PRMT-1 and p120-Catenin as EMT Mediators in Osimertinib Resistance in NSCLC
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Tyrosine Kinase Inhibitors and Epidermal Growth Factor Ligands
2.2. Antibodies
2.3. Cell Lines and Tissue Culture Techniques
2.4. Primers for qPCR
2.5. Immunoblotting
2.6. Immunofluorescence
2.7. SiRNA Transfection
2.8. MTT Cell Viability Assay
2.9. Wound-Healing Assay
2.10. Immunohistochemistry
3. Results
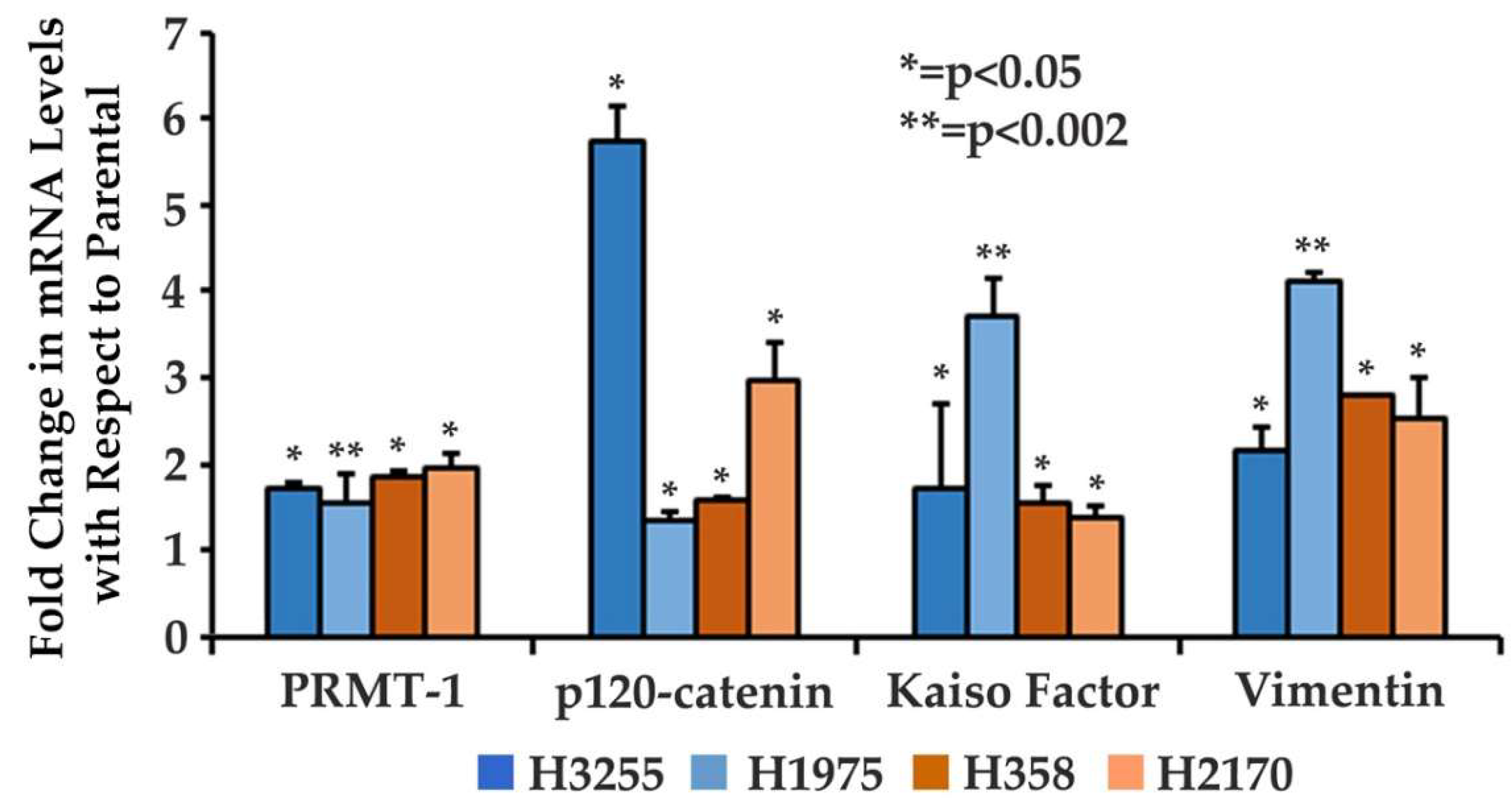
3.1. Increased Gene Expression of EMT-Related Proteins in Osimertinib-Resistant Wild-Type and Mutant EGFR NSCLC Cells
3.2. Increased Expression of Key Protein Biomarkers of the EMT Pathway in H358OR and H1975OR NSCLC Cell Lines
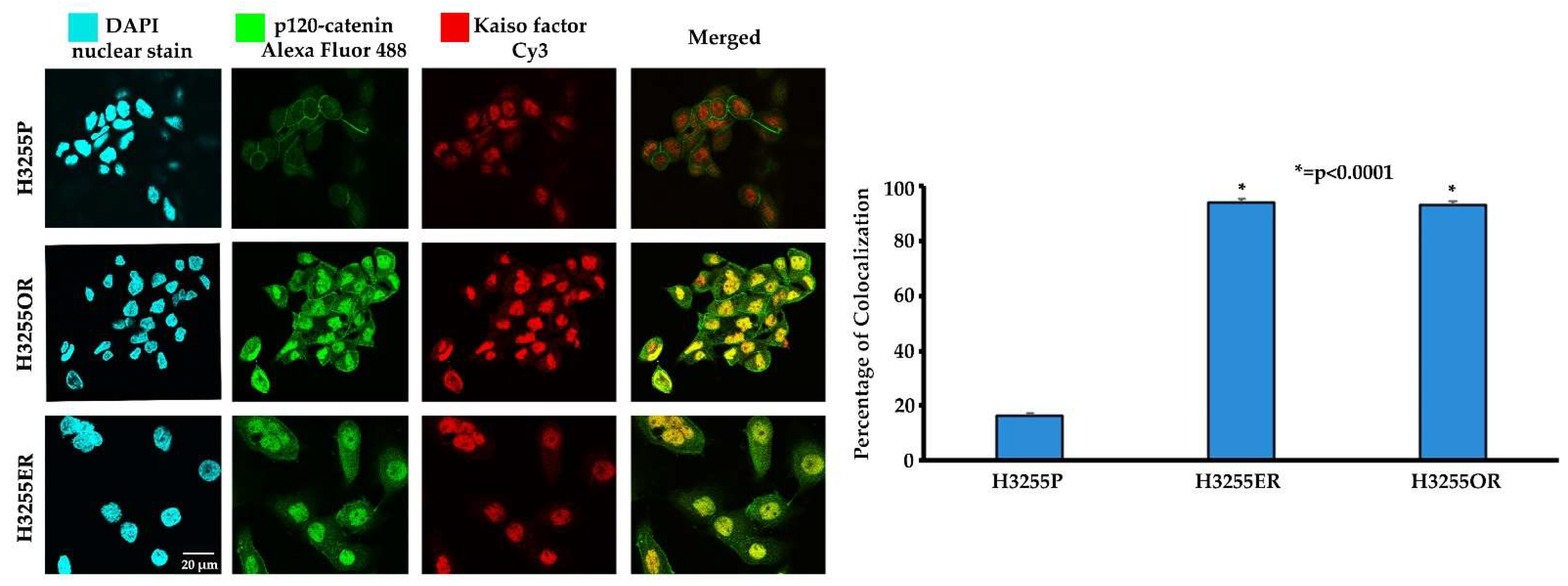
3.3. Colocalization of p120-Catenin and Kaiso Factor in Erlotinib- and Osimertinib-Resistant H3255 NSCLC Cells
3.4. Increased Nuclear Fluorescence of PRMT-1 in the Erlotinib- and Osimertinib-Resistant H3255 and H1975 NSCLC Cells
3.5. Effect of p120-Catenin Knockdown on Reversal of EMT and Osimertinib Resistance in H358OR Cells
3.6. Effect of p120-Catenin Knockdown and Osimertinib Treatment on EMT and Osimertinib Efficacy in H358OR Cells Using a Wound-Healing Assay
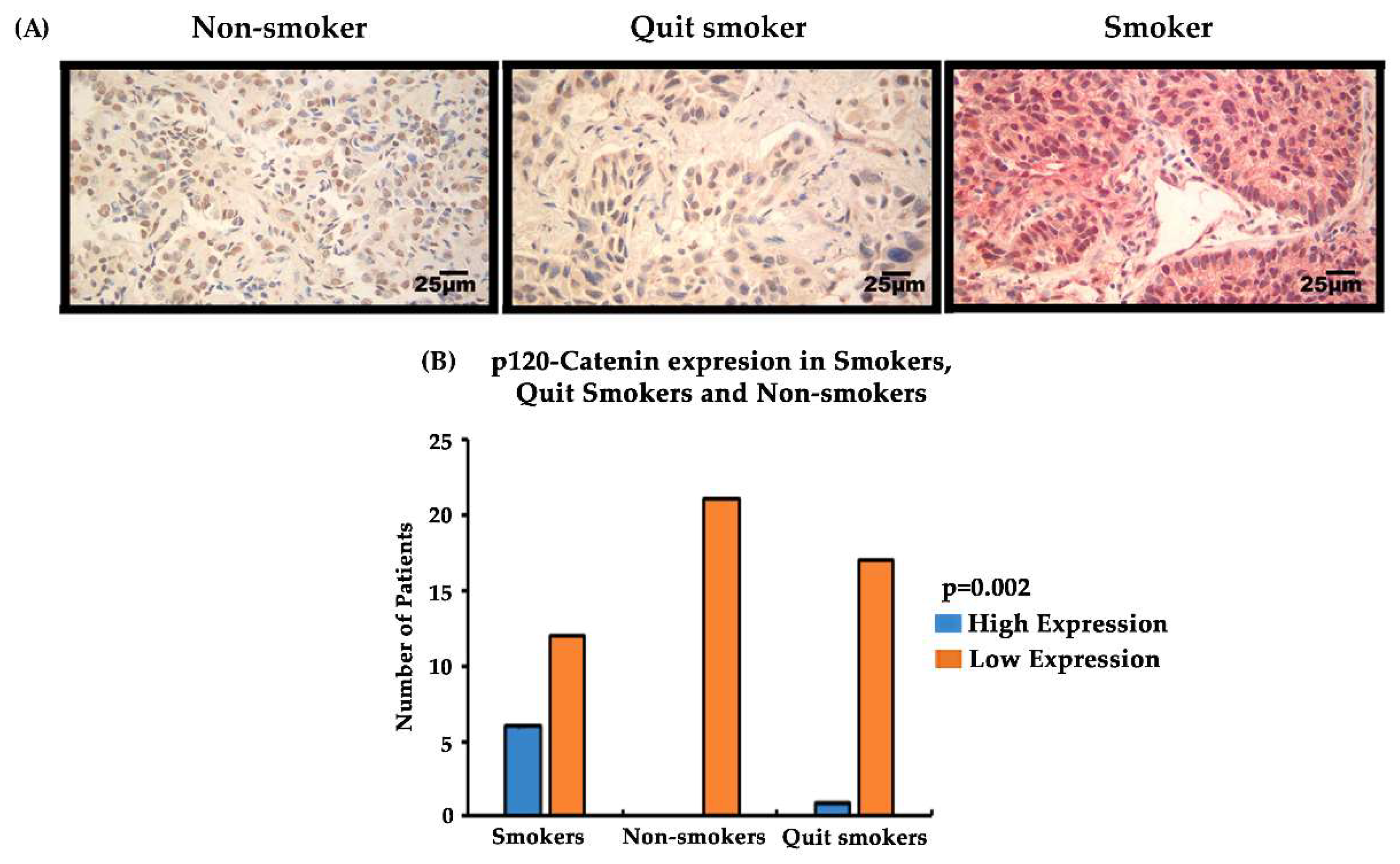
3.7. Expression of EMT-Related Biomarkers p120-Catenin/PRMT-1 in Lung Cancer Patient Tissues at Different Stages (Stage I–IV) and Correlation with the Smoking Status of Patients
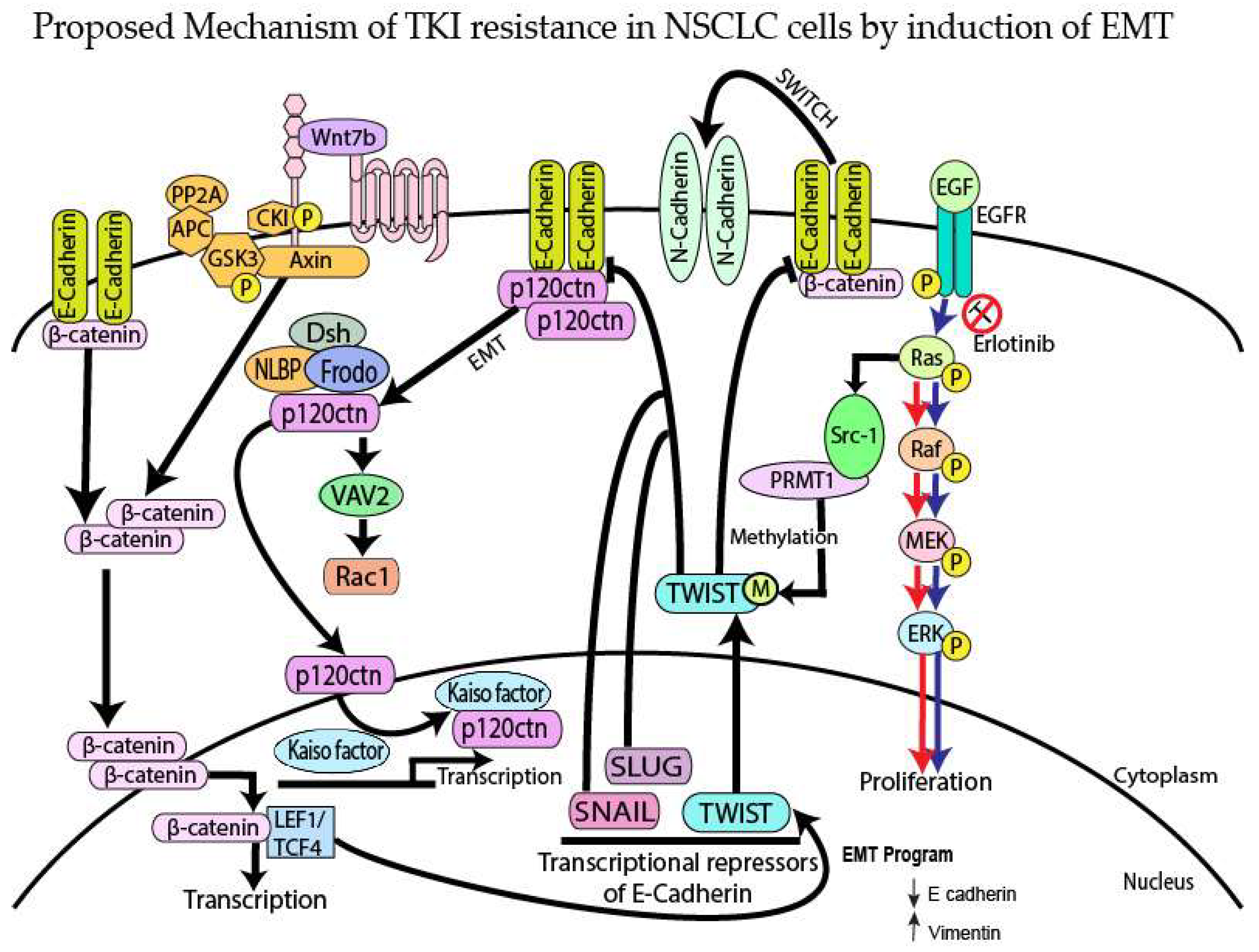
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer statistics, 2023. CA A Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef] [PubMed]
