Pituitary Stalk Morphology as a Predictor of New-Onset Adrenocortical Insufficiency and Arginine Vasopressin Deficiency after Transsphenoidal Resections of Pituitary Macroadenomas: A Retrospective Single-Center Study with a Focus on iMRI
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
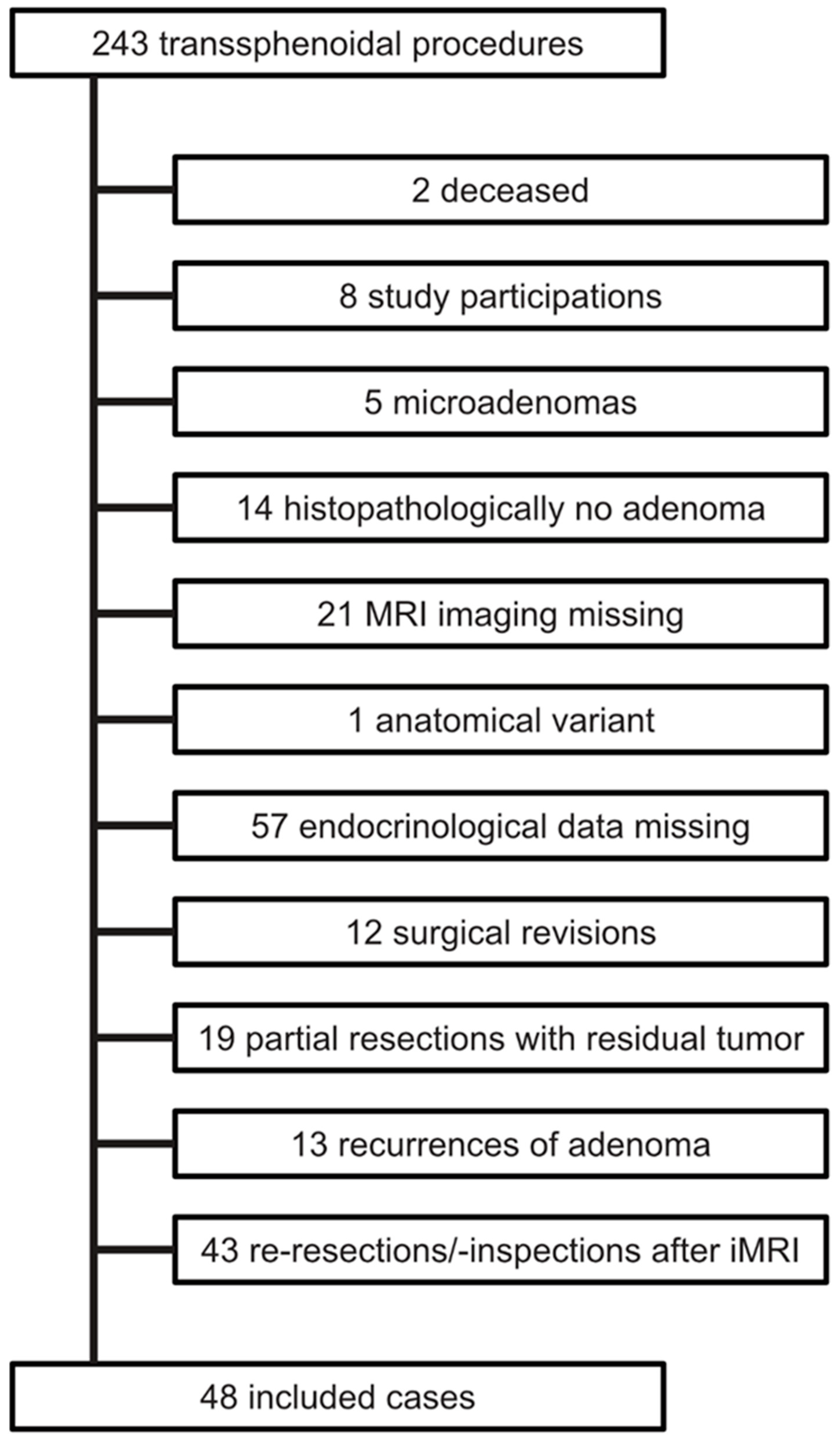
2.1. Patients
2.2. MRI Data
2.3. Endocrinological Data
2.4. Surgical Procedure
2.5. Statistical Analysis
3. Results
3.1. General Characteristics
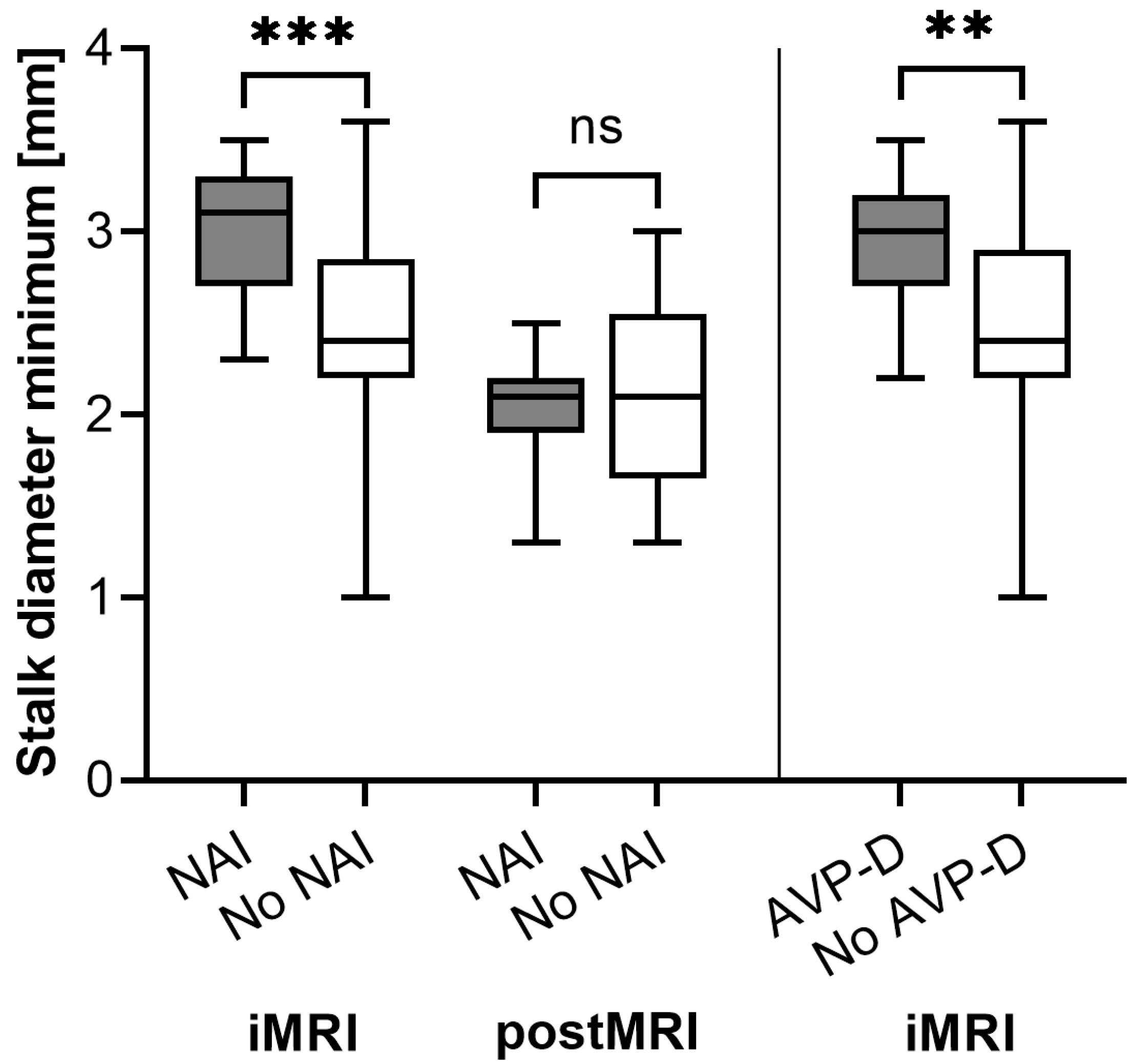
3.2. New-Onset Adrenocortical Insufficiency
3.3. (Transient) AVP-D
4. Discussion
4.1. Key Findings
4.2. Pathophysiological Considerations
4.3. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Famini, P.; Maya, M.M.; Melmed, S. Pituitary Magnetic Resonance Imaging for Sellar and Parasellar Masses: Ten-Year Experience in 2598 Patients. J. Clin. Endocrinol. Metab. 2011, 96, 1633–1641. [Google Scholar] [CrossRef] [PubMed]
- Butenschoen, V.M.; von Werder, A.; Bette, S.; Schmette, V.; Schwendinger, N.; Meyer, B.; Gempt, J. Transsphenoidal Pituitary Adenoma Resection: Do Early Post-Operative Cortisol Levels Predict Permanent Long-Term Hypocortisolism? Neurosurg. Rev. 2022, 45, 1353–1362. [Google Scholar] [CrossRef]
- Coburger, J.; König, R.; Seitz, K.; Bäzner, U.; Wirtz, C.R.; Hlavac, M. Determining the Utility of Intraoperative Magnetic Resonance Imaging for Transsphenoidal Surgery: A Retrospective Study: Clinical Article. J. Neurosurg. 2014, 120, 346–356. [Google Scholar] [CrossRef] [PubMed]
- Little, A.S.; Kelly, D.F.; White, W.L.; Gardner, P.A.; Fernandez-Miranda, J.C.; Chicoine, M.R.; Barkhoudarian, G.; Chandler, J.P.; Prevedello, D.M.; Liebelt, B.D.; et al. Results of a Prospective Multicenter Controlled Study Comparing Surgical Outcomes of Microscopic versus Fully Endoscopic Transsphenoidal Surgery for Nonfunctioning Pituitary Adenomas: The Transsphenoidal Extent of Resection (TRANSSPHER) Study. J. Neurosurg. 2020, 132, 1043–1053. [Google Scholar] [CrossRef]
- Alexander, T.D.; Collopy, S.; Yu, S.; Karsy, M.; Chitguppi, C.; Farrell, C.J.; Evans, J.J. Perioperative Outcomes of a Hydrocortisone Protocol after Endonasal Surgery for Pituitary Adenoma Resection. J. Neurol. Surg. B Skull Base 2022, 83, 383–389. [Google Scholar] [CrossRef] [PubMed]
