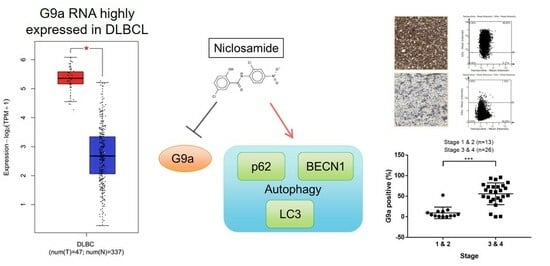
High G9a Expression in DLBCL and Its Inhibition by Niclosamide to Induce Autophagy as a Therapeutic Approach
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
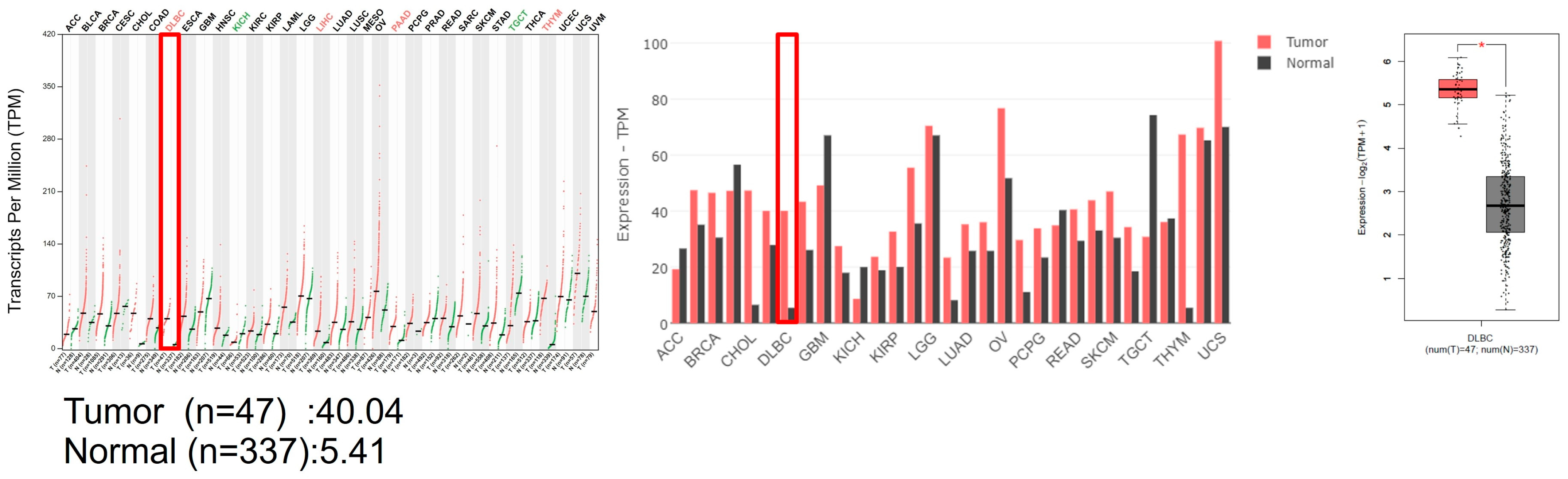
2.1. GEPIA Analysis
2.2. Cell Culture
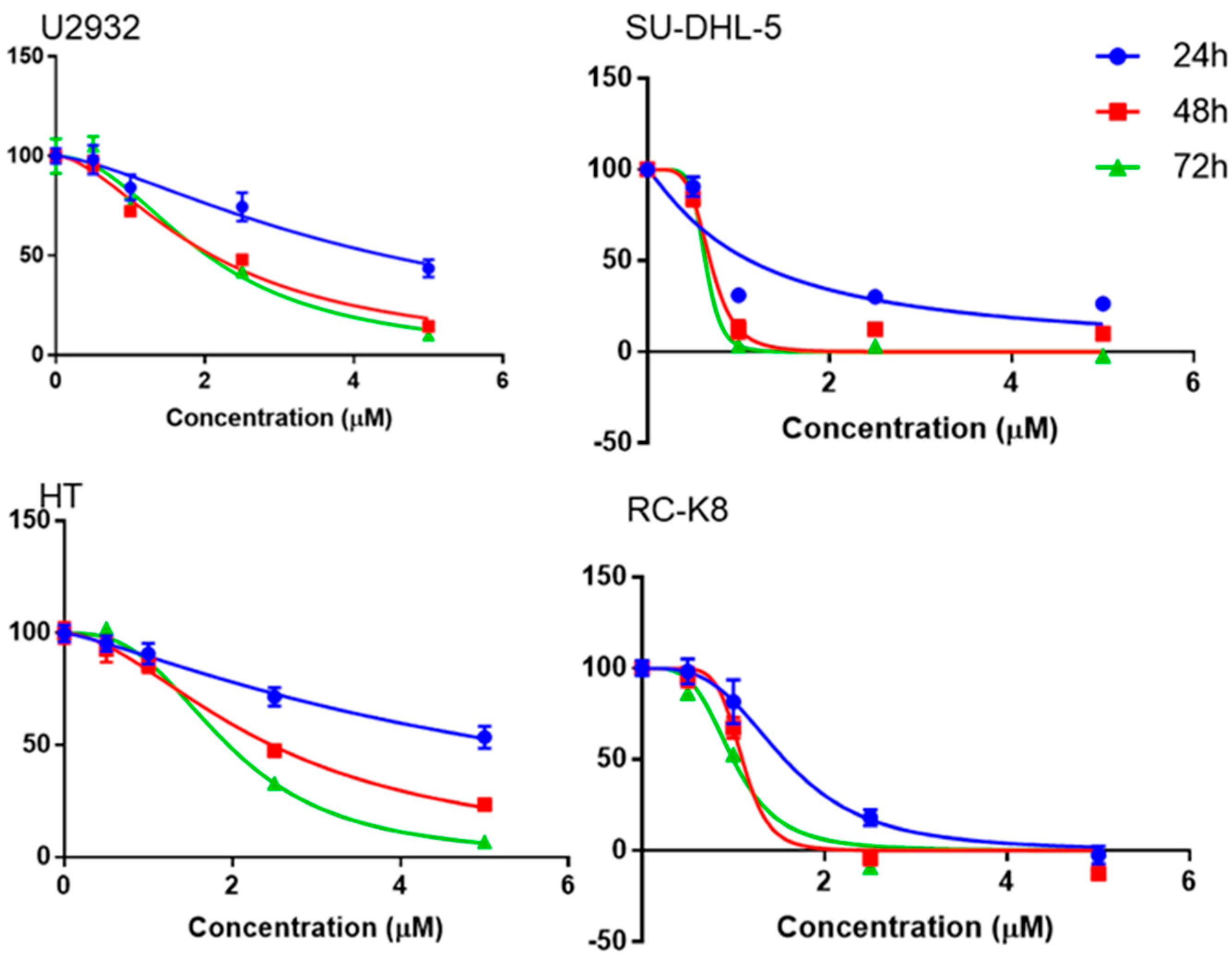
2.3. Cell Viability
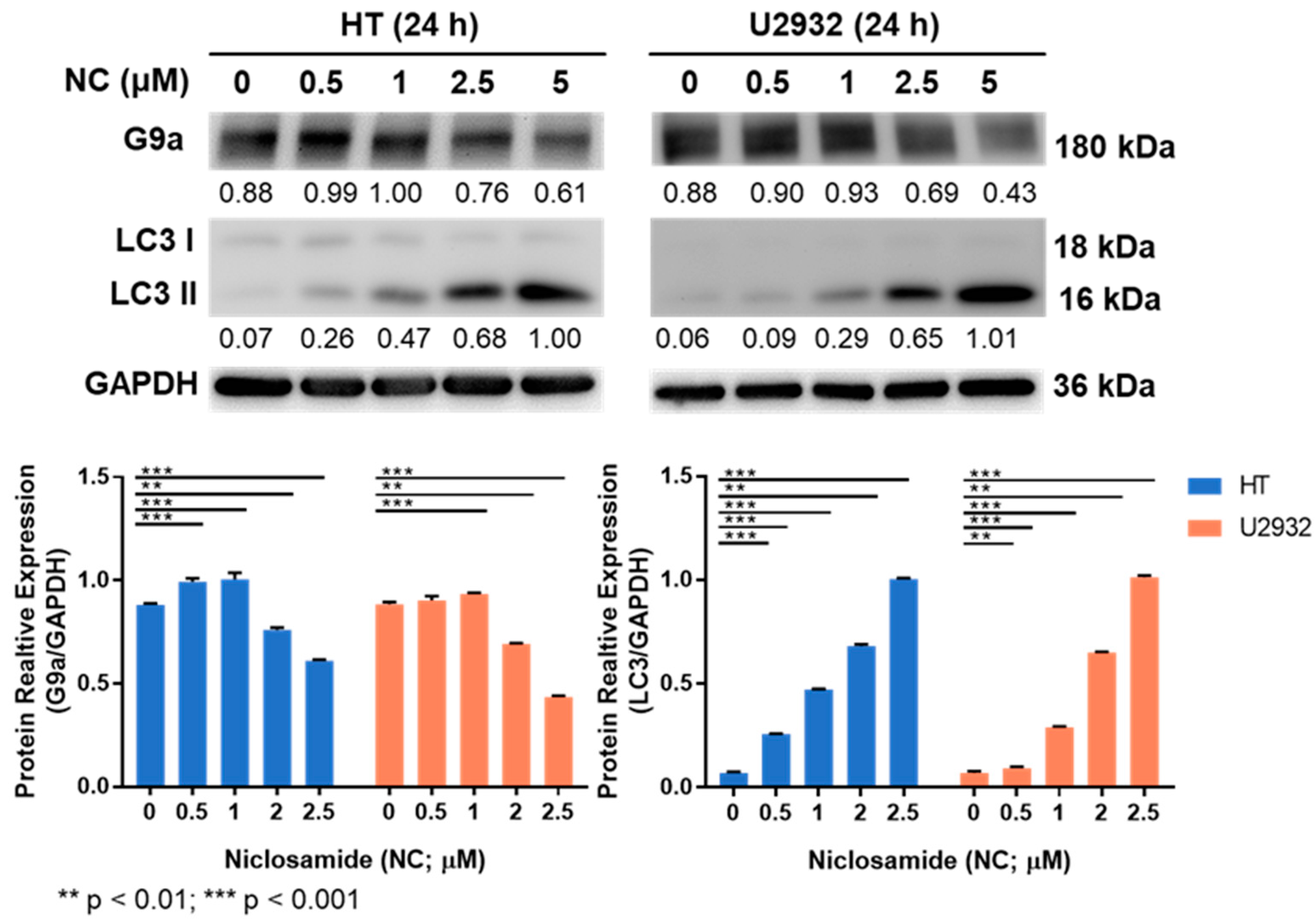
2.4. Immunoblotting
2.5. RT−qPCR
2.6. Collection of Clinical Samples
2.7. Immunohistochemistry
2.8. Image Analysis Using the TissueFAXS PLUS System
2.9. Molecular Docking
2.10. Statistical Analysis
3. Results
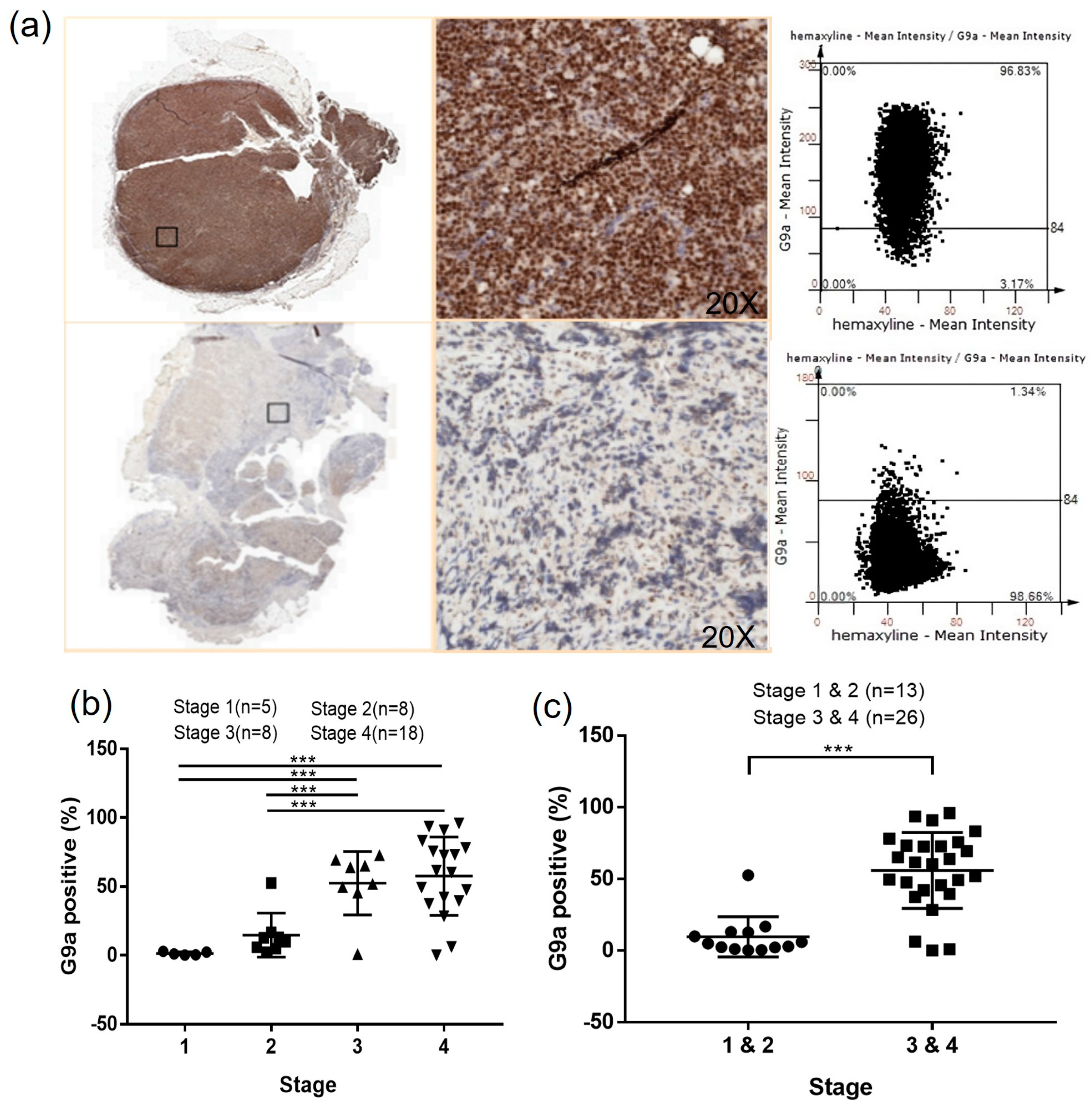
3.1. G9a Is Highly Expressed in DLBCL Patient Samples
3.2. Niclosamide Inhibited DLBCL Cell Line Proliferation in a Time- and Concentration-Dependent Manner and Molecular Docking Study with G9a
3.3. Niclosamide Treatment Reduced G9a and Induced LC3 Proteins
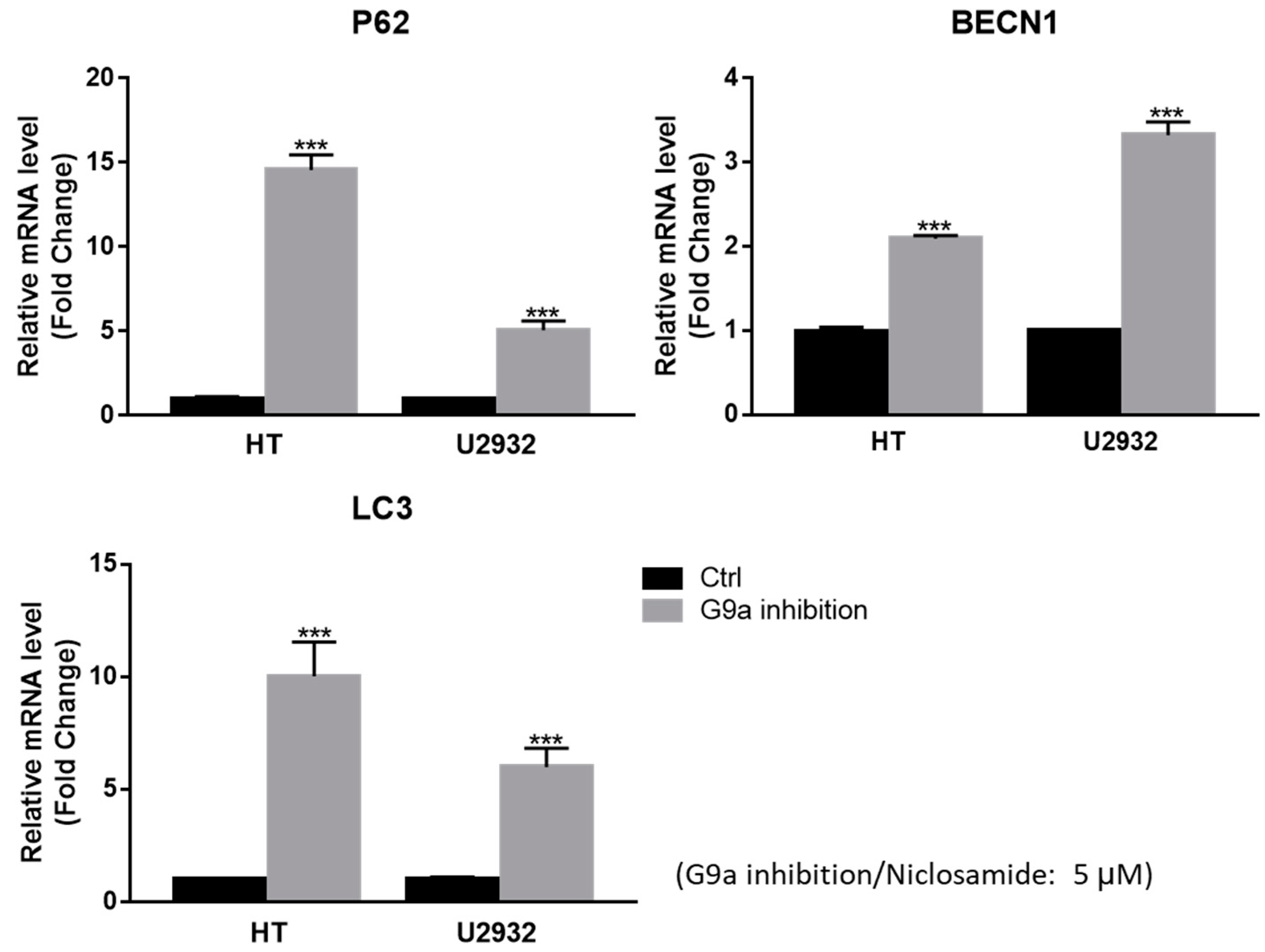
3.4. Niclosamide Induced p62 and BECN1 Genes Expressed in DLBCL Cells
3.5. G9a Expression Is Associated with Advanced-Stage DLBCL
3.6. The Clinical Characteristics Analysis Presents a Significant Difference in the Ann Arbor Stage
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alaggio, R.; Amador, C.; Anagnostopoulos, I.; Attygalle, A.D.; Araujo, I.B.O.; Berti, E.; Bhagat, G.; Borges, A.M.; Boyer, D.; Calaminici, M.; et al. The 5th edition of the World Health Organization Classification of Haematolymphoid Tumours: Lymphoid Neoplasms. Leukemia 2022, 36, 1720–1748. [Google Scholar] [CrossRef] [PubMed]
- Campo, E.; Jaffe, E.S.; Cook, J.R.; Quintanilla-Martinez, L.; Swerdlow, S.H.; Anderson, K.C.; Brousset, P.; Cerroni, L.; de Leval, L.; Dirnhofer, S.; et al. The International Consensus Classification of Mature Lymphoid Neoplasms: A report from the Clinical Advisory Committee. Blood 2022, 140, 1229–1253. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh, A.A.; Eisen, M.B.; Davis, R.E.; Ma, C.; Lossos, I.S.; Rosenwald, A.; Boldrick, J.C.; Sabet, H.; Tran, T.; Yu, X.; et al. Distinct types of diffuse large B-cell lymphoma identified by gene expression profiling. Nature 2000, 403, 503–511. [Google Scholar] [CrossRef] [PubMed]
- Chapuy, B.; Stewart, C.; Dunford, A.J.; Kim, J.; Kamburov, A.; Redd, R.A.; Lawrence, M.S.; Roemer, M.G.M.; Li, A.J.; Ziepert, M.; et al. Molecular subtypes of diffuse large B cell lymphoma are associated with distinct pathogenic mechanisms and outcomes. Nat. Med. 2018, 24, 679–690. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, R.; Wright, G.W.; Huang, D.W.; Johnson, C.A.; Phelan, J.D.; Wang, J.Q.; Roulland, S.; Kasbekar, M.; Young, R.M.; Shaffer, A.L.; et al. Genetics and Pathogenesis of Diffuse Large B-Cell Lymphoma. N. Engl. J. Med. 2018, 378, 1396–1407. [Google Scholar] [CrossRef]
