Conventional Transarterial Chemo embolization Using Streptozocin in Patients with Unresectable Neuroendocrine Liver Metastases
Abstract
Simple Summary
Abstract
1. Introduction
2. Objectives
3. Methods
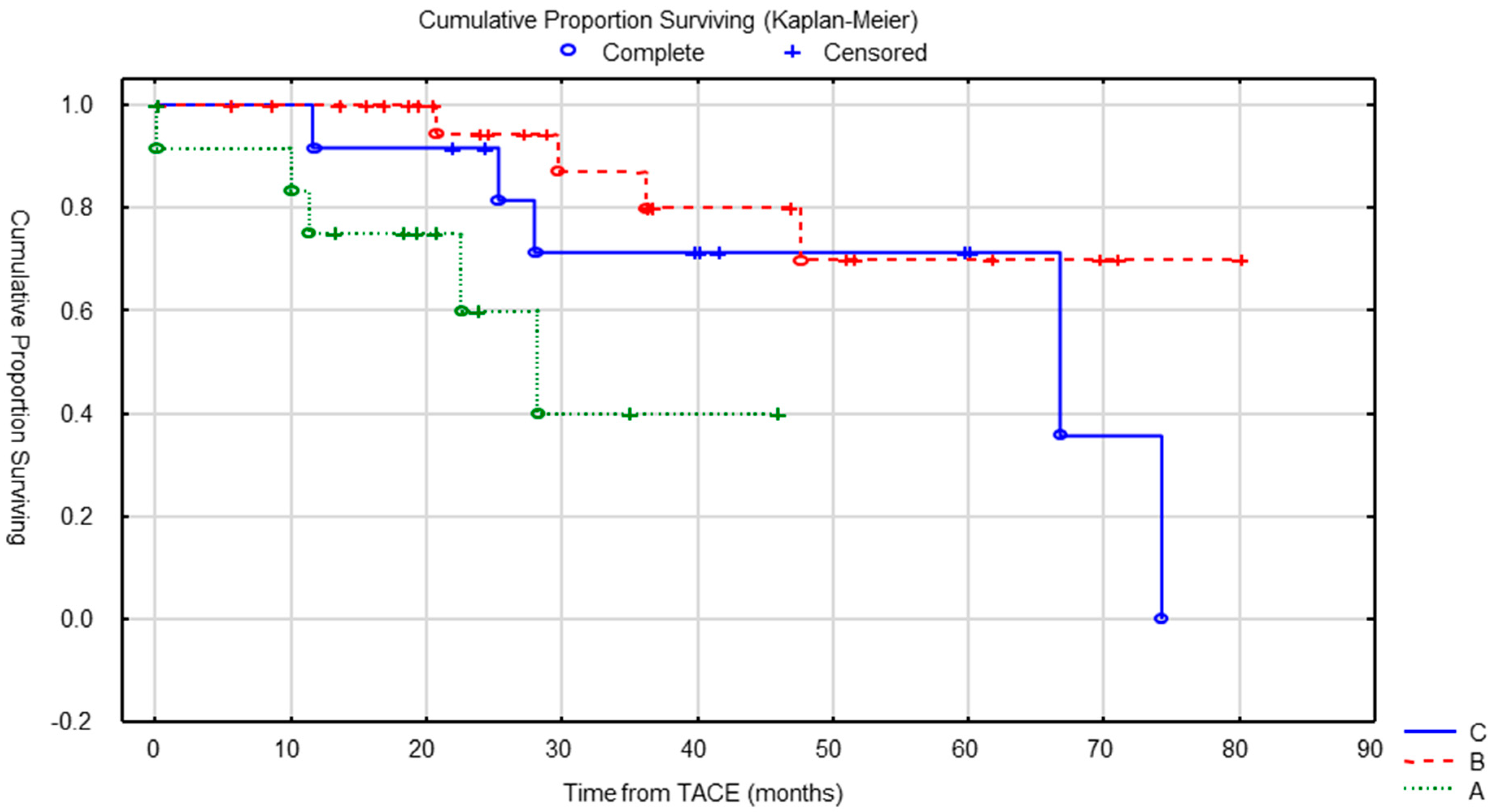
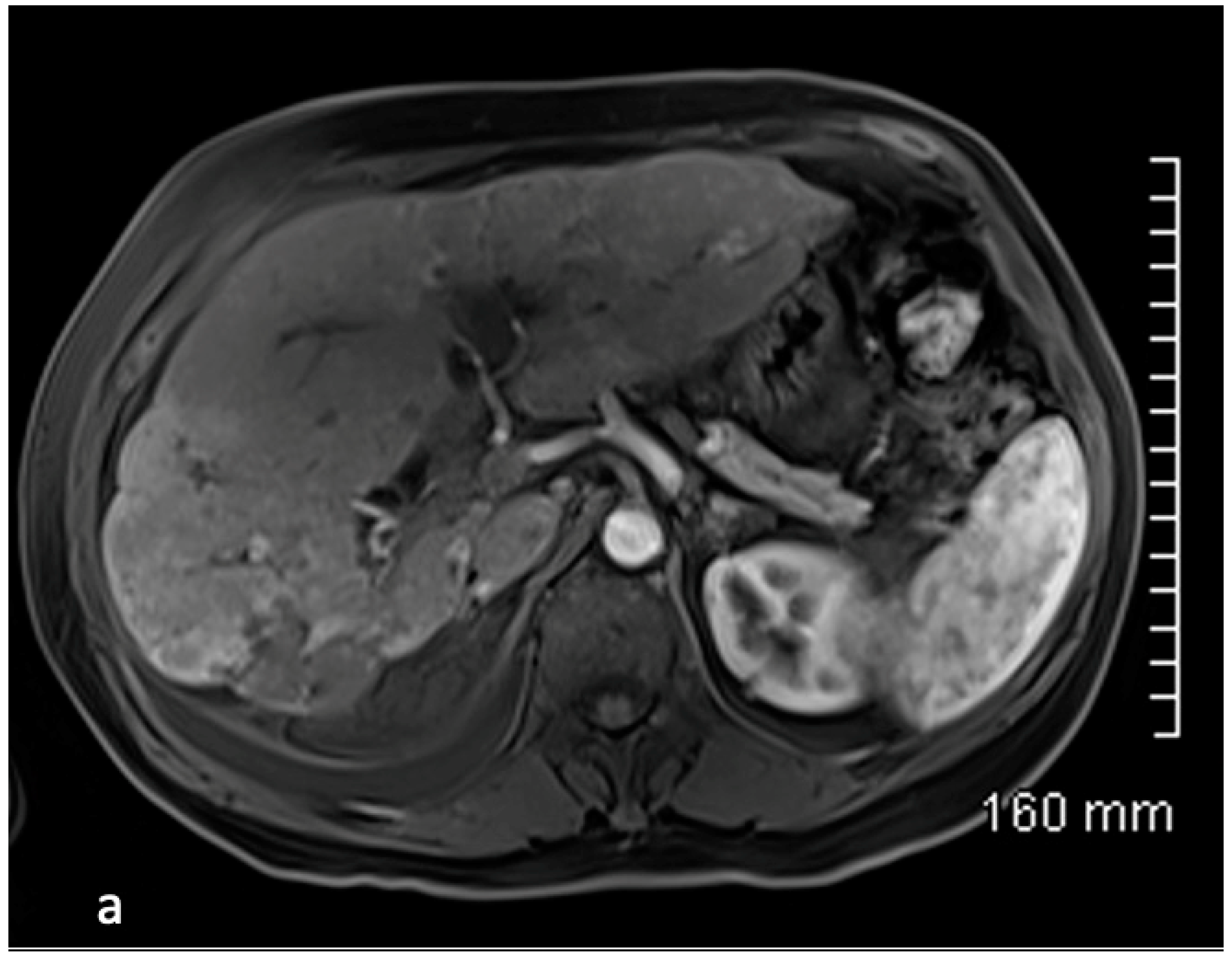
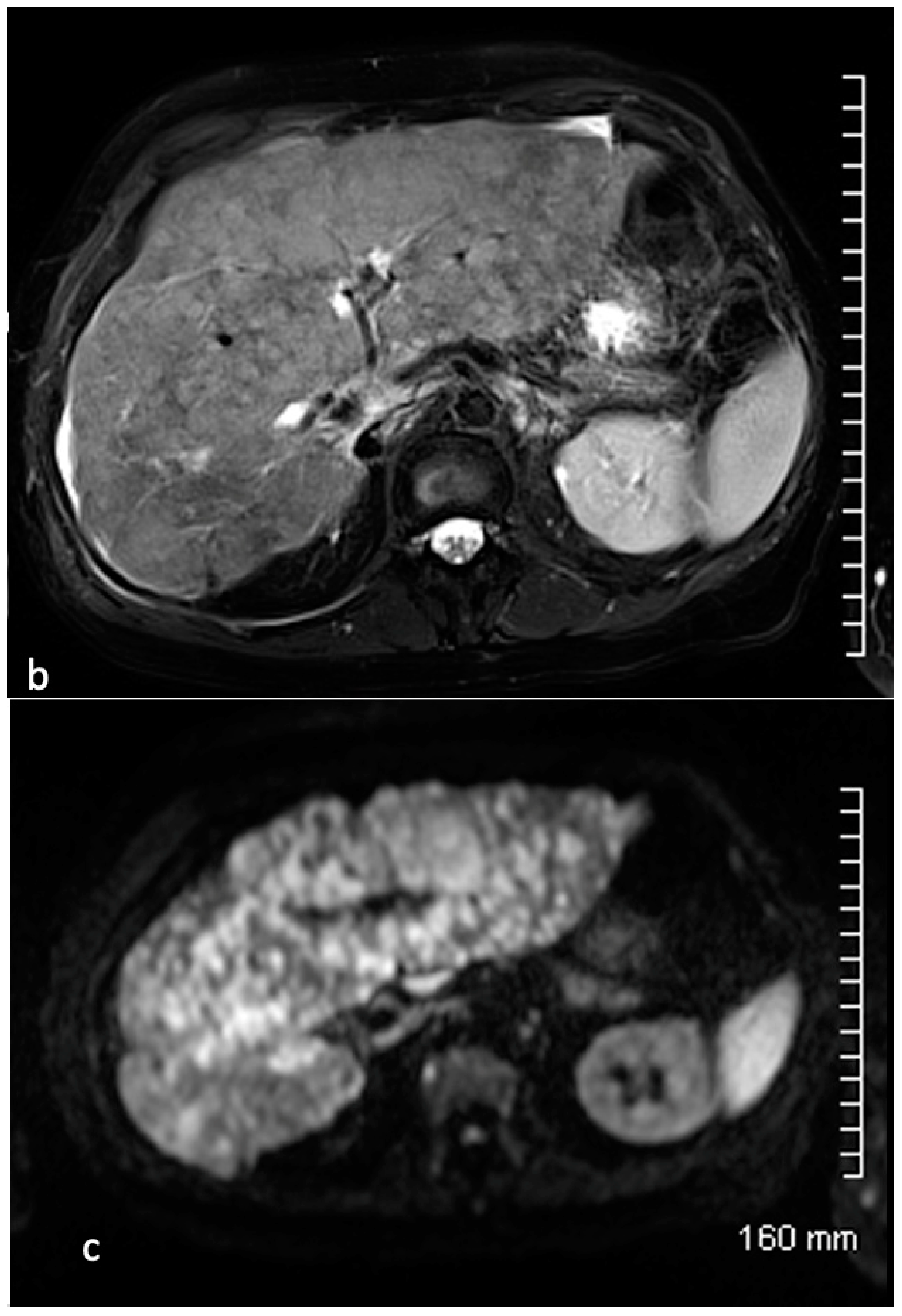
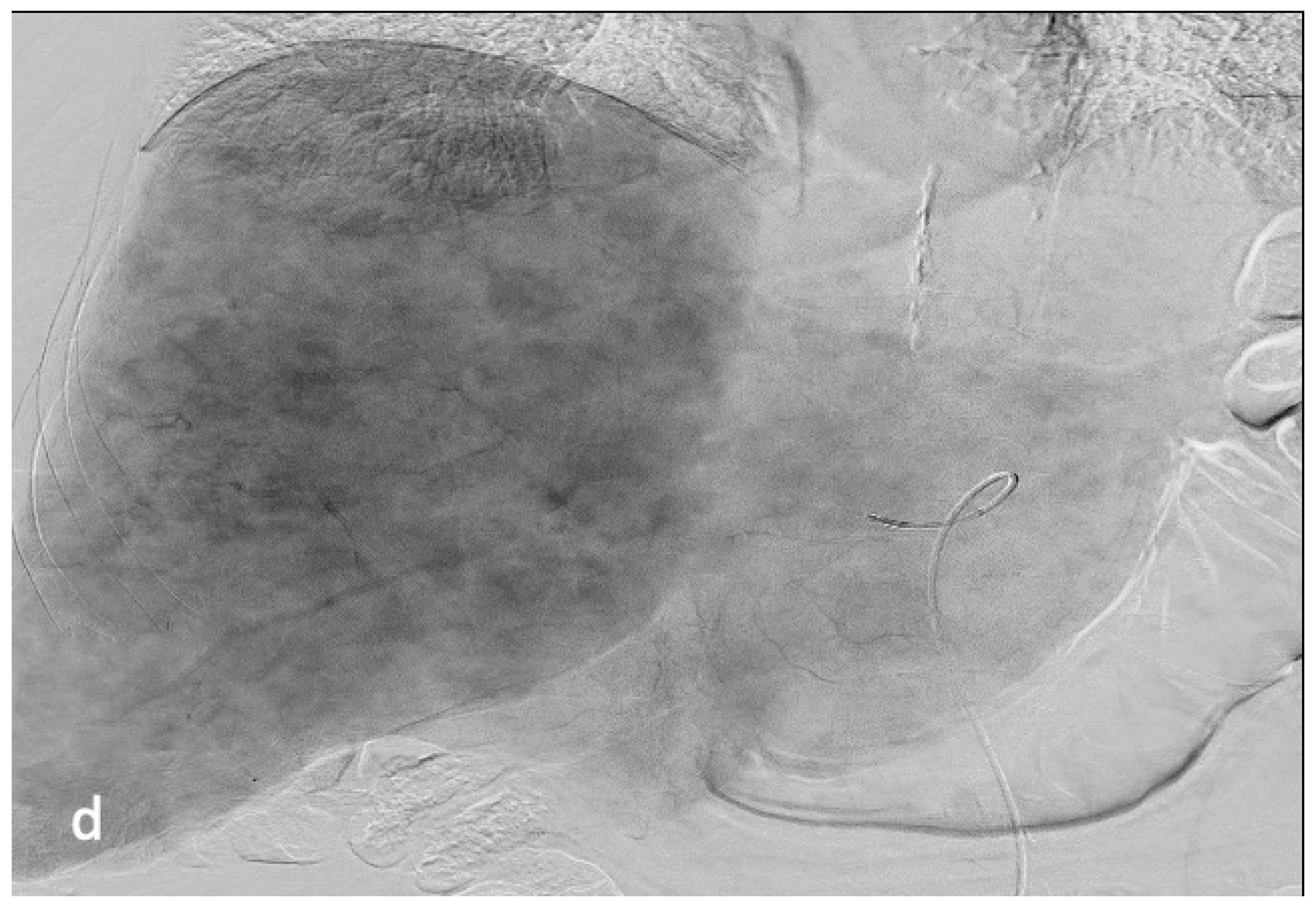
4. Results
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Frilling, A.; Modlin, I.M.; Kidd, M.; Russell, C.; Breitenstein, S.; Salem, R.; Kwekkeboom, D.; Lau, W.-y.; Klersy, C.; Vilgrain, V.; et al. Recommendations for management of patients with neuroendocrine liver metastases. Lancet Oncol. 2014, 15, e8–e21. [Google Scholar] [CrossRef] [PubMed]
- Modlin, I.M.; Sandor, I.A. An analysis of 8305 cases of carcinoid tumors. Cancer 1997, 79, 813–829. [Google Scholar] [CrossRef]
- Kaltsas, G.A.; Besser, G.M.; Grossman, A.B. The diagnosis and medical management of advanced neuroendocrine tumors. Endocr. Rev. 2004, 25, 458–511. [Google Scholar] [CrossRef] [PubMed]
- Oberg, K.; Castellano, D. Current knowledge on diagnosis and staging of neuroendocrine tumors. Cancer Metastasis 2011, 30, 3–7. [Google Scholar] [CrossRef]
- Lawrence, B.; Gustafsson, B.I.; Chan, A.; Svejda, B.; Kidd, M.; Modlin, I.M. The epidemiology of gastroenteropancreatic neuroendocrine tumors. Endocrinol. Metab. Clin. N. Am. 2011, 40, 1–18. [Google Scholar] [CrossRef]
- Hentic, O.; Couvelard, A.; Rebours, V.; Zappa, M.; Dokmak, S.; Hammel, P.; Maire, F.; O’Toole, D.; Lévy, P.; Sauvanet, A.; et al. Ki-67 index, tumor differentiation, and extent of liver involvement are independent prognostic factor in patients with liver metastases of digestive endocrine carcinomas. Endoc. Rel. Cancer 2011, 18, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Riihimaki, M.; Hemminki, A.; Sundquist, K.; Sundquist, J.; Hemminki, K. The epidemiology of metastasis in neuroendocrine tumors. Int. J. Cancer 2016, 139, 2679–2686. [Google Scholar] [CrossRef]
