A Graph-Based Approach to Identify Factors Contributing to Postoperative Lung Cancer Recurrence among Patients with Non-Small-Cell Lung Cancer
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Image Acquisition
2.3. Study Variables
- (1)
- Clinicopathologic features: Clinicodemographic information included age, gender, race, weight, height, smoking status, and surgery details. Race was coded as follows: (1) white, (2) African American, or (3) other. Smoking status was coded as follows: (1) current and prior smokers or (2) no smoking history. The histopathological information included pathological TNM staging and histopathologic subtypes (HPS): (1) adenocarcinoma, (2) squamous cell carcinoma, or (3) other.
- (2)
- Body composition tissues depicted on whole-body CT scans: We developed a convolutional-neural-network (CNN)-based deep learning algorithm to automatically segment five different body tissues depicted on the CT images, including visceral adipose tissue (VAT), subcutaneous adipose tissue (SAT), intermuscular adipose tissue (IMAT), skeletal muscle (SM), and bones [31]. We used this algorithm to identify these body tissues on the whole-body CT scans obtained as part of PET-CT examinations. Compared to chest CT scans, whole-body CT scans enable a more accurate assessment of body composition [32]. Based on the segmentation, volume and mean density (i.e., average Hounsfield (HU) value) were computed for each body tissue.
- (3)
- Tumor features based on dedicated chest CT scans: Lung tumors in the cohort were automatically segmented using our available algorithm [33], and 10 CT image features were quantified: (1) volume, (2) mean density, (3) surface area, (4) maximum diameter, (5) mean diameter, (6) solidness, (7) mean diameter of the solid part, (8) cavity ratio, (9) calcification volume, and (10) irregularity. We used a threshold of −300 HU to determine the solid component of a nodule. A threshold of −910 HU was used to determine the cavitation within a nodule. The irregularity of a nodule was calculated as the ratio between its surface area and volume. The calcification volume was computed as the volume in the nodule with a density greater than 200 HU.
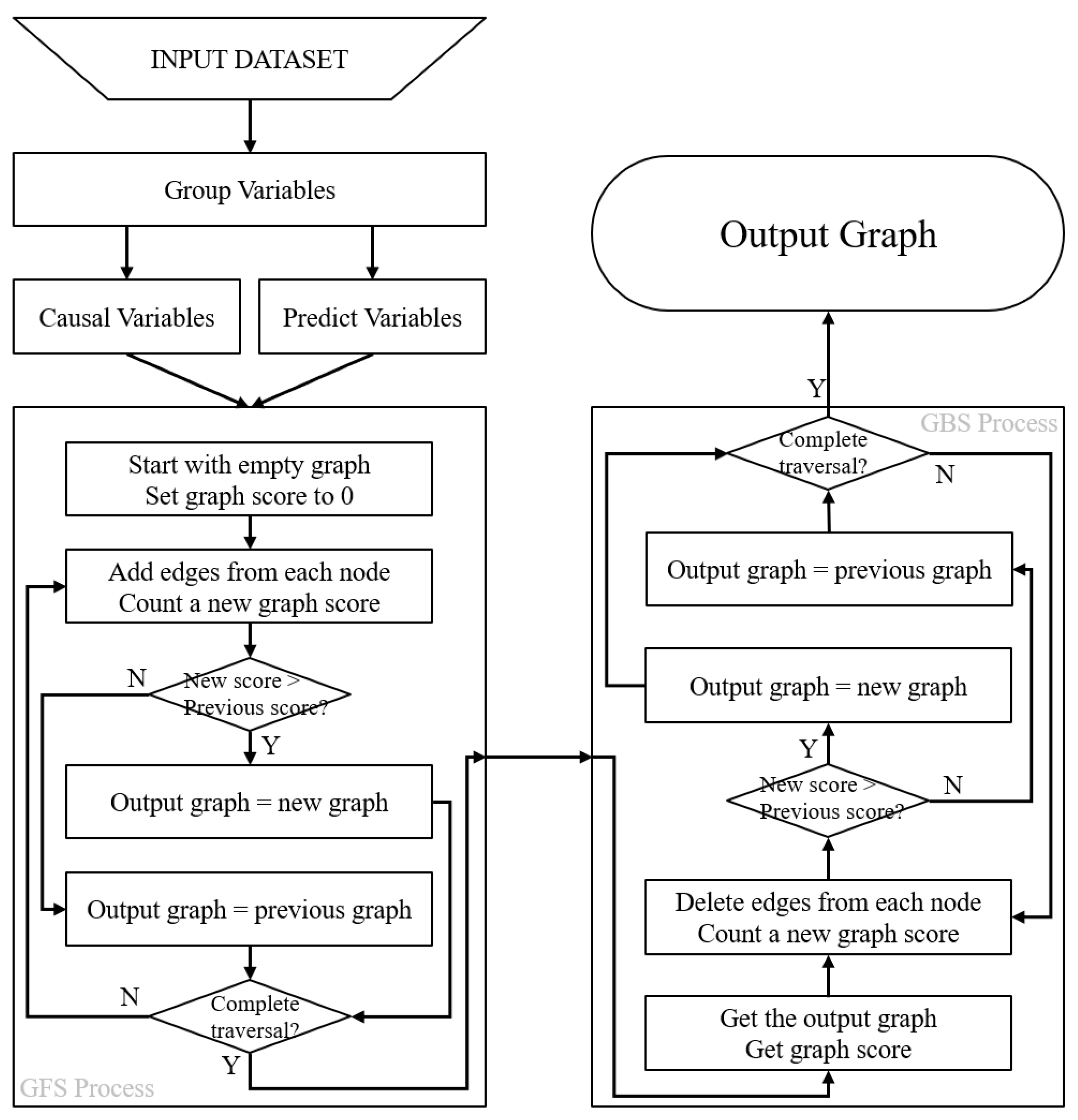
2.4. Causal Discovery Modeling Based on Grouped Greedy Equivalence Search (GGES)
- (1)
