Overexpression of MRP1/ABCC1, Survivin and BCRP/ABCC2 Predicts the Resistance of Diffuse Large B-Cell Lymphoma to R-CHOP Treatment
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Selection
2.2. Ethic Statement
2.3. Treatment
2.4. Immunohistochemistry
2.5. Statistical Analysis
3. Results
3.1. Clinical Characteristics of the Patients
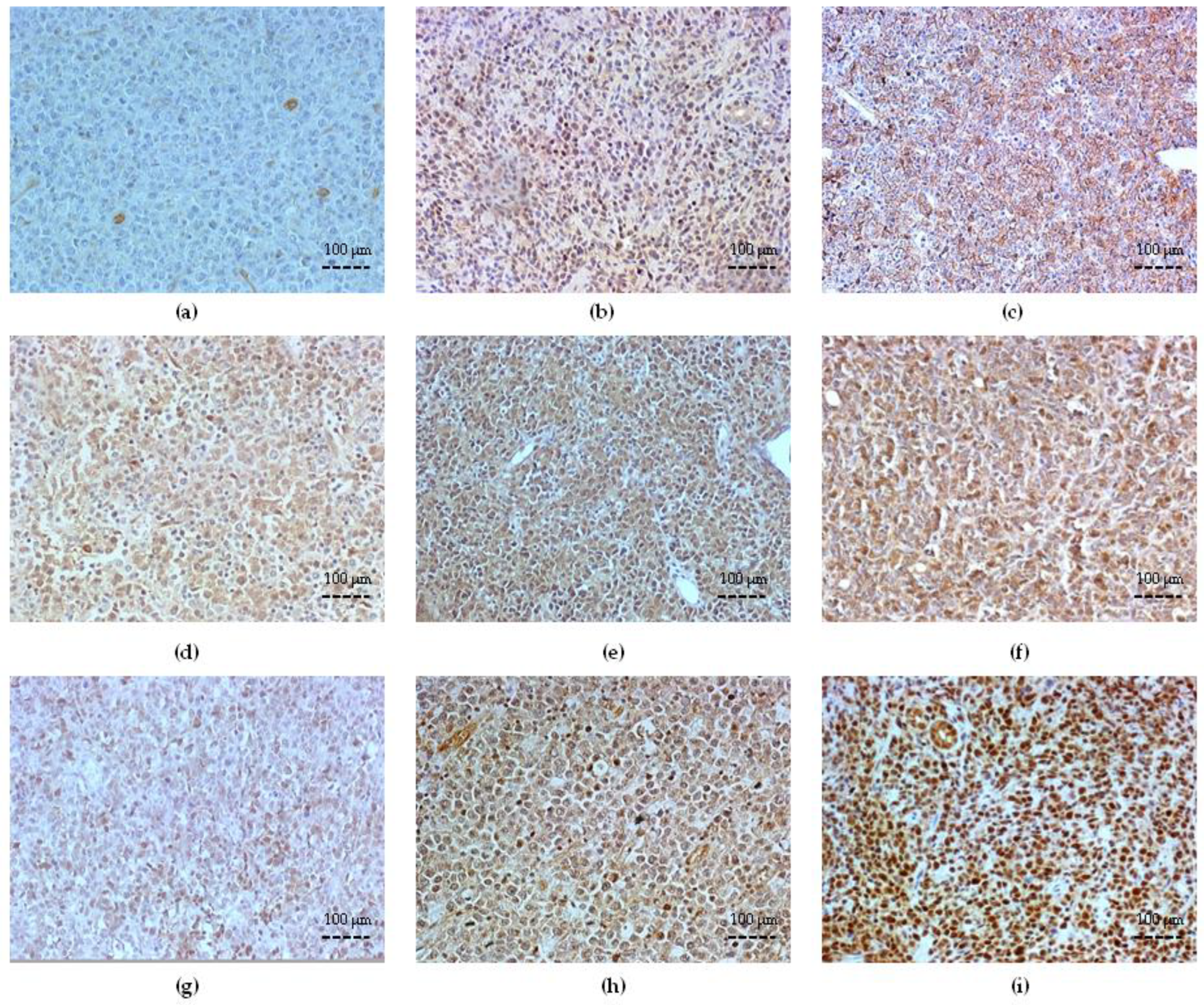
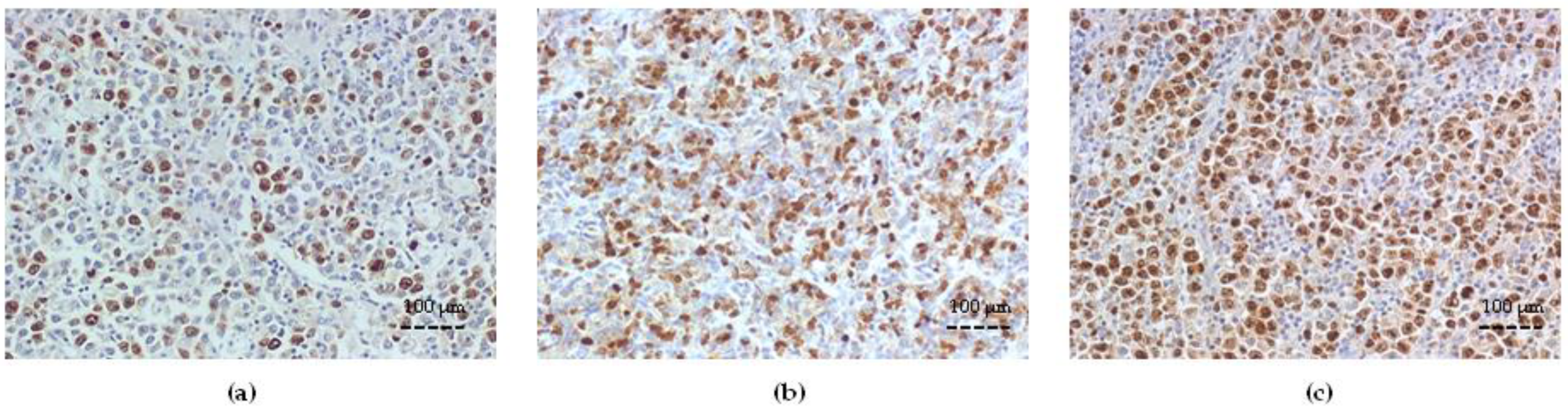
3.2. Bcl-2 Expression and Correlation with Clinicopathologic Variables
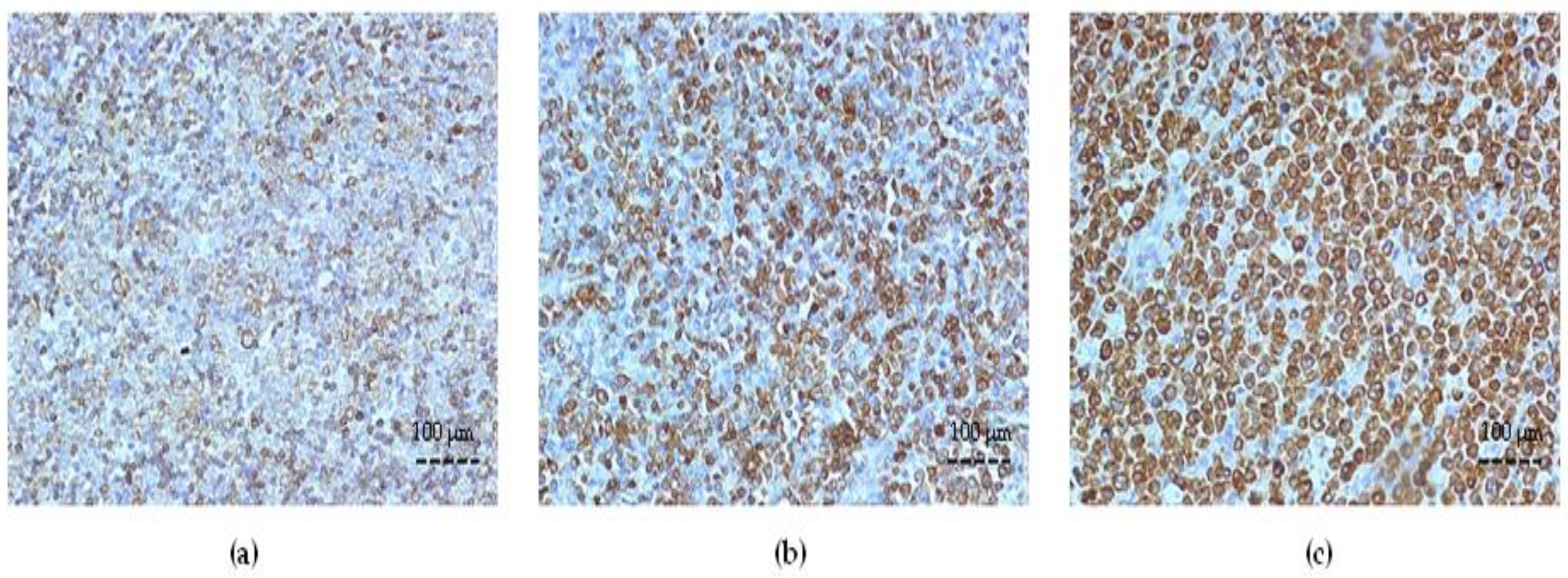
3.3. ABC Transporter Expression in DLBCL
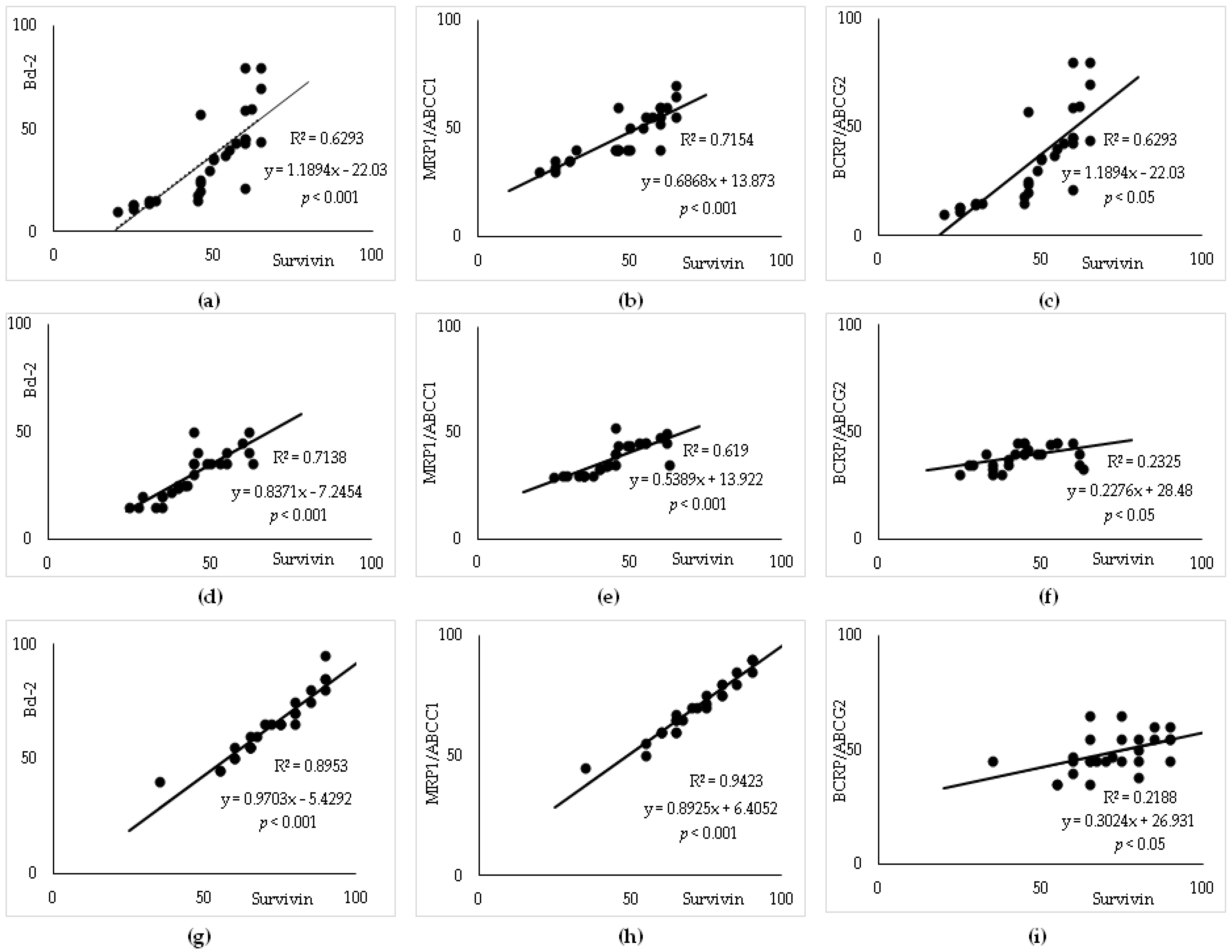
3.4. Survivin Expression and Correlation with Bcl-2 and ABC Transporters
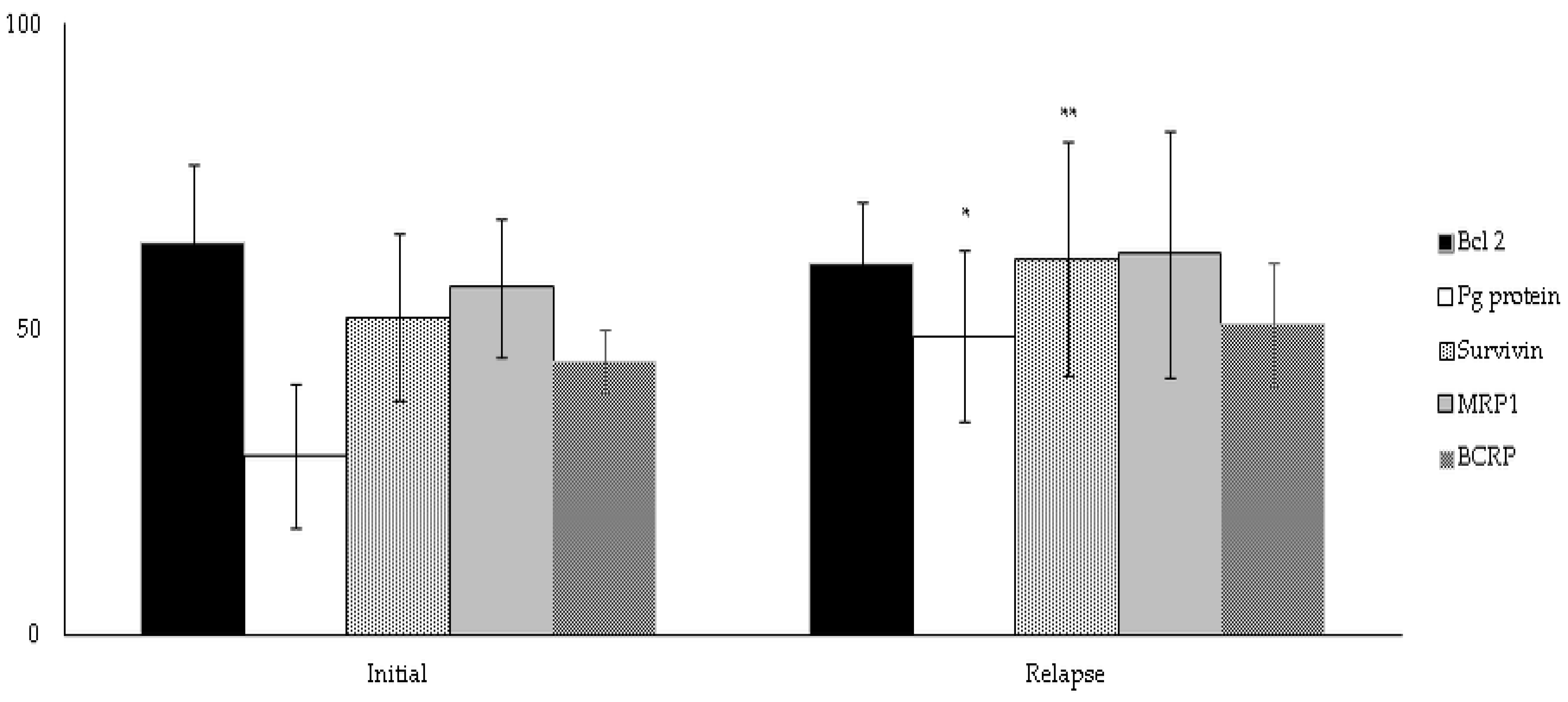
3.5. Subgroup Analysis
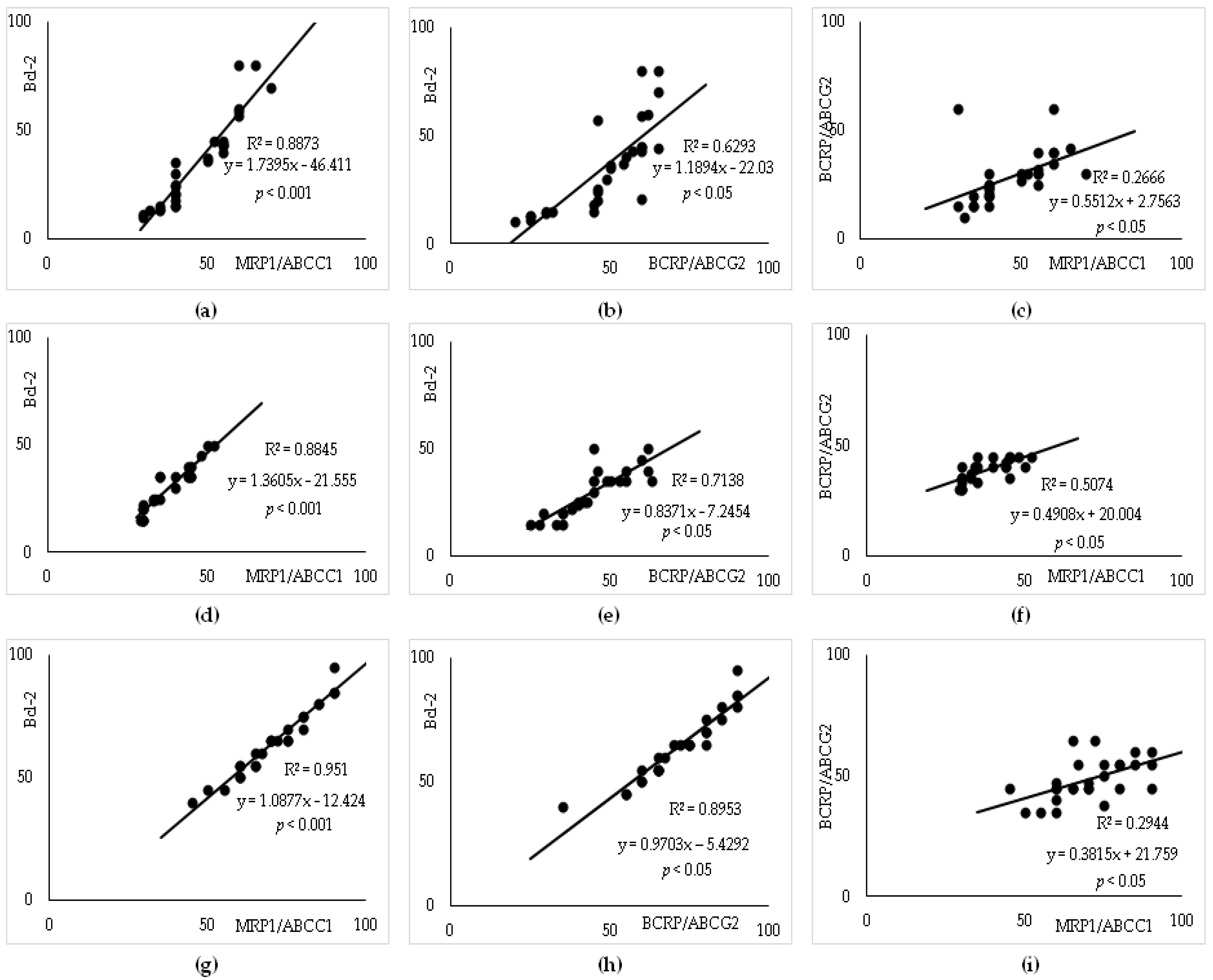
3.6. Linear Regression Analysis to Predict R-CHOP Treatment Resistance
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- He, M.Y.; Kridel, R. Treatment resistance in diffuse large B-cell lymphoma. Leukemia 2021, 35, 2151–2165. [Google Scholar] [CrossRef] [PubMed]
- Thandra, K.C.; Barsouk, A.; Saginala, K.; Padala, S.A.; Barsouk, A.; Rawla, P. Epidemiology of Non-Hodgkin’s Lymphoma. Med. Sci. 2021, 9, 5. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, R.; Wright, G.W.; Huang, D.W.; Johnson, C.A.; Phelan, J.D.; Wang, J.Q.; Roulland, S.; Kasbekar, M.; Young, R.M.; Shaffer, A.L.; et al. Genetics and Pathogenesis of Diffuse Large B-Cell Lymphoma. N. Engl. J. Med. 2018, 378, 1396–1407. [Google Scholar] [CrossRef]
- Choueiry, F.; Singh, S.; Sircar, A.; Laliotis, G.; Sun, X.; Chavdoula, E.; Zhang, S.; Helmig-Mason, J.; Hart, A.; Epperla, N.; et al. Integration of Metabolomics and Gene Expression Profiling Elucidates IL4I1 as Modulator of Ibrutinib Resistance in ABC-Diffuse Large B Cell Lymphoma. Cancers 2021, 13, 2146. [Google Scholar] [CrossRef]
- Salles, G.; Barrett, M.; Foà, R.; Maurer, J.; O’Brien, S.; Valente, N.; Wenger, M.; Maloney, D.G. Rituximab in B-Cell Hematologic Malignancies: A Review of 20 Years of Clinical Experience. Adv. Ther. 2017, 34, 2232–2273. [Google Scholar] [CrossRef] [PubMed]