- Reddy, A.; Zhang, J.; Davis, N.S.; Moffitt, A.B.; Love, C.L.; Waldrop, A.; Leppa, S.; Pasanen, A.; Meriranta, L.; Karjalainen-Lindsberg, M.L.; et al. Genetic and Functional Drivers of Diffuse Large B Cell Lymphoma. Cell 2017, 171, 481–494. [Google Scholar] [CrossRef]
- Zhang, J.; Gu, Y.; Chen, B. Drug-Resistance Mechanism and New Targeted Drugs and Treatments of Relapse and Refractory DLBCL. Cancer Manag. Res. 2023, 15, 245–255. [Google Scholar] [CrossRef]
- Lim, S.K.; Peng, C.C.; Low, S.; Vijay, V.; Budiman, A.; Phang, B.H.; Lim, J.Q.; Jeyasekharan, A.D.; Lim, S.T.; Ong, C.K.; et al. Sustained activation of non-canonical NF-kappaB signalling drives glycolytic reprogramming in doxorubicin-resistant DLBCL. Leukemia 2023, 37, 441–452. [Google Scholar] [CrossRef]
- Sehn, L.H.; Salles, G. Diffuse Large B-Cell Lymphoma. N. Engl. J. Med. 2021, 384, 842–858. [Google Scholar] [CrossRef]
- Sermer, D.; Pasqualucci, L.; Wendel, H.G.; Melnick, A.; Younes, A. Emerging epigenetic-modulating therapies in lymphoma. Nat. Rev. Clin. Oncol. 2019, 16, 494–507. [Google Scholar] [CrossRef]
- Chambwe, N.; Kormaksson, M.; Geng, H.; De, S.; Michor, F.; Johnson, N.A.; Morin, R.D.; Scott, D.W.; Godley, L.A.; Gascoyne, R.D.; et al. Variability in DNA methylation defines novel epigenetic subgroups of DLBCL associated with different clinical outcomes. Blood 2014, 123, 1699–1708. [Google Scholar] [CrossRef]
- San Jose-Eneriz, E.; Agirre, X.; Rabal, O.; Vilas-Zornoza, A.; Sanchez-Arias, J.A.; Miranda, E.; Ugarte, A.; Roa, S.; Paiva, B.; Estella-Hermoso de Mendoza, A.; et al. Discovery of first-in-class reversible dual small molecule inhibitors against G9a and DNMTs in hematological malignancies. Nat. Commun. 2017, 8, 15424. [Google Scholar] [CrossRef] [PubMed]
- Fong, K.-w.; Zhao, J.C.; Lu, X.; Kim, J.; Piunti, A.; Shilatifard, A.; Yu, J. PALI1 promotes tumor growth through competitive recruitment of PRC2 to G9A-target chromatin for dual epigenetic silencing. Mol. Cell 2022, 82, 4611–4626. [Google Scholar] [CrossRef] [PubMed]
- Thng, D.K.H.; Hooi, L.; Toh, C.C.M.; Lim, J.J.; Rajagopalan, D.; Syariff, I.Q.C.; Tan, Z.M.; Rashid, M.; Zhou, L.; Kow, A.W.C.; et al. Histone-lysine N-methyltransferase EHMT2 (G9a) inhibition mitigates tumorigenicity in Myc-driven liver cancer. Mol. Oncol. 2023. [Google Scholar] [CrossRef]
