Tumor Bed Boost Radiotherapy in the Conservative Treatment of Breast Cancer: A Review of Intra-Operative Techniques and Outcomes
Abstract
Simple Summary
Abstract
1. Introduction
2. Bed Boost in Breast Cancer
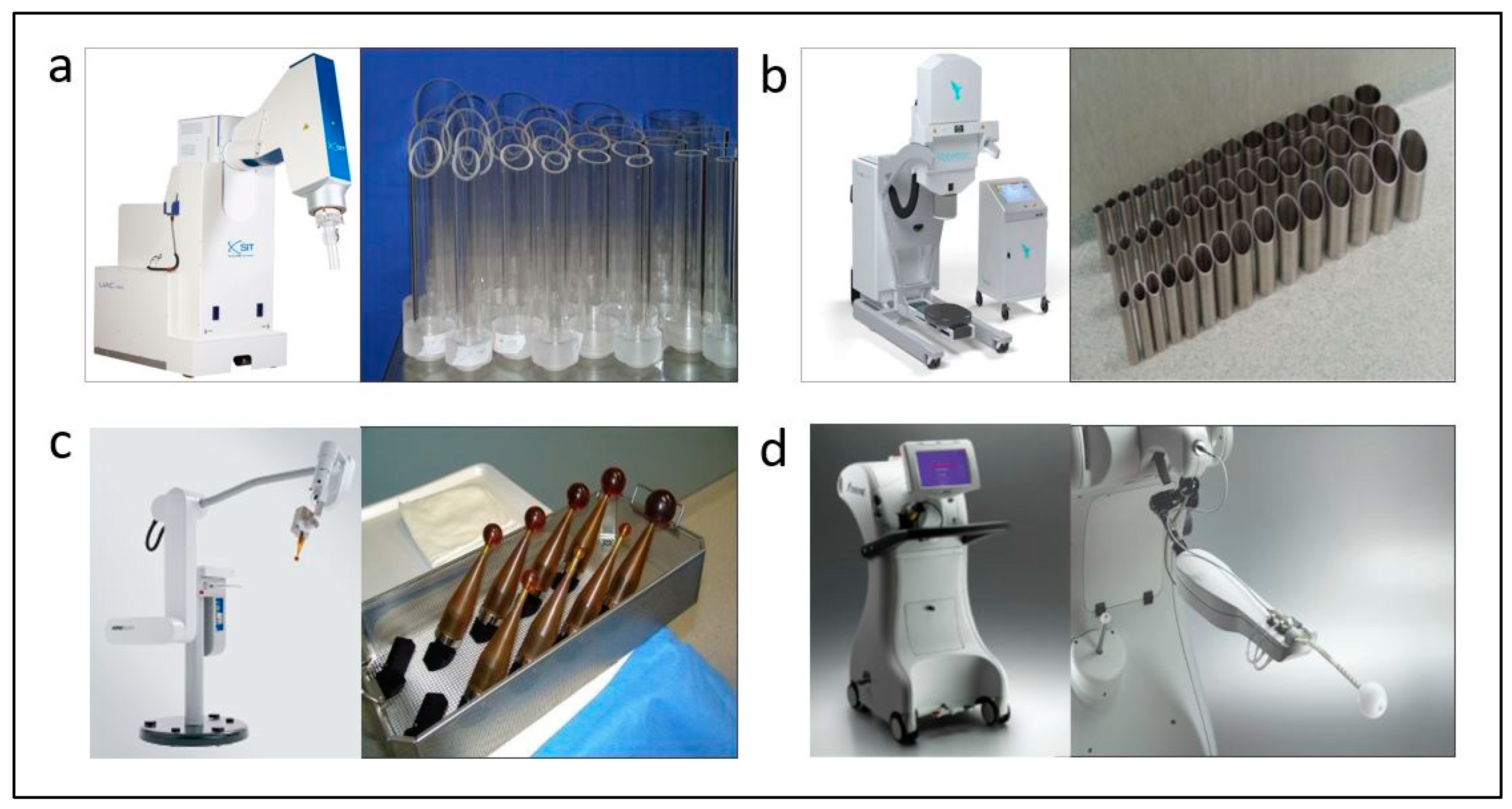
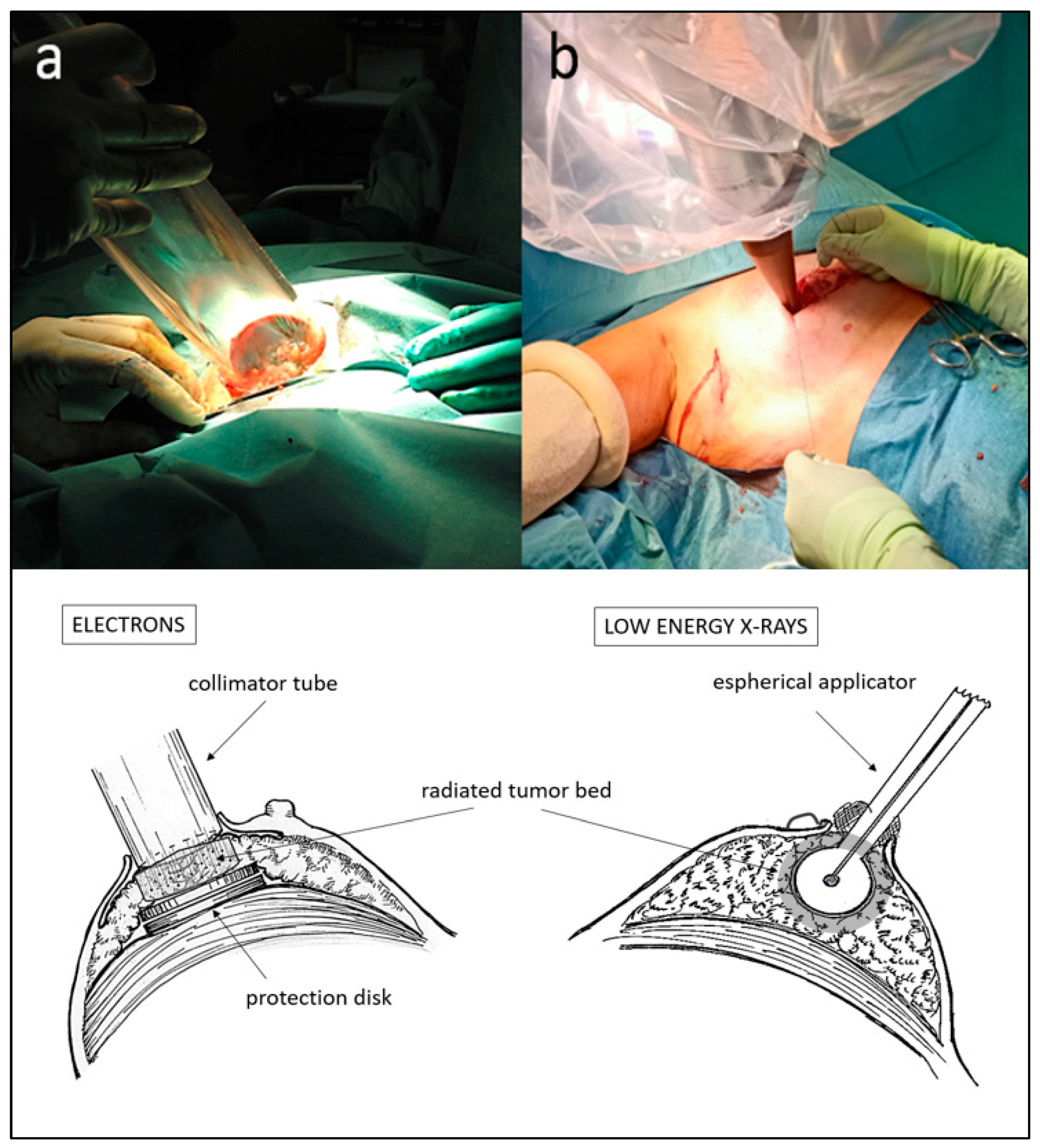
3. IORT in Conservative Breast Cancer Treatment
| Study | No. Patients | IORT Technique | IORT Dose (Single Fraction, Gy) | Median Follow-Up (Years) | Rate of Local Recurrence with IORT (%) | Rate of Local Recurrence with WBI (%) | Hazard Ratio (95% CI, p Value) |
|---|---|---|---|---|---|---|---|
| ELIOT [26] | 1305 | Electrons | 21 | 12.4 | 11 | 2 | 4.62 (2.68–7.95, p < 0.0001) |
| TARGIT-A [31] | 3451 | 50 kV X-rays | 20 | 8.6 | 2.11 | 0.95 | 1.13 (0.91–1.41, p = 0.28) |
4. BED Boost with IORT
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Veronesi, U.; Marubini, E.; Mariani, L.; Galimberti, V.; Luini, A.; Veronesi, P.; Salvadori, B.; Zucali, R. Radiotherapy after breast -conserving surgery in small breast carcinoma: Long-term results of a randomized trial. Ann. Oncol. 2001, 12, 997–1003. [Google Scholar] [CrossRef] [PubMed]
- Fisher, B.; Anderson, S.; Bryant, J.; Margolese, R.G.; Deutsch, M.; Fisher, E.R.; Jeong, J.H.; Wolmark, N. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy and lumpectomy plus irradiation for the treatment of invasive breast cancer. N. Engl. J. Med. 2002, 16, 1233–1241. [Google Scholar] [CrossRef]
- Almahariq, M.F.; Quinn, T.J.; Siddiqui, Z.; Jawad, M.S.; Chen, P.Y.; Gustafson, G.S.; Dilworth, J.T. Breast conserving therapy is associated with improved overall survival compared to mastectomy in early-stage, lymph node-negative breast cancer. Radiother. Oncol. 2020, 142, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Whelan, T.; MacKenzie, R.; Julian, J.; Levine, M.; Shelley, W.; Grimard, L.; Lada, B.; Lukka, H.; Perera, F.; Fyles, A.; et al. Randomized trial of breast irradiation schedules after lumpectomy for women with lymph node-negative breast cancer. J. Natl. Cancer Inst. 2002, 94, 1143–1150. [Google Scholar] [CrossRef] [PubMed]
