Leukemic Involvement Is a Common Feature in Waldenström Macroglobulinemia at Diagnosis
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Samples
2.2. Morphologic, Immunophenotypic, and Molecular Studies
2.3. Statistical Analysis
3. Results
3.1. Patients Characteristics
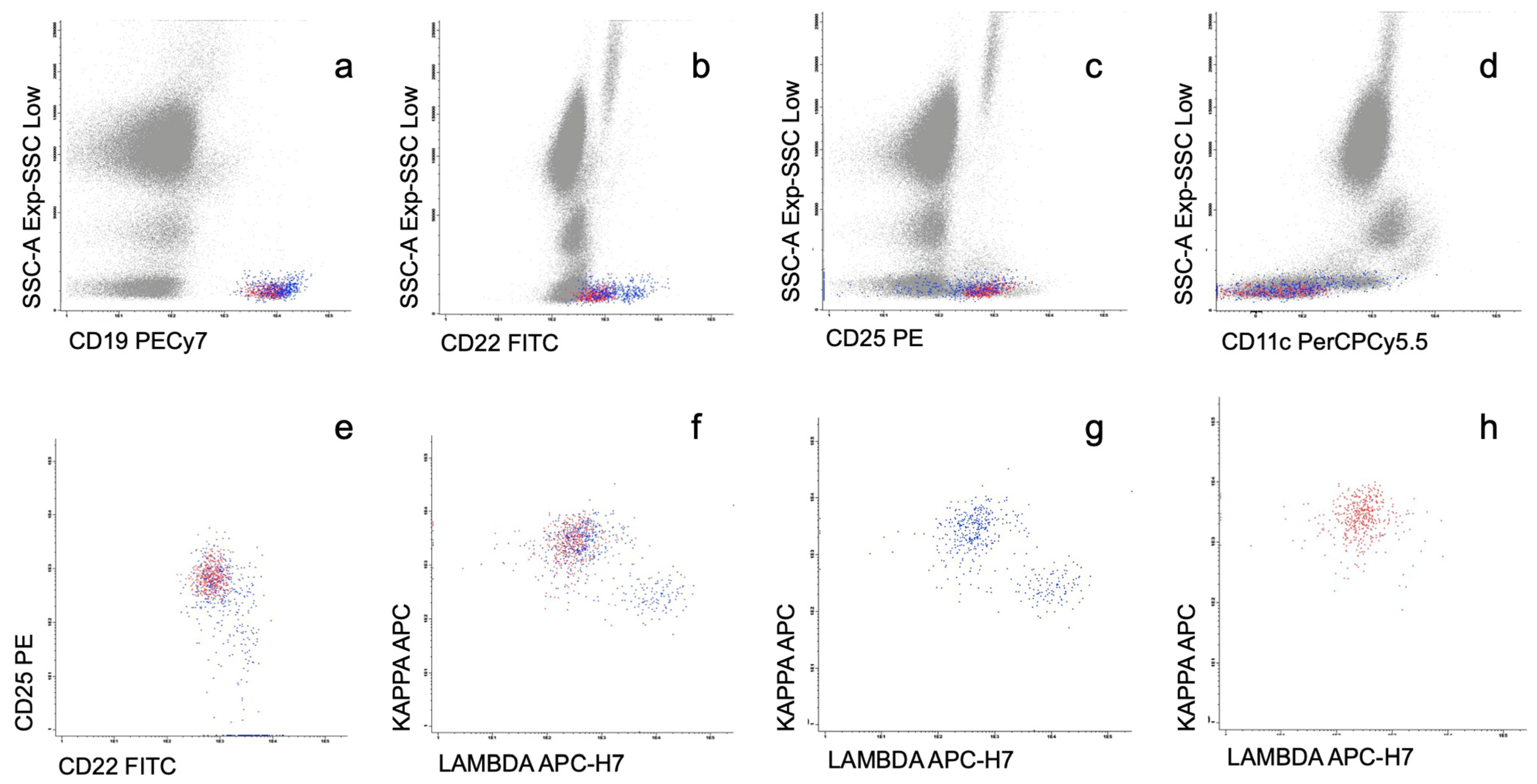
3.2. Phenotypic Characteristics of Circulating WM Cells
3.3. Morphologic Features in WM with PB Involvement
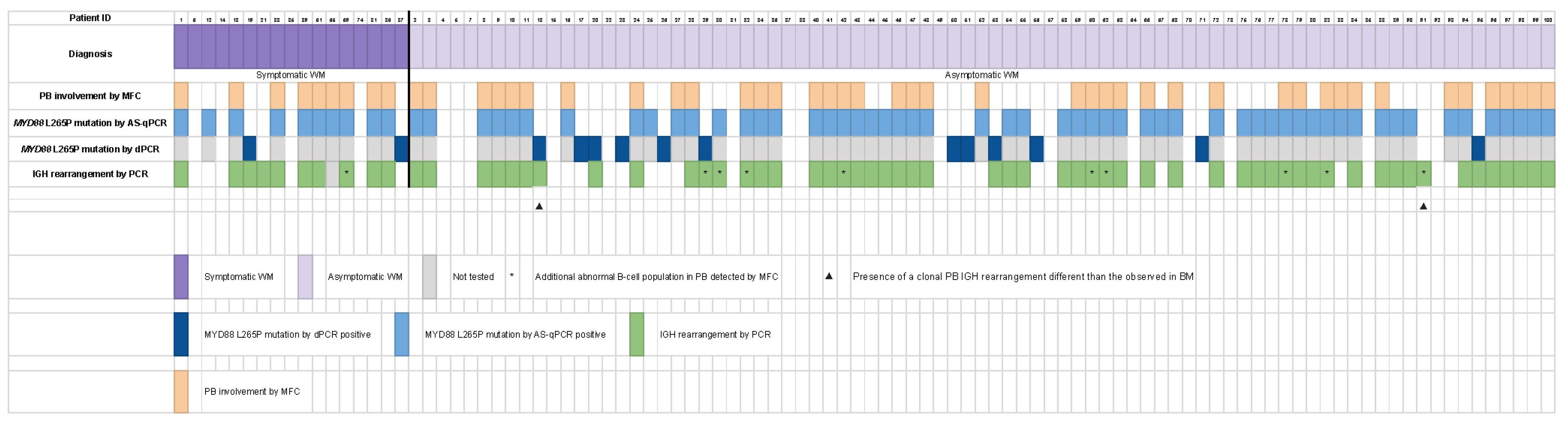
3.4. Molecular Data in Patients with and without Leukemic Expression by MFC
3.5. Correlation between Leukemic Expression, Clinical and Analytical Data, and Bone Marrow Features
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Swerdlow, S.H.; Campo, E.; Harris, N.L.; Jaffe, E.S.; Pileri, S.A.; Stein, H.; Thiele, J. (Eds.) WHO Classification of Tumours of Hematopoietic and Lymphoid Tissues, 4th ed.; IARC: Lyon, France, 2017. [Google Scholar]
- Alaggio, R.; Amador, C.; Anagnostopoulos, I.; Attygalle, A.D.; Barreto de Oliveira Araujo, I.; Berti, E.; Bhagat, G.; Borges, A.M.; Boyer, D.; Calaminici, M.; et al. The 5th edition of the World Health Organization Classification of Haematolymphoid Tumours: Lymphoid Neoplasms. Leukemia 2022, 36, 1720–1748. [Google Scholar] [CrossRef] [PubMed]
- Campo, E.; Jaffe, E.S.; Cook, J.R.; Quintanilla-Martinez, L.; Swerdlow, S.H.; Anderson, K.C.; Brousset, P.; Cerroni, L.; de Leval, L.; Dirnhofer, S.; et al. The International Consensus Classification of Mature Lymphoid Neoplasms: A report from the Clinical Advisory Committee. Blood 2022, 140, 1229–1253. [Google Scholar] [CrossRef] [PubMed]
- Kyle, R.A.; Benson, J.T.; Larson, D.R.; Therneau, T.M.; Dispenzieri, A.; Kumar, S.; Melton, L.J., III; Rajkumar, S.V. Progression in smoldering Waldenström macroglobulinemia: Long-term results. Blood 2012, 119, 4462–4466. [Google Scholar] [CrossRef] [PubMed]
- Bustoros, M.; Sklavenitis-Pistofidis, R.; Kapoor, P.; Liu, C.; Kastritis, E.; Zanwar, S.; Fell, G.; Abeykoon, J.P.; Hornburg, K.; Neuse, C.J.; et al. Progression Risk Stratification of Asymptomatic Waldenström Macroglobulinemia. J. Clin. Oncol. 2019, 37, 1403–1411. [Google Scholar] [CrossRef] [PubMed]