- Group Process: All variables are divided into two groups: the causal variable group and the predicted variable group . The groups have the following constraint condition () (Equation (2)): the predicted variables are assumed to have no influence on other variables within their group and are only influenced by other variables. On the other hand, causal variables can both cause and be influenced by other variables. This grouping process requires some prior knowledge about which variables represent the outcomes.
- (2)
- Greedy Forward Search (GFS) Process: The GGES method starts with an initial empty graph and sets the score of the whole graph to 0. Then, the method proceeds with adding edges between variables in a sequential manner, calculating the graph score after each addition and comparing the scores to select the model with the highest score. Nodes assigned to the outcome variable group are skipped according to the constraint placed at the beginning of the process (Equation (1)).
- (3)
- Greedy Backward Search (GBS) Process: The GGES then performs a backward search, where each edge is deleted sequentially from the selected graph model, and a new graph model is calculated. The graph model with the highest graph score is selected as the final causal graph result, which represents the inferred causal relationship between the variables in the dataset (Equation (1)):
2.5. Training GGES Models
2.6. Variable Selection
2.7. Performance Validation
3. Results
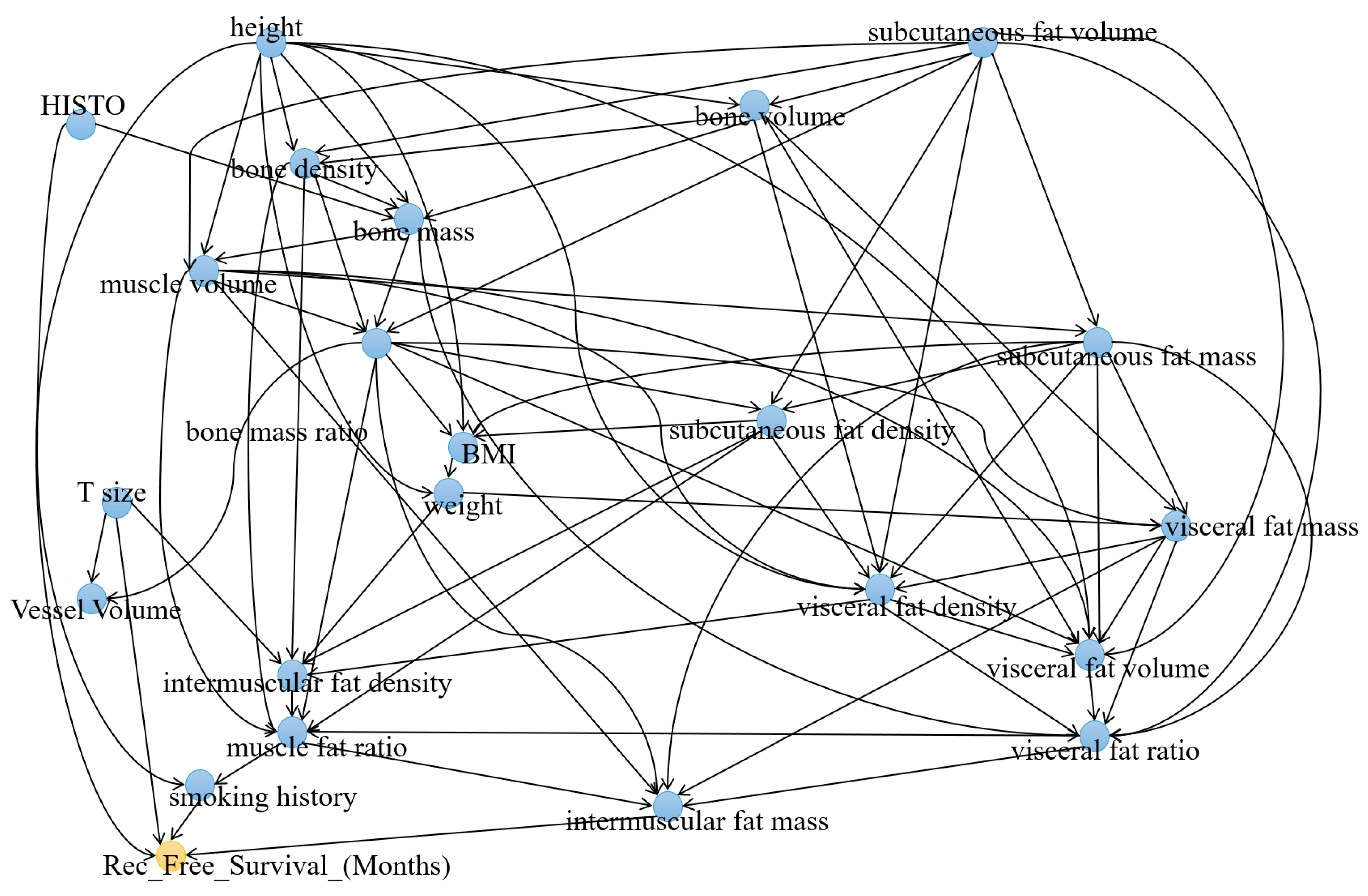
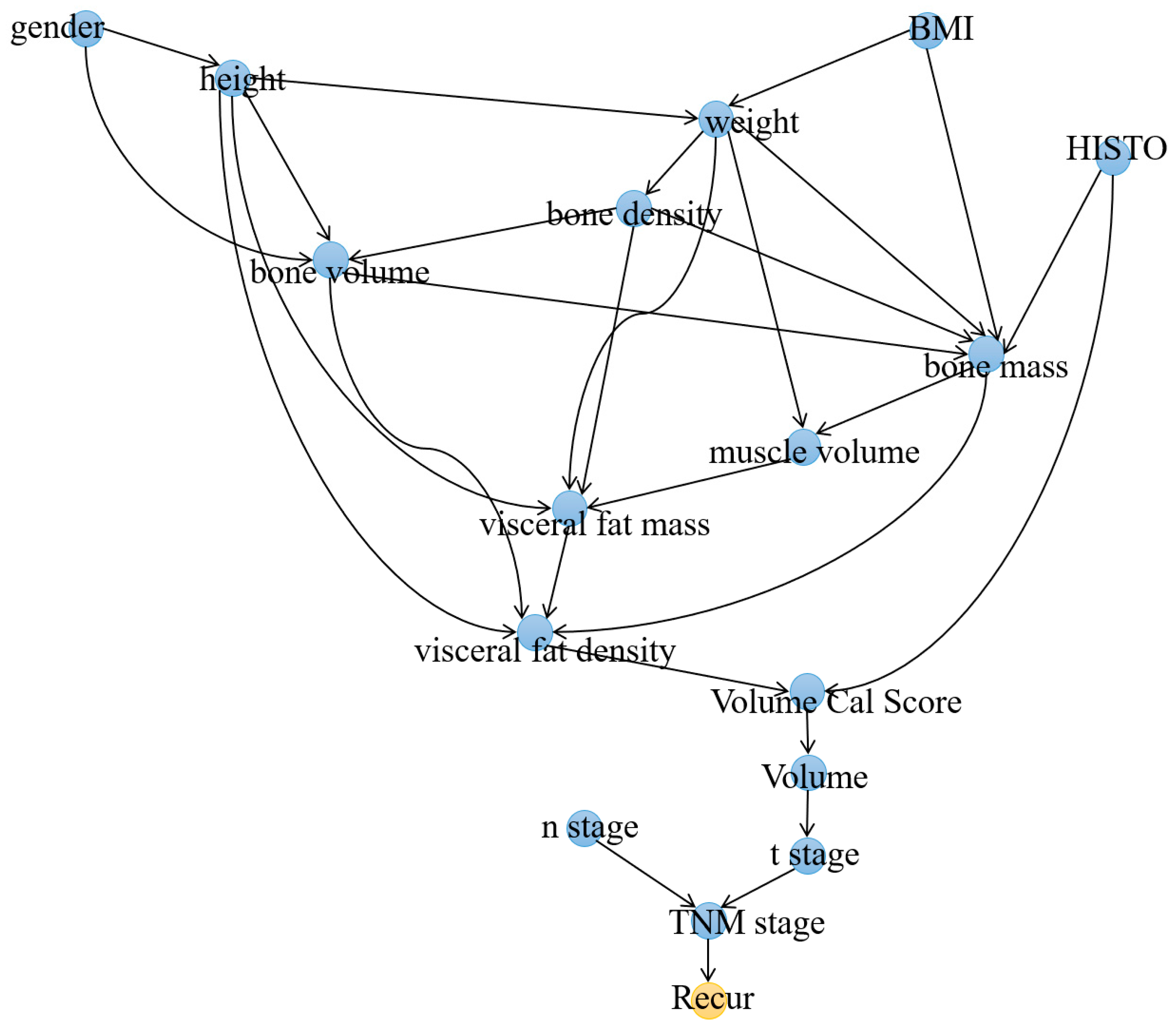
3.1. Causal Analysis of Recurrence-Free Survival
3.2. Causal Analysis of Recurrence
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bade, B.C.; Dela Cruz, C.S. Lung Cancer 2020: Epidemiology, Etiology, and Prevention. Clin. Chest Med. 2020, 41, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Dransfield, M.T.; Lock, B.J.; Garver, R.I., Jr. Improving the lung cancer resection rate in the US Department of Veterans Affairs Health System. Clin. Lung Cancer 2006, 7, 268–272. [Google Scholar] [CrossRef]
- Demicheli, R.; Fornili, M.; Ambrogi, F.; Higgins, K.; Boyd, J.A.; Biganzoli, E.; Kelsey, C.R. Recurrence dynamics for non-small-cell lung cancer: Effect of surgery on the development of metastases. J. Thorac. Oncol. 2012, 7, 723–730. [Google Scholar] [CrossRef] [PubMed]
- Peters, S.; Weder, W.; Dafni, U.; Kerr, K.M.; Bubendorf, L.; Meldgaard, P.; O’Byrne, K.J.; Wrona, A.; Vansteenkiste, J.; Felip, E.; et al. Lungscape: Resected non-small-cell lung cancer outcome by clinical and pathological parameters. J. Thorac. Oncol. 2014, 9, 1675–1684. [Google Scholar] [CrossRef]
