Portrait of the Inflammatory Response to Radioiodine Therapy in Female Patients with Differentiated Thyroid Cancer with/without Type 2 Diabetes Mellitus
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Study Protocol
2.2. Blood Sampling and Radioactivity Quantification
2.3. Biomarker Measurement
2.4. Statistics
3. Results
3.1. Blood Parameters before the 131I Intake
3.2. Characteristics of the Study Population with a Focus on Whole-Blood Radioactivity
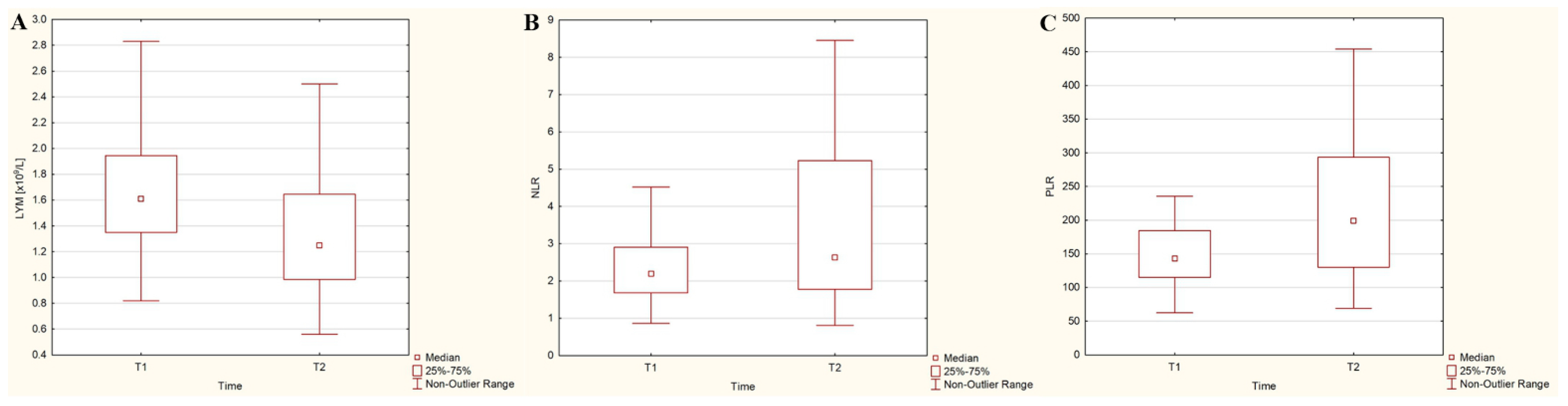
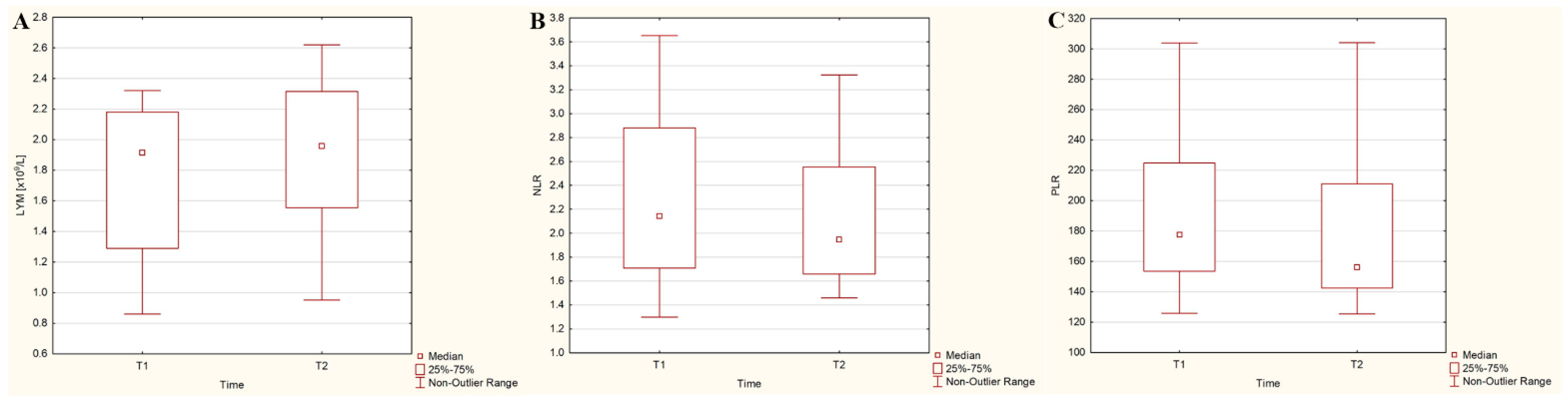
3.3. Dynamics of Hematological Parameters after the 131I Intake
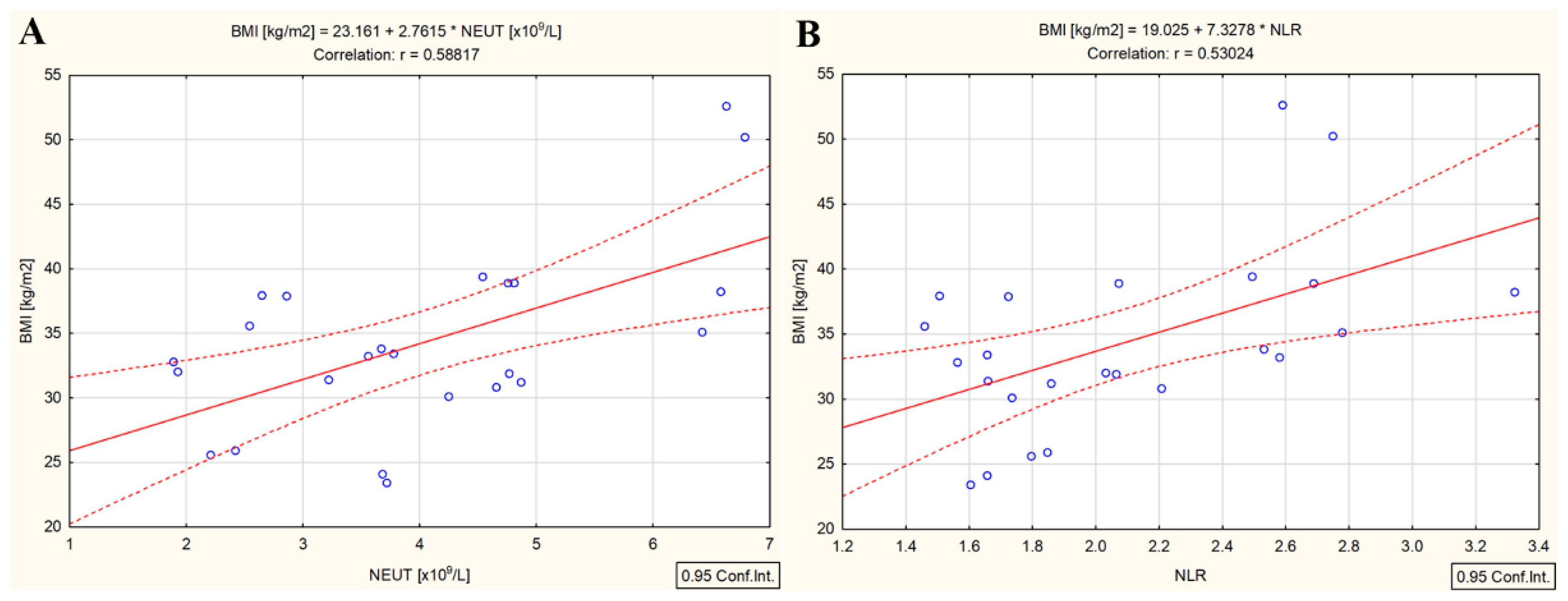
3.4. Correlations in the DTC/−T2DM Group
3.5. Correlations in the DTC/+T2DM Group
4. Discussion
- (i)
- Expression of NIS: NIS expression has been detected in various tissues, including the pancreas, where ductal cells, exocrine parenchymal cells, and islets of Langerhans show positive staining [13,14]). It is possible that, in the presence of T2DM, iodine uptake is relatively high in pancreatic tissues, especially in the islets of Langerhans, which are known to exhibit dysfunction in T2DM [10,14]. It is well known that the thyroid gland concentrates iodine by a factor of 20–40 times compared to plasma [12]. Cumulatively, these factors may contribute to decreased 131I uptake in the bloodstream.
- (ii)
- Changes in biomolecule conformation: The uptake of 131I in the blood is dependent on the iodination of proteins, carbohydrates, and lipids [28]. In the presence of T2DM, structural conformational changes in biomolecules from the blood, resulting from the disease itself, could reduce the number of binding sites and consequently decrease 131I uptake in this group [28].