- Buckley, L.; Humphrey, M.B. Glucocorticoid-Induced Osteoporosis. N. Engl. J. Med. 2018, 379, 2547–2556. [Google Scholar] [CrossRef] [PubMed]
- Prete, A.; Corsello, S.M.; Salvatori, R. Current Best Practice in the Management of Patients after Pituitary Surgery. Ther. Adv. Endocrinol. 2017, 8, 33–48. [Google Scholar] [CrossRef]
- Morin, C.; Fardet, L. Systemic Glucocorticoid Therapy: Risk Factors for Reported Adverse Events and Beliefs about the Drug. A Cross-Sectional Online Survey of 820 Patients. Clin. Rheumatol. 2015, 34, 2119–2126. [Google Scholar] [CrossRef]
- Chanson, P.; Raverot, G.; Castinetti, F.; Cortet-Rudelli, C.; Galland, F.; Salenave, S. Management of Clinically Non-Functioning Pituitary Adenoma. Ann. D’endocrinol. 2015, 76, 239–247. [Google Scholar] [CrossRef]
- Ajlan, A.; Abdulqader, S.; Achrol, A.; Aljamaan, Y.; Feroze, A.; Katznelson, L.; Harsh, G. Diabetes Insipidus Following Endoscopic Transsphenoidal Surgery for Pituitary Adenoma. J. Neurol. Surg. B 2018, 79, 117–122. [Google Scholar] [CrossRef]
- Barker, F.G.; Klibanski, A.; Swearingen, B. Transsphenoidal Surgery for Pituitary Tumors in the United States, 1996–2000: Mortality, Morbidity, and the Effects of Hospital and Surgeon Volume. J. Clin. Endocrinol. Metab. 2003, 88, 4709–4719. [Google Scholar] [CrossRef] [PubMed]
- Sheehan, J.M.; Sheehan, J.P.; Douds, G.L.; Page, R.B. DDAVP Use in Patients Undergoing Transsphenoidal Surgery for Pituitary Adenomas. Acta Neurochir. 2006, 148, 287–291. [Google Scholar] [CrossRef] [PubMed]
- Chabot, J.D.; Chakraborty, S.; Imbarrato, G.; Dehdashti, A.R. Evaluation of Outcomes After Endoscopic Endonasal Surgery for Large and Giant Pituitary Macroadenoma: A Retrospective Review of 39 Consecutive Patients. World Neurosurg. 2015, 84, 978–988. [Google Scholar] [CrossRef]
- Bohl, M.A.; Ahmad, S.; Jahnke, H.; Shepherd, D.; Knecht, L.; White, W.L.; Little, A.S. Delayed Hyponatremia Is the Most Common Cause of 30-Day Unplanned Readmission After Transsphenoidal Surgery for Pituitary Tumors. Neurosurgery 2016, 78, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Asa, S.L.; Mete, O.; Perry, A.; Osamura, R.Y. Overview of the 2022 WHO Classification of Pituitary Tumors. Endocr. Pathol. 2022, 33, 6–26. [Google Scholar] [CrossRef] [PubMed]