- Nemesure, B.; Albano, D.; Bilfinger, T. Lung cancer recurrence and mortality outcomes over a 10-year period using a multidisciplinary team approach. Cancer Epidemiol. 2020, 68, 101804. [Google Scholar] [CrossRef]
- Sekihara, K.; Hishida, T.; Yoshida, J.; Oki, T.; Omori, T.; Katsumata, S.; Ueda, T.; Miyoshi, T.; Goto, M.; Nakasone, S.; et al. Long-term survival outcome after postoperative recurrence of non-small-cell lung cancer: Who is ‘cured’ from postoperative recurrence? Eur. J. Cardiothorac. Surg. 2017, 52, 522–528. [Google Scholar] [CrossRef]
- Morellato, J.B.F.; Guimaraes, M.D.; Medeiros, M.L.L.; Carneiro, H.A.; Oliveira, A.D.; Medici, J.P.O.; Baranauskas, M.V.B.; Gross, J.L. Routine follow-up after surgical treatment of lung cancer: Is chest CT useful? J. Bras. Pneumol. 2021, 47, e20210025. [Google Scholar] [CrossRef]
- Walsh, G.L.; O’Connor, M.; Willis, K.M.; Milas, M.; Wong, R.S.; Nesbitt, J.C.; Putnam, J.B., Jr.; Lee, J.J.; Roth, J.A. Is follow-up of lung cancer patients after resection medically indicated and cost-effective? Ann. Thorac. Surg. 1995, 60, 1563–1570, discussion 1570–1572. [Google Scholar] [CrossRef]
- Forde, P.M.; Spicer, J.; Lu, S.; Provencio, M.; Mitsudomi, T.; Awad, M.M.; Felip, E.; Broderick, S.R.; Brahmer, J.R.; Swanson, S.J.; et al. Neoadjuvant Nivolumab plus Chemotherapy in Resectable Lung Cancer. N. Engl. J. Med. 2022, 386, 1973–1985. [Google Scholar] [CrossRef]
- Wakelee, H.; Liberman, M.; Kato, T.; Tsuboi, M.; Lee, S.H.; Gao, S.; Chen, K.N.; Dooms, C.; Majem, M.; Eigendorff, E.; et al. Perioperative Pembrolizumab for Early-Stage Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2023. Online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Al-Alao, B.S.; Gately, K.; Nicholson, S.; McGovern, E.; Young, V.K.; O’Byrne, K.J. Prognostic impact of vascular and lymphovascular invasion in early lung cancer. Asian Cardiovasc. Thorac. Ann. 2014, 22, 55–64. [Google Scholar] [CrossRef]
- Chen, Y.Y.; Huang, T.W.; Tsai, W.C.; Lin, L.F.; Cheng, J.B.; Chang, H.; Lee, S.C. Risk factors of postoperative recurrences in patients with clinical stage I NSCLC. World J. Surg. Oncol. 2014, 12, 10. [Google Scholar] [CrossRef]
- Kuo, S.W.; Chen, J.S.; Huang, P.M.; Hsu, H.H.; Lai, H.S.; Lee, J.M. Prognostic significance of histologic differentiation, carcinoembryonic antigen value, and lymphovascular invasion in stage I non-small cell lung cancer. J. Thorac. Cardiovasc. Surg. 2014, 148, 1200–1207.e3. [Google Scholar] [CrossRef] [PubMed]