- Dhir, M.; Shrestha, R.; Steel, J.; Marsh, J.W.; Tsung, A.; Tublin, M.E.; Amesur, N.B.; Orons, P.D.; Santos, E.; Geller, D.A.; et al. Initial treatment of unresectable neuroendocrine tumor liver metastases with transarterial chemoembolization using streptozocin: A 20-year experience. Ann. Surg. Oncol. 2017, 24, 450–459. [Google Scholar] [CrossRef]
- Chen, J.X.; Rose, S.; White, S.; El-Haddad, G.; Fidelman, N.; Yarmohammadi, H.; Hwang, W.; Sze, D.Y.; Kothary, N.; Stashek, K.; et al. Embolotherapy for neuroendocrine tumor liver metastases: Prognostic factors for hepatic progression-free survival and overall survival. Cardiovasc. Interv. Radiol. 2017, 40, 69–80. [Google Scholar] [CrossRef]
- Madoff, D.C.; Gupta, S.; Ahrar, K.; Murthy, R.; Yao, J.C. Update on the management of neuroendocrine hepatic metastases. J. Vasc. Interv. Radiol. 2006, 7, 1235–1249. [Google Scholar] [CrossRef]
- Ducreux, M.; Ruszniewski, P.; Chayvialle, J.A.; Blumberg, J.; Cloarec, D.; Michel, H.; Raymond, J.M.; Dupas, J.L.; Gouerou, H.; Jian, R.; et al. The antitumoral effect of the long-acting somatostatin analog lanreotide in neuroendocrine tumors. Am. J. Gastroenterol. 2000, 95, 3276–3781. [Google Scholar] [CrossRef]
- Sutcliffe, R.; Maguire, D.; Ramage, J.; Rela, M.; Heaton, N. Management of neuroendocrine liver metastases. Am. J. Surg. 2004, 187, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Roche, A.; Girish, B.V.; De Baere, T.; Baudin, E.; Boige, V.; Elias, D.; Lasser, P.; Schlumberger, M.; Ducreux, M. Trans-catheter arterial chemoembolization as first-line treatment for hepatic metastases from endocrine tumors. Eur. Radiol. 2003, 13, 136–140. [Google Scholar] [CrossRef]
- Pericleous, M.; Caplin, M.E.; Tsochatzis, E.; Yu, D.; Morgan-Rowe, L.; Toumpanakis, C. Hepatic artery embolization in advanced neuroendocrine tumors: Efficacy and long-term outcomes. J. Clin. Oncol. 2016, 12, 61–69. [Google Scholar] [CrossRef]