- START Trialists’ Group; Bentzen, S.M.; Agrawal, R.K.; Aird, E.G.A.; Barrett, J.M.; Barrett-Lee, P.J.; Bentzen, S.M.; Bliss, J.M.; Brown, J.; Dewar, J.A.; et al. The UK Standardisation of Breast Radiotherapy (START) Trial B of radiotherapy hypofractionation for treatment of early breast cancer: A randomised trial. Lancet 2008, 371, 1098–1107. [Google Scholar]
- Murray Brunt, A.; Haviland, J.S.; Wheatley, D.A.; Sydenham, M.A.; Alhasso, A.; Bloomfield, D.J.; Chan, C.; Churn, M.; Cleator, S.; Coles, C.E.; et al. Hypofractionated breast radiotherapy for 1 week versus 3 weeks (FAST-Forward): 5-year efficacy and late normal tissue effects results from a multicentre, non-inferiority, randomised, phase 3 trial. Lancet 2020, 395, 1613–1626. [Google Scholar] [CrossRef]
- Holland, R.; Veling, S.H.; Mravunac, M.; Hendriks, J.H. Histologic multifocality of Tis, T1-2 breast carcinomas: Implications for clinical trials of breast-conserving surgery. Cancer 1985, 56, 979–990. [Google Scholar] [CrossRef]
- Romestaing, P.; Lehingue, Y.; Carrie, C.; Coquard, R.; Montbarbon, X.; Ardiet, J.M.; Mamelle, N.; Gérard, J.P. Role of 10-Gy boost in the conservative treatment of early breast cancer. Results of a randomizerd clinical trial in Lyon, France. J. Clin. Oncol. 1997, 15, 963–968. [Google Scholar] [CrossRef]
- Bartelink, H.; Maingon, P.; Poortmans, P.; Weltens, C.; Fourquet, A.; Jager, J.; Schinagl, D.; Oei, B.; Rodenhuis, C.; Horiot, J.-C.; et al. European Organisation for Research and Treatment of Cancer Radiation Oncology and Breast Cancer Groups. Whole-breast irradiation with or without a boost for patients treated with breast-conserving surgery for early breast cancer: 20-year follow-up of a randomised phase 3 trial. Lancet Oncol. 2015, 16, 47–56. [Google Scholar]