- Ko, K.H.; Hsu, H.H.; Huang, T.W.; Gao, H.W.; Cheng, C.Y.; Hsu, Y.C.; Chang, W.C.; Chu, C.M.; Chen, J.H.; Lee, S.C. Predictive value of 18F-FDG PET and CT morphologic features for recurrence in pathological stage IA non-small cell lung cancer. Medicine 2015, 94, e434. [Google Scholar] [CrossRef]
- Frank, S.M.; Higgins, M.S.; Breslow, M.J.; Fleisher, L.A.; Gorman, R.B.; Sitzmann, J.V.; Raff, H.; Beattie, C. The catecholamine, cortisol, and hemodynamic responses to mild perioperative hypothermia. A randomized clinical trial. Anesthesiology 1995, 82, 83–93. [Google Scholar] [CrossRef]
- Kurz, A.; Sessler, D.I.; Lenhardt, R. Perioperative normothermia to reduce the incidence of surgical-wound infection and shorten hospitalization. Study of Wound Infection and Temperature Group. N. Engl. J. Med. 1996, 334, 1209–1215. [Google Scholar] [CrossRef]
- Horowitz, M.; Neeman, E.; Sharon, E.; Ben-Eliyahu, S. Exploiting the critical perioperative period to improve long-term cancer outcomes. Nat. Rev. Clin. Oncol. 2015, 12, 213–226. [Google Scholar] [CrossRef]
- Wu, H.L.; Wu, Y.M.; Chen, J.T.; Chang, K.Y.; Cherng, Y.G.; Lin, S.P.; Tsou, M.Y.; Tai, Y.H. A comparison of inflammation markers for predicting oncological outcomes after surgical resection of non-small-cell lung cancer: A validated analysis of 2066 patients. Sci. Rep. 2020, 10, 19523. [Google Scholar] [CrossRef]
- Xu, S.; Zhou, J.; Liu, K.; Chen, Z.; He, Z. A Recurrence-Specific Gene-Based Prognosis Prediction Model for Lung Adenocarcinoma through Machine Learning Algorithm. BioMed Res. Int. 2020, 2020, 9124792. [Google Scholar] [CrossRef]
- Zhong, J.; Chen, J.M.; Chen, S.L.; Yi, Y.F. Constructing a Risk Prediction Model for Lung Cancer Recurrence by Using Gene Function Clustering and Machine Learning. Comb. Chem. High Throughput Screen. 2019, 22, 266–275. [Google Scholar] [CrossRef]
- Jones, G.D.; Brandt, W.S.; Shen, R.; Sanchez-Vega, F.; Tan, K.S.; Martin, A.; Zhou, J.; Berger, M.; Solit, D.B.; Schultz, N.; et al. A Genomic-Pathologic Annotated Risk Model to Predict Recurrence in Early-Stage Lung Adenocarcinoma. JAMA Surg. 2021, 156, e205601. [Google Scholar] [CrossRef]
- Wang, X.; Janowczyk, A.; Zhou, Y.; Thawani, R.; Fu, P.; Schalper, K.; Velcheti, V.; Madabhushi, A. Prediction of recurrence in early stage non-small cell lung cancer using computer extracted nuclear features from digital H&E images. Sci. Rep. 2017, 7, 13543. [Google Scholar]
- Wu, Z.; Wang, L.; Li, C.; Cai, Y.; Liang, Y.; Mo, X.; Lu, Q.; Dong, L.; Liu, Y. DeepLRHE: A Deep Convolutional Neural Network Framework to Evaluate the Risk of Lung Cancer Recurrence and Metastasis from Histopathology Images. Front. Genet. 2020, 11, 768. [Google Scholar] [CrossRef] [PubMed]