- Inder, W.J.; Hunt, P.J. Glucocorticoid Replacement in Pituitary Surgery: Guidelines for Perioperative Assessment and Management. J. Clin. Endocrinol. Metab. 2002, 87, 2745–2750. [Google Scholar] [CrossRef]
- Brooks, E.K.; Inder, W.J. Disorders of Salt and Water Balance After Pituitary Surgery. J. Clin. Endocrinol. Metab. 2022, 108, 198–208. [Google Scholar] [CrossRef] [PubMed]
- Xue, L.; Wu, J.; Chen, J.; Yang, Y. Change in the Pituitary Stalk Deviation Angle after Transsphenoidal Surgery Can Predict the Development of Diabetes Insipidus for Pituitary Adenomas. Endocr. Connect. 2022, 11, e220187. [Google Scholar] [CrossRef]
- Raveendranath, V.; Nagarajan, K.; Umamageswari, A.; Srinidhi, S.; Kavitha, T. Three-Dimensional Magnetic Resonance-Based Morphometry of Pituitary Stalk. Radiol. Med. 2019, 124, 206–210. [Google Scholar] [CrossRef]
- Ho, M.-L.; Rojas, R.; Eisenberg, R.L. Cerebral Edema. Am. J. Roentgenol. 2012, 199, W258–W273. [Google Scholar] [CrossRef]
- de Vries, F.; Lobatto, D.J.; Verstegen, M.J.T.; van Furth, W.R.; Pereira, A.M.; Biermasz, N.R. Postoperative Diabetes Insipidus: How to Define and Grade This Complication? Pituitary 2021, 24, 284–291. [Google Scholar] [CrossRef] [PubMed]
- Cerina, V.; Kruljac, I.; Radosevic, J.M.; Kirigin, L.S.; Stipic, D.; Pecina, H.I.; Vrkljan, M. Diagnostic Accuracy of Perioperative Measurement of Basal Anterior Pituitary and Target Gland Hormones in Predicting Adrenal Insufficiency after Pituitary Surgery. Medicine 2016, 95, e2898. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Polovina, T.S.; Kraljevic, I.; Solak, M.; Balasko, A.; Haxhiu, A.; Haxhiu, A.; Dusek, T.; Poljicanin, T.; Kastelan, D. Early Basal Cortisol Level as a Predictor of Hypothalamic-Pituitary-Adrenal (HPA) Axis Function After Pituitary Tumor Surgery. Exp. Clin. Endocrinol. Diabetes 2020, 128, 709–714. [Google Scholar] [CrossRef] [PubMed]
- Staby, I.; Krogh, J.; Klose, M.; Baekdal, J.; Feldt-Rasmussen, U.; Poulsgaard, L.; Springborg, J.B.; Andreassen, M. Pituitary Function after Transsphenoidal Surgery Including Measurement of Basal Morning Cortisol as Predictor of Adrenal Insufficiency. Endocr. Connect. 2021, 10, 750–757. [Google Scholar] [CrossRef]