- Haviland, J.S.; Owen, J.R.; Dewar, J.A.; Agrawal, R.K.; Barrett, J.; Barrett-Lee, P.J.; Dobbs, J.; Hopwood, P.; Lawton, P.A.; Magee, B.J.; et al. The UK Standardisation of Breast Radiotherapy (START) trials of radiotherapy hypofractionation for treatment of early breast cancer: 10-year follow-up results of two randomised controlled trials. Lancet Oncol. 2013, 14, 1086–1094. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Khan, A.J.; Yegya-Raman, N.; Sayan, M.; Ahlawat, S.; Ohri, N.; Goyal, S.; Moore, D.F.; Eladoumikdachi, F.; Toppmeyer, D.; et al. 5-year results of a prospective phase 2 trial evaluating 3-week hypofractionated whole breast radiation therapy inclusive of a sequential boost. Int. J. Radiat. Oncol. Biol. Phys. 2019, 105, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Sanz, J.; Rodríguez, N.; Foro, P.; Dengra, J.; Reig, A.; Pérez, P.; Membrive, I.; Ortiz, A.; Codinach, M.; Algara, M. Hypofractionated boost after whole breast irradiation in breast carcinoma: Chronic toxicity results and cosmesis. Clin. Transl. Oncol. 2017, 19, 464–469. [Google Scholar] [CrossRef]
- Forster, T.; Köhler, C.; Dorn, M.; Häfner, M.F.; Arians, N.; König, L.; Ben Harrabi, S.; Schlampp, I.; Weykamp, F.; Meixner, E.; et al. Noninferiority of Local Control and Comparable Toxicity of Intensity Modulated Radiation Therapy With Simultaneous Integrated Boost in Breast Cancer: 5-Year Results of the IMRT-MC2 Phase III Trial. Int. J. Radiat. Oncol. Biol. Phys. 2023. in press. Available online: https://linkinghub.elsevier.com/retrieve/pii/S0360301623005230 (accessed on 20 June 2023).