- Molina, J.R.; Yang, P.; Cassivi, S.D.; Schild, S.E.; Adjei, A.A. Non-small cell lung cancer: Epidemiology, risk factors, treatment, and survivorship. Mayo Clin. Proc. 2008, 83, 584–594. [Google Scholar] [CrossRef] [PubMed]
- Lim, E.H.; Zhang, S.L.; Li, J.L.; Yap, W.S.; Howe, T.C.; Tan, B.P.; Lee, Y.S.; Wong, D.; Khoo, K.L.; Seto, K.Y.; et al. Using whole genome amplification (WGA) of low-volume biopsies to assess the prognostic role of EGFR, KRAS, p53, and CMET mutations in advanced-stage non-small cell lung cancer (NSCLC). J. Thorac. Oncol. 2009, 4, 12–21. [Google Scholar] [CrossRef]
- Luo, Y.H.; Luo, L.; Wampfler, J.A.; Wang, Y.; Liu, D.; Chen, Y.M.; Adjei, A.A.; Midthun, D.E.; Yang, P. 5-year overall survival in patients with lung cancer eligible or ineligible for screening according to US Preventive Services Task Force criteria: A prospective, observational cohort study. Lancet Oncol. 2019, 20, 1098–1108. [Google Scholar] [CrossRef]
- Schrank, Z.; Chhabra, G.; Lin, L.; Iderzorig, T.; Osude, C.; Khan, N.; Kuckovic, A.; Singh, S.; Miller, R.J.; Puri, N. Current Molecular-Targeted Therapies in NSCLC and Their Mechanism of Resistance. Cancers 2018, 10, 224. [Google Scholar] [CrossRef] [PubMed]
- da Cunha Santos, G.; Shepherd, F.A.; Tsao, M.S. EGFR mutations and lung cancer. Annu. Rev. Pathol. 2011, 6, 49–69. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Wang, X.; Jin, H. EGFR-TKI resistance in NSCLC patients: Mechanisms and strategies. Am. J. Cancer Res. 2014, 4, 411–435. [Google Scholar]
- Bethune, G.; Bethune, D.; Ridgway, N.; Xu, Z. Epidermal growth factor receptor (EGFR) in lung cancer: An overview and update. J. Thorac. Dis. 2010, 2, 48–51. [Google Scholar]