- Li, R.G.; Deng, H.; Liu, X.H.; Chen, Z.Y.; Wan, S.S.; Wang, L. Histone Methyltransferase G9a Promotes the Development of Renal Cancer through Epigenetic Silencing of Tumor Suppressor Gene SPINK5. Oxid. Med. Cell Longev. 2021, 2021, 6650781. [Google Scholar] [CrossRef]
- Yin, C.; Ke, X.; Zhang, R.; Hou, J.; Dong, Z.; Wang, F.; Zhang, K.; Zhong, X.; Yang, L.; Cui, H. G9a promotes cell proliferation and suppresses autophagy in gastric cancer by directly activating mTOR. FASEB J. 2019, 33, 14036–14050. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Hu, H.; Zhang, X.; Zhang, Y.; Sun, X.; Wang, H.; Kan, W.; Tan, M.J.; Shi, H.; Zang, Y.; et al. Phosphorylation of PHF2 by AMPK releases the repressive H3K9me2 and inhibits cancer metastasis. Signal Transduct. Target. Ther. 2023, 8, 95. [Google Scholar] [CrossRef]
- Sun, T.; Zhang, K.; Pangeni, R.P.; Wu, J.; Li, W.; Du, Y.; Guo, Y.; Chaurasiya, S.; Arvanitis, L.; Raz, D.J. G9a Promotes Invasion and Metastasis of Non-Small Cell Lung Cancer through Enhancing Focal Adhesion Kinase Activation via NF-kappaB Signaling Pathway. Mol. Cancer Res. 2021, 19, 429–440. [Google Scholar] [CrossRef]
- Liu, X.R.; Zhou, L.H.; Hu, J.X.; Liu, L.M.; Wan, H.P.; Zhang, X.Q. UNC0638, a G9a inhibitor, suppresses epithelial-mesenchymal transition-mediated cellular migration and invasion in triple negative breast cancer. Mol. Med. Rep. 2018, 17, 2239–2244. [Google Scholar] [CrossRef]
- Pangeni, R.P.; Yang, L.; Zhang, K.; Wang, J.; Li, W.; Guo, C.; Yun, X.; Sun, T.; Wang, J.; Raz, D.J. G9a regulates tumorigenicity and stemness through genome-wide DNA methylation reprogramming in non-small cell lung cancer. Clin. Epigenetics 2020, 12, 88. [Google Scholar] [CrossRef]
- Liu, C.W.; Hua, K.T.; Li, K.C.; Kao, H.F.; Hong, R.L.; Ko, J.Y.; Hsiao, M.; Kuo, M.L.; Tan, C.T. Histone Methyltransferase G9a Drives Chemotherapy Resistance by Regulating the Glutamate-Cysteine Ligase Catalytic Subunit in Head and Neck Squamous Cell Carcinoma. Mol. Cancer Ther. 2017, 16, 1421–1434. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Dominguez, D.J.; Hajji, N.; Lopez-Alemany, R.; Sanchez-Molina, S.; Figuerola-Bou, E.; Moron Civanto, F.J.; Rello-Varona, S.; Andres-Leon, E.; Benito, A.; Keun, H.C.; et al. Selective histone methyltransferase G9a inhibition reduces metastatic development of Ewing sarcoma through the epigenetic regulation of NEU1. Oncogene 2022, 41, 2638–2650. [Google Scholar] [CrossRef] [PubMed]
- Kwon, W.A.; Seo, H.K. Novel G9a/DNMT first-in-class dual reversible inhibitor has potent antitumor effect in bladder cancer. Transl. Cancer Res. 2020, 9, 1319–1321. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Mook, R.A., Jr.; Premont, R.T.; Wang, J. Niclosamide: Beyond an antihelminthic drug. Cell Signal 2018, 41, 89–96. [Google Scholar] [CrossRef]