- Owen, R.G.; Treon, S.P.; Al-Katib, A.; Fonseca, R.; Greipp, P.R.; McMaster, M.L.; Morra, E.; Pangalis, G.A.; San Miguel, J.F.; Branagan, A.R.; et al. Clinicopathological definition of Waldenstrom’s macroglobulinemia: Consensus panel recommendations from the Second International Workshop on Waldenstrom’s Macroglobulinemia. Semin. Oncol. 2003, 30, 110–115. [Google Scholar] [CrossRef]
- Bassarova, A.; Trøen, G.; Spetalen, S.; Micci, F.; Tierens, A.; Delabie, J. Lymphoplasmacytic lymphoma and marginal zone lymphoma in the bone marrow: Paratrabecular involvement as an important distinguishing feature. Am. J. Clin. Pathol. 2015, 43, 797–806. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Reyero, J.; Martinez Magunacelaya, N.; Gonzalez de Villambrosia, S.; Gomez Mediavilla, A.; Urquieta Lam, M.; Insunza, A.; Tonda, R.; Beltran, S.; Gut, M.; Gonzalez, A.; et al. Diagnostic value of bone marrow core biopsy patterns in lymphoplasmacytic lymphoma/Waldenström macroglobulinaemia and description of its mutational profiles by targeted NGS. J. Clin. Pathol. 2020, 73, 571–577. [Google Scholar] [CrossRef]
- Ocio, E.M.; del Carpio, D.; Caballero, A.; Alonso, J.; Paiva, B.; Pesoa, R.; Villaescusa, T.; López-Anglada, L.; Vidriales, B.; García-Sanz, R. Differential Diagnosis of IgM MGUS and WM According to B-Lymphoid Infiltration by Morphology and Flow Cytometry. Clin. Lymphoma Myeloma Leuk. 2011, 11, 93–95. [Google Scholar] [CrossRef]
- Rosado, F.G.; Morice, W.G.; He, R.; Howard, M.T.; Timm, M.; McPhail, E. Immunophenotypic features by multiparameter flow cytometry can help distinguish low grade B-cell lymphomas with plasmacytic differentiation from plasma cell proliferative disorders with an unrelated clonal B-cell process. Br. J. Haematol. 2015, 169, 368–376. [Google Scholar] [CrossRef]
- Morice, W.G.; Chen, D.; Kurtin, P.J.; Hanson, C.A.; McPhail, E.D. Novel immunophenotypic features of marrow lymphoplasmacytic lymphoma and correlation with Waldenström’s macroglobulinemia. Mod. Pathol. 2009, 22, 807–816. [Google Scholar] [CrossRef]
