Perceptions of Canadian Radiation Oncologists, Medical Physicists, and Radiation Trainees about the Feasibility and Need of Boron Neutron Capture Therapy (BNCT) in Canada: A National Survey
Abstract
Simple Summary
Abstract
1. Introduction
1.1. BNCT’s Emerging Role in Radiotherapy
1.2. BNCT History
1.3. Accessibility of Neutron Sources
1.4. Objectives
2. Methods
3. Results
3.1. Eligibility and Response Rate
3.2. Demographics
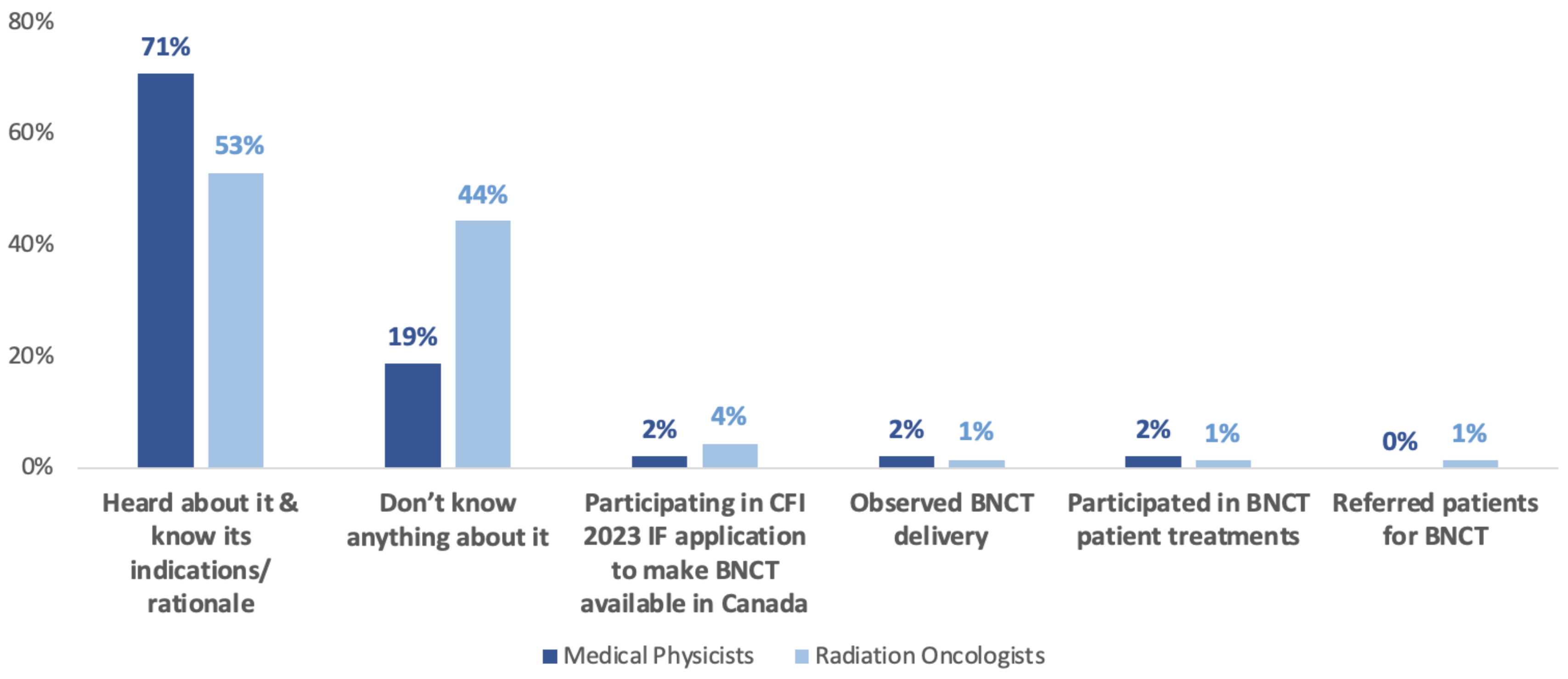
3.3. Current Knowledge about BNCT
3.4. Perceptions on the Failure of Early BNCT Studies
3.5. Radiation Oncologists’ Treatment Recommendations
3.6. Awareness of Current BNCT Development
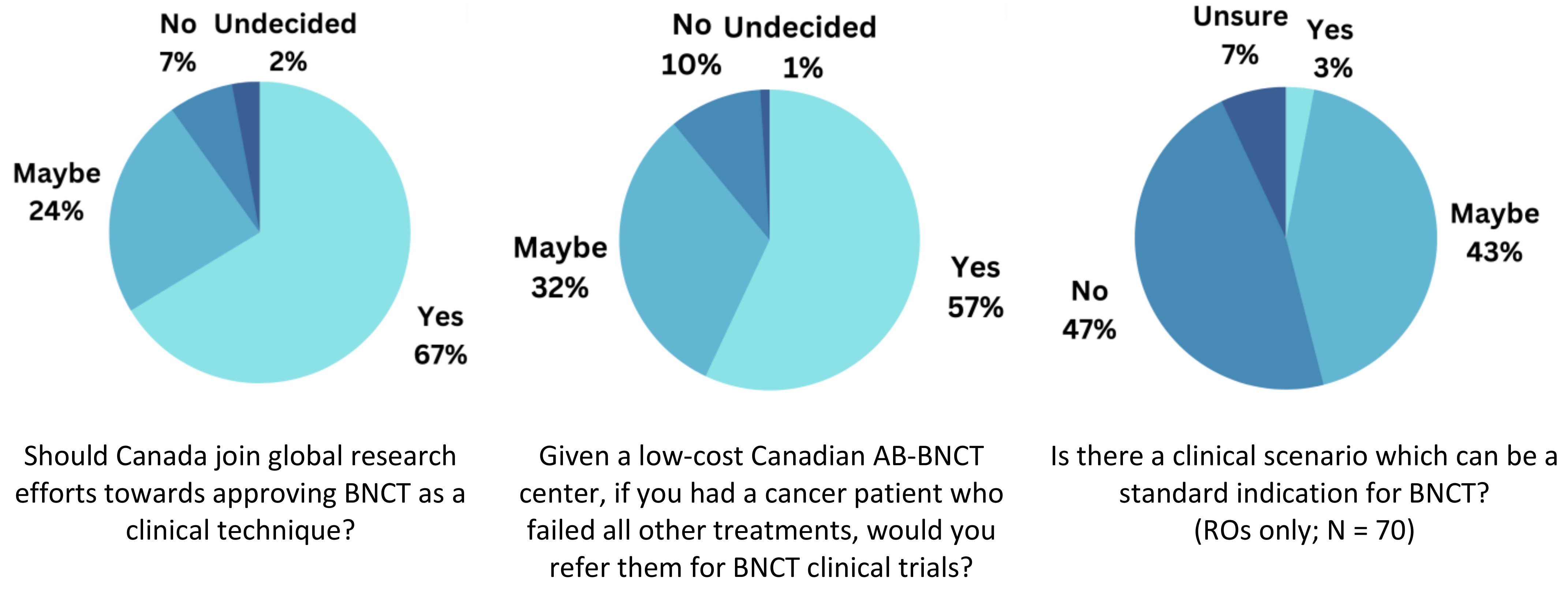
3.7. Opinions on Joining BNCT Research and in the Clinical Context
3.8. Additional Comments
3.8.1. Hesitations towards BNCT Value
3.8.2. Enthusiasm towards the Potential of Canadian BNCT Research
3.8.3. Considerations of BNCT Research
3.8.4. Limited Awareness of BNCT
4. Discussion
4.1. Willingness for BNCT Referrals
4.2. Unanswered Questions in BNCT
4.3. Limited Knowledge on BNCT
4.4. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brenner, D.R.; Weir, H.K.; Demers, A.A.; Ellison, L.F.; Louzado, C.; Shaw, A.; Turner, D.; Woods, R.R.; Smith, L.M. Projected estimates of cancer in Canada in 2020. Can. Med. Assoc. J. 2020, 192, E199–E205. [Google Scholar] [CrossRef] [PubMed]
- Saverwein, W.; Wittig, A.; Moss, R.; Nakagawa, Y. (Eds.) Neutron Capture Therapy: Principles and Applications; Springer: Berlin/Heidelberg, Germany, 2012. [Google Scholar] [CrossRef]
