Real World Experience of Second-Line Treatment Strategies after Palbociclib and Letrozole: Overall Survival in Metastatic Hormone Receptor-Positive Human Epidermal Growth Factor Receptor 2-Negative Breast Cancer
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Statistical Analysis
3. Results
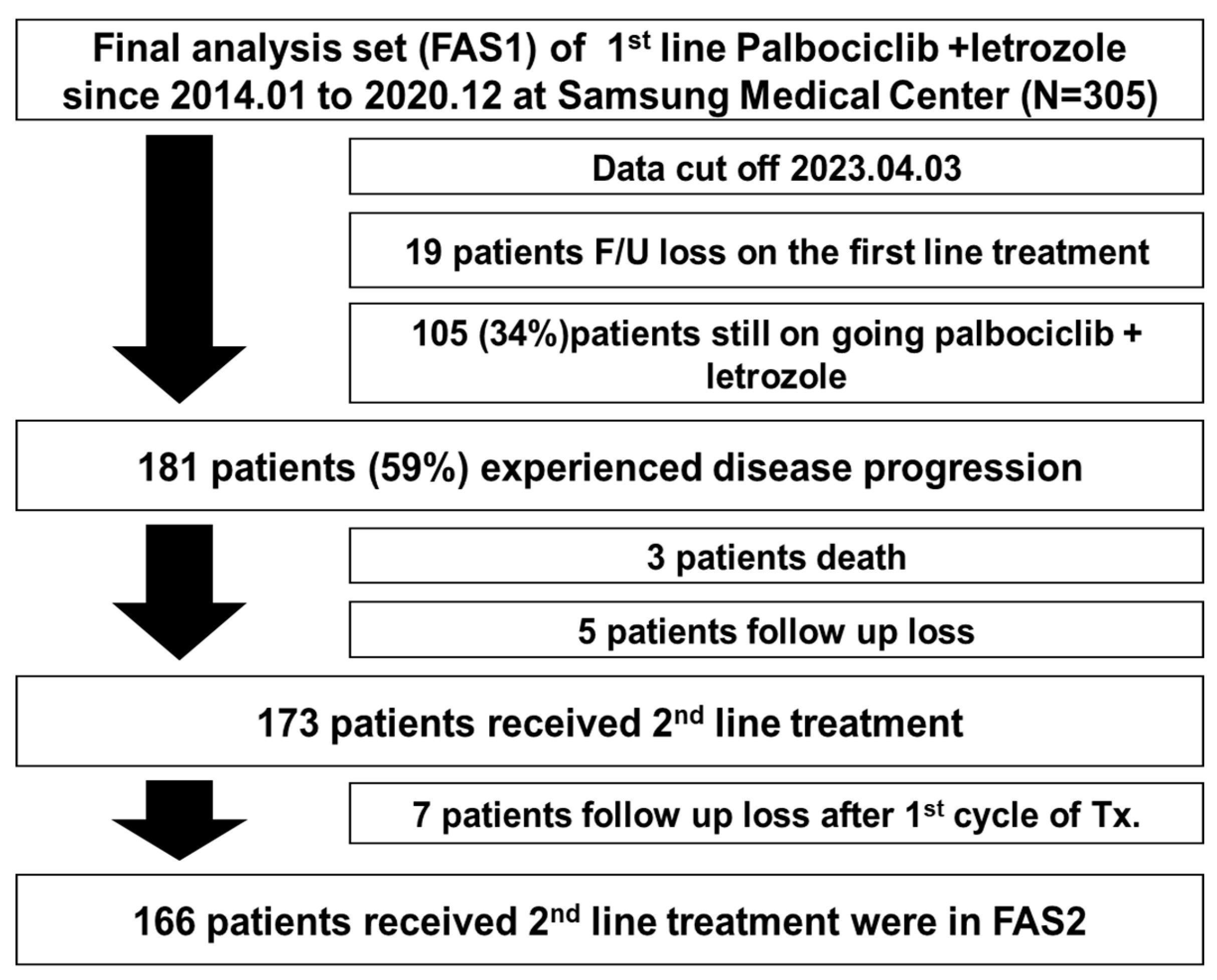
3.1. Patient Baseline Characteristics and Updated Survival Analysis
3.2. Second-Line Treatment after Palbociclib with Letrozole
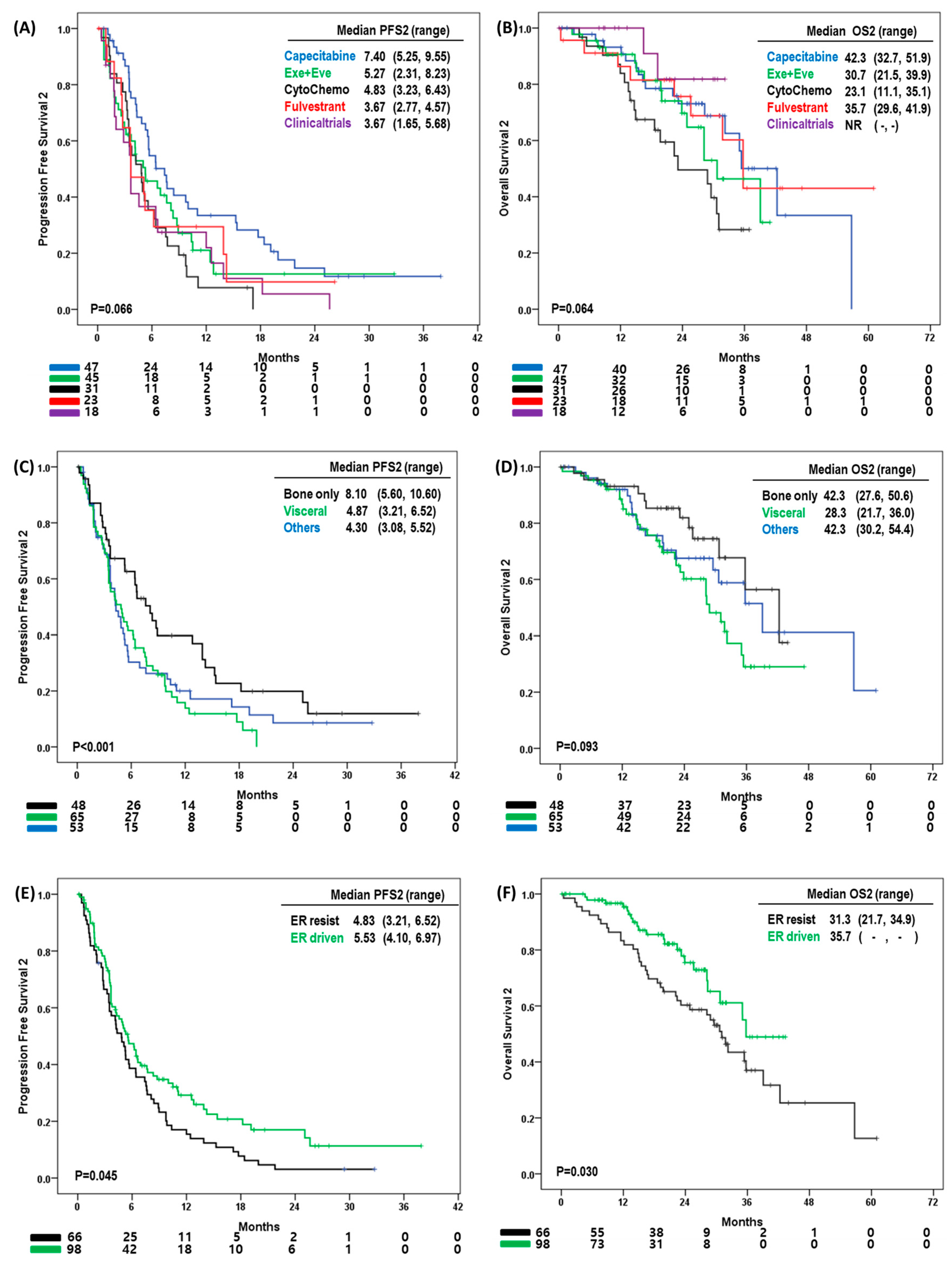
3.3. Progression-Free Survival and Overall Survival with Second-Line Treatment
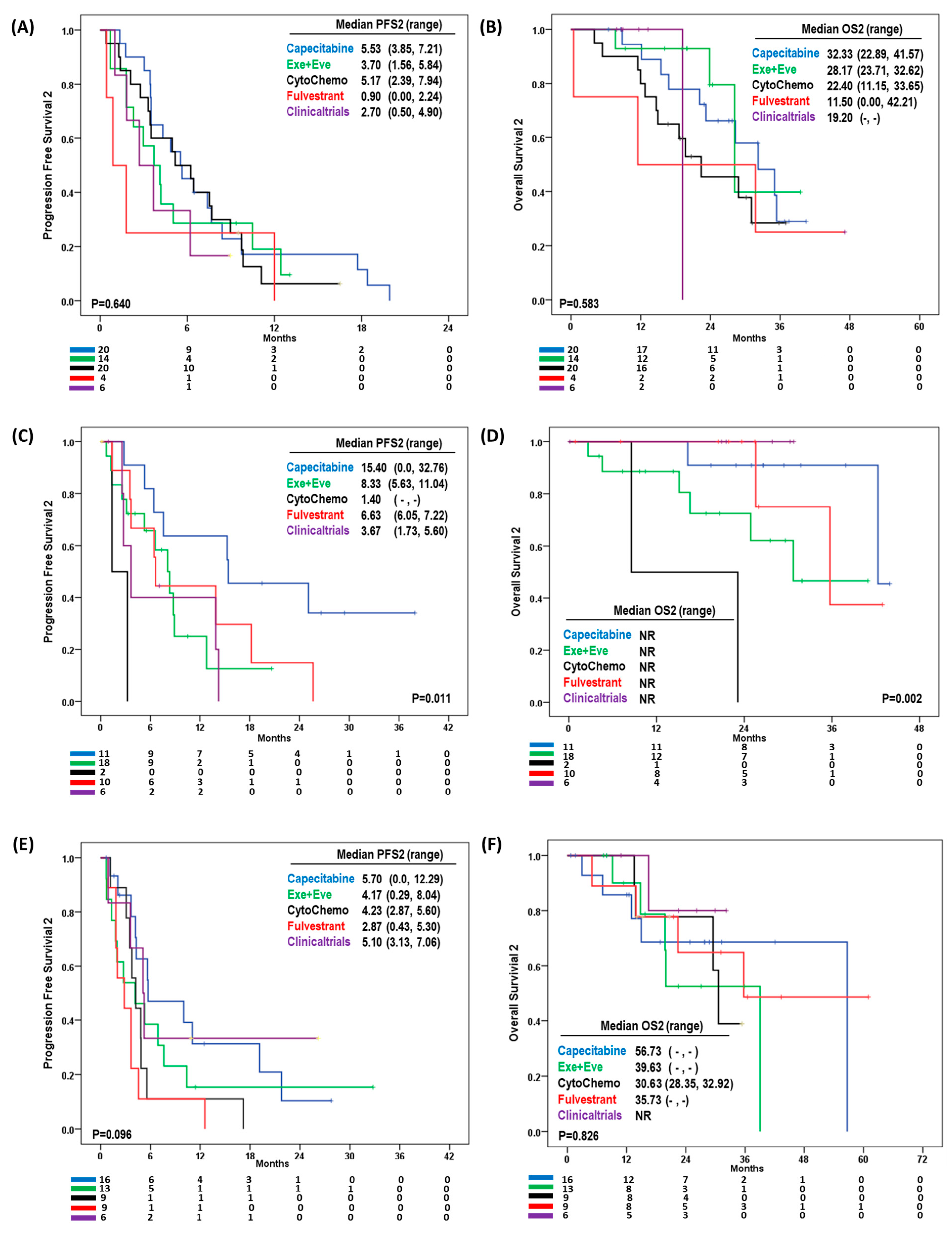
3.4. Effect of Second-Line Treatment According to Site of Disease Progression
3.5. Clinical Characteristics Affecting Overall Survival
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Giaquinto, A.N.; Sung, H.; Miller, K.D.; Kramer, J.L.; Newman, L.A.; Minihan, A.; Jemal, A.; Siegel, R.L. Breast Cancer Statistics, 2022. CA A Cancer J. Clin. 2022, 72, 524–541. [Google Scholar] [CrossRef] [PubMed]
- Gennari, A.; André, F.; Barrios, C.H.; Cortés, J.; de Azambuja, E.; DeMichele, A.; Dent, R.; Fenlon, D.; Gligorov, J.; Hurvitz, S.A.; et al. ESMO Clinical Practice Guideline for the diagnosis, staging and treatment of patients with metastatic breast cancer. Ann. Oncol. 2021, 32, 1475–1495. [Google Scholar] [CrossRef] [PubMed]
- Finn, R.S.; Martin, M.; Rugo, H.S.; Jones, S.; Im, S.-A.; Gelmon, K.; Harbeck, N.; Lipatov, O.N.; Walshe, J.M.; Moulder, S.; et al. Palbociclib and Letrozole in Advanced Breast Cancer. N. Engl. J. Med. 2016, 375, 1925–1936. [Google Scholar] [CrossRef] [PubMed]
- Hortobagyi, G.N.; Stemmer, S.M.; Burris, H.A.; Yap, Y.-S.; Sonke, G.S.; Paluch-Shimon, S.; Campone, M.; Blackwell, K.L.; André, F.; Winer, E.P.; et al. Ribociclib as First-Line Therapy for HR-Positive, Advanced Breast Cancer. N. Engl. J. Med. 2016, 375, 1738–1748. [Google Scholar] [CrossRef] [PubMed]
- Tripathy, D.; Im, S.-A.; Colleoni, M.; Franke, F.; Bardia, A.; Harbeck, N.; Hurvitz, S.A.; Chow, L.; Sohn, J.; Lee, K.S.; et al. Ribociclib plus endocrine therapy for premenopausal women with hormone-receptor-positive, advanced breast cancer (MONALEESA-7): A randomised phase 3 trial. Lancet Oncol. 2018, 19, 904–915. [Google Scholar] [CrossRef] [PubMed]
- Goetz, M.P.; Toi, M.; Campone, M.; Sohn, J.; Paluch-Shimon, S.; Huober, J.; Park, I.H.; Trédan, O.; Chen, S.-C.; Manso, L. MONARCH 3: Abemaciclib as initial therapy for advanced breast cancer. J. Clin. Oncol. 2017, 35, 3638–3646. [Google Scholar] [CrossRef]