- San Miguel, J.F.; Vidriales, M.B.; Ocio, E.; Mateo, G.; Sánchez-Guijo, F.; Sánchez, M.L.; Escribano, L.; Bárez, A.; Moro, M.J.; Hernández, J.; et al. Immunophenotypic Analyisis of Waldenstrom’s Macroglobulinemia. Semin. Oncol. 2003, 30, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Paiva, B.; Montes, M.C.; García-Sanz, R.; Ocio, E.M.; Alonso, J.; de las Heras, N.; Escalante, F.; Cuello, R.; de Coca, A.G.; Galende, J.; et al. Multiparameter flow cytometry for the identification of the Waldenström’s clone in IgM-MGUS and Waldenström’s Macroglobulinemia: New criteria for differential diagnosis and risk stratification. Leukemia. 2014, 28, 166–173. [Google Scholar] [CrossRef]
- Hunter, Z.R.; Branagan, A.R.; Manning, R.; Patterson, C.J.; Santos, D.D.; Torunilhac, O.; Dorfman, D.M.; Treon, S.P. CD5, CD10, and CD23 Expression in Waldenström’s Macroglobulinemia. Clin. Lymphoma 2005, 5, 246–249. [Google Scholar] [CrossRef] [PubMed]
- Konoplev, S.; Medeiros, L.J.; Bueso-Ramos, C.E.; Jorgensen, J.L.; Lin, P. Immunophenotypic profile of lymphoplasmacytic lymphoma/Waldenström macroglobulinemia. Am. J. Clin. Pathol. 2005, 124, 414–420. [Google Scholar] [CrossRef] [PubMed]
- Gascue, A.; Merino, J.; Paiva, B. Flow Cytometry. Hematol. Oncol. Clin. N. Am. 2018, 32, 765–775. [Google Scholar] [CrossRef] [PubMed]
- Harmon, C.M.; Smith, L.B. B-cell Non-Hodgkin Lymphomas with Plasmacytic Differentiation. Surg. Pathol. Clin. 2016, 9, 11–28. [Google Scholar] [CrossRef] [PubMed]
- Poulain, S.; Roumier, C.; Decambron, A.; Renneville, A.; Herbaux, C.; Bertrand, E.; Tricot, S.; Daudignon, A.; Galiègue-Zouitina, S.; Soenen, V.; et al. MYD88 L265P mutation in Waldenstrom macroglobulinemia. Blood 2013, 121, 4504–4511. [Google Scholar] [CrossRef]
- Treon, S.P.; Xu, L.; Yang, G.; Zhou, Y.; Liu, X.; Cao, Y.; Sheehy, P.; Manning, R.J.; Patterson, C.J.; Tripsas, C.; et al. MYD88 L265P Somatic Mutation in Waldenström’s Macroglobulinemia. N. Eng. J. Med. 2012, 367, 826–833. [Google Scholar] [CrossRef] [PubMed]
- Swerdlow, S.H.; Kuzu, I.; Dogan, A.; Dirnhofer, S.; Chan, J.K.C.; Sander, B.; Ott, G.; Xerri, L.; Quintanilla-Martinez, L.; Campo, E. The many faces of small B cell lymphomas with plasmacytic differentiation and the contribution of MYD88 testing. Virchows Arch. 2016, 468, 259–275. [Google Scholar] [CrossRef]