- (iii)
- Increased urination: Obesity, often associated with T2DM, can lead to increased intra-abdominal pressure, resulting in increased urine production or frequency [29]. This, coupled with the common symptom of increased urination in T2DM [30], could lead to a higher excretion of 131I in T2DM patients compared to those without T2DM.
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- International Diabetes Federation. IDF Atlas, 10th ed. Available online: https://diabetesatlas.org/atlas/tenth-edition/ (accessed on 14 February 2023).
- The American Diabetes Association. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes—2021. Diabetes Care 2021, 44, S15–S33. [Google Scholar] [CrossRef]
- Zhu, B.; Qu, S. The Relationship Between Diabetes Mellitus and Cancers and Its Underlying Mechanisms. Front. Endocrinol. 2022, 13, 800995. [Google Scholar] [CrossRef] [PubMed]
- Kalra, S.; Aggarwal, S.; Khandelwal, D. Thyroid Dysfunction and Type 2 Diabetes Mellitus: Screening Strategies and Implications for Management. Diabetes Ther. 2019, 10, 2035–2044. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tuttle, R.M.; Ahuja, S.; Avram, A.M.; Bernet, V.J.; Bourguet, P.; Daniels, G.H.; Dillehay, G.; Draganescu, C.; Flux, G.; Führer, D.; et al. Controversies, Consensus, and Collaboration in the Use of 131I Therapy in Differentiated Thyroid Cancer: A Joint Statement from the American Thyroid Association, the European Association of Nuclear Medicine, the Society of Nuclear Medicine and Molecular Imaging, and the European Thyroid Association. Thyroid 2019, 29, 461–470. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gulec, S.A.; Ahuja, S.; Avram, A.M.; Bernet, V.J.; Bourguet, P.; Draganescu, C.; Elisei, R.; Giovanella, L.; Grant, F.D.; Greenspan, B.; et al. A Joint Statement from the American Thyroid Association, the European Association of Nuclear Medicine, the European Thyroid Association, the Society of Nuclear Medicine and Molecular Imaging on Current Diagnostic and Theranostic Approaches in the Management of Thyroid Cancer. Thyroid 2021, 31, 1009–1019. [Google Scholar] [CrossRef]
- Gherghe, M.; Lazar, A.M.; Mutuleanu, M.D.; Stanciu, A.E.; Martin, S. Radiomics Analysis of [18F]FDG PET/CT Thyroid Incidentalomas: How Can It Improve Patients’ Clinical Management? A Systematic Review from the Literature. Diagnostics 2022, 12, 471. [Google Scholar] [CrossRef]
- Avram, A.M.; Giovanella, L.; Greenspan, B.; Lawson, S.A.; Luster, M.; Van Nostrand, D.; Peacock, J.G.; Ovčariček, P.P.; Silberstein, E.; Tulchinsky, M.; et al. SNMMI Procedure Standard/EANM Practice Guideline for Nuclear Medicine Evaluation and Therapy of Differentiated Thyroid Cancer: Abbreviated Version. J. Nucl. Med. 2022, 63, 15N–35N. [Google Scholar] [PubMed]
- Pacini, F.; Fuhrer, D.; Elisei, R.; Handkiewicz-Junak, D.; Leboulleux, S.; Luster, M.; Schlumberger, M.; Smit, J.W. 2022 ETA Consensus Statement: What are the indications for post-surgical radioiodine therapy in differentiated thyroid cancer? Eur. Thyroid. J. 2022, 11, e210046. [Google Scholar] [CrossRef]
- Roh, E.; Noh, E.; Hwang, S.Y.; Kim, J.A.; Song, E.; Park, M.; Choi, K.M.; Baik, S.H.; Cho, G.J.; Yoo, H.J. Increased Risk of Type 2 Diabetes in Patients with Thyroid Cancer After Thyroidectomy: A Nationwide Cohort Study. J. Clin. Endocrinol. Metab. 2021, 107, e1047–e1056. [Google Scholar] [CrossRef]
- Riedel, C.; Levy, O.; Carrasco, N. Post-transcriptional regulation of the sodium/iodide symporter by thyrotropin. J. Biol. Chem. 2001, 276, 21458–21463. [Google Scholar] [CrossRef] [Green Version]
- Salvatore, D.; Davies, T.F.; Schlumberger, M.J.; Hay, I.D.; Larsen, P.R. Thyroid physiology and diagnostic evaluation of patients with thyroid disorders. In Williams Textbook of Endocrinology, 12th ed.; Melmed, S., Polonsky, K.S., Larsen, P.R., Kronenberg, H.M., Eds.; Elsevier: Philadelphia, PA, USA, 2011; pp. 327–475. [Google Scholar]