- Finn, R.S.; Rugo, H.S.; Dieras, V.C.; Harbeck, N.; Im, S.-A.; Gelmon, K.A.; Walshe, J.M.; Martin, M.; Gregor, M.C.M.; Bananis, E.; et al. Overall survival (OS) with first-line palbociclib plus letrozole (PAL+LET) versus placebo plus letrozole (PBO+LET) in women with estrogen receptor–positive/human epidermal growth factor receptor 2–negative advanced breast cancer (ER+/HER2−ABC): Analyses from PALOMA-2. J. Clin. Oncol. 2022, 40, LBA1003. [Google Scholar] [CrossRef]
- Hortobagyi, G.N.; Stemmer, S.M.; Burris, H.A.; Yap, Y.-S.; Sonke, G.S.; Hart, L.; Campone, M.; Petrakova, K.; Winer, E.P.; Janni, W.; et al. Overall Survival with Ribociclib plus Letrozole in Advanced Breast Cancer. N. Engl. J. Med. 2022, 386, 942–950. [Google Scholar] [CrossRef]
- Im, S.-A.; Lu, Y.-S.; Bardia, A.; Harbeck, N.; Colleoni, M.; Franke, F.; Chow, L.; Sohn, J.; Lee, K.-S.; Campos-Gomez, S.; et al. Overall Survival with Ribociclib plus Endocrine Therapy in Breast Cancer. N. Engl. J. Med. 2019, 381, 307–316. [Google Scholar] [CrossRef]
- Goetz, M.; Toi, M.; Huober, J.; Sohn, J.; Tredan, O.; Park, I.; Campone, M.; Chen, S.; Sanchez, L.M.; Paluch-Shimon, S.; et al. LBA15 MONARCH 3: Interim overall survival (OS) results of abemaciclib plus a nonsteroidal aromatase inhibitor (NSAI) in patients (pts) with HR+, HER2- advanced breast cancer (ABC). Ann. Oncol. 2022, 33, S1384. [Google Scholar] [CrossRef]
- Rugo, H.S.; Brufsky, A.; Liu, X.; Li, B.; McRoy, L.; Chen, C.; Layman, R.M.; Cristofanilli, M.; Torres, M.A.; Curigliano, G.; et al. Real-world study of overall survival with palbociclib plus aromatase inhibitor in HR+/HER2− metastatic breast cancer. NPJ Breast Cancer 2022, 8, 114. [Google Scholar] [CrossRef] [PubMed]
- Burstein, H.J.; Somerfield, M.R.; Barton, D.L.; Dorris, A.; Fallowfield, L.J.; Jain, D.; Johnston, S.R.D.; Korde, L.A.; Litton, J.K.; Macrae, E.R.; et al. Endocrine Treatment and Targeted Therapy for Hormone Receptor–Positive, Human Epidermal Growth Factor Receptor 2–Negative Metastatic Breast Cancer: ASCO Guideline Update. J. Clin. Oncol. 2021, 39, 3959–3977. [Google Scholar] [CrossRef] [PubMed]
- André, F.; Ciruelos, E.; Rubovszky, G.; Campone, M.; Loibl, S.; Rugo, H.S.; Iwata, H.; Conte, P.; Mayer, I.A.; Kaufman, B.; et al. Alpelisib for PIK3CA-Mutated, Hormone Receptor–Positive Advanced Breast Cancer. N. Engl. J. Med. 2019, 380, 1929–1940. [Google Scholar] [CrossRef]
- Rugo, H.S.; Lerebours, F.; Ciruelos, E.; Drullinsky, P.; Ruiz-Borrego, M.; Neven, P.; Park, Y.H.; Prat, A.; Bachelot, T.; Juric, D.; et al. Alpelisib plus fulvestrant in PIK3CA-mutated, hormone receptor-positive advanced breast cancer after a CDK4/6 inhibitor (BYLieve): One cohort of a phase 2, multicentre, open-label, non-comparative study. Lancet Oncol. 2021, 22, 489–498. [Google Scholar] [CrossRef] [PubMed]