- Pfreundschuh, M.; Kuhnt, E.; Trümper, L.; Osterborg, A.; Trneny, M.; Shepherd, L.; Gill, D.S.; Walewski, J.; Pettengell, R.; Jaeger, U.; et al. CHOP-like chemotherapy with or without rituximab in young patients with good-prognosis diffuse large-Bcell lymphoma: 6-year results of an open-label randomised study of the MabThera International Trial (MInT) Group. Lancet Oncol. 2011, 12, 1013–1022. [Google Scholar] [CrossRef] [PubMed]
- Lenz, G. Insights into the Molecular Pathogenesis of Activated B-Cell-like Diffuse Large B-Cell Lymphoma and Its Therapeutic Implications. Cancers 2015, 7, 811–822. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Li, L.R. R-CHOP resistance in diffuse large B-cell lymphoma: Biological and molecular mechanisms. Chin. Med. J. 2020, 134, 253–260. [Google Scholar] [CrossRef]
- Rovira, J.; Valera, A.; Colomo, L.; Setoain, X.; Rodríguez, S.; Martínez-Trillos, A.; Giné, E.; Dlouhy, I.; Magnano, L.; Gaya, A.; et al. Prognosis of Patients with Diffuse Large B Cell Lymphoma Not Reaching Complete Response or Relapsing after Frontline Chemotherapy or Immunochemotherapy. Ann. Hematol. 2015, 94, 803–812. [Google Scholar] [CrossRef]
- Crump, M.; Neelapu, S.S.; Farooq, U.; Van Den Neste, E.; Kuruvilla, J.; Westin, J.; Link, B.K.; Hay, A.; Cerhan, J.R.; Zhu, L.; et al. Outcomes in Refractory Diffuse Large B-Cell Lymphoma: Results from the International SCHOLAR-1 Study. Blood 2017, 130, 1800–1808. [Google Scholar] [CrossRef]
- Berendsen, M.R.; Stevens, W.B.C.; van den Brand, M.; van Krieken, J.H.; Scheijen, B. Molecular Genetics of Relapsed Diffuse Large B-Cell Lymphoma: Insight into Mechanisms of Therapy Resistance. Cancers 2020, 12, 3553. [Google Scholar] [CrossRef] [PubMed]
- Pi, M.; Kuang, H.; Yue, C.; Yang, Q.; Wu, A.; Li, Y.; Assaraf, Y.G.; Yang, D.H.; Wu, S. Targeting metabolism to overcome cancer drug resistance: A promising therapeutic strategy for diffuse large B cell lymphoma. Drug Resist. Updat. 2022, 61, 100822. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Gu, Y.; Chen, B. Drug-Resistance Mechanism and New Targeted Drugs and Treatments of Relapse and Refractory DLBCL. Cancer Manag. Res. 2023, 15, 245–255. [Google Scholar] [CrossRef]