- Rotondo, F.; Butz, H.; Syro, L.V.; Yousef, G.M.; Di Ieva, A.; Restrepo, L.M.; Quintanar-Stephano, A.; Berczi, I.; Kovacs, K. Arginine Vasopressin (AVP): A Review of Its Historical Perspectives, Current Research and Multifunctional Role in the Hypothalamo-Hypophysial System. Pituitary 2016, 19, 345–355. [Google Scholar] [CrossRef] [PubMed]
- Stieg, M.R.; Renner, U.; Stalla, G.K.; Kopczak, A. Advances in Understanding Hypopituitarism. F1000Research 2017, 6, 4–8. [Google Scholar] [CrossRef]
- Cironi, K.A.; Decater, T.; Iwanaga, J.; Dumont, A.S.; Tubbs, R.S. Arterial Supply to the Pituitary Gland: A Comprehensive Review. World Neurosurg. 2020, 142, 206–211. [Google Scholar] [CrossRef]
- Ito, M.; Mitobe, Y.; Hiraka, T.; Kanoto, M.; Sonoda, Y. Changes in Vascular Supply Pattern Associated with Growth of Nonfunctioning Pituitary Adenomas. Surg. Neurol. Int. 2022, 13, e481. [Google Scholar] [CrossRef]
- Staartjes, V.E.; Stricker, S.; Muscas, G.; Maldaner, N.; Holzmann, D.; Burkhardt, J.-K.; Seifert, B.; Schmid, C.; Serra, C.; Regli, L. Intraoperative Unfolding and Postoperative Pruning of the Pituitary Gland after Transsphenoidal Surgery for Pituitary Adenoma: A Volumetric and Endocrinological Evaluation. Endocrine 2019, 63, 231–239. [Google Scholar] [CrossRef]
- Fatemi, N.; Dusick, J.R.; Mattozo, C.; McArthur, D.L.; Cohan, P.; Boscardin, J.; Wang, C.; Swerdloff, R.S.; Kelly, D.F. Pituitary Hormonal Loss and Recovery after Transsphenoidal Adenoma Removal. Neurosurgery 2008, 63, 709–719. [Google Scholar] [CrossRef]
- Iorgi, N.D.; Morana, G.; Gallizia, A.L.; Maghnie, M. Pituitary Gland Imaging and Outcome. Endocr. Dev. 2012, 23, 16–29. [Google Scholar] [CrossRef] [PubMed]
- Satogami, N.; Miki, Y.; Koyama, T.; Kataoka, M.; Togashi, K. Normal Pituitary Stalk: High-Resolution MR Imaging at 3T. Am. J. Neuroradiol. 2010, 31, 355–359. [Google Scholar] [CrossRef] [PubMed]
- Ling, S.; Zhao, Z.; Tao, B.; Zhao, H.; Su, T.; Jiang, Y.; Xie, J.; Sun, Q.; Bian, L.; Sun, K.; et al. Pituitary Stalk Thickening in a Large Cohort: Toward More Accurate Predictors of Pituitary Dysfunction and Etiology. Endocr. Pract. 2019, 25, 534–544. [Google Scholar] [CrossRef] [PubMed]

| Variable | Plane/Sequence | Definition |
|---|---|---|