- Modi, S.; Jacot, W.; Yamashita, T.; Sohn, J.; Vidal, M.; Tokunaga, E.; Tsurutani, J.; Ueno, N.T.; Prat, A.; Chae, Y.S.; et al. Trastuzumab Deruxtecan in Previously Treated HER2-Low Advanced Breast Cancer. N. Engl. J. Med. 2022, 387, 9–20. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Oh, J.M.; Park, Y.H.; Ahn, J.S.; Im, Y.H. Which Clinicopathologic Parameters Suggest Primary Resistance to Palbociclib in Combination With Letrozole as the First-Line Treatment for Hormone Receptor-Positive, HER2-Negative Advanced Breast Cancer? Front. Oncol. 2021, 11, 759150. [Google Scholar] [CrossRef]
- Lin, M.; Chen, Y.; Jin, Y.; Hu, X.; Zhang, J. Comparative Overall Survival of CDK4/6 Inhibitors Plus Endocrine Therapy vs. Endocrine Therapy Alone for Hormone receptor-positive, HER2-negative metastatic breast cancer. J. Cancer 2020, 11, 7127–7136. [Google Scholar] [CrossRef]
- Im, S.A.; Mukai, H.; Park, I.H.; Masuda, N.; Shimizu, C.; Kim, S.B.; Im, Y.H.; Ohtani, S.; Huang Bartlett, C.; Lu, D.R.; et al. Palbociclib Plus Letrozole as First-Line Therapy in Postmenopausal Asian Women With Metastatic Breast Cancer: Results From the Phase III, Randomized PALOMA-2 Study. J. Glob. Oncol. 2019, 5, 1–19. [Google Scholar] [CrossRef]
- Cardoso, F.; Harbeck, N.; Fallowfield, L.; Kyriakides, S.; Senkus, E.; Group, E.G.W. Locally recurrent or metastatic breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2012, 23 (Suppl. S7), vii11–vii19. [Google Scholar] [CrossRef]
- Mouridsen, H.; Gershanovich, M.; Sun, Y.; Perez-Carrion, R.; Boni, C.; Monnier, A.; Apffelstaedt, J.; Smith, R.; Sleeboom, H.P.; Jaenicke, F.; et al. Phase III study of letrozole versus tamoxifen as first-line therapy of advanced breast cancer in postmenopausal women: Analysis of survival and update of efficacy from the International Letrozole Breast Cancer Group. J. Clin. Oncol. 2003, 21, 2101–2109. [Google Scholar] [CrossRef]
- Nabholtz, J.M.; Bonneterre, J.; Buzdar, A.; Robertson, J.F.; Thurlimann, B. Anastrozole (Arimidex) versus tamoxifen as first-line therapy for advanced breast cancer in postmenopausal women: Survival analysis and updated safety results. Eur. J. Cancer 2003, 39, 1684–1689. [Google Scholar] [CrossRef] [PubMed]
- Bonneterre, J.; Thurlimann, B.; Robertson, J.F.; Krzakowski, M.; Mauriac, L.; Koralewski, P.; Vergote, I.; Webster, A.; Steinberg, M.; von Euler, M. Anastrozole versus tamoxifen as first-line therapy for advanced breast cancer in 668 postmenopausal women: Results of the Tamoxifen or Arimidex Randomized Group Efficacy and Tolerability study. J. Clin. Oncol. 2000, 18, 3748–3757. [Google Scholar] [CrossRef] [PubMed]