- Moon, S.; Choi, D.; Lee, J.-Y.; Kim, M.H.; Hong, H.; Kim, B.-S.; Choi, J.-H. Machine Learning-Powered Prediction of Recurrence in Patients with Non-Small Cell Lung Cancer Using Quantitative Clinical and Radiomic Biomarkers. Proc. SPIE 2020, 11314, 220–227. [Google Scholar]
- Piche, M.E.; Poirier, P.; Lemieux, I.; Despres, J.P. Overview of Epidemiology and Contribution of Obesity and Body Fat Distribution to Cardiovascular Disease: An Update. Prog. Cardiovasc. Dis. 2018, 61, 103–113. [Google Scholar] [CrossRef]
- Al-Sofiani, M.E.; Ganji, S.S.; Kalyani, R.R. Body composition changes in diabetes and aging. J. Diabetes Complicat. 2019, 33, 451–459. [Google Scholar] [CrossRef]
- Ahangaran, M.; Jahed-Motlagh, M.R.; Minaei-Bidgoli, B. Causal discovery from sequential data in ALS disease based on entropy criteria. J. Biomed. Inform. 2019, 89, 41–55. [Google Scholar] [CrossRef] [PubMed]
- Cooper, G.F.; Bahar, I.; Becich, M.J.; Benos, P.V.; Berg, J.; Espino, J.U.; Glymour, C.; Jacobson, R.C.; Kienholz, M.; Lee, A.V. The center for causal discovery of biomedical knowledge from big data. J. Am. Med. Inform. Assoc. 2015, 22, 1132–1136. [Google Scholar] [CrossRef]
- Saxe, G.N.; Statnikov, A.; Fenyo, D.; Ren, J.; Li, Z.; Prasad, M.; Wall, D.; Bergman, N.; Briggs, E.C.; Aliferis, C. A complex systems approach to causal discovery in psychiatry. PLoS ONE 2016, 11, e0151174. [Google Scholar] [CrossRef]
- Malinsky, D.; Danks, D. Causal discovery algorithms: A practical guide. Philos. Compass 2018, 13, e12470. [Google Scholar] [CrossRef]
- Pu, L.; Gezer, N.S.; Ashraf, S.F.; Ocak, I.; Dresser, D.E.; Dhupar, R. Automated segmentation of five different body tissues on computed tomography using deep learning. Med. Phys. 2023, 50, 178–191. [Google Scholar] [CrossRef]
- Pu, L.; Ashraf, S.F.; Gezer, N.S.; Ocak, I.; Dresser, D.E.; Leader, J.K.; Dhupar, R. Estimating 3-D whole-body composition from a chest CT scan. Med. Phys. 2022, 49, 7108–7117. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, S.F.; Yin, K.; Meng, C.X.; Wang, Q.; Wang, Q.; Pu, J.; Dhupar, R. Predicting benign, preinvasive, and invasive lung nodules on computed tomography scans using machine learning. J. Thorac. Cardiovasc. Surg. 2021, 163, 1496–1505.e10. [Google Scholar] [CrossRef]
- Hauser, A.; Bühlmann, P. Characterization and greedy learning of interventional Markov equivalence classes of directed acyclic graphs. J. Mach. Learn. Res. 2012, 13, 2409–2464. [Google Scholar]