- Hatanaka, H. Clinical results of boron neutron capture therapy. Basic Life Sci. 1990, 54, 15–21. [Google Scholar] [CrossRef]
- Mishima, Y.; Ichihashi, M.; Hatta, S.; Honda, C.; Yamamura, K.; Nakagawa, T.; Obara, H.; Shirakawa, J.; Hiratsuka, J.; Taniyama, K. First human clinical trial of melanoma neutron capture. Diagnosis and therapy. Strahlenther. Onkol. Organ Dtsch. Rontgenges. Al. 1989, 165, 251–254. [Google Scholar]
- Malouff, T.D.; Seneviratne, D.S.; Ebner, D.K.; Stross, W.C.; Waddle, M.R.; Trifiletti, D.M.; Krishnan, S. Boron Neutron Capture Therapy: A Review of Clinical Applications. Front. Oncol. 2021, 11, 601820. Available online: https://www.frontiersin.org/articles/10.3389/fonc.2021.601820 (accessed on 30 May 2023). [CrossRef] [PubMed]
- Dymova, M.A.; Taskaev, S.Y.; Richter, V.A.; Kuligina, E.V. Boron neutron capture therapy: Current status and future perspectives. Cancer Commun. 2020, 40, 406–421. [Google Scholar] [CrossRef] [PubMed]
- Locher, G.L. Biological effects and therapeutic possibilities of neutron. Am. J. Roentgenol. 1936, 36, 1. Available online: https://cir.nii.ac.jp/crid/1571417124880031104 (accessed on 30 May 2023).
- Farr, L.E.; Sweet, W.H.; Locksley, H.B.; Robertson, J.S. Neutron capture therapy of gliomas using boron. Trans. Am. Neurol. Assoc. 1954, 13, 110–113. [Google Scholar]