- Luo, F.; Luo, M.; Rong, Q.X.; Zhang, H.; Chen, Z.; Wang, F.; Zhao, H.Y.; Fu, L.W. Niclosamide, an antihelmintic drug, enhances efficacy of PD-1/PD-L1 immune checkpoint blockade in non-small cell lung cancer. J. Immunother. Cancer 2019, 7, 245. [Google Scholar] [CrossRef]
- Jin, B.; Wang, C.; Li, J.; Du, X.; Ding, K.; Pan, J. Anthelmintic Niclosamide Disrupts the Interplay of p65 and FOXM1/beta-catenin and Eradicates Leukemia Stem Cells in Chronic Myelogenous Leukemia. Clin. Cancer Res. 2017, 23, 789–803. [Google Scholar] [CrossRef]
- Wang, C.; Zhou, X.; Xu, H.; Shi, X.; Zhao, J.; Yang, M.; Zhang, L.; Jin, X.; Hu, Y.; Li, X.; et al. Niclosamide Inhibits Cell Growth and Enhances Drug Sensitivity of Hepatocellular Carcinoma Cells via STAT3 Signaling Pathway. J. Cancer 2018, 9, 4150–4155. [Google Scholar] [CrossRef]
- Al-Kuraishy, H.M.; Al-Gareeb, A.I.; Alzahrani, K.J.; Alexiou, A.; Batiha, G.E. Niclosamide for COVID-19: Bridging the gap. Mol. Biol. Rep. 2021, 48, 8195–8202. [Google Scholar] [CrossRef]
- Tharmalingam, N.; Port, J.; Castillo, D.; Mylonakis, E. Repurposing the anthelmintic drug niclosamide to combat Helicobacter pylori. Sci. Rep. 2018, 8, 3701. [Google Scholar] [CrossRef]
- Satoh, K.; Zhang, L.; Zhang, Y.; Chelluri, R.; Boufraqech, M.; Nilubol, N.; Patel, D.; Shen, M.; Kebebew, E. Identification of Niclosamide as a Novel Anticancer Agent for Adrenocortical CarcinomaNiclosamide in Adrenal Cancer. Clin. Cancer Res. 2016, 22, 3458–3466. [Google Scholar] [CrossRef]
- Yin, L.; Gao, Y.; Zhang, X.; Wang, J.; Ding, D.; Zhang, Y.; Zhang, J.; Chen, H. Niclosamide sensitizes triple-negative breast cancer cells to ionizing radiation in association with the inhibition of Wnt/beta-catenin signaling. Oncotarget 2016, 7, 42126–42138. [Google Scholar] [CrossRef]
- Li, R.; You, S.; Hu, Z.; Chen, Z.G.; Sica, G.L.; Khuri, F.R.; Curran, W.J.; Shin, D.M.; Deng, X. Inhibition of STAT3 by niclosamide synergizes with erlotinib against head and neck cancer. PLoS ONE 2013, 8, e74670. [Google Scholar] [CrossRef]
- Liu, J.; Chen, X.; Ward, T.; Pegram, M.; Shen, K. Combined niclosamide with cisplatin inhibits epithelial-mesenchymal transition and tumor growth in cisplatin-resistant triple-negative breast cancer. Tumour Biol. 2016, 37, 9825–9835. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; Kang, B.; Li, C.; Chen, T.; Zhang, Z. GEPIA2: An enhanced web server for large-scale expression profiling and interactive analysis. Nucleic Acids Res. 2019, 47, W556–W560. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yang, X.; Gan, J.; Chen, S.; Xiao, Z.X.; Cao, Y. CB-Dock2: Improved protein-ligand blind docking by integrating cavity detection, docking and homologous template fitting. Nucleic Acids Res. 2022, 50, W159–W164. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Min, J.; Lunin, V.V.; Antoshenko, T.; Dombrovski, L.; Zeng, H.; Allali-Hassani, A.; Campagna-Slater, V.; Vedadi, M.; Arrowsmith, C.H.; et al. Structural biology of human H3K9 methyltransferases. PLoS ONE 2010, 5, e8570. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Chen, J.; Cheng, T.; Gindulyte, A.; He, J.; He, S.; Li, Q.; Shoemaker, B.A.; Thiessen, P.A.; Yu, B.; et al. PubChem 2023 update. Nucleic Acids Res 2023, 51, D1373–D1380. [Google Scholar] [CrossRef]
- Huang, H.H.; Ko, B.S.; Chen, H.M.; Chen, L.J.; Wang, C.Y.; Hsiao, F.Y. Frontline treatments in extremely elderly patients with diffuse large B-cell lymphoma: A population-based study in Taiwan, 2010–2015. Immun. Ageing 2020, 17, 17. [Google Scholar] [CrossRef]
- Pei, S.N.; Wang, M.C.; Ma, M.C.; Kuo, C.Y.; Liao, C.K.; Qiu, H.; Rothwell, L.A.; Liu, Y. A comprehensive retrospective cohort study of the journey of B-cell lymphoma in Taiwan. Sci. Rep. 2021, 11, 10069. [Google Scholar] [CrossRef]
- Tsai, Y.F.; Liu, Y.C.; Yang, C.I.; Chuang, T.M.; Ke, Y.L.; Yeh, T.J.; Gau, Y.C.; Du, J.S.; Wang, H.C.; Cho, S.F.; et al. Poor Prognosis of Diffuse Large B-Cell Lymphoma with Hepatitis C Infection. J. Pers. Med. 2021, 11, 844. [Google Scholar] [CrossRef]
- Hans, C.P.; Weisenburger, D.D.; Greiner, T.C.; Gascoyne, R.D.; Delabie, J.; Ott, G.; Muller-Hermelink, H.K.; Campo, E.; Braziel, R.M.; Jaffe, E.S.; et al. Confirmation of the molecular classification of diffuse large B-cell lymphoma by immunohistochemistry using a tissue microarray. Blood 2004, 103, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.T.; Chen, S.W.; Ho, C.H.; Kuo, C.C.; Sakata, S.; Takeuchi, K.; Chuang, S.S. Immunophenotypic and genetic characteristics of diffuse large B-cell lymphoma in Taiwan. J. Formos. Med. Assoc. 2016, 115, 961–967. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Xu, P.; Sun, X.; Song, X.; Peng, Y.; He, B.; Wu, Z.; Zhu, J. Prognostic value of lymphocyte-to-monocyte ratio and histone methyltransferase G9a histone methyltransferase in patients with double expression lymphoma: A retrospective observational study. Medicine 2021, 100, e24449. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.H.; Lee, K.F.; Chen, C.C.; Chen, Y.Y.; Huang, C.E.; Tsai, P.S.; Tsou, H.Y.; Chou, H.J.; Chen, M.F.; Chen, P.T.; et al. Clinical characteristics and treatment outcome in a Taiwanese population of patients with Epstein-Barr virus-positive diffuse large B-cell lymphoma. Jpn. J. Clin. Oncol. 2014, 44, 1164–1171. [Google Scholar] [CrossRef]
