Tumor-Free Resection Margin Distance in the Surgical Treatment of Node-Negative Squamous Cell Cancer of the Vulva Has No Impact on Survival: Analysis of a Large Patient Cohort in a Tertiary Care Center
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
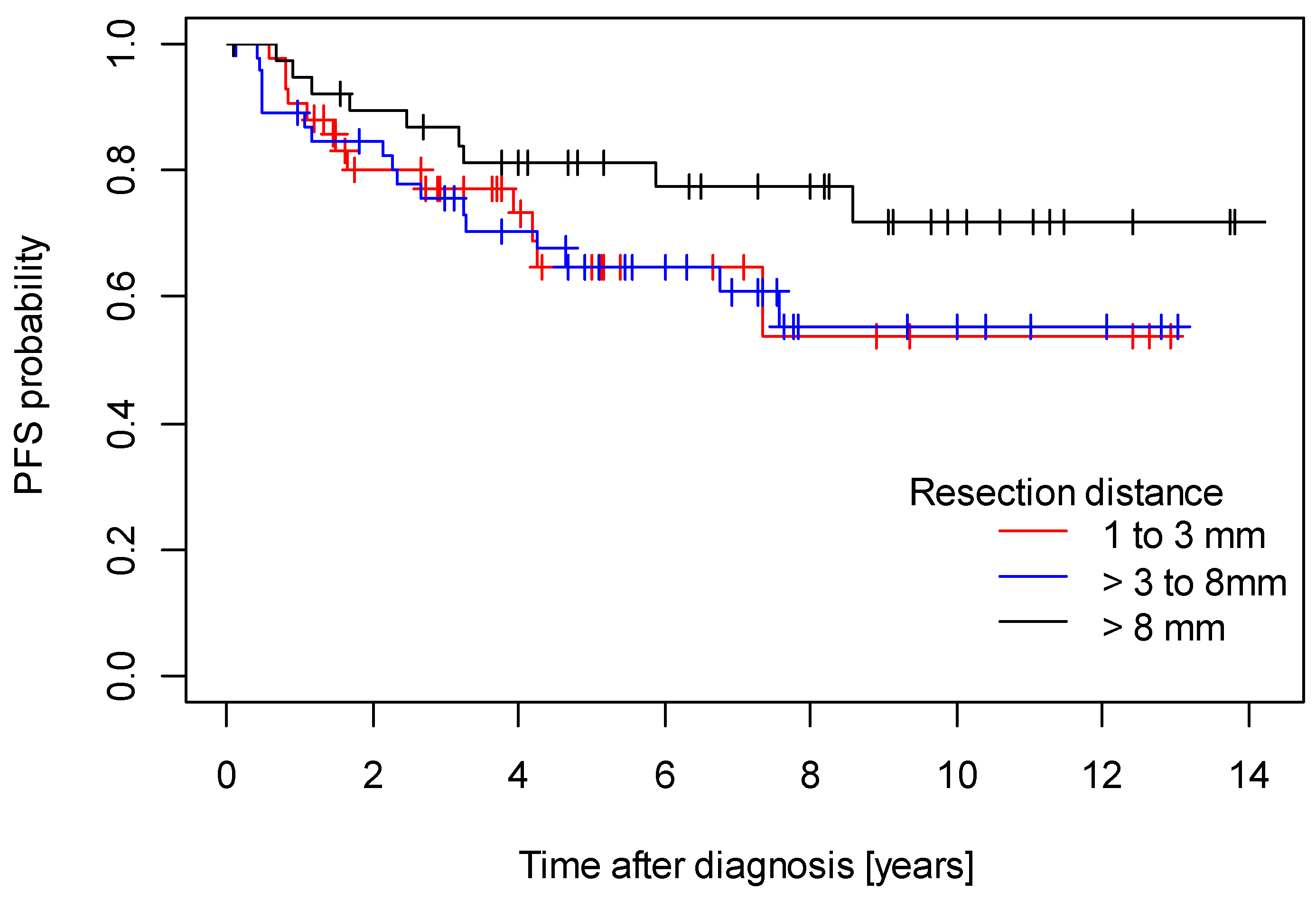
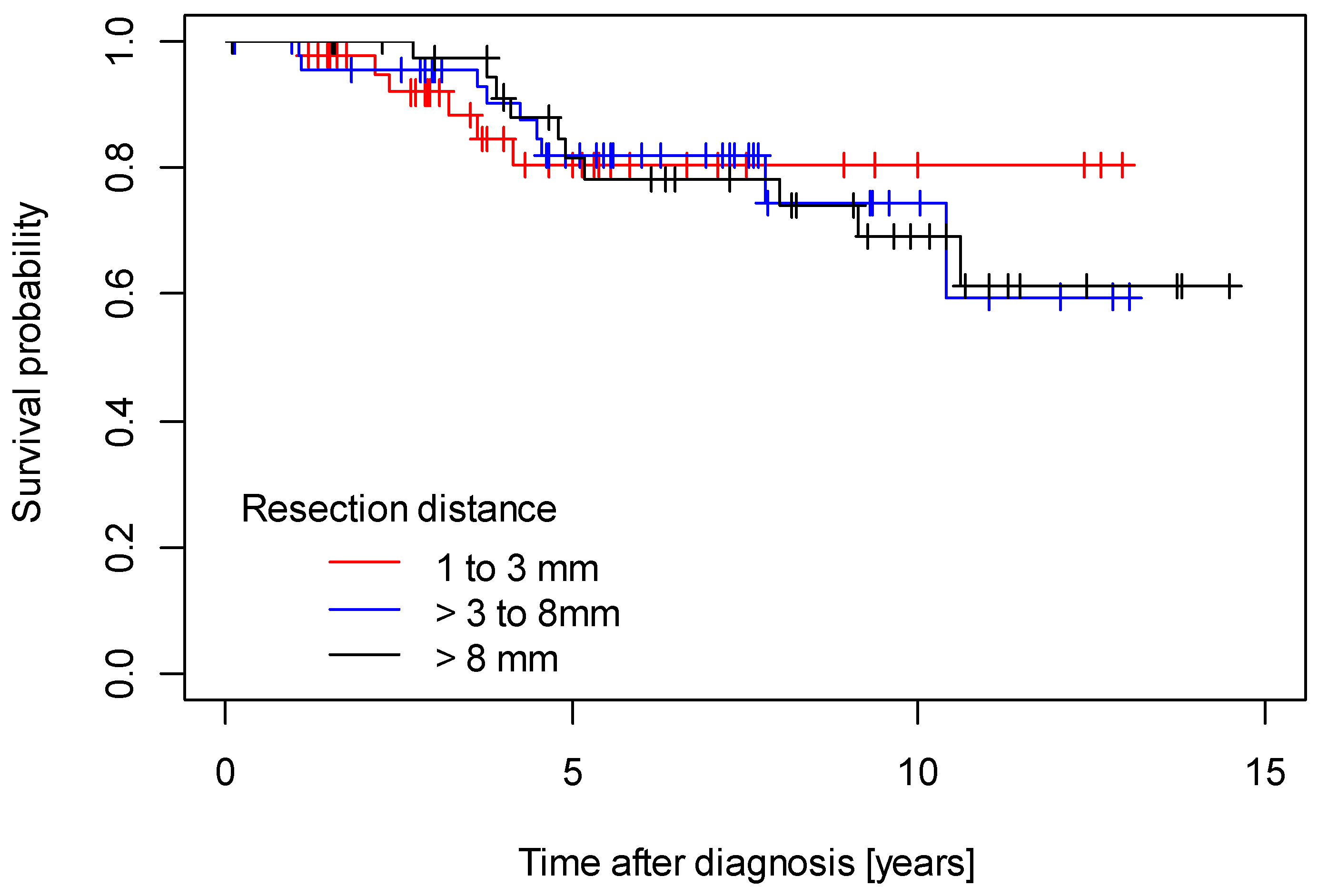
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Available online: www.krebsdaten.de (accessed on 28 June 2023).