- Savolainen, S.; Kortesniemi, M.; Timonen, M.; Reijonen, V.; Kuusela, L.; Uusi-Simola, J.; Salli, E.; Koivunoro, H.; Seppälä, T.; Lönnroth, N.; et al. Boron neutron capture therapy (BNCT) in Finland: Technological and physical prospects after 20 years of experiences. Phys. Medica. 2013, 29, 233–248. [Google Scholar] [CrossRef]
- Kankaanranta, L.; Seppälä, T.; Koivunoro, H.; Saarilahti, K.; Atula, T.; Collan, J.; Salli, E.; Kortesniemi, M.; Uusi-Simola, J.; Välimäki, P.; et al. Boron Neutron Capture Therapy in the Treatment of Locally Recurred Head-and-Neck Cancer: Final Analysis of a Phase I/II Trial. Int. J. Radiat. Oncol. Biol. Phys. 2012, 82, e67–e75. [Google Scholar] [CrossRef]
- Miyatake, S.-I.; Kawabata, S.; Hiramatsu, R.; Kuroiwa, T.; Suzuki, M.; Kondo, N.; Ono, K. Boron Neutron Capture Therapy for Malignant Brain Tumors. Neurol. Med. Chir. 2016, 56, 361–371. [Google Scholar] [CrossRef]
- Moss, R.L. Critical review, with an optimistic outlook, on Boron Neutron Capture Therapy (BNCT). Appl. Radiat. Isot. 2014, 88, 2–11. [Google Scholar] [CrossRef]
- Dziura, D.; Tabbassum, S.; MacNeil, A.; Maharaj, D.D.; Laxdal, R.; Kester, O.; Pan, M.; Kumada, H.; Marquardt, D. Boron neutron capture therapy in the new age of accelerator-based neutron production and preliminary progress in Canada. Can. J. Phys. 2023. [Google Scholar] [CrossRef]
- Taskaev, S.Y. Boron Neutron Capture Therapy for Cancer: At the Finish Line. SCIENCE First Hand. Available online: http://scfh.ru/en/papers/boron-neutron-capture-therapy-for-cancer/ (accessed on 30 May 2023).