- Meegdes, M.; Geurts, S.M.E.; Erdkamp, F.L.G.; Dercksen, M.W.; Vriens, B.; Aaldering, K.N.A.; Pepels, M.; van de Winkel, L.M.H.; Peters, N.; Tol, J.; et al. Real-world time trends in overall survival, treatments and patient characteristics in HR+/HER2− metastatic breast cancer: An observational study of the SONABRE Registry. Lancet Reg. Health Eur. 2023, 26, 100573. [Google Scholar] [CrossRef] [PubMed]
- Valachis, A.; Carlqvist, P.; Ma, Y.; Szilcz, M.; Freilich, J.; Vertuani, S.; Holm, B.; Lindman, H. Overall survival of patients with metastatic breast cancer in Sweden: A nationwide study. Br. J. Cancer 2022, 127, 720–725. [Google Scholar] [CrossRef]
- Blum, J.L.; Jones, S.E.; Buzdar, A.U.; LoRusso, P.M.; Kuter, I.; Vogel, C.; Osterwalder, B.; Burger, H.U.; Brown, C.S.; Griffin, T. Multicenter phase II study of capecitabine in paclitaxel-refractory metastatic breast cancer. J. Clin. Oncol. 1999, 17, 485–493. [Google Scholar] [CrossRef]
- Oshaughnessy, J.A.; Blum, J.; Moiseyenko, V.; Jones, S.E.; Miles, D.; Bell, D.; Rosso, R.; Mauriac, L.; Osterwalder, B.; Burger, H.U.; et al. Randomized, open-label, phase II trial of oral capecitabine (Xeloda) vs. a reference arm of intravenous CMF (cyclophosphamide, methotrexate and 5-fluorouracil) as first-line therapy for advanced/metastatic breast cancer. Ann. Oncol. 2001, 12, 1247–1254. [Google Scholar] [CrossRef]
- Park, Y.H.; Kim, T.Y.; Kim, G.M.; Kang, S.Y.; Park, I.H.; Kim, J.H.; Lee, K.E.; Ahn, H.K.; Lee, M.H.; Kim, H.J.; et al. Palbociclib plus exemestane with gonadotropin-releasing hormone agonist versus capecitabine in premenopausal women with hormone receptor-positive, HER2-negative metastatic breast cancer (KCSG-BR15-10): A multicentre, open-label, randomised, phase 2 trial. Lancet Oncol. 2019, 20, 1750–1759. [Google Scholar] [CrossRef]
- Martin, M.; Zielinski, C.; Ruiz-Borrego, M.; Carrasco, E.; Turner, N.; Ciruelos, E.M.; Munoz, M.; Bermejo, B.; Margeli, M.; Anton, A.; et al. Palbociclib in combination with endocrine therapy versus capecitabine in hormonal receptor-positive, human epidermal growth factor 2-negative, aromatase inhibitor-resistant metastatic breast cancer: A phase III randomised controlled trial-PEARL. Ann. Oncol. 2021, 32, 488–499. [Google Scholar] [CrossRef]
- Razavi, P.; Chang, M.T.; Xu, G.; Bandlamudi, C.; Ross, D.S.; Vasan, N.; Cai, Y.; Bielski, C.M.; Donoghue, M.T.A.; Jonsson, P.; et al. The Genomic Landscape of Endocrine-Resistant Advanced Breast Cancers. Cancer Cell 2018, 34, 427–438.e426. [Google Scholar] [CrossRef]