- Mix, J.M.; Gopalani, S.V.; Simko, S.; Saraiya, M. Trends in HPV- and non-HPV-associated vulvar cancer incidence, United States, 2001–2017. Prev. Med. 2022, 164, 107302. [Google Scholar] [CrossRef]
- Magrina, J.F.; Gonzalez-Bosquet, J.; Weaver, A.L.; Gaffey, T.A.; Webb, M.J.; Podratz, K.C.; Cornella, J.L. Primary squamous cell cancer of the vulva: Radical versus modified radical vulvar surgery. Gynecol. Oncol. 1998, 71, 116–121. [Google Scholar] [CrossRef]
- Podratz, K.C.; Symmonds, R.E.; Taylor, W.F.; Williams, T.J. Carcinoma of the vulva: Analysis of treatment and survival. Obstet. Gynecol. 1983, 61, 63–74. [Google Scholar]
- Viswanathan, A.N.; Pinto, A.P.; Schultz, D.; Berkowitz, R.; Crum, C.P. Relationship of margin status and radiation dose to recurrence in post-operative vulvar carcinoma. Gynecol. Oncol. 2013, 130, 545–549. [Google Scholar] [CrossRef] [PubMed]
- Di Donna, M.C.; Quartuccio, N.; Giallombardo, V.; Sturiale, L.; Arnone, A.; Ricapito, R.; Sozzi, G.; Arnone, G.; Chiantera, V. Detection of sentinel lymph node in vulvar cancer using 99mTc-labeled colloid lymphoscintigraphy, blue dye, and indocyanine-green fluorescence: A meta-analysis of studies published in 2010–2020. Arch. Gynecol. Obstet. 2023, 307, 1677–1686. [Google Scholar]
- Laas, E.; Fourchotte, V.; Gaillard, T.; Pauly, L.; Reyal, F.; Feron, J.G.; Lécuru, F. Sentinel Lymph Node Biopsy in Uterine Cancer: Time for a Modern Approach. Cancers 2023, 15, 389. [Google Scholar] [CrossRef] [PubMed]
- Barry, P.N.; Ling, D.C.; Beriwal, S. Definitive chemoradiation or radiation therapy alone for the management of vulvar cancer. Int. J. Gynecol. Cancer 2022, 32, 332–337. [Google Scholar] [CrossRef] [PubMed]
- Pedrão, P.G.; Guimarães, Y.M.; Godoy, L.R.; Possati-Resende, J.C.; Bovo, A.C.; Andrade, C.E.M.C.; Longatto-Filho, A.; Dos Reis, R. Management of Early-Stage Vulvar Cancer. Cancers 2022, 14, 4184. [Google Scholar] [CrossRef]
- Chan, J.K.; Sugiyama, V.; Pham, H.; Gu, M.; Rutgers, J.; Osann, K.; Cheung, M.K.; Berman, M.L.; DiSaia, P.J. Margin distance and other clinicopathologic prognostic factors in vulvar carcinoma: A multivariate analysis. Gynecol. Oncol. 2007, 104, 636–641. [Google Scholar] [CrossRef]
- Woelber, L.; Choschzick, M.; Eulenburg, C.; Hager, M.; Jaenicke, F.; Gieseking, F.; Kock, L.; Ihnen, M.; Petersen, C.; Schwarz, J.; et al. Prognostic value of pathological resection margin distance in squamous cell cancer of the vulva. Ann. Surg. Oncol. 2011, 18, 3811–3818. [Google Scholar] [CrossRef]
- Milliken, S.; May, J.; Sanderson, P.A.; Congiu, M.A.; D’Oria, O.; Caruso, G.; Giannini, A. Reducing the radicality of surgery for vulvar cancer: Are smaller margins safer? Minerva Obstet. Gynecol. 2021, 73, 160–165. [Google Scholar] [CrossRef] [PubMed]
- Barlow, E.L.; Jackson, M.; Hacker, N.F. The Prognostic Role of the Surgical Margins in Squamous Vulvar Cancer: A Retrospective Australian Study. Cancers 2020, 12, 3375. [Google Scholar] [CrossRef] [PubMed]
- Gentileschi, S.; Servillo, M.; Garganese, G.; Fragomeni, S.; De Bonis, F.; Scambia, G.; Salgarello, M. Surgical therapy of vulvar cancer: How to choose the correct reconstruction? J. Gynecol. Oncol. 2016, 27, e60. [Google Scholar] [CrossRef] [PubMed]
- National Comprehensive Cancer Network Guidelines. Version 1.2023 Vulvar Cancer (Squamos Cell Carcinoma). Available online: https://www.nccn.org/guidelines/guidelines-detail?category=1&id=1476 (accessed on 16 May 2023).