- Kusaka, S. Enhancing the effectiveness of boron neutron capture therapy (BNCT) for cancer treatment. Impact 2021, 2021, 19–21. [Google Scholar] [CrossRef]
- Bayanov, B.; Kashaeva, E.; Makarov, A.; Malyshkin, G.; Samarin, S.; Taskaev, S. A neutron producing target for BINP accelerator-based neutron source. Appl. Radiat. Isot. 2009, 67, S282–S284. [Google Scholar] [CrossRef]
- Tanaka, H.; Sakurai, Y.; Suzuki, M.; Masunaga, S.; Mitsumoto, T.; Fujita, K.; Kashino, G.; Kinashi, Y.; Liu, Y.; Takada, M.; et al. Experimental verification of beam characteristics for cyclotron-based epithermal neutron source (C-BENS). Appl. Radiat. Isot. 2011, 69, 1642–1645. [Google Scholar] [CrossRef]
- Tanaka, H.; Sakurai, Y.; Suzuki, M.; Masunaga, S.; Kinashi, Y.; Kashino, G.; Liu, Y.; Mitsumoto, T.; Yajima, S.; Tsutsui, H.; et al. Characteristics comparison between a cyclotron-based neutron source and KUR-HWNIF for boron neutron capture therapy. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2009, 267, 1970–1977. [Google Scholar] [CrossRef]
- Kiyanagi, Y.; Sakurai, Y.; Kumada, H.; Tanaka, H. Status of accelerator-based BNCT projects worldwide. AIP Conf. Proc. 2019, 2160, 050012. [Google Scholar] [CrossRef]
- Kreiner, A.J.; Bergueiro, J.; Cartelli, D.; Baldo, M.; Castell, W.; Asoia, J.G.; Padulo, J.; Sandín, J.C.S.; Igarzabal, M.; Erhardt, J.; et al. Present status of Accelerator-Based BNCT. Rep. Pract. Oncol. Radiother. 2016, 21, 95–101. [Google Scholar] [CrossRef]
- ISNCT. Accelerator-Based BNCT Projects. Available online: https://isnct.net/bnct-boron-neutron-capture-therapy/accelerator-based-bnct-projects-2021/ (accessed on 22 May 2023).
- Matsumoto, Y.; Fukumitsu, N.; Ishikawa, H.; Nakai, K.; Sakurai, H. A Critical Review of Radiation Therapy: From Particle Beam Therapy (Proton, Carbon, and BNCT) to Beyond. J. Pers. Med. 2021, 11, 825. [Google Scholar] [CrossRef]
- Jin, W.H.; Seldon, C.; Butkus, M.; Sauerwein, W.; Giap, H.B. A Review of Boron Neutron Capture Therapy: Its History and Current Challenges. Int. J. Part. Ther. 2022, 9, 71–82. [Google Scholar] [CrossRef]
- Laxdal, R.; Maharaj, D.D.; Abbaslou, M.; Tun, Z.; Banks, D.; Gottberg, A.; Marchetto, M.; Rodriguez, E.; Yamani, Z.; Fritzsche, H.; et al. A prototype compact accelerator-based neutron source (CANS) for Canada. J. Neutron Res. 2021, 23, 1–19. [Google Scholar] [CrossRef]
- Maharaj, D.D.; Abbaslou, M.; Tabbassum, S.; Gottberg, A.; Marchetto, M.; Tun, Z.; Nie, L.; Kester, O.; Marquardt, D.; Laxdal, R. A Prototype Compact Accelerator Driven Neutron Source for Canada Supporting Medical and Scientific Applications. arXiv 2022, arXiv:2205.01662. [Google Scholar] [CrossRef]
- ISNCT. Advances in Boron Neutron Capture Therapy. In Proceedings of the IAEA Virtual Technical Meeting, Vienna, Austria, 27–31 July 2020; Available online: https://isnct.net/blog/2020/11/16/iaea-bnct-technical-meeting/ (accessed on 22 May 2023).