- Insuasti-Beltran, G.; Gale, J.M.; Wilson, C.S.; Foucar, K.; Czuchlewski, D.R. Significance of MYD88L265P Mutation Status in the Subclassification of Low-Grade B-Cell Lymphoma/Leukemia. Arch. Pathol. Lab. Med. 2015, 139, 1035–1041. [Google Scholar] [CrossRef]
- Varettoni, M.; Arcaini, L.; Zibellini, S.; Boveri, E.; Rattotti, S.; Riboni, R.; Corso, A.; Orlandi, E.; Bonfichi, M.; Gotti, M.; et al. Prevalence and clinical significance of the MYD88 (L265P) somatic mutation in Waldenström’s macroglobulinemia and related lymphoid neoplasms. Blood 2013, 121, 2522–2528. [Google Scholar] [CrossRef]
- Jiménez, C.; Chillón, M.C.; Balanzategui, A.; Puig, N.; Sebastián, E.; Alcoceba, M.; Sarasquete, M.E.; Conde, I.P.; Corral, R.; Marín, L.A.; et al. Detection of MYD88 L265P mutation by real-time allele-specific oligonucleotide polymerase chain reaction. Appl. Immunohistochem. Mol. Morphol. 2014, 22, 768–773. [Google Scholar] [CrossRef]
- Xu, Y.; Hunter, Z.R.; Yang, G.; Zhou, Y.; Cao, Y.; Liu, X.; Morra, E.; Trojani, A.; Greco, A.; Arcaini, L.; et al. MYD88 L265P in Waldenström’s macroglobulinemia, IgM monoclonal gammopathy, and other B-cell lymphoproliferative disorders using conventional and quantitative allele-specific polymerase chain reaction. Blood 2013, 121, 2051–2058. [Google Scholar] [CrossRef]
- van Dongen, J.J.M.; Langerak, A.W.; Brüggemann, M.; Evans, P.A.S.; Hummel, M.; Lavender, F.L.; Delabesse, E.; Davi, F.; Schuuring, E.; García-Sanz, R.; et al. Design and standardization of PCR primers and protocols for detection of clonal immunoglobulin and T-cell receptor gene recombinations in suspect lymphoproliferations: Report of the BIOMED-2 Concerted Action BMH4-CT98-3936. Leukemia 2003, 17, 2257–2317. [Google Scholar] [CrossRef]
- Ferrante, M.; Furlan, D.; Zibellini, S.; Borriero, M.; Candido, C.; Sahnane, N.; Uccella, S.; Genuardi, E.; Alessandria, B.; Bianchi, B.; et al. MYD88 L265P Detection in IgM Monoclonal Gammopathies: Methodological Considerations for Routine Implementation. Diagnostics 2021, 11, 779. [Google Scholar] [CrossRef]
- Drandi, D.; Genuardi, E.; Dogliotti, I.; Ferrante, M.; Jiménez, C.; Guerrini, F.; Lo Schirico, M.; Mantoan, B.; Muccio, V.; Lia, G.; et al. Highly sensitive MYD88 L265P mutation detection by droplet digital PCR in Waldenström Macroglobulinemia. Haematologica 2018, 103, 1029–1037. [Google Scholar] [CrossRef]