- Oonk, M.H.M.; Planchamp, F.; Baldwin, P.; Mahner, S.; Mirza, M.R.; Fischerová, D.; Creutzberg, C.L.; Guillot, E.; Garganese, G.; Lax, S.; et al. European Society of Gynaecological Oncology Guidelines for the Management of Patients with Vulvar Cancer—Update 2023. Int. J. Gynecol. Cancer. 2023, 33, 1023–1043. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://register.awmf.org/de/leitlinien/detail/015-059OL (accessed on 16 May 2023).
- Raimond, E.; Delorme, C.; Ouldamer, L.; Carcopino, X.; Bendifallah, S.; Touboul, C.; Daraï, E.; Ballester, M.; Graesslin, O.; Research group FRANCOGYN. Surgical treatment of vulvar cancer: Impact of tumor-free margin distance on recurrence and survival. A multicentre cohort analysis from the francogyn study group. Eur. J. Surg. Oncol. 2019, 45, 2109–2114. [Google Scholar] [CrossRef]
- Nomura, H.; Omi, M.; Netsu, S.; Aoki, Y.; Tanigawa, T.; Kurita, T.; Matoda, M.; Okamoto, S.; Omatsu, K.; Kanao, H. Positive surgical margin is an independent predictor of overall survival of patients with vulvar squamous cell carcinoma. J. Obstet. Gynaecol. Res. 2021, 47, 3990–3997. [Google Scholar] [CrossRef]
- Woelber, L.; Griebel, L.F.; Eulenburg, C.; Sehouli, J.; Jueckstock, J.; Hilpert, F.; de Gregorio, N.; Hasenburg, A.; Ignatov, A.; Hillemanns, P.; et al. Role of tumour-free margin distance for loco-regional control in vulvar cancer-a subset analysis of the Arbeitsgemeinschaft Gynäkologische Onkologie CaRE-1 multicenter study. Eur. J. Cancer. 2016, 69, 180–188. [Google Scholar] [CrossRef]
- Baiocchi, G.; Mantoan, H.; de Brot, L.; Badiglian-Filho, L.; Kumagai, L.Y.; Faloppa, C.C.; da Costa, A.A. How important is the pathological margin distance in vulvar cancer? Eur. J. Surg. Oncol. 2015, 41, 1653–1658. [Google Scholar] [CrossRef]
- Nooij, L.S.; van der Slot, M.A.; Dekkers, O.M.; Stijnen, T.; Gaarenstroom, K.N.; Creutzberg, C.L.; Smit, V.T.; Bosse, T.; van Poelgeest, M.I. Tumour-free margins in vulvar squamous cell carcinoma: Does distance really matter? Eur. J. Cancer. 2016, 65, 139–149. [Google Scholar] [CrossRef]
- Jaeger, A.; Prieske, K.; Mathey, S.; Fischer, I.; Vettorazzi, E.; Kuerti, S.; Reuter, S.; Dieckmann, J.; Schmalfeldt, B.; Woelber, L. Pelvic lymphadenectomy in vulvar cancer and its impact on prognosis and outcome. Arch. Gynecol. Obstet. 2022, 305, 233–240. [Google Scholar] [CrossRef]
- Ignatov, T.; Eggemann, H.; Burger, E.; Costa, S.D.; Ignatov, A. Adjuvant radiotherapy for vulvar cancer with close or positive surgical margins. J. Cancer Res. Clin. Oncol. 2016, 142, 489–495. [Google Scholar] [CrossRef] [PubMed]