- Interactive Map of Accelerators. Nucleus. Available online: https://nucleus.iaea.org/sites/accelerators (accessed on 30 May 2023).
- Busby, S.M. The cobalt bomb in the treatment of bladder tumours; A preliminary report. Can. Med. Assoc. J. 1955, 73, 872–875. [Google Scholar] [PubMed]
- Gillan, C.; Briggs, K.; Pazos, A.G.; Maurus, M.; Harnett, N.; Catton, P.; Wiljer, D. Barriers to accessing radiation therapy in Canada: A systematic review. Radiat. Oncol. 2012, 7, 167. [Google Scholar] [CrossRef]
- Hirose, K.; Konno, A.; Hiratsuka, J.; Yoshimoto, S.; Kato, T.; Ono, K.; Otsuki, N.; Hatazawa, J.; Tanaka, H.; Takayama, K.; et al. Boron neutron capture therapy using cyclotron-based epithermal neutron source and borofalan (10B) for recurrent or locally advanced head and neck cancer (JHN002): An open-label phase II trial. Radiother. Oncol. 2021, 155, 182–187. [Google Scholar] [CrossRef]
- He, H.; Li, J.; Jiang, P.; Tian, S.; Wang, H.; Fan, R.; Liu, J.; Yang, Y.; Liu, Z.; Wang, J. The basis and advances in clinical application of boron neutron capture therapy. Radiat. Oncol. 2021, 16, 216. [Google Scholar] [CrossRef]
- Kusaka, S.; Morizane, Y.; Tokumaru, Y.; Tamaki, S.; Maemunah, I.R.; Akiyama, Y.; Sato, F.; Murata, I. Boron Delivery to Brain Cells via Cerebrospinal Fluid (CSF) Circulation for BNCT in a Rat Melanoma Model. Biology 2022, 11, 397. [Google Scholar] [CrossRef]
- Sköld, K.; H-Stenstam, B.; Diaz, A.Z.; Giusti, V.; Pellettieri, L.; Hopewell, J.W. Boron Neutron Capture Therapy for glioblastoma multiforme: Advantage of prolonged infusion of BPA-f. Acta Neurol. Scand. 2010, 122, 58–62. [Google Scholar] [CrossRef]
- International Atomic Energy Agency. Advances in Boron Neutron Capture Therapy; Non-Serial Publications; IAEA: Vienna, Austria, 2023; Available online: https://www.iaea.org/publications/15339/advances-in-boron-neutron-capture-therapy. (accessed on 23 June 2023).