- Garassino, M.C.; Broggini, M. Chemotherapy versus tyrosine kinase inhibitor in EGFR unselected population advanced non-small cell lung cancer still matter of debate?-An update incorporating the DELTA trial data. J. Thorac. Dis. 2015, 7, 224–226. [Google Scholar] [CrossRef]
- Remon, J.; Steuer, C.E.; Ramalingam, S.S.; Felip, E. Osimertinib and other third-generation EGFR TKI in EGFR-mutant NSCLC patients. Ann. Oncol. 2018, 29, i20–i27. [Google Scholar] [CrossRef]
- Qin, Q.; Li, X.; Liang, X.; Zeng, L.; Wang, J.; Sun, L.; Zhong, D. Targeting the EMT transcription factor Snail overcomes resistance to osimertinib in EGFR-mutant non-small cell lung cancer. Thorac. Cancer 2021, 12, 1708–1715. [Google Scholar] [CrossRef] [PubMed]
- Rastogi, I.; Rajanna, S.; Webb, A.; Chhabra, G.; Foster, B.; Webb, B.; Puri, N. Mechanism of c-Met and EGFR tyrosine kinase inhibitor resistance through epithelial mesenchymal transition in non-small cell lung cancer. Biochem. Biophys. Res. Commun. 2016, 477, 937–944. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.; Eckburg, A.; Gantiwala, S.; Hart, Z.; Dein, J.; Lam, K.; Puri, N. Resistance to Molecularly Targeted Therapies in Melanoma. Cancers 2021, 13, 1115. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Ng, A.S.; Cai, S.; Li, Q.; Yang, L.; Kerr, D. Novel therapeutic strategies: Targeting epithelial-mesenchymal transition in colorectal cancer. Lancet Oncol. 2021, 22, e358–e368. [Google Scholar] [CrossRef] [PubMed]
- Xie, C.; Zhu, J.; Yang, X.; Huang, C.; Zhou, L.; Meng, Z.; Li, X.; Zhong, C. TAp63alpha Is Involved in Tobacco Smoke-Induced Lung Cancer EMT and the Anti-cancer Activity of Curcumin via miR-19 Transcriptional Suppression. Front. Cell Dev. Biol. 2021, 9, 645402. [Google Scholar] [CrossRef] [PubMed]