- Ignatov, T.; Gaßner, J.; Bozukova, M.; Ivros, S.; Mészáros, J.; Ortmann, O.; Eggemann, H.; Ignatov, A. Contralateral lymph node metastases in patients with vulvar cancer and unilateral sentinel lymph node metastases. Acta Obstet. Gynecol. Scand. 2021, 100, 1520–1525. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age at Diagnosis Years | Tumor Stage | Tumor Diameter mm | Depth of Invasion mm | Grading | Type of Vulvar Surgery | Resection Margin mm | |||||||
| Median (Range) | pT1 (n, %) | pT2 (n, %) | pT3 (n, %) | Median (Range) | Median (Range) | G1 (n, %) | G2 (n, %) | G3 (n, %) | Wide Excision (n, %) | Partial Vulvectomy (n, %) | Complete Vulvectomy (n, %) | Median (Range) | |
| Total patients (n = 128) | 68 (25–88) | 105 (82.1%) | 22 (17.2%) | 1 (0.8%) | 16 (1–95) | 2 (1–15) | 19 (14.8%) | 97 (75.8%) | 12 (9.4%) | 7 (5.5%) | 78 (60.9%) | 43 (33.6%) | 5 (1–11) |
| Resection margin category | |||||||||||||
| 1 to 3 mm (n = 42) | 71 (41–84) | 32 (76.2%) | 10 (23.8%) | 0 | 22 (1–95) | 3 (1–15) | 5 (11.9%) | 30 (71.4%) | 7 (16.7%) | 2 (4.8%) | 27 (64.3%) | 13 (31.0%) | 2 (1–3) |
| >3 to 8mm (n = 47) | 65 (25–88) | 41 (87.2%) | 5 (10.6%) | 1 (2.1%) | 17 (1–85) | 2 (1–25) | 7 (14.9%) | 37 (78.7%) | 3 (6.4%) | 3 (5.6%) | 30 (55.6%) | 21 (38.9%) | 5 ( 4–8) |
| >8mm (n = 39) | 67 (33–84) | 32 (82.1%) | 7 (17.9%) | 0 | 10 (1–80) | 1 (1–7) | 7 (17.9%) | 30 (76.9%) | 2 (5.1%) | 2 (5.1%) | 21 (53.8%) | 16 (41.0%) | 9 (9–11) |
| p-Value | 0.202 a | 0.336 b | 0.023 a | 0.021 a | 0.439 b | 0.828 b | <0.001 a | ||||||
| Type of Lymph Node Surgery | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Inguinal Lymphadenectomy * | Sentinel Lymphadenectomy/ Sentinel-Backed Inguinal Lymphadenectomy | Systematic Inguinal Lymphadenectomy | Systematic Pelvic Lymphadenectomy | |||||||||
| Yes n (%) | No n (%) | Number of Lymph Nodes: Median (Range) | Yes n (%) | No n (%) | Number of Lymph Nodes: Median (range) | Yes n (%) | No n (%) | Number of Lymph Nodes: Median (Range) | Yes n (%) | No n (%) | Number of Lymph Nodes: Median (Range) | |
| Total (n = 128) | 128 (100%) | 0 | 6 (1–67) | 110 (85.9%) | 18 (14.0%) | 3 (0–14) | 64 (50.0%) | 64 (50.0%) | 0 (0–30) | 5 (4.0%) | 123 (96.0%) | 0 (0–53) |
| Resection margin category | ||||||||||||
| 1 to 3 mm (n = 42) | 42 (100%) | 0 | 6 (1–30) | 36 (85.7%) | 6 (14.2%) | 3 (0–14) | 19 (45.2%) | 23 (54.8%) | 0 (0–30) | 2 (5.0%) | 40 (95.0%) | 0 (0–8) |
| >3 to 8 mm (n = 47) | 47 (100%) | 0 | 6 (1–67) | 40 (85.1%) | 7 (14.9%) | 3 (0–8) | 27 (57.4%) | 20 (42.5%) | 3 (0–26) | 2 (4.2%) | 45 (95.7%) | 0 (0–53) |
| >8 mm (n = 39) | 39 (100%) | 0 | 6 (1–19) | 34 (87.1%) | 5 (12.8%) | 3 (0–11) | 18 (46.1%) | 21 (53.8%) | 0 (0–19) | 1 (2.5%) | 38 (97.4%) | 0 (0–1) |
| p-value | 1.000 b | 1.000 b | 0.463 b | 1.000 b | ||||||||
| 0.859 a | 0.957 a | 0.532 a | 0.854 a | |||||||||
| Margin Cutoff | Margin < Cutoff | Margin ≥ Cutoff | p-Value (Gray’s Test) | |||