- Moertel, C.G.; Johnson, C.M.; McKusick, M.A.; Martin, J.K., Jr.; Nagorney, D.M.; Kvols, L.K.; Rubin, J.; Kunselman, S. The management of patients with advanced carcinoid tumors and islet cell carcinomas. Ann. Intern. Med. 1994, 120, 302–309. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Johnson, M.M.; Murthy, R.; Ahrar, K.; Wallace, M.J.; Madoff, D.C.; McRae, S.E.; Hicks, M.E.; Rao, S.; Vauthey, J.-N.; et al. Hepatic arterial embolization and chemoembolization for the treatment of patients with metastatic neuroendocrine tumors: Variables affecting response rate and survival. Cancer 2005, 104, 1590–1602. [Google Scholar] [CrossRef]
- Rusniewski, P.; O’Toole, D. Ablative therapies for liver metastases of gastroenteropancreatic endocrine tumors. Neuroendocrinology 2004, 80 (Suppl. S1), 74–78. [Google Scholar] [CrossRef]
- Ruutiainen, A.T.; Soulen, M.C.; Tuite, C.M.; Clark, T.W.I.; Mondschein, J.I.; Stavropoulos, S.W.; Trerotola, S.O. Chemoembolization and bland embolization of neuroendocrine tumor metastases to the liver. J. Vasc. Interv. Radiol. 2007, 18, 847–855. [Google Scholar] [CrossRef]
- Pitt, S.C.; Knuth, J.; Keily, J.M.; McDermott, J.C.; Weber, S.M.; Chen, H.; Rilling, W.S.; Quebbeman, E.J.; Agarwal, D.M.; Pitt, H.A. Hepatic neuroendocrine metastases: Chemo or bland-embolization? J. Gastroint. Surg. 2008, 12, 1951–1960. [Google Scholar] [CrossRef] [PubMed]
- De Baere, T.; Deschamps, F.; Teriitheau, C.; Rao, P.; Conengrapht, K.; Schlumberger, M.; Leboulleux, S.; Baudin, E.; Hechellhammer, L. Transarterial chemoembolization of liver metastases from well differenciated gastroenteropancreatic endocrine tumors with doxorubicin-eluting beads: Preliminary results. J. Vasc. Interv. Radiol. 2008, 19, 855–861. [Google Scholar] [CrossRef]
