | ||
|---|---|---|---|---|---|
| (n = 32) | Low (n = 26) | High (n = 6) | |||
| Age (years) | ≤60 | 11 (34%) | 7 (27%) | 4 (67%) | 0.07 |
| >60 | 21 (66%) | 19 (73%) | 2 (33%) | ||
| Sex | Female | 10 (31%) | 8 (31%) | 2 (33%) | 0.90 |
| Male | 22 (69%) | 18 (69%) | 4 (67%) | ||
| ECOG performance status | 0 | 27 (84%) | 22 (85%) | 5 (83%) | 0.94 |
| 1 | 5 (16%) | 4 (15%) | 1 (17%) | ||
| Smoking status | Current or former smoker | 29 (91%) | 24 (92%) | 5 (83%) | 0.50 |
| Never smoked | 3 (9%) | 2 (8%) | 1 (17%) | ||
| Histologic type | Non-squamous | 24 (75%) | 22 (85%) | 2 (33%) | 0.009 |
| Squamous | 8 (25%) | 4 (15%) | 4 (67%) | ||
| PD-L1 status (%) * | ≤10 | 11 (37%) | 7 (29%) | 4 (67%) | 0.09 |
| >10 | 19 (63%) | 17 (71%) | 2 (33%) | ||
| Disease stage | Ib or II | 5 (16%) | 2 (8%) | 3 (50%) | 0.01 |
| IIIa | 27 (84%) | 24 (92%) | 3 (50%) | ||
| Largest tumor size at baseline (mm) | ≤50 | 19 (59%) | 16 (62%) | 3 (50%) | 0.60 |
| >50 | 13 (41%) | 10 (38%) | 3 (50%) | ||
| Nodal stage | N1/2 | 28 (88%) | 23 (88%) | 5 (83%) | 0.73 |
| N0 | 4 (13%) | 3 (12%) | 1 (17%) | ||
| Nivolumab-based neoadjuvant treatment | Present | 23 (72%) | 18 (69%) | 5 (83%) | 0.49 |
| Absent | 9 (28%) | 8 (31%) | 1 (17%) | ||
| RECIST objective response | Present | 14 (44%) | 12 (46%) | 2 (33%) | 0.57 |
| Absent | 18 (56%) | 14 (54%) | 4 (67%) | ||
| Major pathological response | Present | 15 (58%) | 15 (63%) | 0 (0%) | 0.09 |
| Absent | 11 (42%) | 9 (38%) | 2 (100%) | ||
| Variable | Univariable Analysis of EFS | Multivariable Analysis of EFS | ||
|---|---|---|---|---|
| HR (95% CI) | p-Value | HR (95% CI) | p-Value | |
| Age (years), >60 vs. ≤60 | 0.6 (0.2–1.8) | 0.33 | ||
| Sex, female vs. male | 0.8 (0.2–3.2) | 0.80 | ||
| Smoking status, never smoked vs. current or former smoker | 4.7 (0.9–25.9) | 0.07 | ||
| Histologic type, non-squamous vs. squamous | 0.3 (0.1–0.9) | 0.047 | 0.6 (0.1–4.2) | 0.58 |
| PD-L1 (%), >10 vs. ≤10 | 0.2 (0.1–0.7) | 0.01 | 0.3 (0.1–1.4) | 0.13 |
| Disease stage, IIIa vs. Ib/II | 0.3 (0.1–0.9) | 0.04 | 5.2 (0.5–58.9) | 0.18 |
| Largest tumor size at baseline (mm), >50 vs. ≤50 | 0.8 (0.2–2.9) | 0.78 | ||
| Nodal stage, N1/2 vs. N0 | 1.2 (0.1–9.3) | 0.88 | ||
| Nivolumab-based treatment, present vs. absent | 1.3 (0.3–4.9) | 0.72 | ||
| RECIST objective response, present vs. absent | 0.2 (0.1–1.1) | 0.07 | ||
| TGR0 (%/month), ≤30 vs. >30 | 0.04 (0.01–0.2) | <0.001 | 0.04 (0.01–0.3) | 0.003 |
| Variable | Univariable Analysis of OS | Multivariable Analysis of OS | ||
|---|---|---|---|---|
| HR (95% CI) | p-Value | HR (95% CI) | p-Value | |
| Age (years), >60 vs. ≤60 | 0.9 (0.3–3.1) | 0.90 | ||
| Sex, female vs. male | 1.2 (0.4–4.0) | 0.76 | ||
| Smoking status, never smoked vs. current or former smoker | - | >0.99 | ||