- Turner, N.C.; Swift, C.; Kilburn, L.; Fribbens, C.; Beaney, M.; Garcia-Murillas, I.; Budzar, A.U.; Robertson, J.F.R.; Gradishar, W.; Piccart, M.; et al. ESR1 Mutations and Overall Survival on Fulvestrant versus Exemestane in Advanced Hormone Receptor-Positive Breast Cancer: A Combined Analysis of the Phase III SoFEA and EFECT Trials. Clin. Cancer Res. 2020, 26, 5172–5177. [Google Scholar] [CrossRef]
- Lindeman, G.J.; Fernando, T.M.; Bowen, R.; Jerzak, K.J.; Song, X.; Decker, T.; Boyle, F.; McCune, S.; Armstrong, A.; Shannon, C.; et al. VERONICA: Randomized Phase II Study of Fulvestrant and Venetoclax in ER-Positive Metastatic Breast Cancer Post-CDK4/6 Inhibitors—Efficacy, Safety, and Biomarker Results. Clin. Cancer Res. 2022, 28, 3256–3267. [Google Scholar] [CrossRef] [PubMed]
- Turner, N.; Oliveira, M.; Howell, S.J.; Dalenc, F.; Cortés, J.; Gomez, H.; Hu, X.; Jhaveri, K.; Loibl, S.; Murillo, S.M.; et al. GS3-04 Capivasertib and fulvestrant for patients with aromatase inhibitor-resistant hormone receptor-positive/human epidermal growth factor receptor 2-negative advanced breast cancer: Results from the Phase III CAPItello-291 trial [abstract]. Cancer Res. 2023, 83 (Suppl. S7), GS3–GS4. [Google Scholar] [CrossRef]
- Malone, K.E.; Daling, J.R.; Doody, D.R.; Hsu, L.; Bernstein, L.; Coates, R.J.; Marchbanks, P.A.; Simon, M.S.; McDonald, J.A.; Norman, S.A.; et al. Prevalence and Predictors of BRCA1 and BRCA2 Mutations in a Population-Based Study of Breast Cancer in White and Black American Women Ages 35 to 64 Years. Cancer Res. 2006, 66, 8297–8308. [Google Scholar] [CrossRef] [PubMed]
- Cancer Genome Atlas, N. Comprehensive molecular portraits of human breast tumours. Nature 2012, 490, 61–70. [Google Scholar] [CrossRef]
- O’Leary, B.; Cutts, R.J.; Liu, Y.; Hrebien, S.; Huang, X.; Fenwick, K.; Andre, F.; Loibl, S.; Loi, S.; Garcia-Murillas, I.; et al. The Genetic Landscape and Clonal Evolution of Breast Cancer Resistance to Palbociclib plus Fulvestrant in the PALOMA-3 Trial. Cancer Discov. 2018, 8, 1390–1403. [Google Scholar] [CrossRef]
- Bertucci, F.; Ng, C.K.Y.; Patsouris, A.; Droin, N.; Piscuoglio, S.; Carbuccia, N.; Soria, J.C.; Dien, A.T.; Adnani, Y.; Kamal, M.; et al. Genomic characterization of metastatic breast cancers. Nature 2019, 569, 560–564. [Google Scholar] [CrossRef]