- Gaur, S.K.; Friese, J.L.; Sadow, C.A.; Ayyagari, R.; Binkert, C.A.; Schenker, M.P.; Kulke, M.; Baum, R. Hepatic arterial chemoembolization using drug-eluting beads in gastrointestinal neuroendocrine tumor metastatic to the liver. Cardiovasc. Interv. Radiol. 2011, 34, 566–572. [Google Scholar] [CrossRef] [PubMed]
- Marrache, F.; Vullierme, M.P.; Roy, C.; El Assoued, Y.; Couvelard, A.; O’Toole, D.; Mitry, E.; Hentic, O.; Hammel, P.; Lévy, P.; et al. Arterial phase enhancement and body mass index are predictors of response to chemoembolization for liver metastases of endocrine tumours. Br. J. Cancer 2006, 96, 49–55. [Google Scholar] [CrossRef]
- Zappa, M.; Hentic, O.; Vullierme, M.P.; Lagadec, M.; Ronot, M.; Ruszniewski, P.; Vilgrain, V. Is visual radiological evolution of liver tumour burden in patients with neuroendocrine tumors reproductible? Endocr. Connect 2017, 6, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Ameli, S.; Pandey, A.; Khoshpouri, P.; Ghasabeh, M.A.; Pandey, P.; Li, Z.; Hu, D.; Kamel, I.R. Semi quantitative visual assessment of hepatic tumor burden can reliably predict survival in neuroendocrine liver metastases treated with transarterial chemoembolization. Eur. Radiol. 2019, 29, 5804–5812. [Google Scholar] [CrossRef] [PubMed]
- Pelage, J.P.; Fohlen, A.; Mitry, E.; Lagrange, C.; Beauchet, A.; Rougier, P. Chemoembolization of neuroendocrine liver metastases using streptozocin and tris-acryl microspheres: Embozar (EMBOsphere + ZAnosaR) study. Cardiovasc. Interv. Radiol. 2016, 40, 394–400. [Google Scholar] [CrossRef]
- Dominguez, S.; Denys, A.; Madeira, I.; Hammel, P.; Vilgrain, V.; Menu, Y.; Bernades, P.; Ruszniewski, P. Hepatic arterial chemoembolization with streptozocin in patients with metastatic digestive endocrine tumours. Eur. J. Gastroenterol. Hepatol. 2000, 12, 151–157. [Google Scholar] [CrossRef]