- Kathawala, R.J.; Gupta, P.; Ashby, C.R.; Chen, Z.-S. The modulation of ABC transporter-mediated multidrug resistance in cancer: A review of the past decade. Drug Resist. Updat. 2015, 18, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.R.; Kunkalla, K.; Qu, C.; Schlette, E.; Neelapu, S.S.; Samaniego, F.; Vega, F. ABCG2 is a direct transcriptional target of hedgehog signaling and involved in stroma-induced drug tolerance in diffuse large B-cell lymphoma. Oncogene 2011, 30, 4874–4886. [Google Scholar] [CrossRef]
- Ohsawa, M.; Ikura, Y.; Fukushima, H.; Shirai, N.; Sugama, Y.; Suekane, T.; Hirayama, M.; Hino, M.; Ueda, M. Immunohistochemical expression of multidrug resistance proteins as a predictor of poor response to chemotherapy and prognosis in patients with nodal diffuse large B-cell lymphoma. Oncology 2005, 68, 422–431. [Google Scholar] [CrossRef]
- Greaves, W.; Xiao, L.; Sanchez-Espiridion, B.; Kunkalla, K.; Dave, K.S.; Liang, C.S.; Singh, R.R.; Younes, A.; Medeiros, L.J.; Vega, F. Detection of ABCC1 expression in classical Hodgkin lymphoma is associated with increased risk of treatment failure using standard chemotherapy protocols. J. Hematol. Oncol. 2012, 5, 7. [Google Scholar] [CrossRef]
- Wang, J.-Q.; Li, J.Y.; Teng, Q.-X.; Lei, Z.-N.; Ji, N.; Cui, Q.; Zeng, L.; Pan, Y.; Yang, D.-H.; Chen, Z.-S. Venetoclax, a BCL-2 inhibitor, enhances the efficacy of chemotherapeutic agents in wild-type ABCG2-overexpression-mediated MDR cancer cells. Cancers 2020, 12, 466. [Google Scholar] [CrossRef]
- Liu, K.; Song, J.; Yan, Y.; Zou, K.; Che, Y.; Wang, B.; Li, Z.; Yu, W.; Guo, W.; Zou, L.; et al. Melatonin increases the chemosensitivity of diffuse large B-cell lymphoma cells to epirubicin by inhibiting P-glycoprotein expression via the NF-κB pathway. Transl. Oncol. 2021, 14, 100876. [Google Scholar] [CrossRef]
- Liu, M.; Gao, H.; He, Y.; Sun, X.; Zhang, L. Upregulation of miR-101-3p Overcomes Ibrutinib Resistance by Targeting ABCC5 in Diffuse Large B-Cell Lymphoma (DLBCL). J. Hard. Tissue. Biol. 2023, 32, 11–20. [Google Scholar] [CrossRef]