- Bidard, F.C.; Kaklamani, V.G.; Neven, P.; Streich, G.; Montero, A.J.; Forget, F.; Mouret-Reynier, M.A.; Sohn, J.H.; Taylor, D.; Harnden, K.K.; et al. Elacestrant (oral selective estrogen receptor degrader) Versus Standard Endocrine Therapy for Estrogen Receptor-Positive, Human Epidermal Growth Factor Receptor 2-Negative Advanced Breast Cancer: Results From the Randomized Phase III EMERALD Trial. J. Clin. Oncol. 2022, 40, 3246–3256. [Google Scholar] [CrossRef]

| Capecitabine | Eve/Exe | Cytotoxic | Fulvestrant | Clinical Trial | p-Value | |
|---|---|---|---|---|---|---|
| Age | 0.292 | |||||
| <50YO 1 (n = 74) | 19 | 26 | 12 | 8 | 9 | |
| >50YO (n = 90) | 28 | 19 | 19 | 15 | 19 | |
| ECOG PS 2 | 0.001 | |||||
| 0 (n = 98) | 30 | 32 | 23 | 9 | 4 | |
| 1–2 (n = 66) | 17 | 13 | 8 | 14 | 14 | |
| Initial ER 3 score | 0.971 | |||||
| Strong (n = 147) | 42 | 40 | 29 | 20 | 16 | |
| Weak (n = 17) | 5 | 5 | 2 | 3 | 2 | |
| Initial PgR 4 score | 0.477 | |||||
| Strong (n = 64) | 17 | 13 | 17 | 6 | 11 | |
| Weak (n = 63) | 19 | 19 | 10 | 11 | 4 | |
| No (n = 37) | 11 | 13 | 4 | 6 | 3 | |
| Initial Ki-67 score | 0.326 | |||||
| 1+ (n = 103) | 31 | 28 | 15 | 16 | 13 | |
| 2+ (n = 46) | 12 | 14 | 13 | 4 | 3 | |
| 3+ (n = 12) | 3 | 2 | 2 | 3 | 2 | |
| 4+ (n = 3) | 1 | 1 | 1 | 0 | 0 | |
| Initial visceral metastasis | 0.006 | |||||
| No (n = 120) | 26 | 39 | 21 | 20 | 14 | |
| Yes (n = 44) | 21 | 6 | 10 | 3 | 4 | |
| Number of metastatic sites | 0.262 | |||||
| 1 (n = 74) | 16 | 20 | 13 | 15 | 10 | |
| 2 (n = 61) | 18 | 20 | 12 | 5 | 6 | |
| 3 or more (n = 29) | 13 | 5 | 6 | 3 | 2 | |
| Endocrine resistance in adjuvant setting | 0.011 | |||||
| De novo (n = 70) | 22 | 14 | 11 | 12 | 11 | |
| Primary resistance 5 (n = 28) | 14 | 4 | 4 | 5 | 1 | |
| Secondary resistance 6 (n = 27) | 4 | 12 | 6 | 1 | 4 | |
| No ET resistance (n = 39) | 7 | 15 | 10 | 5 | 2 | |
| Disease progression sites | 0.008 | |||||
| Others (n = 53) | 16 | 13 | 9 | 9 | 6 | |
| Visceral meta (n = 64) | 20 | 14 | 20 | 4 | 6 | |
| Bone only (n = 47) | 11 | 18 | 2 | 10 | 6 | |
| PFS 7 of the first line treatment | 0.209 | |||||
| ER driven (n = 98) | 25 | 28 | 15 | 16 | 14 | |
| Not driven (n = 66) | 22 | 17 | 16 | 7 | 4 | |
| Factors for PFS2 | Ref | N | Hazard Ratio | 95% CI | p-Value | |
|---|---|---|---|---|---|---|
| ECOG PS 1 | 0 | 98 | 0.793 | |||
| 1–2 | 66 | 1.054 | 0.710 | 1.566 | ||
| Endocrine resistance | De novo | 70 | 0.778 | |||
| Primary resistance | 28 | 1.000 | 0.603 | 1.660 | ||
| Secondary resistance | 27 | 0.814 | 0.488 | 1.358 | ||
| No resistance | 39 | 0.817 | 0.511 | 1.307 | ||
| Initial visceral metastasis | No | 120 | 0.596 | |||
| Yes | 44 | 1.166 | 0.717 | 1.896 | ||
| ER 2-driven BC 3 | 66 | 0.078 | ||||
| Yes | No | 98 | 0.725 | 0.507 | 1.036 | |