- Scutari, M.; Graafland, C.E.; Gutiérrez, J.M. Who Learns Better Bayesian Network Structures: Constraint-Based, Score-Based or Hybrid Algorithms? In Proceedings of the International Conference on Probabilistic Graphical Models, Prague, Czech Republic, 11–14 September 2018; pp. 416–427. [Google Scholar]
- Cartwright, N. Causal diversity and the Markov condition. Synthese 1999, 121, 3–27. [Google Scholar] [CrossRef]
- Ebert-Uphoff, I.; Deng, Y. Causal discovery for climate research using graphical models. J. Clim. 2012, 25, 5648–5665. [Google Scholar] [CrossRef]
- Chickering, D.M. Optimal structure identification with greedy search. J. Mach. Learn. Res. 2002, 3, 507–554. [Google Scholar]
- Huang, B.; Zhang, K.; Lin, Y.; Schölkopf, B.; Glymour, C. Generalized score functions for causal discovery. In Proceedings of the 24th ACM SIGKDD International Conference on Knowledge Discovery & Data Mining, London, UK, 19–23 August 2018; pp. 1551–1560. [Google Scholar]
- Malinsky, D.; Spirtes, P. Estimating bounds on causal effects in high-dimensional and possibly confounded systems. Int. J. Approx. Reason. 2017, 88, 371–384. [Google Scholar] [CrossRef]
- Raghu, V.K.; Poon, A.; Benos, P.V. Evaluation of causal structure learning methods on mixed data types. In Proceedings of the 2018 ACM SIGKDD Workshop on Causal Discovery, London, UK, 20 August 2018; pp. 48–65. [Google Scholar]
- Mayer, A.; Thoemmes, F.; Rose, N.; Steyer, R.; West, S.G. Theory and analysis of total, direct, and indirect causal effects. Multivar. Behav. Res. 2014, 49, 425–442. [Google Scholar] [CrossRef]
- Fujiwara, N.; Nakagawa, H.; Kudo, Y.; Tateishi, R.; Taguri, M.; Watadani, T.; Nakagomi, R.; Kondo, M.; Nakatsuka, T.; Minami, T.; et al. Sarcopenia, intramuscular fat deposition, and visceral adiposity independently predict the outcomes of hepatocellular carcinoma. J. Hepatol. 2015, 63, 131–140. [Google Scholar] [CrossRef]
- Park, J.W.; Chang, S.Y.; Lim, J.S.; Park, S.J.; Park, J.J.; Cheon, J.H.; Kim, W.H.; Kim, T.I. Impact of Visceral Fat on Survival and Metastasis of Stage III Colorectal Cancer. Gut Liver 2022, 16, 53–61. [Google Scholar] [CrossRef]
- Leung, C.C.; Lam, T.H.; Yew, W.W.; Chan, W.M.; Law, W.S.; Tam, C.M. Lower lung cancer mortality in obesity. Int. J. Epidemiol. 2011, 40, 174–182. [Google Scholar] [CrossRef]
- Nimri, L.; Saadi, J.; Peri, I.; Yehuda-Shnaidman, E.; Schwartz, B. Mechanisms linking obesity to altered metabolism in mice colon carcinogenesis. Oncotarget 2015, 6, 38195–38209. [Google Scholar] [CrossRef]