- Tsang, T.J.; Hsueh, Y.C.; Wei, E.I.; Lundy, D.J.; Cheng, B.; Chen, Y.T.; Wang, S.S.; Hsieh, P.C.H. Subcellular localization of survivin determines its function in cardiomyocytes. Theranostics 2017, 7, 4577–4590. [Google Scholar] [CrossRef]
- Sah, N.K.; Seniya, C. Survivin splice variants and their diagnostic significance. Tumor Biol. 2015, 36, 6623–6631. [Google Scholar] [CrossRef]
- Bernardo, P.S.; Lemos, L.G.T.; de Moraes, G.N.; Maia, R.C. Unraveling survivin expression in chronic myeloid leukemia: Molecular interactions and clinical implications. Blood Rev. 2020, 43, 100671. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, J.; Sui, X.; Li, Y.; Lu, K.; Fang, X.; Jiang, Y.; Wang, X. Prognostic and Clinicopathological Value of Survivin in Diffuse Large B-cell Lymphoma: A Meta-Analysis. Medicine 2015, 94, e1432. [Google Scholar] [CrossRef]
- Mitrović, Z.; Ilić, I.; Aurer, I.; Kinda, S.B.; Radman, I.; Dotlić, S.; Ajdukovic, R.; Labar, B. Prognostic Significance of Survivin and Caspase-3 Immunohistochemical Expression in Patients with Diffuse Large B-cell Lymphoma Treated with Rituximab and CHOP. Pathol. Oncol. Res. 2011, 17, 243–247. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Xu-Monette, Z.Y.; Cao, X.; Manyam, G.C.; Wang, X.; Tzankov, A.; Xia, Y.; Li, X.; Visco, C.; Sun, R.; et al. Prognostic and biological significance of survivin expression in patients with diffuse large B-cell lymphoma treated with rituximab-CHOP therapy. Mod. Pathol. 2015, 28, 1297–1314. [Google Scholar] [CrossRef]
- Swerdlow, S.H.; Campo, E.; Pileri, S.A.; Harris, N.L.; Stein, H.; Siebert, R.; Advani, R.; Ghielmini, M.; Salles, G.A.; Zelenetz, A.D.; et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood 2016, 127, 2375–2390. [Google Scholar] [CrossRef]
- Lister, T.A.; Crowther, D.; Sutcliffe, S.B.; Glatstein, E.; Canellos, G.P.; Young, R.C.; Rosenberg, S.; Coltman, C.A.; Tubiana, M. Report of a committee convened to discuss the evaluation and staging of patients with Hodgkin’s disease: Cotswolds meeting. J. Clin. Oncol. 1989, 7, 1630–1636. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, L.H.; Litière, S.; de Vries, E.; Ford, R.; Gwyther, S.; Mandrekar, S.; Shankar, L.; Bogaerts, J.; Chen, A.; Dancey, J.; et al. RECIST 1.1-Update and clarification: From the RECIST committee. Eur. J. Cancer 2016, 62, 132–137. [Google Scholar] [CrossRef] [PubMed]