- Dasgupta, P.; Rizwani, W.; Pillai, S.; Kinkade, R.; Kovacs, M.; Rastogi, S.; Banerjee, S.; Carless, M.; Kim, E.; Coppola, D.; et al. Nicotine induces cell proliferation, invasion and epithelial-mesenchymal transition in a variety of human cancer cell lines. Int. J. Cancer 2009, 124, 36–45. [Google Scholar] [CrossRef]
- Hou, W.; Hu, S.; Li, C.; Ma, H.; Wang, Q.; Meng, G.; Guo, T.; Zhang, J. Cigarette Smoke Induced Lung Barrier Dysfunction, EMT, and Tissue Remodeling: A Possible Link between COPD and Lung Cancer. BioMed. Res. Int. 2019, 2019, 2025636. [Google Scholar] [CrossRef]
- Zhao, Y.; Lu, Q.; Li, C.; Wang, X.; Jiang, L.; Huang, L.; Wang, C.; Chen, H. PRMT1 regulates the tumour-initiating properties of esophageal squamous cell carcinoma through histone H4 arginine methylation coupled with transcriptional activation. Cell Death Dis. 2019, 10, 359. [Google Scholar] [CrossRef]
- Avasarala, S.; Van Scoyk, M.; Karuppusamy Rathinam, M.K.; Zerayesus, S.; Zhao, X.; Zhang, W.; Pergande, M.R.; Borgia, J.A.; DeGregori, J.; Port, J.D.; et al. PRMT1 Is a Novel Regulator of Epithelial-Mesenchymal-Transition in Non-small Cell Lung Cancer. J. Biol. Chem. 2015, 290, 13479–13489. [Google Scholar] [CrossRef]
- Gao, Y.; Zhao, Y.; Zhang, J.; Lu, Y.; Liu, X.; Geng, P.; Huang, B.; Zhang, Y.; Lu, J. The dual function of PRMT1 in modulating epithelial-mesenchymal transition and cellular senescence in breast cancer cells through regulation of ZEB1. Sci. Rep. 2016, 6, 19874. [Google Scholar] [CrossRef]
- Iderzorig, T.; Kellen, J.; Osude, C.; Singh, S.; Woodman, J.A.; Garcia, C.; Puri, N. Comparison of EMT mediated tyrosine kinase inhibitor resistance in NSCLC. Biochem. Biophys. Res. Commun. 2018, 496, 770–777. [Google Scholar] [CrossRef] [PubMed]