- Spitzweg, C.; Joba, W.; Schriever, K.; Goellner, J.R.; Morris, J.C.; Heufelder, A.E. Analysis of human sodium iodide symporter immunoreactivity in human exocrine glands. J. Clin. Endocrinol. Metab. 1999, 84, 4178–4184. [Google Scholar] [CrossRef]
- Samadi, R.; Shafiei, B.; Azizi, F.; Ghasemi, A. Radioactive Iodine Therapy and Glucose Tolerance. Cell J. 2017, 19, 184–193. [Google Scholar] [CrossRef] [PubMed]
- Prattichizzo, F.; De Nigris, V.; Spiga, R.; Mancuso, E.; La Sala, L.; Antonicelli, R.; Testa, R.; Procopio, A.D.; Olivieri, F.; Ceriello, A. Inflammageing and metaflammation: The yin and yang of type 2 diabetes. Ageing Res. Rev. 2018, 41, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Kuryłowicz, A.; Koźniewski, K. Anti-Inflammatory Strategies Targeting Metaflammation in Type 2 Diabetes. Molecules 2020, 25, 2224. [Google Scholar] [CrossRef] [PubMed]
- Chew, V.; Lee, Y.H.; Pan, L.; Nasir, N.J.M.; Lim, C.J.; Chua, C.; Lai, L.; Hazirah, S.N.; Lim, T.K.H.; Goh, B.K.P.; et al. Immune activation underlies a sustained clinical response to Yttrium-90 radioembolisation in hepatocellular carcinoma. Gut 2019, 68, 335–346. [Google Scholar] [CrossRef]
- Rui, Z.; Wu, R.; Zheng, W.; Wang, X.; Meng, Z.; Tan, J. Effect of 131I Therapy on Complete Blood Count in Patients with Differentiated Thyroid Cancer. Med. Sci. Monit. 2021, 27, e929590. [Google Scholar] [CrossRef]
- Du, W.; Dong, Q.; Lu, X.; Liu, X.; Wang, Y.; Li, W.; Pan, Z.; Gong, Q.; Liang, C.; Gao, G. Iodine-131 therapy alters the immune/inflammatory responses in the thyroids of patients with Graves’ disease. Exp. Ther. Med. 2017, 13, 1155–1159. [Google Scholar] [CrossRef] [Green Version]
- Sabri, O.; Zimny, M.; Schulz, G.; Schreckenberger, M.; Reinartz, P.; Willmes, K.; Buell, U. Success Rate of Radioiodine Therapy in Graves’ Disease: The Influence of Thyrostatic Medication. J. Clin. Endocrinol. Metab. 1999, 84, 1229–1233. [Google Scholar] [CrossRef]
- Stanciu, A.E.; Verzia, A.; Stanciu, M.M.; Zamfirescu, A.; Gheorghe, D.C. Analysis of the Correlation between the Radioactive Iodine Activity and Neutrophil-to-Lymphocyte Ratio in Patients with Differentiated Thyroid Cancer. Cancers 2022, 14, 1899. [Google Scholar] [CrossRef]
- Yi, W.; Kim, B.H.; Kim, M.; Ryang, S.R.; Jang, M.H.; Kim, J.M.; Kim, E.H.; Jeon, Y.K.; Kim, S.S.; Kim, I.J. Short-term bone marrow suppression in differentiated thyroid cancer patients after radioactive iodine treatment. Endocr. J. 2020, 67, 1193–1198. [Google Scholar] [CrossRef]
- Stanciu, A.E.; Stanciu, M.M.; Zamfirescu, A.; Gheorghe, D.C. Cardiovascular Effects of Cumulative Doses of Radioiodine in Differentiated Thyroid Cancer Patients with Type 2 Diabetes Mellitus. Cancers 2022, 14, 2359. [Google Scholar] [CrossRef]
- The American Thyroid Association Taskforce on Radioiodine Safety; Sisson, J.C.; Freitas, J.; McDougall, I.R.; Dauer, L.T.; Hurley, J.R.; Brierley, J.D.; Edinboro, C.H.; Rosenthal, D.; Thomas, M.J.; et al. Radiation safety in the treatment of patients with thyroid diseases by radioiodine 131I: Practice recommendations of the American Thyroid Association. Thyroid 2011, 21, 335–346. [Google Scholar] [CrossRef] [Green Version]
- Bedel, C.; Korkut, M.; Armağan, H.H. NLR, d-NLR and PLR can be affected by many factors. Int. Immunopharmacol. 2021, 90, 107154. [Google Scholar] [CrossRef]
- Weir, C.B.; Jan, A. BMI Classification Percentile and Cut off Points. In StatPearls; [Updated 29 June 2021]; StatPearls Publishing: Treasure Island, FL, USA, 2022. Available online: https://www.ncbi.nlm.nih.gov/books/NBK541070/ (accessed on 10 March 2023).