- Kress, O.; Wagner, H.J.; Wied, M.; Klose, K.J.; Arnold, R.; Alfke, A. Transarterial chemoembolization of advanced liver metastases of neuroendocrine tumors—A retrospective single-center analysis. Digestion 2003, 68, 94–101. [Google Scholar] [CrossRef]
- Vogl, T.J.; Gruber, T.; Naguib, N.; Hammerstingl, R.; Nour-Eldin, N.E. Liver metastases of neuroendocrine tumors: Treatment with hepatic transarterial chemotherapy using two therapeutic protocols. Am. J. Roentgenol. 2009, 193, 941–947. [Google Scholar] [CrossRef][Green Version]
- Makary, M.S.; Kapke, J.; Yildiz, V.; Pan, X.; Dowell, J.D. Conventional versus drug-eluting bead transarterial chemoembolization for neuroendocrine tumor liver metastases. J. Vasc. Interv. Radiol. 2016, 27, 1298–1304. [Google Scholar] [CrossRef] [PubMed]
- Joskin, J.; De Baere, T.; Auperin, A.; Tselikas, L.; Guiu, B.; Farouil, G.; Boige, V.; Malka, D.; Leboulleux, S.; Ducreux, M.; et al. Predisposing factors of liver necrosis after transcatheter arterial chemoembolization in liver metastases from neuroendocrine tumor. Cardiovasc. Interv. Radiol. 2015, 38, 372–380. [Google Scholar] [CrossRef]
- Fan, K.Y.; Wild, A.T.; Halappe, V.G.; Kumar, R.; Ellsworth, S.; Ziegler, M.; Garg, T.; Rosati, L.M.; Su, Z.; Hacker-Prietz, A.; et al. Neuroendocrine tumor liver metastases treated with yttrium-90 radioembolization. Contemp. Clin. Trials 2016, 50, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Frilling, A.; Clift, A.; Braat, A.J.A.T.; Alsafi, A.; Wasan, H.S.; Al-Nahhas, A.; Thomas, R.; Drymousis, P.; Habib, N.; Tait, P.N. Radioembolisation with 90Y microspheres for neuroendocrine liver metastases: An institutional case series, systematic review and meta-analysis. HBP 2018, 21, 773–783. [Google Scholar] [CrossRef] [PubMed]
- Braat, A.J.A.T.; Kappadath, S.C.; Ahmadzadehfar, H.; Stothers, C.L.; Frilling, A.; Deroose, C.M.; Flamen, P.; Brown, D.B.; Sze, D.Y.; Mahvash, A.; et al. Radioembolization with 90 yttrium resin microspheres of neuroendocrine liver metastases: International multicenter study on efficacy and toxicity. Cardiovasc. Interv. Radiol. 2019, 42, 413–425. [Google Scholar] [CrossRef] [PubMed]