- Franco, P.; Cante, D.; Sciacero, P.; Girelli, G.; La Porta, M.R.; Ricardi, U. Tumor bed boost integration during whole breast radiotherapy: A review of the current evidence. Breast Care 2015, 10, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Paunesku, T.; Woloschak, G.E. Future Directions of Intraoperative Radiation Therapy: A Brief Review. Front. Oncol. 2017, 7, 300. [Google Scholar] [CrossRef] [PubMed]
- Ling, J.; Luo, Y.; Sun, C.; Dong, Z.; Wu, R.; Tang, X.; Du, N.; Zhu, R.; Chen, S.; Liu, M.; et al. Live intraoperative diagnostis of hepatic metastasis via HDACs targeting molecular theranostic agent. Chem. Eng. J. 2021, 406, 126900. [Google Scholar] [CrossRef]
- Livi, L.; Meattini, I.; Marrazzo, L.; Simontacchi, G.; Pallotta, S.; Saieva, C.; Paiar, F.; Scotti, V.; Cardillo, C.D.L.; Bastiani, P.; et al. Accelerated partial breast irradiation using intensity-modulated radiotherapy versus whole breast irradiation: 5-year survival analysis of a phase 3 randomised controlled trial. Eur. J. Cancer 2015, 51, 451–463. [Google Scholar] [CrossRef] [PubMed]
- Polgár, C.; Van Limbergen, E.; Pötter, R.; Kovács, G.; Polo, A.; Lyczek, J.; Hildebrandt, G.; Niehoff, P.; Guinot, J.L.; Guedea, F.; et al. Patient selection for accelerated partial-breast irradiation (APBI) after breast conserving surgery: Recommendations of the Groupe European de Curie therapy-European Society for Therapy Radiology and Oncology (GEC-ESTRO) breast cancer working group based on clinical evidence (2009). Radiother. Oncol. 2010, 94, 264–273. [Google Scholar] [PubMed]
- Correa, C.; Harris, E.E.; Leonardi, M.C.; Smith, B.D.; Taghian, A.G.; Thompson, A.M.; White, J.; Harris, J.R. Accelerated partial breast irradiation: Executive summary for the update of ASTRO evidence-based consensus statement. Pract. Radiat. Oncol. 2017, 7, 73–79. [Google Scholar] [CrossRef]
- Strnad, V.; Ott, O.J.; Hildebrandt, G.; Kauer-Dorner, D.; Knauerhase, H.; Major, T.; Lyczek, J.; Guinot, J.L.; Dunst, J.; Miguelez, C.G.; et al. 5-year results of accelerated partial breast irradiation using sole interstitial multicatheter brachytherapy versus whole-breast irradiation with boost after breast-conserving surgery for low-risk invasive and in situ carcinoma of the female breast: A randomised, phase 3, non-inferiority trial. Lancet 2016, 387, 229–238. [Google Scholar]
- Mózsa, E.; Mészáros, N.; Major, T.; Fröhlich, G.; Stelczer, G.; Sulyok, Z.; Fodor, J.; Polgár, C. Accelerated partial breast irradiation with external beam three-dimensional conformal radiotherapy. Five-year results of a prospective phase II clinical study. Strahlenther. Onkol. 2014, 190, 444–450. [Google Scholar] [CrossRef] [PubMed]
- Holmes, D.R. Intraoperative radiotherapy in breast conserving surgery. J. Surg. Oncol. 2014, 110, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Veronesi, U.; Orecchia, R.; Luini, A.; Galimberti, V.; Gatti, G.; Intra, M.; Veronesi, P.; Leonardi, M.C.; Ciocca, M.; Lazzari, R.; et al. Full-dose intraoperative radiotherapy with electrons during breast-conserving surgery: Experience with 590 cases. Ann. Surg. 2005, 242, 101–106. [Google Scholar] [CrossRef]
- Massarut, S.; Belletti, B.; Segatto, I.; Piccoli, E.; Baldassarre, G. Wound response after intraoperative radiotherapy. Transl. Cancer Res. 2015, 4, 161–172. [Google Scholar]
- Veronesi, U.; Orecchia, R.; Maisonneuve, P.; Viale, G.; Rotmensz, N.; Sangalli, C.; Luini, A.; Veronesi, P.; Galimberti, V.; Zurrida, S.; et al. Intraoperative radiotherapy versus external radiotherapy for early breast cancer (ELIOT): A randomised controlled equivalence trial. Lancet Oncol. 2013, 14, 1269–1277. [Google Scholar] [CrossRef] [PubMed]