- Lassmann, M.; Hanscheid, H.; Chiesa, C.; Hindorf, C.; Flux, G.; Luster, M.; EANM Dosimetry Committee. EANM Dosimetry Committee series on standard operational procedures for pretherapeutic dosimetry I: Blood and bone marrow dosimetry in differentiated thyroid cancer therapy. Eur. J. Nucl. Med. Mol. Imaging 2008, 35, 1405–1412. [Google Scholar] [CrossRef]
- Ravindra, M.; Vivek, R.J.; Ayaz, K.M.; Gouda, B.K.M.; Jeevan, K.S.; Prakash, M.; Revathi, P.S.; Shivaraj, B. A comparative study on iodination of normal and diabetic serum. Int. J. Appl. Biol. Pharm. Technol. 2011, 2, 328–333. [Google Scholar]
- Hagovska, M.; Švihra, J.; Buková, A.; Horbacz, A.; Dračková, D.; Lupták, J.; Švihra, J., Jr. The Relationship between Overweight and Overactive Bladder Symptoms. Obes. Facts. 2020, 13, 297–306. [Google Scholar] [CrossRef]
- Ali, J.; Haider, S.M.S.; Ali, S.M.; Haider, T.; Anwar, A.; Hashmi, A.A. Overall Clinical Features of Type 2 Diabetes Mellitus with Respect to Gender. Cureus 2023, 15, e35771. [Google Scholar] [CrossRef]
- Lahfi, Y.; Anjak, O. The impact of body mass index on the external dose rate from patients treated with radioiodine-131: A preliminary study. Iran. J. Nucl. Med. 2015, 23, 89–95. [Google Scholar]
- Pickering, C.A.; Mas, J.; Dykes, J.N.; Domingo, M.T.; Yamauchi, D.M.; Lopatin, G. Williams LE. Exposure levels associated with Na(131)I thyroid cancer patients: Correlation with initial activity and clinical physical parameters. Health Phys. 2014, 107, S163–S165. [Google Scholar] [CrossRef] [PubMed]
- Elgazar-Carmon, V.; Rudich, A.; Hadad, N.; Levy, R. Neutrophils transiently infiltrate intra-abdominal fat early in the course of high-fat feeding. J. Lipid Res. 2008, 49, 1894–1903. [Google Scholar] [CrossRef] [Green Version]
- Zernecke, A.; Bot, I.; Djalali-Talab, Y.; Shagdarsuren, E.; Bidzhekov, K.; Meiler, S.; Krohn, R.; Schober, A.; Sperandio, M.; Soehnlein, O.; et al. Protective role of CXC receptor 4/CXC ligand 12 unveils the importance of neutrophils in atherosclerosis. Circ. Res. 2008, 102, 209–217. [Google Scholar] [CrossRef]
- Watanabe, Y.; Nagai, Y.; Honda, H.; Okamoto, N.; Yanagibashi, T.; Ogasawara, M.; Yamamoto, S.; Imamura, R.; Takasaki, I.; Hara, H.; et al. Bidirectional crosstalk between neutrophils and adipocytes promotes adipose tissue inflammation. FASEB J. 2019, 33, 11821–11835. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heylmann, D.; Ponath, V.; Kindler, T.; Kaina, B. Comparison of DNA repair and radiosensitivity of different blood cell populations. Sci. Rep. 2021, 11, 2478. [Google Scholar] [CrossRef] [PubMed]
- Srikakulapu, P.; McNamara, C.A.B. Lymphocytes and Adipose Tissue Inflammation. Arter. Thromb. Vasc. Biol. 2020, 40, 1110–1122. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.; Chang, A.R. Neutrophil to Lymphocyte Ratio and Platelet to Lymphocyte Ratio in Hepatocellular Carcinoma Treated with Stereotactic Body Radiotherapy. Korean J. Gastroenterol. 2022, 79, 252–259. [Google Scholar] [CrossRef]