- Loh, C.Y.; Chai, J.Y.; Tang, T.F.; Wong, W.F.; Sethi, G.; Shanmugam, M.K.; Chong, P.P.; Looi, C.Y. The E-Cadherin and N-Cadherin Switch in Epithelial-to-Mesenchymal Transition: Signaling, Therapeutic Implications, and Challenges. Cells 2019, 8, 1118. [Google Scholar] [CrossRef] [PubMed]
- Schackmann, R.C.; Tenhagen, M.; van de Ven, R.A.; Derksen, P.W. p120-catenin in cancer - mechanisms, models and opportunities for intervention. J. Cell Sci. 2013, 126, 3515–3525. [Google Scholar] [CrossRef] [PubMed]
- Kourtidis, A.; Ngok, S.P.; Anastasiadis, P.Z. p120 catenin: An essential regulator of cadherin stability, adhesion-induced signaling, and cancer progression. Prog. Mol. Biol. Transl. Sci. 2013, 116, 409–432. [Google Scholar] [CrossRef]
- Peglion, F.; Etienne-Manneville, S. p120catenin alteration in cancer and its role in tumour invasion. Philos. Trans. R Soc. Lond B Biol. Sci. 2013, 368, 20130015. [Google Scholar] [CrossRef]
- Botting, G.M.; Rastogi, I.; Chhabra, G.; Nlend, M.; Puri, N. Mechanism of Resistance and Novel Targets Mediating Resistance to EGFR and c-Met Tyrosine Kinase Inhibitors in Non-Small Cell Lung Cancer. PLoS ONE 2015, 10, e0136155. [Google Scholar] [CrossRef]
- Fawwaz, M.; Mishiro, K.; Nishii, R.; Makino, A.; Kiyono, Y.; Shiba, K.; Kinuya, S.; Ogawa, K. A Radiobrominated Tyrosine Kinase Inhibitor for EGFR with L858R/T790M Mutations in Lung Carcinoma. Pharmaceuticals 2021, 14, 256. [Google Scholar] [CrossRef]
- Tracy, S.; Mukohara, T.; Hansen, M.; Meyerson, M.; Johnson, B.E.; Janne, P.A. Gefitinib induces apoptosis in the EGFRL858R non-small-cell lung cancer cell line H3255. Cancer Res. 2004, 64, 7241–7244. [Google Scholar] [CrossRef]