| Diameter chiasm (mm) | sagittal T1c 1 | The maximal pituitary stalk diameter is perpendicular to the longitudinal axis of the stalk at the optic chiasm, the level of the insertion into the pituitary gland, and the middle level in between. |
| Diameter middle (mm) | sagittal T1c 1 | |
| Diameter gland (mm) | sagittal T1c 1 | |
| Diameter minimum (mm) | sagittal T1c 1 | The minimal value of the three levels listed above. |
| Length (mm) | sagittal T1c 1 | The length of the pituitary stalk from the inflection point to the chiasmatic recess and the insertion into the gland. |
| Coronal angle (°) | coronal T1c 1 | The absolute value of the angle of the pituitary stalk against the vertical. The pivot point is the infundibulum. |
| T1 signal ratio | sagittal T1 | The ratio of the average T1/T2 signal intensity of a seizable region of interest in the pituitary stalk divided by a seizable region of interest in the callosal body. |
| T2 signal ratio | sagittal T2 |
| Variable | Unit | Value |
|---|---|---|
| Females: Males | N (%) | 23 (48): 25 (52) |
| Age | Years (+/− SD) | 55 (+/−16) |
| Endoscopic: Microscopic | N (%) | 36 (75): 12 (25) |
| Tumor size 1 | mm (+/− SD) | 22 (+/−6) |
| Knosp classification | ||
| 1 | N (%) | 3 (6) |
| 2 | N (%) | 13 (27) |
| 3 | N (%) | 21 (44) |
| 4 | N (%) | 11 (23) |
| Concomitant prolactinemia | N (%) | 20 (42) |
| Preoperative endocrinological status | ||
| No insufficiency | N (%) | 23 (48) |
| Isolated adrenocortical insufficiency | N (%) | 10 (38) |
| Isolated growth hormone insufficiency | N (%) | 5 (10) |
| Isolated gonadotropin insufficiency | N (%) | 0 (0) |
| Mixed insufficiency | N (%) | 10 (21) 2 |
| New-onset adrenocortical insufficiency | N (%) | 11 (38) 3 |
| New-onset arginine vasopressin deficiency (AVP-D) | ||
| Transient | N (%) | 12 (25) |
| Permanent | N (%) | 1 (2) |
| Variable | No NAI | NAI 1 | p-Value | No AVP-D | AVP-D | p-Value |
|---|---|---|---|---|---|---|
| Diameter chiasm (mm) | 3.5 (+/−0.6) | 3.6 (+/−0.6) | 0.253 | 3.3 (+/−0.7) | 3.7 (+/−0.8) | 0.032 |
| Diameter middle (mm) | 2.9 (+/−0.6) | 3.4 (+/−0.7) | 0.016 | 3.0 (+/−0.6) | 3.4 (+/−0.7) | 0.035 |
| Diameter gland (mm) | 2.7 (+/−0.7) | 3.2 (+/−0.4) | 0.028 | 2.7 (+/−0.6) | 3.2 (+/−0.6) | 0.007 |
| Diameter minimum (mm) | 2.5 (+/−0.4) | 3.0 (+/−0.4) | <0.001 | 2.5 (+/−0.6) | 2.9 (+/−0.3) | 0.008 |