| Histologic type, non-squamous vs. squamous | 0.4 (0.1–1.2) | 0.10 | ||
| PD-L1 (%), >10 vs. ≤10 | 0.2 (0.1–0.8) | 0.02 | 0.4 (0.1–1.6) | 0.19 |
| Disease stage, IIIa vs. Ib/II | 0.2 (0.1–0.7) | 0.02 | 0.5 (0.1–1.9) | 0.29 |
| Largest tumor size at baseline (mm), >50 vs. ≤50 | 1.0 (0.3–3.1) | 0.97 | ||
| Nodal stage, N1/2 vs. N0 | 0.8 (0.1–6.2) | 0.82 | ||
| Nivolumab-based treatment, present vs. absent | 1.2 (0.4–4.1) | 0.76 | ||
| RECIST objective response, present vs. absent | 0.4 (0.1–1.4) | 0.15 | ||
| TGR0 (%/month), ≤30 vs. >30 | 0.1 (0.02–0.4) | 0.001 | 0.2 (0.03–0.7) | 0.01 |
| Variable | Univariable Analysis of MPR | Multivariable Analysis of MPR | ||
|---|---|---|---|---|
| OR (95% CI) | p-Value | OR (95% CI) | p-Value | |
| Age (years), >60 vs. ≤60 | 1.1 (0.2–5.8) | 0.87 | ||
| Sex, female vs. Male | 0.4 (0.1–2.3) | 0.32 | ||
| Smoking status, never smoked vs. current or former smoker | - | >0.99 | ||
| Histologic type, non-squamous vs. squamous | 1.5 (0.2, 9.4) | 0.66 | ||
| PD-L1 (%), >10 vs. ≤10 | 3.1 (0.6–17.3) | 0.20 | ||
| Disease stage, IIIa vs. Ib/II | 5.2 (0.5–59.3) | 0.18 | ||
| Largest tumor size at baseline (mm), >50 vs. ≤50 | 3.9 (0.6–24.7) | 0.14 | ||
| Nodal stage, N1/2 vs. N0 | 0.7 (0.1–8.2) | 0.74 | ||
| Nivolumab-based treatment, present vs. absent | 2.3 (0.4–13.3) | 0.36 | ||
| RECIST objective response, present vs. absent | 27.5 (2.6–289.1) | 0.006 | 27.5 (2.6–289.1) | 0.006 |
| TGR0 (%/month), ≤30 vs. >30 | - | >0.99 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramtohul, T.; Challier, L.; Servois, V.; Girard, N. Pretreatment Tumor Growth Rate and Radiological Response as Predictive Markers of Pathological Response and Survival in Patients with Resectable Lung Cancer Treated by Neoadjuvant Treatment. Cancers 2023, 15, 4158. https://doi.org/10.3390/cancers15164158
Ramtohul T, Challier L, Servois V, Girard N. Pretreatment Tumor Growth Rate and Radiological Response as Predictive Markers of Pathological Response and Survival in Patients with Resectable Lung Cancer Treated by Neoadjuvant Treatment. Cancers. 2023; 15(16):4158. https://doi.org/10.3390/cancers15164158
Chicago/Turabian StyleRamtohul, Toulsie, Léa Challier, Vincent Servois, and Nicolas Girard. 2023. "Pretreatment Tumor Growth Rate and Radiological Response as Predictive Markers of Pathological Response and Survival in Patients with Resectable Lung Cancer Treated by Neoadjuvant Treatment" Cancers 15, no. 16: 4158. https://doi.org/10.3390/cancers15164158
APA StyleRamtohul, T., Challier, L., Servois, V., & Girard, N. (2023). Pretreatment Tumor Growth Rate and Radiological Response as Predictive Markers of Pathological Response and Survival in Patients with Resectable Lung Cancer Treated by Neoadjuvant Treatment. Cancers, 15(16), 4158. https://doi.org/10.3390/cancers15164158