- Molina, T.J.; Lin, P.; Swerdlow, S.H.; Cook, J.R. Marginal Zone Lymphomas with Plasmacytic Differentiation and Related Disorders. Am. J. Clin. Pathol. 2011, 136, 211–225. [Google Scholar] [CrossRef]
- Ocio, E.M.; Hernández, J.M.; Mateo, G.; Sánchez, M.L.; González, B.; Vidriales, B.; Gutiérrez, N.C.; Orfao, A.; San Miguel, J.F. Immunophenotypic and cytogenetic comparison of Waldenstrom’s macroglobulinemia with splenic marginal zone lymphoma. Clin. Lymphoma 2005, 5, 241–245. [Google Scholar] [CrossRef]
- Salido, M.; Baró, C.; Oscier, D.; Stamatopoulos, K.; Dierlamm, J.; Matutes, E.; Traverse-Glehen, A.; Berger, F.; Felman, P.; Thieblemont, C.; et al. Cytogenetic aberrations and their prognostic value in a series of 330 splenic marginal zone B-cell lymphomas: A multicenter study of the Splenic B-Cell Lymphoma Group. Blood 2010, 116, 1479–1488. [Google Scholar] [CrossRef]
- Ocio, E.M.; Schop, R.F.J.; Gonzalez, B.; Van Wier, S.A.; Hernandez-Rivas, J.M.; Gutierrez, N.C.; Garcia-Sanz, R.; Moro, M.J.; Aguilera, C.; Hernandez, J.; et al. 6q deletion in Waldenström macroglobulinemia is associated with features of adverse prognosis. Br. J. Haematol. 2007, 136, 80–86. [Google Scholar] [CrossRef]
- Eichhorst, B.; Robak, T.; Montserrat, E.; Ghia, P.; Niemann, C.U.; Kater, A.P.; Gregor, M.; Cymbalista, F.; Buske, C.; Hillmen, P.; et al. Chronic lymphocytic leukaemia: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2021, 32, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Hunter, Z.R.; Yang, G.; Cao, Y.; Liu, X.; Manning, R.; Tripsas, C.; Chen, J.; Patterson, C.J.; Kluk, L.; et al. Detection of MYD88 L265P in peripheral blood of patients with Waldenström’s Macroglobulinemia and IgM monoclonal gammopathy of undetermined significance. Leukemia 2014, 28, 1698–1704. [Google Scholar] [CrossRef] [PubMed]




| All Patients N = 100 | Patients with PB Involvement by MFC N = 50 (50%) | Patients without PB Involvement by MFC N = 50 (50%) | Significance (p) | |
|---|---|---|---|---|