- Strulov Shachar, S.; Williams, G.R. The Obesity Paradox in Cancer-Moving Beyond BMI. Cancer Epidemiol. Biomark. Prev. 2017, 26, 13–16. [Google Scholar] [CrossRef] [PubMed]
- Qu, X.; Huang, X.; Jin, F.; Wang, H.; Hao, Y.; Tang, T.; Dai, K. Bone mineral density and all-cause, cardiovascular and stroke mortality: A meta-analysis of prospective cohort studies. Int. J. Cardiol. 2013, 166, 385–393. [Google Scholar] [CrossRef]
- Tseng, O.L.; Dawes, M.G.; Spinelli, J.J.; Gotay, C.C.; McBride, M.L. Utilization of bone mineral density testing among breast cancer survivors in British Columbia, Canada. Osteoporos. Int. 2017, 28, 3439–3449. [Google Scholar] [CrossRef]
- Ganry, O.; Lapotre-Ledoux, B.; Fardellone, P.; Dubreuil, A. Bone mass density, subsequent risk of colon cancer and survival in postmenopausal women. Eur. J. Epidemiol. 2008, 23, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Ilic, I.; Potthoff, A.L.; Borger, V.; Heimann, M.; Paech, D.; Giordano, F.A.; Schmeel, L.C.; Radbruch, A.; Schuss, P.; Schafer, N.; et al. Bone Mineral Density as an Individual Prognostic Biomarker in Patients with Surgically-Treated Brain Metastasis from Lung Cancer (NSCLC). Cancers 2022, 14, 4633. [Google Scholar] [CrossRef]

| Characteristics | Value 1 |
|---|---|
| Age | 68.3 ± 9.45 |
| Height (cm) | 174.4 ± 43.0 |
| Weight (kg) | 66.3 ± 4.02 |
| BMI | 27.8 ± 5.98 |
| Sex | |
| Female | 168 (46.28%) |
| Male | 195 (53.72%) |
| Race | |
| White | 326 (89.81%) |
| Black | 34 (9.37%) |
| Asian | 3 (0.82%) |
| Surgical method | |
| lobectomy | 292 (80.44%) |
| Segmentectomy and wedge resection | 62 (17.08%) |
| Pneumonectomy | 9 (2.48%) |
| Tumor site | |
| RUL | 147 (40.50%) |
| RML | 26 (7.16%) |
| RLL | 50 (13.77%) |
| LUL | 91 (25.07%) |
| LLL | 49 (13.50%) |
| Overall pathological stage | |
| 0 (NED) | 6 (1.65%) |
| 1A1, 1A2, 1A3, 1B | 188 (51.79%) |
| 2A, B | 103 (28.37%) |
| 3 | 66 (18.18%) |
| T stage | |
| 0 | 6 (1.65%) |
| 1A, B, C | 138 (38.02%) |
| 2A, B | 157 (43.25%) |
| 3 | 47 (12.95%) |
| 4 | 15 (4.13%) |
| N stage | |
| 0 | 252 (69.42%) |
| 1 | 68 (18.73%) |
| 2 | 43 (11.85%) |
| Recurrence | 0.67 ± 0.47 |
| Rec_free_survival_(months) | 12.53 ± 16.29 |
| Variables | IDA Score | Variables | IDA Score |
|---|---|---|---|
| density_intermuscular_fat | −0.008 | vis_fat_ratio | 1.569 |
| Weight | −0.033 | mass_intermuscular_fat | −1.600 |
| volume_muscle | 0.231 | height | 1.696 |
| Vessel_Volume.ml. | −0.299 | HISTO_CODED | −1.822 |