- Hoffmann, M.S.; Hunter, B.D.; Cobb, P.W.; Varela, J.C.; Munoz, J. Overcoming Barriers to Referral for Chimeric Antigen Receptor T Cell Therapy in Patients with Relapsed/Refractory Diffuse Large B Cell Lymphoma. Transplant. Cell Ther. 2023, 29, 440–448. [Google Scholar] [CrossRef]
- Jo, T.; Yoshihara, S.; Okuyama, Y.; Fujii, K.; Henzan, T.; Kahata, K.; Yamazaki, R.; Takeda, W.; Umezawa, Y.; Fukushima, K. Risk factors for CAR-T cell manufacturing failure among DLBCL patients: A nationwide survey in Japan. Br. J. Haematol. 2023, 202, 256–266. [Google Scholar] [CrossRef]
- Mei, M.G.; Masucci, L.; Jain, M.D. Cellular therapy: Great promise, but at what cost? Mol. Ther. 2023, 31, 5–6. [Google Scholar] [CrossRef]
- Turabi, K.S.; Deshmukh, A.; Paul, S.; Swami, D.; Siddiqui, S.; Kumar, U.; Naikar, S.; Devarajan, S.; Basu, S.; Paul, M.K.; et al. Drug repurposing—An emerging strategy in cancer therapeutics. Naunyn Schmiedebergs Arch. Pharmacol. 2022, 395, 1139–1158. [Google Scholar] [CrossRef]
- Jara, M.O.; Williams, R.O., III. The challenge of repurposing niclosamide: Considering pharmacokinetic parameters, routes of administration, and drug metabolism. J. Drug Deliv. Sci. Technol. 2023, 81, 104187. [Google Scholar] [CrossRef]
- Jiang, H.; Li, A.M.; Ye, J. The magic bullet: Niclosamide. Front. Oncol. 2022, 12, 1004978. [Google Scholar] [CrossRef]
- Tao, H.; Zhang, Y.; Zeng, X.; Shulman, G.I.; Jin, S. Niclosamide ethanolamine-induced mild mitochondrial uncoupling improves diabetic symptoms in mice. Nat. Med. 2014, 20, 1263–1269. [Google Scholar] [CrossRef] [PubMed]
- You, S.; Li, R.; Park, D.; Xie, M.; Sica, G.L.; Cao, Y.; Xiao, Z.Q.; Deng, X. Disruption of STAT3 by niclosamide reverses radioresistance of human lung cancer. Mol. Cancer Ther. 2014, 13, 606–616. [Google Scholar] [CrossRef] [PubMed]
- Londono-Joshi, A.I.; Arend, R.C.; Aristizabal, L.; Lu, W.; Samant, R.S.; Metge, B.J.; Hidalgo, B.; Grizzle, W.E.; Conner, M.; Forero-Torres, A.; et al. Effect of niclosamide on basal-like breast cancers. Mol. Cancer Ther. 2014, 13, 800–811. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Lu, Z.; Ding, K.; Li, J.; Du, X.; Chen, C.; Sun, X.; Wu, Y.; Zhou, J.; Pan, J. Antineoplastic mechanisms of niclosamide in acute myelogenous leukemia stem cells: Inactivation of the NF-kappaB pathway and generation of reactive oxygen species. Cancer Res. 2010, 70, 2516–2527. [Google Scholar] [CrossRef] [PubMed]