| Median age, years (range, IQR) | 73 (36–97) (IQR: 12.75) | 73 (54–89) (IQR: 14) | 73.5 (36–97) (IQR: 12.5) | p = 0.303 2 |
| Sex female/male | 69.5% | 78.6% | 61.3% | p = 0.685 1 |
| ECOG > 1 | 3% | 2% | 4% | p = 1.000 1 |
| Lymphadenopathy (by physical examination and/or CT scan) | 8% | 8% | 8% | p = 1.000 1 |
| Splenomegaly (by physical examination and/or CT scan) | 1% | 0% | 2% | p = 1.000 1 |
| Leukocytes (×109/L), median (range, IQR) | 7.08 (3.37–17.58) (IQR: 3.10) | 7.29 (3.97–17.58) (IQR: 3.08) | 6.93 (3.37–17.53) (IQR: 3.63) | p = 0.135 2 |
| Lymphocytes (×109/L), median (range, IQR) | 2.00 (0.61–8.81) (IQR: 0.97) | 2.3 (0.77–7.28) (IQR: 1.09) | 1.87 (0.61–8.81) (IQR: 0.89) | p = 0.110 2 |
| Lymphocytes ≥ 4 × 109/L | 6% | 8% | 4% | p = 0.678 1 |
| Hemoglobin (g/L), median (range, IQR) | 133 (82–178) (IQR: 23.75) | 135 (82–167) (IQR: 20.00) | 131 (88–178) (IQR: 26.25) | p = 0.701 2 |
| Platelets (×109/L), median (range, IQR) | 247 (110–669) (IQR: 104.75) | 254 (110–503) (IQR: 109.00) | 243 (124–669) (IQR: 101.25) | p = 0.849 2 |
| Increased serum LDH levels | 5% | 2% | 8% | p = 0.362 1 |
| Increased serum β2-microglobulin levels | 40.8% | 50% | 32% | p = 0.100 1 |
| Serum monoclonal component (g/L), median (range, IQR) | 12.72 (1.89–72.80) (IQR: 6.36) | 12.36 (5.93–43.50) (IQR: 5.38) | 13.63 (1.89–72.80) (IQR: 7.30) | p = 0.397 2 |
| Serum monoclonal component ≥ 15 g/L | 33% | 26% | 40% | p = 0.202 1 |
| Serum monoclonal component ≥ 30 g/L | 5% | 6% | 4% | p = 1.000 1 |
| Positive urine immunofixation | 25% | 30.3% | 19.3% | p = 0.550 1 |
| PB involvement by MFC | 50% | - | - | - |
| Median % CD19 from total lymphocytes in PB (range, IQR) | 6.00 (0.6–47) (IQR: 6.75) | 7.5 (0.6–47) (IQR: 11.10) | 5.00 (0.7–32) (IQR: 4.70) | p = 0.001 2 |
| Median % clonal CD19 from total CD19 in PB (range, IQR) | - | 60 (8–99) (IQR: 60) | - | - |
| CD5 expression in PB in leukemic cases | - | 24% | - | - |
| Median % CD19 from total lymphocytes in BM (range, IQR) | 34 (4–76) (IQR: 25) | 35 (5–76) (IQR: 22) | 23,50 (4–60) (IQR: 23) | p = 0.006 2 |
| Median % clonal CD19 from total CD19 in BM (range, IQR) | 80 (0–100) (IQR: 45) | 90 (6–100) (IQR: 34) | 70 (0–100) (IQR: 52) | p = 0.046 2 |
| BM lymphocytes, % (range) | 22 (8–91) | 28 (8–91) | 18 (10–47) | p = 0.008 2 |