| Disease progression site | 53 | 0.039 | ||||
| Visceral organ | Other | 64 | 1.169 | 0.773 | 1.768 | |
| Bone only | 47 | 0.633 | 0.397 | 1.000 | ||
| Second-line treatment | Capecitabine | 47 | 0.031 | |||
| Everolimus/exemestane | 45 | 1.665 | 1.038 | 2.670 | ||
| Other cytotoxic chemo | 31 | 1.655 | 0.995 | 2.753 | ||
| Fulvestrant | 23 | 2.383 | 1.364 | 4.163 | ||
| Clinical trials | 18 | 1.713 | 0.904 | 3.246 | ||
| Factors for OS2 | Ref | N | Hazard Ratio | 95% CI | p-Value | |
| ECOG PS 1 | 0 | 98 | 0.720 | |||
| 1–2 | 66 | 0.897 | 0.494 | 1.628 | ||
| Endocrine resistance | De novo | 70 | 0.759 | |||
| Primary resistance | 28 | 0.770 | 0.373 | 1.593 | ||
| Secondary resistance | 27 | 0.687 | 0.297 | 1.590 | ||
| No resistance | 39 | 0.980 | 0.465 | 2.065 | ||
| Initial visceral metastasis | No | 120 | 0.039 | |||
| Yes | 44 | 2.097 | 1.039 | 4.234 | ||
| ER 2-driven BC 3 | No | 66 | 0.019 | |||
| Yes | 98 | 0.525 | 0.306 | 0.901 | ||
| Disease progression site | Other | 53 | 0.026 | |||
| Visceral organ | 64 | 2.339 | 1.166 | 4.234 | ||
| Bone only | 47 | 0.967 | 0.461 | 2.027 | ||
| Second-line treatment | Capecitabine | 47 | 0.316 | |||
| Everolimus/exemestane | 45 | 0.890 | 0.421 | 1.878 | ||
| Other cytotoxic chemo | 31 | 1.253 | 0.600 | 2.617 | ||
| Fulvestrant | 23 | 0.669 | 0.274 | 1.631 | ||
| Clinical trials | 18 | 0.292 | 0.064 | 1.328 | ||
| PFS2 4 | ≤5.2 months | 88 | <0.001 | |||
| >5.2 months | 76 | 0.323 | 0.188 | 0.589 |
| Factors | Ref | N | Hazard Ratio | 95% CI | p-Value | |
|---|---|---|---|---|---|---|
| Age | <50 | 130 | 0.131 | |||
| >50 years old | 175 | 1.493 | 0.888 | 2.510 | ||
| ECOG PS 1 | 0 | 177 | 0.658 | |||
| 1 | 122 | 0.793 | 0.483 | 1.301 | ||
| 2 | 5 | 1.543 | 0.434 | 5.484 | ||
| Unknown | 1 | - | - | - | ||
| Visceral metastasis | No | 239 | 0.068 | |||
| Yes | 66 | 1.599 | 0.967 | 2.645 | ||
| Initial CA-15-3 | Normal | 272 | 0.019 | |||
| Elevation | 31 | 1.922 | 1.184 | 3.120 | ||
| Unknown | 2 | 0.706 | 0.67 | 2.986 | ||
| Initial CEA | Normal | 272 | 0.908 | |||
| Elevation | 31 | 0.987 | 0.550 | 1.771 | ||
| Unknown | 2 | 1.505 | 0.231 | 9.790 | ||
| Endocrine resistance | De novo | 70 | 0.031 | |||
| Primary resistance | 28 | 2.250 | 1.171 | 4.323 | ||
| Secondary resistance | 27 | 0.854 | 0.427 | 1.705 | ||
| No resistance | 39 | 0.898 | 0.499 | 1.578 | ||
| Germline BRCA status | Normal | 92 | 0.040 | |||
| Mutation | 6 | 3.989 | 1.311 | 12.139 | ||
| Not tested | 207 | 1.460 | 0.867 | 2.459 | ||
| Number of meta organs | 1 | 157 | 0.021 | |||
| 2 or more | 148 | 1.774 | 1.091 | 2.886 |