- Orecchia, R.; Veronesi, U.; Maisonneuve, P.; Galimberti, V.E.; Lazzari, R.; Veronesi, P.; Jereczek-Fossa, B.A.; Cattani, F.; Sangalli, C.; Luini, A.; et al. Intraoperative irradiation for early breast cancer (ELIOT): Long-term recurrence and survival outcomes from a single-centre, randomised, phase 3 equivalence trial. Lancet Oncol. 2021, 22, 597–608. [Google Scholar] [CrossRef] [PubMed]
- Veronesi, U.; Orecchia, R.; Luini, A.; Galimberti, V.; Zurrida, S.; Intra, M.; Veronesi, P.; Arnone, P.; Leonardi, M.C.; Ciocca, M.; et al. Intraoperative radiotherapy during breast conserving surgery: A study on 1,822 cases treated with electrons. Breast Cancer Res. Treat. 2010, 124, 141–151. [Google Scholar] [CrossRef] [PubMed]
- Vaidya, J.S.; Wenz, F.; Bulsara, M.; Tobias, J.S.; Joseph, D.J.; Keshtgar, M.; Flyger, H.L.; Massarut, S.; Alvarado, M.; Saunders, C.; et al. Risk-adapted targeted intraoperative radiotherapy versus whole-breast radiotherapy for breast cancer: 5-year results for local control and overall survival from the TARGIT-A randomised trial. Lancet 2014, 383, 603–613. [Google Scholar] [CrossRef] [PubMed]
- Corica, T.; Nowak, A.K.; Saunders, C.M.; Bulsara, M.K.; Taylor, M.; Williams, N.R.; Keshtgar, M.; Joseph, D.J.; Vaidya, J.S. Cosmetic outcome as rated by patients, doctors, nurses and BCCT.core software assessed over 5 years in a subset of patients in the TARGIT-A Trial. Radiat. Oncol. 2018, 13, 68–77. [Google Scholar] [CrossRef]
- Welzel, G.; Boch, A.; Sperk, E.; Hofmann, F.; Kraus-Tiefenbacher, U.; Gerhardt, A.; Suetterlin, M.; Wenz, F. Radiation-related quality of life parameters after targeted intraoperative radiotherapy versus whole breast radiotherapy in patients with breast cancer: Results from the randomized phase III trial TARGIT-A. Radiat. Oncol. 2013, 8, 9. [Google Scholar] [CrossRef]
- Vaidya, J.S.; Bulsara, M.; Baum, M.; Wenz, F.; Massarut, S.; Pigorsch, S.; Alvarado, M.; Douek, M.; Saunders, C.; Flyger, H.L.; et al. Long term survival and local control outcomes from single dose targeted intraoperative radiotherapy during lumpectomy (TARGIT-IORT) for early breast cancer: TARGIT-A randomised clinical trial. BMJ 2020, 370, m2836. [Google Scholar] [CrossRef] [PubMed]
- Eraso, A.; Sanz, J.; Ibáñez, R.; Alonso, L.M.; Calin, A.; Casamayor, M.C.; Pla, M.J.; Piñero, A.; Ripoll, F.; Algara, M. Primer consenso español sobre el uso de la radioterapia intraoperatoria en el cáncer de mama. Conclusiones del panel de expertos. Rev. Senol. Patol. Mamar. 2023, 36, 100502. [Google Scholar] [CrossRef]
- Sedlmayer, F.; Reitsamer, R.; Wenz, F.; Sperk, E.; Fussl, C.; Kaiser, J.; Ziegler, I.; Zehentmayr, F.; Deutschmann, H.; Kopp, P.; et al. Intraoperative radiotherapy (IORT) as boost in breast cancer. Radiat. Oncol. 2017, 12, 23. [Google Scholar] [CrossRef] [PubMed]
- Lemanski, C.; Azria, D.; Thezenas, S.; Gutowski, M.; Saint-Aubert, B.; Rouanet, P.; Fenoglietto, P.; Ailleres, N.; Dubois, J.-B. Intraoperative radiotherapy given as a boost for early breast cancer: Long-term clinical and cosmetic results. Int. J. Radiat. Oncol. Biol. Phys. 2006, 64, 1410–1415. [Google Scholar] [CrossRef] [PubMed]
- Reitsamer, R.; Sedlmayer, F.; Kopp, M.; Kametriser, G.; Menzel, C.; Deutschmann, H.; Nairz, O.; Hitzl, W.; Peintinger, F. The Salzburg concept of intraoperative radiotherapy for breast cancer: Results and considerations. Int. J. Cancer 2006, 118, 2882–2887. [Google Scholar] [CrossRef] [PubMed]
- Battle, J.A.; Dubois, J.B.; Merrick, H.W.; Dobelbower, R.R. Intraoperative Irradiation: Techniques and Results; IORT for Breast Cancer Current Clinical Oncology; Gunderson, L.L., Willett, C.G., Calvo, F.A., Harrison, L.B., Eds.; Humana Press, Inc.: Totowa, NJ, USA, 1997; 521p. [Google Scholar]