| BM plasma cells, % (range) | 4 (0–18) | 3 (1–15) | 4 (0–18) | p = 0.811 2 |
| Treatment at presentation | 13% | 14% | 12% | p = 1.000 1 |
| PB Involvement by MFC Detected 50/100 (50%) | PB Involvement by MFC Not Detected 50/100 (50%) | ||
|---|---|---|---|
| MYD88 L265P mutation by AS-qPCR in PB | MYD88mut | 50/50 (100%) | 15/50 (30%) |
| MYD88wt | 0/50 (0%) | 35/50 (70%) | |
| IGH gene rearrangement in PB | IGH rearranged | 44/49 * (90%) | 20 **/50 (40%) |
| IGH non rearranged | 5/49 * (10%) | 30/50 (60%) |
| FITC | PE | PerCPCy5.5 | PECy7 | APC | APC-H7 | V-450 | V-500 |
|---|---|---|---|---|---|---|---|
| CD22 | CD25 | CD11c | CD19 | Kappa | Lambda | CD20 | CD45 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Montesdeoca, S.; García-Gisbert, N.; Calvo, X.; Arenillas, L.; Román, D.; Fernández-Rodríguez, C.; Navarro, R.; Costan, B.; Vela, M.d.C.; Camacho, L.; et al. Leukemic Involvement Is a Common Feature in Waldenström Macroglobulinemia at Diagnosis. Cancers 2023, 15, 4152. https://doi.org/10.3390/cancers15164152
Montesdeoca S, García-Gisbert N, Calvo X, Arenillas L, Román D, Fernández-Rodríguez C, Navarro R, Costan B, Vela MdC, Camacho L, et al. Leukemic Involvement Is a Common Feature in Waldenström Macroglobulinemia at Diagnosis. Cancers. 2023; 15(16):4152. https://doi.org/10.3390/cancers15164152
Chicago/Turabian StyleMontesdeoca, Sara, Nieves García-Gisbert, Xavier Calvo, Leonor Arenillas, David Román, Concepción Fernández-Rodríguez, Rosa Navarro, Beatriz Costan, María del Carmen Vela, Laura Camacho, and et al. 2023. "Leukemic Involvement Is a Common Feature in Waldenström Macroglobulinemia at Diagnosis" Cancers 15, no. 16: 4152. https://doi.org/10.3390/cancers15164152
APA StyleMontesdeoca, S., García-Gisbert, N., Calvo, X., Arenillas, L., Román, D., Fernández-Rodríguez, C., Navarro, R., Costan, B., Vela, M. d. C., Camacho, L., Abella, E., Colomo, L., Salido, M., Puiggros, A., Florensa, L., Espinet, B., Bellosillo, B., & Ferrer del Álamo, A. (2023). Leukemic Involvement Is a Common Feature in Waldenström Macroglobulinemia at Diagnosis. Cancers, 15(16), 4152. https://doi.org/10.3390/cancers15164152