- Osude, C.; Lin, L.; Patel, M.; Eckburg, A.; Berei, J.; Kuckovic, A.; Dube, N.; Rastogi, A.; Gautam, S.; Smith, T.J.; et al. Mediating EGFR-TKI Resistance by VEGF/VEGFR Autocrine Pathway in Non-Small Cell Lung Cancer. Cells 2022, 11, 1694. [Google Scholar] [CrossRef]
- Crees, Z.D.; Shearrow, C.; Lin, L.; Girard, J.; Arasi, K.; Bhoraskar, A.; Berei, J.; Eckburg, A.; Anderson, A.D.; Garcia, C.; et al. EGFR/c-Met and mTOR signaling are predictors of survival in non-small cell lung cancer. Adv. Med. Oncol. 2020, 12, 1758835920953731. [Google Scholar] [CrossRef]
- Dunach, M.; Del Valle-Perez, B.; Garcia de Herreros, A. p120-catenin in canonical Wnt signaling. Crit. Rev. Biochem. Mol. Biol. 2017, 52, 327–339. [Google Scholar] [CrossRef] [PubMed]
- Park, J.I.; Ji, H.; Jun, S.; Gu, D.; Hikasa, H.; Li, L.; Sokol, S.Y.; McCrea, P.D. Frodo links Dishevelled to the p120-catenin/Kaiso pathway: Distinct catenin subfamilies promote Wnt signals. Dev. Cell 2006, 11, 683–695. [Google Scholar] [CrossRef] [PubMed]
- Vu, T.; Jin, L.; Datta, P.K. Effect of Cigarette Smoking on Epithelial to Mesenchymal Transition (EMT) in Lung Cancer. J. Clin. Med. 2016, 5. [Google Scholar] [CrossRef]
- Yochum, Z.A.; Cades, J.; Wang, H.; Chatterjee, S.; Simons, B.W.; O′Brien, J.P.; Khetarpal, S.K.; Lemtiri-Chlieh, G.; Myers, K.V.; Huang, E.H.; et al. Targeting the EMT transcription factor TWIST1 overcomes resistance to EGFR inhibitors in EGFR-mutant non-small-cell lung cancer. Oncogene 2019, 38, 656–670. [Google Scholar] [CrossRef] [PubMed]
- Weng, C.H.; Chen, L.Y.; Lin, Y.C.; Shih, J.Y.; Lin, Y.C.; Tseng, R.Y.; Chiu, A.C.; Yeh, Y.H.; Liu, C.; Lin, Y.T.; et al. Epithelial-mesenchymal transition (EMT) beyond EGFR mutations per se is a common mechanism for acquired resistance to EGFR TKI. Oncogene 2019, 38, 455–468. [Google Scholar] [CrossRef] [PubMed]