- Cheson, B.D.; Horning, S.J.; Coiffier, B.; Shipp, M.A.; Fisher, R.I.; Connors, J.M.; Lister, T.A.; Vose, J.; Grillo-López, A.; Hagenbeek, A.; et al. Report of an international workshop to standardize response criteria for non-Hodgkin’s lymphomas. NCI Sponsored International Working Group. J. Clin. Oncol. 1999, 17, 1244. [Google Scholar] [CrossRef]
- Hans, C.P.; Weisenburger, D.D.; Greiner, T.C.; Gascoyne, R.D.; Delabie, J.; Ott, G.; Müller-Hermelink, H.; Campo, E.; Braziel, R.M.; Jaffe, E.S.; et al. Confirmation of the molecular classification of diffuse large B-cell lymphoma by immunohistochemistry using a tissue microarray. Blood 2004, 103, 275–282. [Google Scholar] [CrossRef]
- International Non-Hodgkin’s Lymphoma Prognostic Factors Project. A predictive model for aggressive non-Hodgkin’s lymphoma. N. Engl. J. Med. 1993, 329, 987–994. [Google Scholar] [CrossRef] [PubMed]
- Nežić, L.; Škrbić, R.; Amidžić, L.; Gajanin, R.; Kuča, K.; Jaćević, V. Simvastatin Protects Cardiomyocytes Against Endotoxin-induced Apoptosis and Up-regulates Survivin/NF-kB/p65 Expression. Sci. Rep. 2018, 8, 14652. [Google Scholar] [CrossRef] [PubMed]
- Nežić, L.; Amidžić, L.; Škrbić, R.; Gajanin, R.; Nepovimova, E.; Vališ, M.; Kuča, K.; Jaćević, V. Simvastatin Inhibits Endotoxin-Induced Apoptosis in Liver and Spleen Through Up-Regulation of Survivin/NF-kB/p65 Expression. Front. Pharmacol. 2019, 10, 54. [Google Scholar] [CrossRef] [PubMed]
- Szczuraszek, K.; Materna, V.; Halon, A.; Mazur, G.; Wróbel, T.; Kuliczkowski, K.; Maciejczyk, A.; Zabel, M.; Drag, M.; Dietel, M.; et al. Positive correlation between cyclooxygenase-2 and ABC-transporter expression in non-Hodgkin’s lymphomas. Oncol. Rep. 2009, 22, 1315–1323. [Google Scholar] [CrossRef][Green Version]
- Tsuyama, N.; Sakata, S.; Baba, S.; Mishima, Y.; Nishimura, N.; Ueda, K.; Yokoyama, M.; Terui, Y.; Hatake, K.; Kitagawa, M.; et al. BCL2 expression in DLBCL: Reappraisal of immunohistochemistry with new criteria for therapeutic biomarker evaluation. Blood 2017, 130, 489–500. [Google Scholar] [CrossRef]