| Medical Physicists (40.7%; N = 48) | Radiation Oncologists (59.3%; N = 70) | Total % (N = 118) | |
|---|---|---|---|
| Age | |||
| ≤35 Years | 7 | 12 | 16.1% |
| >35 Years, and ≤45 years | 22 | 26 | 40.7% |
| >45 Years, and ≤55 years | 9 | 13 | 18.6% |
| >55 Years, and ≤65 years | 6 | 10 | 13.6% |
| >65 Years | 3 | 5 | 6.8% |
| Prefer not to say | 1 | 4 | 4.2% |
| Gender | |||
| Male | 35 | 50 | 72% |
| Female | 10 | 16 | 22% |
| Prefer not to say | 3 | 4 | 5.9% |
| Region of Practice | |||
| Ontario | 20 | 34 | 45.8% |
| Quebec | 10 | 12 | 18.6% |
| British Columbia | 2 | 5 | 5.9% |
| Saskatchewan | 4 | 3 | 5.9% |
| Nova Scotia | 1 | 4 | 4.2% |
| Alberta | 2 | 4 | 5.1% |
| Manitoba | 1 | 1 | 1.7% |
| New Brunswick | 1 | 0.8% | |
| Newfoundland and Labrador | 1 | 0.8% | |
| Prince Edward Island | 2 | 1 | 2.5% |
| Canada | 1 | 0.8% | |
| Outside Canada | 5 | 4 | 7.6% |
| Years in Practice | |||
| >10 Years, and ≤20 years | 16 | 20 | 30.5% |
| >20 Years | 12 | 17 | 24.6% |
| >5 Years, and ≤10 years | 11 | 11 | 18.6% |
| 0–5 Years | 7 | 15 | 18.6% |
| I am a resident | 2 | 7 | 7.6% |
| Current Knowledge Status of BNCT | Number of Respondents | % of Total Respondents (N = 118) |
|---|---|---|
| I have heard about it and know its indications or rationale | 71 | 60.2% |
| Don’t know anything about it | 40 | 33.9% |
| I am participating in the CFI 2023 IF application to make BNCT available in Canada | 4 | 3.4% |
| I have observed the delivery of BNCT | 2 | 1.7% |
| I have participated in BNCT patient treatments | 2 | 1.7% |
| I have experience in neutron physics and BNCT related technology devlopment | 2 | 1.7% |
| I have read about it | 2 | 1.7% |
| I have referred patients for BNCT | 1 | 0.8% |
| Heard about it once | 1 | 0.8% |
| I have engaged in or published research on BNCT | 1 | 0.8% |
| I recall learning about it during training | 1 | 0.8% |
| Reasons for Failure of Success of Early BNCT Studies between 1950–2000 | Number of Respondents | % of Total Respondents (N = 118) |
|---|---|---|
| I do not know | 52 | 44.1% |
| Lack of large clinical trials due to limited availability of neutron sources or BNCT centres | 50 | 42.4% |
| The fact that nuclear reactors are not suited to perform clinical procedures | 41 | 34.7% |
| Lack of modern treatment planning systems for delivery of optimal neutron dose | 41 | 34.7% |
| Lack of precision in measuring boron concentration in the patient | 34 | 28.8% |
| Need for effective boron compounds | 28 | 23.7% |
| The presence of undesired radiation in the reactor’s neutron beam | 20 | 16.9% |
| Lack of infrastructure | 3 | 2.5% |
| Need for precise dosimetry information | 2 | 1.7% |
| Budget vs benefits/risks | 1 | 0.8% |
| Insufficient efficacy data to merit overcoming logistical barriers associated with theranostic interventions | 1 | 0.8% |
| Lack of motivation from current centers to develop proper evidence | 1 | 0.80% |
| Relative ease and availability of IMRT and not cost effective by comparison | 1 | 0.80% |
| Probably a combination of multiple above | 1 | 0.80% |
| Unresectable Cancers | Number of Respondents | % of Respondents (N = 70) |
|---|---|---|
| Glioblastoma (60 years old, maximal dose chemoradiation 6000 cGy or higher) | ||
| 1. Palliative chemo such as temozolomide | 48 | 68.6% |