- Nagathihalli, N.S.; Massion, P.P.; Gonzalez, A.L.; Lu, P.; Datta, P.K. Smoking induces epithelial-to-mesenchymal transition in non-small cell lung cancer through HDAC-mediated downregulation of E-cadherin. Mol. Cancer 2012, 11, 2362–2372. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.J.; Sun, Y.H.; Zhang, S.J.; Jiang, J.X.; Dong, X.W.; Jia, Y.L.; Shen, J.; Guan, Y.; Zhang, L.H.; Li, F.F.; et al. Cigarette smoke-induced alveolar epithelial-mesenchymal transition is mediated by Rac1 activation. Biochim. Biophys. Acta 2014, 1840, 1838–1849. [Google Scholar] [CrossRef]
- Elakoum, R.; Gauchotte, G.; Oussalah, A.; Wissler, M.P.; Clement-Duchene, C.; Vignaud, J.M.; Gueant, J.L.; Namour, F. CARM1 and PRMT1 are dysregulated in lung cancer without hierarchical features. Biochimie 2014, 97, 210–218. [Google Scholar] [CrossRef]
- Wang, E.H.; Liu, Y.; Xu, H.T.; Dai, S.D.; Liu, N.; Xie, C.Y.; Yuan, X.M. Abnormal expression and clinicopathologic significance of p120-catenin in lung cancer. Histol. Histopathol. 2006, 21, 841–847. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, Y.; Jiang, G.; Zhang, X.; Zhao, H.; Wu, J.; Xu, K.; Wang, E. Impact of p120-catenin isoforms 1A and 3A on epithelial mesenchymal transition of lung cancer cells expressing E-cadherin in different subcellular locations. PLoS ONE 2014, 9, e88064. [Google Scholar] [CrossRef]
- Liu, Y.; Lai, M.; Li, S.; Wang, Y.; Feng, F.; Zhang, T.; Tong, L.; Zhang, M.; Chen, H.; Chen, Y.; et al. LS-106, a novel EGFR inhibitor targeting C797S, exhibits antitumor activities both in vitro and in vivo. Cancer Sci. 2022, 113, 709–720. [Google Scholar] [CrossRef] [PubMed]




| Gene | Sequence | Melting Point | DNA Bases |