- Do Minh, D.; Chapiro, J.; Gorodetski, B.; Huang, Q.; Liu, A.; Smolka, S.; Savic, L.J.; Wainstejn, D.; Lin, M.; Schlachter, T.; et al. Intra-arterial therapy of neuroendocrine tumour liver metastases: Comparing conventional TACE, drug-eluting beads TACE and yttrium-90 radioembolisation as treatment options using a propensity score analysis model. Eur. Radiol. 2017, 27, 4995–5005. [Google Scholar] [CrossRef] [PubMed]




| n = 52 | |
|---|---|
| Gender, male/female n | 25/27 |
| Mean age (years) | |
| Age at diagnosis (years) | 61.0 ± 11.0 (med 63.0, min–max 31.9–80.8) |
| Age at first TACE (years) | 63.8 ± 10.4 (med 65.1, 33.3–82.6) |
| Body mass index (BMI) n = 50 | 24.6 ± 4.5 (med 23.6, 17.2–36.0) |
| BMI ≤ 20, n (%) | 7 (14) |
| 20 ≤ BMI ≤ 25, n (%) | 23 (46) |
| 25 ≤ BMI ≤ 30, n (%) | 12 (24) |
| BMI > 30, n (%) | 8 (16) |
| Primary tumour, n (%) | |
| GI tract | 29 (56) |
| Pancreas | 14 (27) |
| Lung | 4 (8) |
| Unknown | 5 (8) |
| Surgical resection | 30 (58) |
| Grade, n (%) (n = 49) | |
| Well-differentiated (grade 1) | 26 (53) |
| Grade 2 | 22 (45) |
| Grade 3 | 1 (2) |
| Mean Ki67 index (n = 40) | 5.7 ± 7.1 (med 2.0, min–max 0–30) |
| Liver metastases, n (%) | |
| Synchronous liver metastases | 41 (79) |
| Metachronous liver metastases | 11 (21) |
| Previous treatment, n (%) * | |
| Hepatic surgery | 12 (23) |
| Radiofrequency ablation | 2 (4) |
| Systemic chemotherapy | 14 (27) |
| Somatostatin analogues | 37 (71) |
| TACE | 8 (15) |
| Peptide receptor radionuclide therapy | 2 (4) |
| Liver tumour burden, n (%) | |
| <10 | 9 (17) |
| 10–25 | 15 (29) |
| 25–50 | 13 (25) |
| 50–75 | 8 (16) |
| >75% | 7 (13) |
| Extrahepatic disease | 20 (38) |
| Discovery mode, n (%) | |
| Carcinoid syndrome | 12 (23) |
| Abdominal or lumbar pain | 22 (42) |