|---|---|---|---|---|---|---|
| N | N Recurrence | N | N Recurrence | Death | Recurrence | |
| 2 mm | 17 | 5 | 111 | 34 | 0.280 | 0.713 |
| 3 mm | 30 | 9 | 98 | 30 | 0.916 | 0.674 |
| 4 mm | 42 | 13 | 86 | 26 | 0.476 | 0.522 |
| 5 mm | 53 | 16 | 75 | 23 | 0.947 | 0.599 |
| 6 mm | 68 | 22 | 60 | 17 | 0.428 | 0.272 |
| 7 mm | 74 | 24 | 54 | 15 | 0.516 | 0.330 |
| 8 mm | 82 | 26 | 46 | 13 | 0.240 | 0.352 |
| 9 mm | 111 | 36 | 17 | 3 | 0.156 | 0.146 |
| 10 mm | 113 | 37 | 15 | 2 | 0.090 | 0.084 |
| 11 mm | 127 | 39 | 1 | 0 | 0.716 | 0.475 |
| Variable | p-Value | Coefficient | 95 %CI | ||
|---|---|---|---|---|---|
| Age (per year) | continuously | 0.304 | −0.02 | −0.06 | 0.02 |
| Type of surgery | partial vulvectomy versus wide excision | 0.506 | 0.80 | −1.57 | 3.16 |
| complete vulvectomy versus wide excision | 0.305 | 1.27 | −1.17 | 3.71 | |
| pT | pT2 versus pT1 | 0.675 | 0.39 | −1.43 | 2.20 |
| pT3 versus pT1 | 0.966 | −0.13 | −6.13 | 5.87 | |
| Depth of invasion (mm) | continuously | 0.016 | −0.22 | −0.41 | −0.04 |
| Tumor grade | G2 versus G1 | 0.784 | 0.21 | −1.32 | 1.75 |
| G3 versus G1 | 0.502 | −0.77 | −3.03 | 1.50 | |
| Tumor diameter (mm) | continuously | 0.323 | −0.02 | −0.06 | 0.02 |
| Characteristics | Total (n = 128) | Resection Margin Category | p-Value | ||
|---|---|---|---|---|---|
| 1 to 3 mm (n = 42) | >3 to 8 mm (n = 47) | >8 mm (n = 39) | |||
| Recurrent disease | |||||
| No | 89 69.5% | 29 69.0% | 30 63.8% | 30 76.9% | 0.431 a |
| Yes | 39 30.5% | 13 31.0% | 17 36.2% | 9 23.1% | |
| Recurrent Disease/Death (50 Events) | Hazard Ratio | p-Value | 95% CI | |
|---|---|---|---|---|
| Age [every year] | 1.04 | 0.003 | 1.01 | 1.07 |
| pT2 versus pT1 | 0.89 | 0.791 | 0.38 | 2.10 |
| pT3 versus pT1 | 0.58 | 0.612 | 0.07 | 4.84 |
| Tumor diameter [mm] | 1.01 | 0.247 | 0.99 | 1.03 |
| Depth of Invasion [mm] | 1.01 | 0.817 | 0.92 | 1.11 |
| Grade 2 versus 1 | 0.75 | 0.445 | 0.35 | 1.58 |
| Grade 3 versus 1 | 1.92 | 0.273 | 0.60 | 6.19 |
| Resection margin >3–8 mm versus 1–3 mm | 1.26 | 0.515 | 0.63 | 2.53 |
| Resection margin >8 mm versus 1–3 mm | 0.93 | 0.865 | 0.43 | 2.04 |
| Recurrent disease (38 events) | Hazard Ratio | p-value | 95% CI | |
| Age [per year] | 1.04 | 0.011 | 1.01 | 1.07 |
| pT2 versus pT1 | 0.99 | 0.986 | 0.37 | 2.65 |
| pT3 versus pT1 | 0.00 | 0.997 | 0.00 | Inf |
| Tumor diameter [mm] | 1.01 | 0.496 | 0.98 | 1.03 |
| Depth of Invasion [mm] | 0.97 | 0.611 | 0.87 | 1.08 |
| Grade 2 versus 1 | 0.82 | 0.639 | 0.35 | 1.91 |
| Grade 3 versus 1 | 1.28 | 0.735 | 0.31 | 5.22 |
| Resection margin >3–8 mm versus 1–3 mm | 1.27 | 0.533 | 0.60 | 2.66 |
| Resection > 8 mm versus 1–3 mm | 0.55 | 0.208 | 0.22 | 1.39 |
| Recurrent Disease/Death (50 events) | Hazard Ratio | p-Value | 95% CI | |