- Merrick, H.W.; Hager, E.; Dobelbower, R.R. Intraoperative radiation therapy for breast cancer. Surg. Oncol. Clin. N. Am. 2003, 12, 1065–1078. [Google Scholar] [CrossRef] [PubMed]
- Ivaldi, G.B.; Leonardi, M.C.; Orecchia, R.; Zerini, D.; Morra, A.; Galimberti, V.; Gatti, G.; Luini, A.; Veronesi, P.; Ciocca, M.; et al. Preliminary Results of Electron Intraoperative Therapy Boost and Hypofractionated External Beam Radiotherapy After Breast-Conserving Surgery in Premenopausal Women. Int. J. Radiat. Oncol. Biol. Phys. 2008, 72, 485–493. [Google Scholar] [CrossRef]
- Fastner, G.; Sedlmayer, F.; Merz, F.; Deutschmann, H.; Reitsamer, R.; Menzel, C.; Stierle, C.; Farmini, A.; Fischer, T.; Ciabattoni, A.; et al. IORT with electrons as boost strategy during breast conserving therapy in limited stage breast cancer: Long term results of an ISIORT pooled analysis. Radiother. Oncol. 2013, 108, 279–286. [Google Scholar] [CrossRef] [PubMed]
- Fastner, G.; Reitsamer, R.; Ziegler, I.; Zehentmayr, F.; Fussl, C.; Kopp, P.; Peintinger, F.; Greil, R.; Fischer, T.; Deutschmann, H.; et al. IOERT as anticipated tumor bed boost during breast-conserving surgery after neoadjuvant chemotherapy in locally advanced breast cancer-Results of a case series after 5-year follow-up. Int. J. Cancer 2015, 136, 1193–1201. [Google Scholar] [CrossRef] [PubMed]
- Fastner, G.; Hauser-Kronberger, C.; Moder, A.; Reitsamer, R.; Zehentmayr, F.; Kopp, P.; Fussl, C.; Fischer, T.; Deutschmann, H.; Sedlmayer, F. Überlebens-und Lokalkontrollraten bei triple-negativen Mammakarzinomen nach brusterhaltender Operation und IOERT als vorgezogenem Tumorbettboost. Strahlenther. Onkol. 2016, 192, 1–7. [Google Scholar] [CrossRef]
- Kaiser, J.; Kronberger, C.; Moder, A.; Kopp, P.; Wallner, M.; Reitsamer, R.; Fischer, T.; Fussl, C.; Zehentmayr, F.; Sedlmayer, F.; et al. Intraoperative Tumor Bed Boost with Electrons in Breast Cancer of Clinical Stages I Through III: Updated 10-Year Results. Int. J. Radiat. Oncol. Biol. Phys. 2018, 102, 92–101. [Google Scholar] [CrossRef] [PubMed]
- Machiels, M.; Weytjens, R.; Erven, K.; Westerhoff, J.M.; Amrouch, S.; Bosiers, J.; Verkinderen, L.; Hauspy, J.; van Dam, P.; Stevens, P.; et al. Oncological outcome, postoperative complications, and mammographic changes after intraoperative radiotherapy with electrons (IOERT) as a boost in a large single-institution cohort of breast cancer patients. Breast J. 2020, 26, 1937–1945. [Google Scholar] [CrossRef] [PubMed]
- Ciabattoni, A.; Gregucci, F.; Llange, K.; Alessandro, M.; Corazzi, F.; Ivaldi, G.B.; Zuccoli, P.; Stefanelli, A.; Cristaudo, A.; Fusco, V.; et al. Intra-Operative Electron Radiation Therapy (IOERT) Anticipated Boost in Breast Cancer Treatment: An Italian Multicenter Experience. Cancers 2022, 14, 292. [Google Scholar] [CrossRef] [PubMed]
- Schumacher, L.; Tio, J.; Eich, H.T.; Reinartz, G. Efficacy and Tolerance of IMRT Boost Compared to IORT Boost in Early Breast Cancer: A German Monocenter Study. Cancers 2022, 14, 6196. [Google Scholar] [CrossRef] [PubMed]
- Leonardi, M.C.; Cormio, C.F.; Frassoni, S.; Dicuonzo, S.; Fodor, C.; Intra, M.; Zerella, M.A.; Morra, A.; Cattani, F.; Comi, S.; et al. Ten-year results of hypofractionated whole breast radiotherapy and intraoperative electron boost in premenopausal women. Radiother. Oncol. 2022, 177, 71–80. [Google Scholar] [CrossRef]
- Ciabattoni, A.; Gregucci, F.; Fastner, G.; Cavuto, S.; Spera, A.; Drago, S.; Ziegler, I.; Mirri, M.A.; Consorti, R.; Sedlmayer, F. IOERT versus external beam electrons for boost radiotherapy in stage I/II breast cancer: 10-year results of a phase III randomized study. Breast Cancer Res. 2021, 23, 46. [Google Scholar] [CrossRef] [PubMed]