| Fortuitous | 11 (21) |
| Other | 4 (8) |
| Unknown | 3 (6) |
| Carcinoid dominant symptoms, n (%) | |
| Diarrhea | 29 (56) |
| Flushes | 23 (44) |
| Baseline Chromogranin A (n = 45) | 1030.1 ± 1644.6 (med 350.0, min–max 25.0–7629.0) |
| ng/mL | |
| Elevated (>100) n (%) | 37 (82) |
| Normal | 8 (18) |
| Urinary 5-HIAA (n = 20) | 70.0 ± 92.6 (med 35.5, 3.4–280.0) |
| µmol/24 h | |
| Elevated (>50) n (%) | 6 (30) |
| Normal (<50) | 14 (70) |
| Baseline liver and renal function (n = 51) | |
| AST (mean ± SD, normal < 35), IU/L | 29.6 ± 20.0 (med 25.0, min–max 12.0–144.0) |
| ALT (mean ± SD, normal < 45), IU/L | 31.1 ± 31.3 (med 20.0, min–max 7.0–216.0) |
| Bilirubin (mean ± SD, normal < 16) IU/L | 11.4 ± 4.7 (med 10.0, min–max 5.0–31.0) |
| Creatinine clearance (MDRD) mL/min | 80.3 ± 18.8 (85, 26–117) |
| Clearance >60 mL/min, | 44 (86) |
| Clearance 30–60 mL/min, | 7 (14) |
| Radiological features of liver metastases | |
| Longest diameter (mean SD) mm | |
| Target 1 (n = 52) | 55.9 ± 35.1 (48.5, 10–158.0) |
| Target 2 (n = 43) | 34.1 ± 22.8 (30.0, 6.0–100.0) |
| Sum of longest disease (mean SD) | 84.1 ± 50.9 (77.0, 12.0–195.0) |
| List of Side Effects | n = 127 |
|---|---|
| Post embolization syndrome | 99 (78%) |
| Serious adverse events | 10 (8%) |
| Pneumopathy | 2 (1.6%) |
| Heart failure | 2 (1.6%) |
| Bowel obstruction | 2 (1.6%) |
| ARDS | 1 (0.8%) |
| Liver abscess | 1 (0.8%) |
| Death | 2 (1.6%) |
| Toxicity | Normal | Grade 1 | Grade 2 | Grade 3 | Grade 4 |
|---|---|---|---|---|---|
| AST (IU/L) | (>ULN-2.5× ULN) | (2.5–5× ULN) | (5–20× ULN) | (>20× ULN) | |
| Baseline (n = 52) | 40 (77%) | 11 (21%) | 1 (2%) | 0 (0%) | 0 (0%) |
| Day 1 (n = 51) | 0 (0%) | 3 (6%) | 3 (6%) | 20 (39%) | 25 (49%) |
| Day 5 (n = 49) | 0 (0%) | 11 (23%) | 10 (20%) | 24 (49%) | 4 (8%) |
| At 2 months (n = 38) | 25 (66%) | 10 (26%) | 3 (8%) | 0 (0%) | 0 (0%) |
| ALT (IU/L) | (>ULN-2.5× ULN) | (2.5–5× ULN) | (5–20× ULN) | (>20× ULN) | |