- Ray, E.; Vaghasiya, K.; Sharma, A.; Shukla, R.; Khan, R.; Kumar, A.; Verma, R.K. Autophagy-Inducing Inhalable Co-crystal Formulation of Niclosamide-Nicotinamide for Lung Cancer Therapy. AAPS PharmSciTech 2020, 21, 260. [Google Scholar] [CrossRef]
- Huang, F.L.; Yu, S.J.; Liao, E.C.; Li, L.Y.; Shen, P.W.; Li, C.L. Niclosamide suppresses T-cell acute lymphoblastic leukemia growth through activation of apoptosis and autophagy. Oncol. Rep. 2022, 47, 30. [Google Scholar] [CrossRef]
- Xiang, D.; Yuan, Y.; Chen, L.; Liu, X.; Belani, C.; Cheng, H. Niclosamide, an anti-helminthic molecule, downregulates the retroviral oncoprotein Tax and pro-survival Bcl-2 proteins in HTLV-1-transformed T lymphocytes. Biochem. Biophys. Res. Commun. 2015, 464, 221–228. [Google Scholar] [CrossRef][Green Version]
- Ansari, J.; Polk, P.; Aufman, J.; Herrera, G.A.; Cardelli, J.; Munker, R. Potent Inhibition of the Growth and Induction of Apoptosis in Lymphoma By the Anthelminthic Drug Niclosamide: In Vitro Data. Blood 2015, 126, 5131. [Google Scholar] [CrossRef]
- Artal-Martinez de Narvajas, A.; Gomez, T.S.; Zhang, J.S.; Mann, A.O.; Taoda, Y.; Gorman, J.A.; Herreros-Villanueva, M.; Gress, T.M.; Ellenrieder, V.; Bujanda, L.; et al. Epigenetic regulation of autophagy by the methyltransferase G9a. Mol. Cell Biol. 2013, 33, 3983–3993. [Google Scholar] [CrossRef]
- De Smedt, E.; Devin, J.; Muylaert, C.; Robert, N.; Requirand, G.; Vlummens, P.; Vincent, L.; Cartron, G.; Maes, K.; Moreaux, J.; et al. G9a/GLP targeting in MM promotes autophagy-associated apoptosis and boosts proteasome inhibitor-mediated cell death. Blood Adv. 2021, 5, 2325–2338. [Google Scholar] [CrossRef]
- De Santi, M.; Baldelli, G.; Diotallevi, A.; Galluzzi, L.; Schiavano, G.F.; Brandi, G. Metformin prevents cell tumorigenesis through autophagy-related cell death. Sci. Rep. 2019, 9, 66. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Huang, H.P.; Wang, Y.; Jin, J.; Long, W.G.; Chen, K.; Zhao, X.H.; Chen, C.G.; Li, J. Curcumin overcome primary gefitinib resistance in non-small-cell lung cancer cells through inducing autophagy-related cell death. J. Exp. Clin. Cancer Res. 2019, 38, 254. [Google Scholar] [CrossRef] [PubMed]
| Forward Primer | Reverse Primer | |
|---|---|---|
| p62 | 5′-GTGAATTTCCTGAAGAACGTTGG-3′ | 5′-TGGAACTCTCTGGAGAGACGG-3′ |
| BECN1 | 5′-CTGGACACGAGTTTCAAGATCCT-3′ | 5′- GTTAGTCTCTTCCTCCTGGGTCTCT-3′ |
| LC3 | 5′-TCCTGGACAAGACCAAGTTTTTG-3′ | 5′-ACCATGCTGTGCTGGTTCAC-3′ |
| GAPDH | 5′-CTTAGCACCCCTGGCCAAG-3′ | 5′-ATGTTCTGGAGAGCCCCG-3′ |
| G9a > 40% | G9a < 40% | ||
|---|---|---|---|
| N = 21 | N = 18 | p Value | |