- Fastner, G.; Reitsamer, R.; Urbański, B.; Kopp, P.; Murawa, D.; Adamczyk, B.; Karzcewska, A.; Milecki, P.; Hager, E.; Reiland, J.; et al. Toxicity and cosmetic outcome after hypofractionated whole breast irradiation and boost-IOERT in early stage breast cancer (HIOB): First results of a prospective multicenter trial (NCT 01343459). Radiother Oncol. 2020, 146, 136–142. [Google Scholar] [CrossRef]
- Blank, E.; Kraus-Tiefenbacher, U.; Welzel, G.; Keller, A.; Bohrer, M.; Sütterlin, M.; Wenz, F. Single-center long-term follow-up after intraoperative radiotherapy as a boost during breast-conserving surgery using low-kilovoltage X-rays. Ann. Surg. Oncol. 2010, 17, 352–358. [Google Scholar] [CrossRef]
- Li, X.; Sanz, J.; Argudo, N.; Vernet-Tomas, M.; Rodríguez, N.; Torrent, L.; Fernández-Velilla, E.; Pera, O.; Huang, Y.; Nicolau, P.; et al. Intraoperative irradiation in breast cancer: Preliminary results in 80 patients as partial breast irradiation or anticipated boots prior to hypofractionated whole breast irradiation. Clin. Trnasl. Oncol. 2022, 24, 829–835. [Google Scholar] [CrossRef] [PubMed]
- Vaidya, J.S.; Baum, M.; Tobias, J.S.; Wenz, F.; Massarut, S.; Keshtgar, M.; Hilaris, B.; Saunders, C.; Williams, N.R.; Brew-Graves, C.; et al. Long-term results of Targeted Intraoperative radioTherapy (Targit) boost during breast-conserving surgery. Int. J. Radiat. Oncol. Biol. Phys. 2011, 81, 1091–1097. [Google Scholar] [CrossRef]
- Pez, M.; Keller, A.; Welzel, G.; Abo-Madyan, Y.; Ehmann, M.; Tuschy, B.; Berlit, S.; Sütterlin, M.; Wenz, F.; Giordano, F.A.; et al. Long-term outcome after intraoperative radiotherapy as a boost in breast cancer. Strahlenther. Onkol. 2020, 196, 349–355. [Google Scholar] [CrossRef] [PubMed]
- Wenz, F.; Welzel, G.; Blank, E.; Hermann, B.; Steil, V.; Sütterlin, M.; Kraus-Tiefenbacher, U. Intraoperative radiotherapy as a boost during breast-conserving surgery using low-kilovoltage X-rays: The first 5 years of experience with a novel approach. Int. J. Radiat. Oncol. Biol. Phys. 2010, 77, 1309–1314. [Google Scholar] [CrossRef] [PubMed]
- Tallet, A.; Racadot, S.; Boher, J.; Cohen, M.; Barrou, J.; Houvenaeghel, G.; Gutowski, M.; Delmond, L.; Lemanski, C. The actual benefit of intraoperative radiation therapy using 50 kV X-rays in early breast cancer: A retrospective study of 676 patients. Breast J. 2020, 26, 2145–2150. [Google Scholar] [CrossRef] [PubMed]
- Hochhertz, F.; Hass, P.; Röllich, B.; Ochel, H.J.; Gawish, A. A single-institution retrospective analysis of intraoperative radiation boost during breast-conservation treatment for breast cancer. J. Cancer Res. Clin. Oncol. 2022, 149, 5743–5749. [Google Scholar] [CrossRef] [PubMed]
- Kolberg, H.C.; Lövey, G.; Akpolat-Basci, L.; Stephanou, M.; Fasching, P.; Untch, M.; Hoffmann, O.; Bulsara, M.; Vaidya, J.; Liedtke, C. Targeted Intraoperative Radiotherapy Tumour Bed Boost during Breast-Conserving Surgery after Neoadjuvant Chemotherapy—A Subgroup Analysis of Hormone Receptor-Positive HER2-Negative Breast Cancer. Breast Care 2017, 12, 318–323. [Google Scholar] [CrossRef] [PubMed]
- Cracco, S.; Semprini, G.; Cattin, F.; Gregoraci, G.; Zeppieri, M.; Isola, M.; Ceschia, T.; Cedolini, C.; Parodi, P.C. Impact of intraoperative radiotherapy on cosmetic outcome and complications after oncoplastic breast surgery. Breast J. 2015, 21, 285–290. [Google Scholar] [CrossRef] [PubMed]
- Malter, W.; Kirn, V.; Richters, L.; Fridrich, C.; Markiefka, B.; Bongartz, R.; Semrau, R.; Mallmann, P.; Kraemer, S. Intraoperative Boost Radiotherapy during Targeted Oncoplastic Breast Surgery: Overview and Single Center Experiences. Int. J. Breast Cancer 2014, 2014, 637898. [Google Scholar] [CrossRef]
- Available online: https://clinicaltrials.gov/ct2/show/NCT01792726 (accessed on 15 June 2023).