|---|---|---|---|
| Vimentin | F: TGTCCAAATCGATGTGGATGTTTC | 55.7 °C | 24 |
| R: TTGTACCATTCTTCTGCCTCCTG | 56.8 °C | 23 | |
| PRMT-1 | F: CCTTCACCTCCCCGTTCTG | 57.8 °C | 19 |
| R: CCAGGGCGTGCACGTAGT | 60.6 °C | 18 | |
| p120-catenin | F: CGGCATACGTCATCCCCATT | 60.25 °C | 20 |
| R: TCTTCCCTCAGCCCTCAAGT | 60.18 °C | 20 | |
| Kaiso factor | F: GGAAGCAGTGTCTGTTGACT | 54.9 °C | 21 |
| R: CCATGCCCTTTCCTCTTTCT | 54.9 °C | 23 | |
| GAPDH | F: ATGACATCAAGAAGGTGGTG | 52.4 °C | 20 |
| R: CAGGAAATGAGCTTGACAAA | 50.9 °C | 20 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Racherla, K.S.; Dovalovsky, K.; Patel, M.; Harper, E.; Barnard, J.; Nasifuzzaman, S.M.; Smith, M.; Sikand, R.; Drinka, E.; Puri, N. PRMT-1 and p120-Catenin as EMT Mediators in Osimertinib Resistance in NSCLC. Cancers 2023, 15, 3461. https://doi.org/10.3390/cancers15133461
Racherla KS, Dovalovsky K, Patel M, Harper E, Barnard J, Nasifuzzaman SM, Smith M, Sikand R, Drinka E, Puri N. PRMT-1 and p120-Catenin as EMT Mediators in Osimertinib Resistance in NSCLC. Cancers. 2023; 15(13):3461. https://doi.org/10.3390/cancers15133461
Chicago/Turabian StyleRacherla, Kavya Sri, Katrina Dovalovsky, Meet Patel, Emma Harper, Jacob Barnard, S M Nasifuzzaman, Mason Smith, Riya Sikand, Eva Drinka, and Neelu Puri. 2023. "PRMT-1 and p120-Catenin as EMT Mediators in Osimertinib Resistance in NSCLC" Cancers 15, no. 13: 3461. https://doi.org/10.3390/cancers15133461
APA StyleRacherla, K. S., Dovalovsky, K., Patel, M., Harper, E., Barnard, J., Nasifuzzaman, S. M., Smith, M., Sikand, R., Drinka, E., & Puri, N. (2023). PRMT-1 and p120-Catenin as EMT Mediators in Osimertinib Resistance in NSCLC. Cancers, 15(13), 3461. https://doi.org/10.3390/cancers15133461