- Li, L.; Li, Y.; Que, X.; Gao, X.; Gao, Q.; Yu, M.; Ma, K.; Xi, Y.; Wang, T. Prognostic significances of overexpression MYC and/or BCL2 in R-CHOP-treated diffuse large B-cell lymphoma: A Systematic review and meta-analysis. Sci. Rep. 2018, 8, 6267. [Google Scholar] [CrossRef]
- Krull, J.E.; Wenzl, K.; Hartert, K.T.; Manske, M.K.; Sarangi, V.; Maurer, M.J.; Larson, M.C.; Nowakowski, G.S.; Ansell, S.M.; McPhail, E.; et al. Somatic copy number gains in MYC, BCL2, and BCL6 identifies a subset of aggressive alternative-DH/TH DLBCL patients. Blood Cancer J. 2020, 10, 117. [Google Scholar] [CrossRef]
- Alaggio, R.; Amador, C.; Anagnostopoulos, I.; Attygalle, A.D.; Araujo, I.B.O.; Berti, E.; Bhagat, G.; Borges, A.M.; Boyer, D.; Calaminici, M.; et al. The 5th edition of the World Health Organization Classification of Haematolymphoid Tumours: Lymphoid Neoplasms. Leukemia 2022, 36, 1720–1748. [Google Scholar] [CrossRef]
- Li, L.; Zhang, X.; Zhang, T.; Song, Z.; Hu, G.; Li, W.; Li, L.; Qiu, L.; Qian, Z.; Zhou, S.; et al. Prognostic Significance of BCL-2 and BCL-6 Expression in MYC-positive DLBCL. Clin. Lymphoma Myeloma Leuk. 2018, 18, e381–e389. [Google Scholar] [CrossRef]
- Barraclough, A.; Alzahrani, M.; Ettrup, M.S.; Bishton, M.; van Vliet, C.; Farinha, P.; Gould, C.; Birch, S.; Sehn, L.H.; Sovani, V.; et al. COO and MYC/BCL2 status do not predict outcome among patients with stage I/II DLBCL: A retrospective multicenter study. Blood Adv. 2019, 3, 2013–2021. [Google Scholar] [CrossRef] [PubMed]
- Camicia, R.; Winkler, H.C.; Hassa, P.O. Novel drug targets for personalized precision medicine in relapsed/refractory diffuse large B-cell lymphoma: A comprehensive review. Mol. Cancer 2015, 14, 207. [Google Scholar] [CrossRef] [PubMed]
- Andreadis, C.; Gimotty, P.A.; Wahl, P.; Hammond, R.; Houldsworth, J.; Schuster, S.J.; Rebbeck, T.R. Members of the glutathione and ABC-transporter families are associated with clinical outcome in patients with diffuse large B-cell lymphoma. Blood 2007, 109, 3409–3416. [Google Scholar] [CrossRef]