| 2. Supportive care only | 41 | 58.6% |
| 3. Bevacizumab or other systemic agents | 33 | 47.1% |
| 4. SRS | 16 | 22.9% |
| 5. Palliative low dose external beam radiation | 13 | 18.6% |
| 6. BNCT | 12 | 17.1% |
| 7. I do not know | 10 | 14.3% |
| 8. Clinical trials | 5 | 7.1% |
| 9. Re-irradiation | 2 | 2.9% |
| 10. Tumor Treating Fields | 2 | 2.9% |
| 11. Refer to CNS colleague | 1 | 1.4% |
| 12. BCNU | 1 | 1.4% |
| Malignant Meningioma (50 years old, maximal dose chemoradiation 6000 cGy or higher) | ||
| 1. Supportive care only | 27 | 38.6% |
| 2. Immunotherapy or other systemic agents | 25 | 35.7% |
| 3. I do not know | 22 | 31.4% |
| 4. SRS | 19 | 27.1% |
| 5. Palliative chemo | 17 | 24.3% |
| 6. BNCT | 11 | 15.7% |
| 7. Palliative low dose external beam radiation | 10 | 14.3% |
| 8. Clinical trials | 3 | 4.3% |
| 9. Referral for outside opinion | 2 | 2.9% |
| 10. Surgery | 1 | 1.4% |
| Head and Neck (65-years old, maximal dose chemoradiation 7000 cGy or higher) | ||
| 1. Palliative chemo | 47 | 67.1% |
| 2. Immunotherapy or other systemic agents | 47 | 67.1% |
| 3. Supportive care only | 35 | 50% |
| 4. Palliative low dose external beam radiation | 33 | 47.1% |
| 5. I do not know | 22 | 31.4% |
| 6. SRS or SBRT | 18 | 25.7% |
| 7. BNCT | 13 | 18.6% |
| 8. Re-irradiation with IMRT | 1 | 1.4% |
| 9. Repeat radical RT may be feasible. | 1 | 1.4% |
| 10. Refer to H&N colleague | 1 | 1.4% |
| 11. Depends on region of recurrence and extent, local vs distal and performance status | 1 | 1.4% |
| Melanoma (30 years old, recurred after multiple surgeries, high dose adjuvant radiation therapy, and third line systemic treatment had to stop due to severe toxicity) | ||
| 1. Fourth line targeting therapy or immunotherapy | 43 | 61.4% |
| 2. Supportive care only | 36 | 51.4% |
| 3. Palliative low dose external beam radiation | 23 | 32.9% |
| 4. SRS or SBRT | 20 | 28.6% |
| 5. Palliative chemo | 19 | 27.1% |
| 6. BNCT | 11 | 15.7% |
| 7. I do not know | 7 | 10% |
| 8. Clinical trials | 7 | 10% |
| 9. Embolization | 1 | 1.4% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Bader, A.-R.; Agapito, J.; Pan, M. Perceptions of Canadian Radiation Oncologists, Medical Physicists, and Radiation Trainees about the Feasibility and Need of Boron Neutron Capture Therapy (BNCT) in Canada: A National Survey. Cancers 2023, 15, 3626. https://doi.org/10.3390/cancers15143626
Al-Bader A-R, Agapito J, Pan M. Perceptions of Canadian Radiation Oncologists, Medical Physicists, and Radiation Trainees about the Feasibility and Need of Boron Neutron Capture Therapy (BNCT) in Canada: A National Survey. Cancers. 2023; 15(14):3626. https://doi.org/10.3390/cancers15143626
Chicago/Turabian StyleAl-Bader, Al-Retage, John Agapito, and Ming Pan. 2023. "Perceptions of Canadian Radiation Oncologists, Medical Physicists, and Radiation Trainees about the Feasibility and Need of Boron Neutron Capture Therapy (BNCT) in Canada: A National Survey" Cancers 15, no. 14: 3626. https://doi.org/10.3390/cancers15143626
APA StyleAl-Bader, A.-R., Agapito, J., & Pan, M. (2023). Perceptions of Canadian Radiation Oncologists, Medical Physicists, and Radiation Trainees about the Feasibility and Need of Boron Neutron Capture Therapy (BNCT) in Canada: A National Survey. Cancers, 15(14), 3626. https://doi.org/10.3390/cancers15143626