| Age, (mean ± SD) | 59.76 ± 18.86 | 61.22 ± 12.69 | 0.776 |
| Gender, N (%) | 0.878 | ||
| Male | 11 (52.38%) | 7 (38.89%) | |
| Female | 10 (47.62%) | 11 (61.11%) | |
| ECOG, N (%) | 0.717 | ||
| 1 + 2 | 20 (95.24%) | 17 (100%) | |
| 3 + 4 | 1 (4.76%) | 1 (0.00%) | |
| Ann Arbor stage, N (%) | 0.00166 ** | ||
| I | 0 (0%) | 5 (27.78%) | |
| II | 1 (4.76%) | 7 (38.89%) | |
| III | 7 (33.33%) | 1 (5.55%) | |
| IV | 13 (61.91%) | 5 (27.78%) | |
| I-II | 1 (4.76%) | 12 (66.67%) | 0.0158 * |
| III-IV | 20 (95.24%) | 6 (33.33%) | |
| BW (mean ± SD) | 60.85 ± 9.27 | 61.50 ± 11.94 | 0.855 |
| BH (mean ± SD) | 162.94 ± 8.52 | 158.71 ± 6.38 | 0.105 |
| BMI (mean ± SD) | 23.02 ± 3.69 | 24.12 ± 4.01 | 0.397 |
| WBC (mean ± SD) | 6453.68 ± 2459.36 | 7365.88 ± 3487.83 | 0.367 |
| Platelet (×103, mean ± SD) | 217.91 ± 105.5 | 259 ± 141.4 | 0.327 |
| LDH (mean ± SD) | 484.84 ± 474.77 | 255.59 ± 271.25 | 0.089 |
| Beta-2 microglobulin (mean ± SD) | 387.47 ± 347.70 | 404.75 ± 502.06 | 0.905 |
| Albumin (mean ± SD) | 3.89 ± 0.41 | 3.91 ± 0.64 | 0.922 |
| GOT (mean ± SD) | 32.63 ± 22.80 | 31.59 ± 12.00 | 0.867 |
| GPT (mean ± SD) | 24.11 ± 25.78 | 30.18 ± 18.30 | 0.426 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hsu, C.-M.; Chang, K.-C.; Chuang, T.-M.; Chu, M.-L.; Lin, P.-W.; Liu, H.-S.; Kao, S.-Y.; Liu, Y.-C.; Huang, C.-T.; Wang, M.-H.; et al. High G9a Expression in DLBCL and Its Inhibition by Niclosamide to Induce Autophagy as a Therapeutic Approach. Cancers 2023, 15, 4150. https://doi.org/10.3390/cancers15164150
Hsu C-M, Chang K-C, Chuang T-M, Chu M-L, Lin P-W, Liu H-S, Kao S-Y, Liu Y-C, Huang C-T, Wang M-H, et al. High G9a Expression in DLBCL and Its Inhibition by Niclosamide to Induce Autophagy as a Therapeutic Approach. Cancers. 2023; 15(16):4150. https://doi.org/10.3390/cancers15164150
Chicago/Turabian StyleHsu, Chin-Mu, Kung-Chao Chang, Tzer-Ming Chuang, Man-Ling Chu, Pei-Wen Lin, Hsiao-Sheng Liu, Shih-Yu Kao, Yi-Chang Liu, Chien-Tzu Huang, Min-Hong Wang, and et al. 2023. "High G9a Expression in DLBCL and Its Inhibition by Niclosamide to Induce Autophagy as a Therapeutic Approach" Cancers 15, no. 16: 4150. https://doi.org/10.3390/cancers15164150
APA StyleHsu, C.-M., Chang, K.-C., Chuang, T.-M., Chu, M.-L., Lin, P.-W., Liu, H.-S., Kao, S.-Y., Liu, Y.-C., Huang, C.-T., Wang, M.-H., Yeh, T.-J., Gau, Y.-C., Du, J.-S., Wang, H.-C., Cho, S.-F., Hsiao, C.-E., Tsai, Y., Hsiao, S. Y., Hung, L.-C., ... Hsiao, H.-H. (2023). High G9a Expression in DLBCL and Its Inhibition by Niclosamide to Induce Autophagy as a Therapeutic Approach. Cancers, 15(16), 4150. https://doi.org/10.3390/cancers15164150