- Rujirojindakul, P.; Aiempanakit, K.; Kayasut, K.; Lekhakula, A.; Sriplung, H. No Prognostic Impact of p53 and P-Glycoprotein Expression in Patients with Diffuse Large B-Cell Lymphoma. ISRN Oncol. 2011, 2011, 670358. [Google Scholar] [CrossRef]
- Tabata, M.; Tsubaki, M.; Takeda, T.; Tateishi, K.; Tsurushima, K.; Imano, M.; Satou, T.; Ishizaka, T.; Nishida, S. Dasatinib reverses drug resistance by downregulating MDR1 and Survivin in Burkitt lymphoma cells. BMC Complement. Med. Ther. 2020, 20, 84. [Google Scholar] [CrossRef]
- Steidl, C.; Telenius, A.; Shah, S.P.; Farinha, P.; Barclay, L.; Boyle, M.; Connors, J.M.; Horsman, D.E.; Gascoyne, R.D. Genome-wide copy number analysis of Hodgkin Reed-Sternberg cells identifies recurrent imbalances with correlations to treatment outcome. Blood 2010, 116, 418–427. [Google Scholar] [CrossRef]
- Gündüz, E.; Dinçer, M.; Yıldız, G.; Bal, C.; Gülbaş, Z. The frequency and clinical relevance of multidrug resistance protein expression in patients with lymphoma. Turk. J. Haematol. 2012, 29, 120–129. [Google Scholar] [CrossRef] [PubMed]
- Markovic, O.; Marisavljevic, D.; Cemerikic-Martinovic, V.; Martinovic, T.; Filipovic, B.; Stanisavljevic, D.; Zivković, R.; Hajder, J.; Stanisavljevic, N.; Mihaljevic, B. Survivin expression in patients with newly diagnosed nodal diffuse large B cell lymphoma (DLBCL). Med. Oncol. 2012, 29, 3515–3521. [Google Scholar] [CrossRef]
- Liu, R.; Chen, Y.; Liu, G.; Li, C.; Song, Y.; Cao, Z.; Li, W.; Hu, J.; Lu, C.; Liu, Y. PI3K/AKT pathway as a key link modulates the multidrug resistance of cancers. Cell Death Dis. 2020, 11, 797. [Google Scholar] [CrossRef]
| Characteristics | Patients with DLBCL, n = 73 (%) |
|---|---|
| Age (median) | 61 |
| range | 19–83 |
| ≥ 60 years | (46.6) |
| Gender | |
| Male/female | 45 (61.6)/28 (38.4) |
| Treatment response | |
| Complete remission | 23 (31.5) |
| Relapsed disease | 30 (41.1) |
| Primary refractory disease | 20 (27.4) |
| Stage | |
| I | 4 (5.5) |
| II | 4 (5.5) |
| III | 14 (19.2) |
| IV | 51 (69.8) |
| PS (ECOG) > 1 | 38 (52.1) |
| B symptoms | 47 (64.4) |
| R-IPI score | |
| Low (0) | 1 (1.4) |
| Intermediate (1–2) | 35 (47.9) |
| High (>2) | 37 (50.7) |