|---|---|---|---|---|
| Age [per year] | 1.04 | 0.004 | 1.01 | 1.07 |
| pT2 versus pT1 | 0.85 | 0.705 | 0.37 | 1.97 |
| pT3 versus pT1 | 0.64 | 0.680 | 0.08 | 5.29 |
| Tumor diameter [mm] | 1.01 | 0.238 | 0.99 | 1.03 |
| Depth of Invasion [mm] | 1.02 | 0.746 | 0.93 | 1.11 |
| Grade 2 versus 1 | 0.77 | 0.487 | 0.36 | 1.62 |
| Grade 3 versus 1 | 1.87 | 0.299 | 0.57 | 6.07 |
| Resection margin [mm] | 0.99 | 0.806 | 0.89 | 1.09 |
| Recurrent disease (38 events) | Hazard Ratio | p-value | 95% CI | |
| Age [per year] | 1.04 | 0.013 | 1.01 | 1.07 |
| pT2 versus pT1 | 0.87 | 0.770 | 0.33 | 2.26 |
| pT3 versus pT1 | 0.00 | 0.997 | 0.00 | Inf |
| Tumor diameter [mm] | 1.01 | 0.419 | 0.99 | 1.03 |
| Depth of Invasion [mm] | 0.98 | 0.730 | 0.88 | 1.09 |
| Grade 2 versus 1 | 0.86 | 0.720 | 0.37 | 2.00 |
| Grade 3 versus 1 | 1.23 | 0.775 | 0.30 | 5.10 |
| Resection margin [mm] | 0.95 | 0.318 | 0.85 | 1.06 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taran, F.A.; Pasternak, J.; Staebler, A.; Rohner, A.; Neis, F.; Engler, T.; Oberlechner, E.; Schönfisch, B.; Juhasz-Böss, I.; Hartkopf, A.D.; et al. Tumor-Free Resection Margin Distance in the Surgical Treatment of Node-Negative Squamous Cell Cancer of the Vulva Has No Impact on Survival: Analysis of a Large Patient Cohort in a Tertiary Care Center. Cancers 2023, 15, 4110. https://doi.org/10.3390/cancers15164110
Taran FA, Pasternak J, Staebler A, Rohner A, Neis F, Engler T, Oberlechner E, Schönfisch B, Juhasz-Böss I, Hartkopf AD, et al. Tumor-Free Resection Margin Distance in the Surgical Treatment of Node-Negative Squamous Cell Cancer of the Vulva Has No Impact on Survival: Analysis of a Large Patient Cohort in a Tertiary Care Center. Cancers. 2023; 15(16):4110. https://doi.org/10.3390/cancers15164110
Chicago/Turabian StyleTaran, Florin Andrei, Jana Pasternak, Annette Staebler, Annika Rohner, Felix Neis, Tobias Engler, Ernst Oberlechner, Birgitt Schönfisch, Ingolf Juhasz-Böss, Andreas Daniel Hartkopf, and et al. 2023. "Tumor-Free Resection Margin Distance in the Surgical Treatment of Node-Negative Squamous Cell Cancer of the Vulva Has No Impact on Survival: Analysis of a Large Patient Cohort in a Tertiary Care Center" Cancers 15, no. 16: 4110. https://doi.org/10.3390/cancers15164110
APA StyleTaran, F. A., Pasternak, J., Staebler, A., Rohner, A., Neis, F., Engler, T., Oberlechner, E., Schönfisch, B., Juhasz-Böss, I., Hartkopf, A. D., Brucker, S., & Walter, C. B. (2023). Tumor-Free Resection Margin Distance in the Surgical Treatment of Node-Negative Squamous Cell Cancer of the Vulva Has No Impact on Survival: Analysis of a Large Patient Cohort in a Tertiary Care Center. Cancers, 15(16), 4110. https://doi.org/10.3390/cancers15164110