- Ren, B.; Zhu, Y. A New Perspective on Thyroid Hormones: Crosstalk with Reproductive Hormones in Females. Int. J. Mol. Sci. 2022, 23, 2708. [Google Scholar] [CrossRef] [PubMed]
- Irelli, A.; Sirufo, M.M.; D’Ugo, C.; Ginaldi, L.; De Martinis, M. Sex and Gender Influences on Cancer Immunotherapy Response. Biomedicines 2020, 8, 232. [Google Scholar] [CrossRef]
- Chakraborty, B.; Byemerwa, J.; Krebs, T.; Lim, F.; Chang, C.Y.; McDonnell, D.P. Estrogen Receptor Signaling in the Immune System. Endocr. Rev. 2023, 44, 117–141. [Google Scholar] [CrossRef]



| Variables | DTC/−T2DM | DTC/+T2DM | p-Value |
|---|---|---|---|
| n = 56 | n = 24 | ||
| Lymphocytes (×109/L) a | 1.6 (1.3–1.9) | 1.9 (1.3–2.2) | 0.015 |
| Neutrophils (×109/L) a | 3.6 (3.1–4.5) | 3.7 (3.0–4.6) | 0.731 |
| Platelets (×109/L) a | 233.5 (199.0–273.0) | 333.0 (312.0–378.0) | <0.001 |
| NLR a | 2.2 (1.7–2.9) | 2.1 (1.7–2.8) | 0.712 |
| PLR a | 143.1 (115.1–184.5) | 177.5 (153.5–224.8) | 0.035 |
| TSH (mIU/L) a | 81.2 (62.7–98.5) | 81.3 (62.9–99.1) | 0.752 |
| Variables | DTC/−T2DM | DTC/+T2DM | p-Value |
|---|---|---|---|
| n = 56 | n = 24 | ||
| Age (years) a | 57.3 ± 9.1 | 62.7 ± 6.5 | 0.153 |
| Height (m) b | 1.64 (1.60–1.65) | 1.63 (1.60–1.65) | 0.754 |
| Weight (kg) b | 81.8 (69.05–90.74) | 91.48 (80.12–101.44) | 0.032 |
| BMI (kg/m2) b | 29.6 (26.2–33.9) | 33.3 (30.8–37.9) | <0.001 |
| Blood Volume (mL) b | 4364.1 (3976.3–4825.1) | 4880.1 (4613.8–5112.3) | 0.018 |
| Administered Activity of 131I (mCi) b | 88.7 (60.7–129.4) | 107.9 (88.9–137) | 0.035 |
| Whole-blood radioactivity (mCi) b | 1.5 (0.7–2.3) | 0.7 (0.4–0.9) | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stanciu, A.E.; Hurduc, A.; Stanciu, M.M.; Gherghe, M.; Gheorghe, D.C.; Prunoiu, V.M.; Zamfir-Chiru-Anton, A. Portrait of the Inflammatory Response to Radioiodine Therapy in Female Patients with Differentiated Thyroid Cancer with/without Type 2 Diabetes Mellitus. Cancers 2023, 15, 3793. https://doi.org/10.3390/cancers15153793
Stanciu AE, Hurduc A, Stanciu MM, Gherghe M, Gheorghe DC, Prunoiu VM, Zamfir-Chiru-Anton A. Portrait of the Inflammatory Response to Radioiodine Therapy in Female Patients with Differentiated Thyroid Cancer with/without Type 2 Diabetes Mellitus. Cancers. 2023; 15(15):3793. https://doi.org/10.3390/cancers15153793
Chicago/Turabian StyleStanciu, Adina Elena, Anca Hurduc, Marcel Marian Stanciu, Mirela Gherghe, Dan Cristian Gheorghe, Virgiliu Mihail Prunoiu, and Adina Zamfir-Chiru-Anton. 2023. "Portrait of the Inflammatory Response to Radioiodine Therapy in Female Patients with Differentiated Thyroid Cancer with/without Type 2 Diabetes Mellitus" Cancers 15, no. 15: 3793. https://doi.org/10.3390/cancers15153793
APA StyleStanciu, A. E., Hurduc, A., Stanciu, M. M., Gherghe, M., Gheorghe, D. C., Prunoiu, V. M., & Zamfir-Chiru-Anton, A. (2023). Portrait of the Inflammatory Response to Radioiodine Therapy in Female Patients with Differentiated Thyroid Cancer with/without Type 2 Diabetes Mellitus. Cancers, 15(15), 3793. https://doi.org/10.3390/cancers15153793