| Baseline (n = 52) | 45 (86%) | 6 (12%) | 1 (2%) | 0 (0%) | 0 (0%) |
| Day 1 (n = 51) | 2 (4%) | 8 (16%) | 4 (8%) | 19 (37%) | 18 (35%) |
| Day 5 (n = 49) | 3 (6%) | 9 (19%) | 7 (14%) | 25 (51%) | 5 (10%) |
| At 2 months (n = 38) | 27 (71%) | 10 (26%) | 1 (3%) | 0 (0%) | 0 (0%) |
| Bilirubin (IU/L) | (>ULN-1.5× ULN) | (1.5–3× ULN) | (3–10× ULN) | (>10× ULN) | |
| Baseline (n = 52) | 45 (86%) | 6 (12%) | 1 (2%) | 0 (0%) | 0 (0%) |
| Day 1 (n = 51) | 16 (31%) | 20 (39%) | 9 (18%) | 6 (12%) | 0 (0%) |
| Day 5 (n = 49) | 16 (32%) | 15 (31%) | 13 (27%) | 5 (10%) | 0 (0%) |
| At 2 months (n = 36) | 32 (89%) | 4 (11%) | 0 (0%) | 0 (0%) | 0 (0%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fohlen, A.; Beaudouin, R.; Alvès, A.; Bouhier-Leporrier, K.; Pasik, C.; Pelage, J.-P. Conventional Transarterial Chemo embolization Using Streptozocin in Patients with Unresectable Neuroendocrine Liver Metastases. Cancers 2023, 15, 4021. https://doi.org/10.3390/cancers15164021
Fohlen A, Beaudouin R, Alvès A, Bouhier-Leporrier K, Pasik C, Pelage J-P. Conventional Transarterial Chemo embolization Using Streptozocin in Patients with Unresectable Neuroendocrine Liver Metastases. Cancers. 2023; 15(16):4021. https://doi.org/10.3390/cancers15164021
Chicago/Turabian StyleFohlen, Audrey, Remi Beaudouin, Arnaud Alvès, Karine Bouhier-Leporrier, Christophe Pasik, and Jean-Pierre Pelage. 2023. "Conventional Transarterial Chemo embolization Using Streptozocin in Patients with Unresectable Neuroendocrine Liver Metastases" Cancers 15, no. 16: 4021. https://doi.org/10.3390/cancers15164021
APA StyleFohlen, A., Beaudouin, R., Alvès, A., Bouhier-Leporrier, K., Pasik, C., & Pelage, J.-P. (2023). Conventional Transarterial Chemo embolization Using Streptozocin in Patients with Unresectable Neuroendocrine Liver Metastases. Cancers, 15(16), 4021. https://doi.org/10.3390/cancers15164021