| Extranodal localization > 1 | 18 (24.7) |
| LDH elevated | 46 (63.0) |
| β2 microglobulin | 31 (41.5) |
| Treatment | |
| R-CHOP/ Equivalent R-CHOP | 63 (86.3)/10 (13.7) |
| GBC/non-GBC subtype | 38 (52.1)/35(47.9) |
| Model | Unstandardized Coefficients | Standardized Coefficients | p | ||
|---|---|---|---|---|---|
| B | S.E. | Beta | t | ||
| All Patients with DLBCL (n = 73) | |||||
| Constant | −0.774 | 0.309 | −2.505 | 0.014 | |
| ECOG | 0.412 | 0.102 | 0.316 | 4.062 | <0.001 |
| MRP1/ABCC1 | 0.024 | 0.006 | 0.327 | 3.907 | <0.001 |
| Survivin | 0.027 | 0.006 | 0.332 | 3.988 | <0.001 |
| BCRP/ABCG2 | 0.033 | 0.007 | 0.374 | 4.740 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mandić, D.; Nežić, L.; Amdžić, L.; Vojinović, N.; Gajanin, R.; Popović, M.; Đeri, J.; Balint, M.T.; Dumanović, J.; Milovanović, Z.; et al. Overexpression of MRP1/ABCC1, Survivin and BCRP/ABCC2 Predicts the Resistance of Diffuse Large B-Cell Lymphoma to R-CHOP Treatment. Cancers 2023, 15, 4106. https://doi.org/10.3390/cancers15164106
Mandić D, Nežić L, Amdžić L, Vojinović N, Gajanin R, Popović M, Đeri J, Balint MT, Dumanović J, Milovanović Z, et al. Overexpression of MRP1/ABCC1, Survivin and BCRP/ABCC2 Predicts the Resistance of Diffuse Large B-Cell Lymphoma to R-CHOP Treatment. Cancers. 2023; 15(16):4106. https://doi.org/10.3390/cancers15164106
Chicago/Turabian StyleMandić, Danijela, Lana Nežić, Ljiljana Amdžić, Nataša Vojinović, Radoslav Gajanin, Miroslav Popović, Jugoslav Đeri, Milena Todorović Balint, Jelena Dumanović, Zoran Milovanović, and et al. 2023. "Overexpression of MRP1/ABCC1, Survivin and BCRP/ABCC2 Predicts the Resistance of Diffuse Large B-Cell Lymphoma to R-CHOP Treatment" Cancers 15, no. 16: 4106. https://doi.org/10.3390/cancers15164106
APA StyleMandić, D., Nežić, L., Amdžić, L., Vojinović, N., Gajanin, R., Popović, M., Đeri, J., Balint, M. T., Dumanović, J., Milovanović, Z., Grujić-Milanović, J., Škrbić, R., & Jaćević, V. (2023). Overexpression of MRP1/ABCC1, Survivin and BCRP/ABCC2 Predicts the Resistance of Diffuse Large B-Cell Lymphoma to R-CHOP Treatment. Cancers, 15(16), 4106. https://doi.org/10.3390/cancers15164106