| Factors for OS | Ref | N | Hazard Ratio | 95% CI | p-Value | |
|---|---|---|---|---|---|---|
| Visceral metastasis | No | 120 | 0.062 | |||
| Yes | 44 | 1.935 | 0.968 | 3.867 | ||
| Initial CA-15-3 | Normal | 81 | 0.609 | |||
| Elevation | 74 | 1.295 | 0.762 | 2.200 | ||
| Unknown | 9 | 0.924 | 0.208 | 4.095 | ||
| Germline BRCA status | Wild type | 54 | 0.604 | |||
| Mutation | 6 | 1.571 | 0.475 | 5.199 | ||
| Not tested | 104 | 0.899 | 0.482 | 1.674 | ||
| Number of meta organs | 1 | 74 | 0.048 | |||
| 2 or more | 90 | 1.705 | 1.004 | 2.894 | ||
| ER-driven BC | No | 66 | <0.001 | |||
| Yes | 98 | 0.180 | 0.103 | 0.316 | ||
| Disease progression site | Other | 53 | 0.034 | |||
| Visceral organ | 64 | 2.191 | 1.099 | 4.368 | ||
| Bone only | 47 | 0.932 | 0.444 | 1.956 | ||
| Second-line treatment | Capecitabine | 47 | 0.373 | |||
| Everolimus/exemestane | 45 | 0.984 | 0.469 | 2.063 | ||
| Other cytotoxic chemo | 31 | 1.318 | 0.624 | 2.786 | ||
| Fulvestrant | 23 | 0.829 | 0.353 | 1.947 | ||
| Clinical trials | 18 | 0.292 | 0.065 | 1.318 | ||
| PFS2 1 | ≤5.2 months | 88 | <0.001 | |||
| >5.2 months | 76 | 0.340 | 0.194 | 0.598 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.-Y.; Shin, J.; Ahn, J.S.; Park, Y.H.; Im, Y.-H. Real World Experience of Second-Line Treatment Strategies after Palbociclib and Letrozole: Overall Survival in Metastatic Hormone Receptor-Positive Human Epidermal Growth Factor Receptor 2-Negative Breast Cancer. Cancers 2023, 15, 3431. https://doi.org/10.3390/cancers15133431
Kim J-Y, Shin J, Ahn JS, Park YH, Im Y-H. Real World Experience of Second-Line Treatment Strategies after Palbociclib and Letrozole: Overall Survival in Metastatic Hormone Receptor-Positive Human Epidermal Growth Factor Receptor 2-Negative Breast Cancer. Cancers. 2023; 15(13):3431. https://doi.org/10.3390/cancers15133431
Chicago/Turabian StyleKim, Ji-Yeon, Junghoon Shin, Jin Seok Ahn, Yeon Hee Park, and Young-Hyuck Im. 2023. "Real World Experience of Second-Line Treatment Strategies after Palbociclib and Letrozole: Overall Survival in Metastatic Hormone Receptor-Positive Human Epidermal Growth Factor Receptor 2-Negative Breast Cancer" Cancers 15, no. 13: 3431. https://doi.org/10.3390/cancers15133431
APA StyleKim, J.-Y., Shin, J., Ahn, J. S., Park, Y. H., & Im, Y.-H. (2023). Real World Experience of Second-Line Treatment Strategies after Palbociclib and Letrozole: Overall Survival in Metastatic Hormone Receptor-Positive Human Epidermal Growth Factor Receptor 2-Negative Breast Cancer. Cancers, 15(13), 3431. https://doi.org/10.3390/cancers15133431