| Length (mm) | 10.5 (+/−1.4) | 11.7 (+/−3.1) | 0.245 | 10.7 (+/−1.8) | 11.0 (+/−3.2) | 0.789 |
| Coronal angle (°) | 13 (+/−14) | 14 (+/−15) | 0.869 | 13 (+/−12) | 12 (+/−16) | 0.416 |
| T1 signal ratio | 1.2 (+/−0.2) | 1.2 (+/−0.3) | 0.884 | 1.2 (+/−0.2) | 1.1 (+/−0.1) | 0.178 |
| T2 signal ratio | 2.6 (+/−0.6) | 2.4 (+/−0.6) | 0.339 | 2.5 (+/−0.5) | 2.4 (+/−0.7) | 0.461 |
| Variable | Sensitivity | Specificity | PPV | NPV |
|---|---|---|---|---|
| Diameter minimum > 2.9 mm and NAI | 73 | 89 | 80 | 84 |
| Diameter minimum > 2.7 mm and AVP-D | 92 | 66 | 50 | 96 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Becker, R.; Hlavac, M.; Etzrodt-Walter, G.; Sommer, F.; Wirtz, C.R.; Schmitz, B.; Pala, A. Pituitary Stalk Morphology as a Predictor of New-Onset Adrenocortical Insufficiency and Arginine Vasopressin Deficiency after Transsphenoidal Resections of Pituitary Macroadenomas: A Retrospective Single-Center Study with a Focus on iMRI. Cancers 2023, 15, 3929. https://doi.org/10.3390/cancers15153929
Becker R, Hlavac M, Etzrodt-Walter G, Sommer F, Wirtz CR, Schmitz B, Pala A. Pituitary Stalk Morphology as a Predictor of New-Onset Adrenocortical Insufficiency and Arginine Vasopressin Deficiency after Transsphenoidal Resections of Pituitary Macroadenomas: A Retrospective Single-Center Study with a Focus on iMRI. Cancers. 2023; 15(15):3929. https://doi.org/10.3390/cancers15153929
Chicago/Turabian StyleBecker, Ralf, Michal Hlavac, Gwendolin Etzrodt-Walter, Fabian Sommer, Christian Rainer Wirtz, Bernd Schmitz, and Andrej Pala. 2023. "Pituitary Stalk Morphology as a Predictor of New-Onset Adrenocortical Insufficiency and Arginine Vasopressin Deficiency after Transsphenoidal Resections of Pituitary Macroadenomas: A Retrospective Single-Center Study with a Focus on iMRI" Cancers 15, no. 15: 3929. https://doi.org/10.3390/cancers15153929
APA StyleBecker, R., Hlavac, M., Etzrodt-Walter, G., Sommer, F., Wirtz, C. R., Schmitz, B., & Pala, A. (2023). Pituitary Stalk Morphology as a Predictor of New-Onset Adrenocortical Insufficiency and Arginine Vasopressin Deficiency after Transsphenoidal Resections of Pituitary Macroadenomas: A Retrospective Single-Center Study with a Focus on iMRI. Cancers, 15(15), 3929. https://doi.org/10.3390/cancers15153929