| density_subcutaneous_fat | −0.314 | Tsize | −2.371 |
| BMI | −0.384 | volume_bone | −2.421 |
| volume_subcutaneous_fat | −0.740 | SMOKE_HX_CODED | −3.106 |
| density_bone | 0.798 | muscle_fat_ratio | −3.776 |
| bone_mass_ratio | 0.964 | mass_bone | 41.781 |
| density_visceral_fat | 1.121 | volume_visceral_fat | −43.176 |
| mass_visceral_fat | −1.224 | mass_subcutaneous_fat | −76.002 |
| Variables | IDA Score |
|---|---|
| density_visceral_fat | 0.002 |
| density_bone | 0.004 |
| mass_subcutaneous_fat | −0.010 |
| density_subcutaneous_fat | 0.011 |
| density_intermuscular_fat | 0.017 |
| volume_bone | −0.030 |
| BMI | −0.032 |
| SEX | 0.033 |
| volume_muscle | 0.041 |
| height | 0.054 |
| mass_visceral_fat | −0.062 |
| density_muscle | 0.127 |
| weight | −0.237 |
| mass_bone | 0.665 |
| volume_subcutaneous_fat | 1.230 |
| volume_visceral_fat | 6.338 |
| Variables | IDA Score |
|---|---|
| density_visceral_fat | 0.002 |
| density_bone | 0.004 |
| volume_bone | −0.030 |
| BMI | −0.032 |
| Gender | 0.033 |
| volume_muscle | 0.041 |
| Volume_Cal._Score.mm3. | 0.047 |
| HISTO_CODED | 0.048 |
| height | 0.054 |
| t_stage | 0.057 |
| mass_visceral_fat | −0.062 |
| Volume.ml. | 0.065 |
| n_stage | 0.109 |
| weight | −0.237 |
| TNM_stage | 0.281 |
| mass_bone | 0.291 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iyer, K.; Ren, S.; Pu, L.; Mazur, S.; Zhao, X.; Dhupar, R.; Pu, J. A Graph-Based Approach to Identify Factors Contributing to Postoperative Lung Cancer Recurrence among Patients with Non-Small-Cell Lung Cancer. Cancers 2023, 15, 3472. https://doi.org/10.3390/cancers15133472
Iyer K, Ren S, Pu L, Mazur S, Zhao X, Dhupar R, Pu J. A Graph-Based Approach to Identify Factors Contributing to Postoperative Lung Cancer Recurrence among Patients with Non-Small-Cell Lung Cancer. Cancers. 2023; 15(13):3472. https://doi.org/10.3390/cancers15133472
Chicago/Turabian StyleIyer, Kartik, Shangsi Ren, Lucy Pu, Summer Mazur, Xiaoyan Zhao, Rajeev Dhupar, and Jiantao Pu. 2023. "A Graph-Based Approach to Identify Factors Contributing to Postoperative Lung Cancer Recurrence among Patients with Non-Small-Cell Lung Cancer" Cancers 15, no. 13: 3472. https://doi.org/10.3390/cancers15133472
APA StyleIyer, K., Ren, S., Pu, L., Mazur, S., Zhao, X., Dhupar, R., & Pu, J. (2023). A Graph-Based Approach to Identify Factors Contributing to Postoperative Lung Cancer Recurrence among Patients with Non-Small-Cell Lung Cancer. Cancers, 15(13), 3472. https://doi.org/10.3390/cancers15133472