| Author/Year | Number of Patients | IORT Dose (Gy) | WBI Total Dose/ Number of Fractions | Follow-Up (Years) | Local Control (%) | Overall Survival (%) | Toxicities |
|---|---|---|---|---|---|---|---|
| Retrospective | |||||||
| Batlle/1997 [36] | 51 | 10 | 45/25 | >2 | 100 | NR | 13.3% grade 2 fibrosis |
| Merrick/2003 [37] | 21 | 10–15 | 45–50/25 | 5.9 | 100 | 90.5 | NR |
| Lemanski/2006 [34] | 50 | 9–20 | 50/25 | 9 | 96 | 94 | 12% grade 2 fibrosis |
| Reitsamer/2006 [35] | 190 (IORT) 118 (EBRT) | 9 (IORT) 12 (EBRT) | 51–56/28 | 4.25 (IORT) 6.75 (EBRT) | 100 (IORT), 95.7 (EBRT) | NR | NR |
| Ivaldi/2008 [38] | 204 | 13.3 | 37.05/13 | 8.9 | 100 | 98.2% grade ≤ 2 late skin toxicity | |
| Fastner/2013 (ISIORT registry) [39] | 1109 | 6–15 | 50–54/25–28 | 6 | 99.2 | 91.4 | NR |
| Fastner/2015 [40] | 83 (IORT) 26 (EBRT) | 9 (IORT) 12 (EBRT) | 51–57/25–28 | 5 (IORT) 5.6 (EBRT) | 98.5 (IORT) 88.1 (EBRT) | 86.4 (IORT) 92 (EBRT) | NR |
| Fastner/2016 [41] | 71 | 7–12 | 54/27 | 8 | 89 | 75 | NR |
| Kaiser/2018 [42] | 770 | 10 | 54/24–28 | 10 | 97.2 | 85.7 | NR |
| Machiels/2020 [43] | 763 | 9 | 40/15 or 50/25 | 5.2 | 98.4 | 97.2 | 3.5% postoperative complications |
| Ciabattoni/2022 [44] | 797 | 9–12 | 40.5/15 or 42.56/16 | 5 | 99.25 | 98.6 | 22.5% ≥ grade 2 acute toxicity |
| Schumacher/2022 [45] | 76 (IORT) 76 (EBRT) | 9 (IORT) 8.4 (SIB EBRT) | 50.4/28 or 50/25 | 7.92 | 100 (IORT) 98.7 (EBRT) | 92 (IORT) 97.4 (EBRT) | 37% grade 1 acute dermatitis 21% grade 1 chronic fibrosis |
| Leonardi/2022 [46] | 481 | 12 | 37.05/13 | 9.6 | 95.9 | 96.5 | <2% acute grade 3 dermatitis 40.8% moderate/severe fibrosis |
| Prospective/Randomized | |||||||
| Ciabattoni/2021 [47] | 133 (IORT) 112 (EBRT) | 10 | 50/25 | 12 | 95.7 (IORT) 94.7(EBRT) | 91.6 (IORT) 94.3 (EBRT) | No late complications |
| Fastner/2022 (HIOB Trial) [48] | 1119 | 11.1 | 40.5/15 | 4.2 | 98.3 | 97.9 | 33% grade 1 fibrosis, 9.9% grade 2 fibrosis |
| Author/Year | Number of Patients | IORT Dose (Gy) | WBI Total Dose/Number of Fractions | Follow-Up (Years) | Local Control (%) | Overall Survival (%) | Toxicities |
|---|---|---|---|---|---|---|---|
| Retrospective | |||||||
| Blank/2010 [49] | 197 | 20 | 45–50/25 | 3 | 96.9 | 91.35 | 13.8% chronic dermatitis 34.6% grade 2 fibrosis |
| Wenz/2010 [53] | 154 | 20 | 45/25 | 2.83 | 98.5 | 87% | 30% grade 2 fibrosis 22% grade 1 fibrosis |
| Vaidja/2011 [51] | 299 | 20 | 50/25 | 5 | 97.4 | NR | NR |
| Pez/2020 [52] | 400 | 20 | 46–50/23–25 | 6.5 | 96.25 | 81.8 | 19% and 21.1% chronic fibrosis at 5 and ≥8 years |
| Tallet/2020 [54] | 240 | 20 | 46–50/23–25 | 4.5 | 99.6 | NR | 34% grade 1–2 chronic toxicity |
| Hochertz/2022 [55] | 68 | 20 | 40.5/15 (16%) 50.4/28 (84%) | 7.6 | 92.6 | 86.7 | 1.4 grade 3 acute dermatitis |
| Prospective/Randomized | |||||||
| Li/2022 [50] | 49 | 20 | 40.5/15 | 1.5 | 100 | 100 | 36.7% grade 1–2 fibrosis |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sanz, J.; Eraso, A.; Ibáñez, R.; Williams, R.; Algara, M. Tumor Bed Boost Radiotherapy in the Conservative Treatment of Breast Cancer: A Review of Intra-Operative Techniques and Outcomes. Cancers 2023, 15, 4025. https://doi.org/10.3390/cancers15164025
Sanz J, Eraso A, Ibáñez R, Williams R, Algara M. Tumor Bed Boost Radiotherapy in the Conservative Treatment of Breast Cancer: A Review of Intra-Operative Techniques and Outcomes. Cancers. 2023; 15(16):4025. https://doi.org/10.3390/cancers15164025
Chicago/Turabian StyleSanz, Javier, Arantxa Eraso, Reyes Ibáñez, Rachel Williams, and Manuel Algara. 2023. "Tumor Bed Boost Radiotherapy in the Conservative Treatment of Breast Cancer: A Review of Intra-Operative Techniques and Outcomes" Cancers 15, no. 16: 4025. https://doi.org/10.3390/cancers15164025
APA StyleSanz, J., Eraso, A., Ibáñez, R., Williams, R., & Algara, M. (2023). Tumor Bed Boost Radiotherapy in the Conservative Treatment of Breast Cancer: A Review of Intra-Operative Techniques and Outcomes. Cancers, 15(16), 4025. https://doi.org/10.3390/cancers15164025






