Photodynamic Therapy for Glioblastoma: Illuminating the Path toward Clinical Applicability
Abstract
Simple Summary
Abstract
1. Introduction
2. Photodynamic Therapy Principles: The Math and Biology
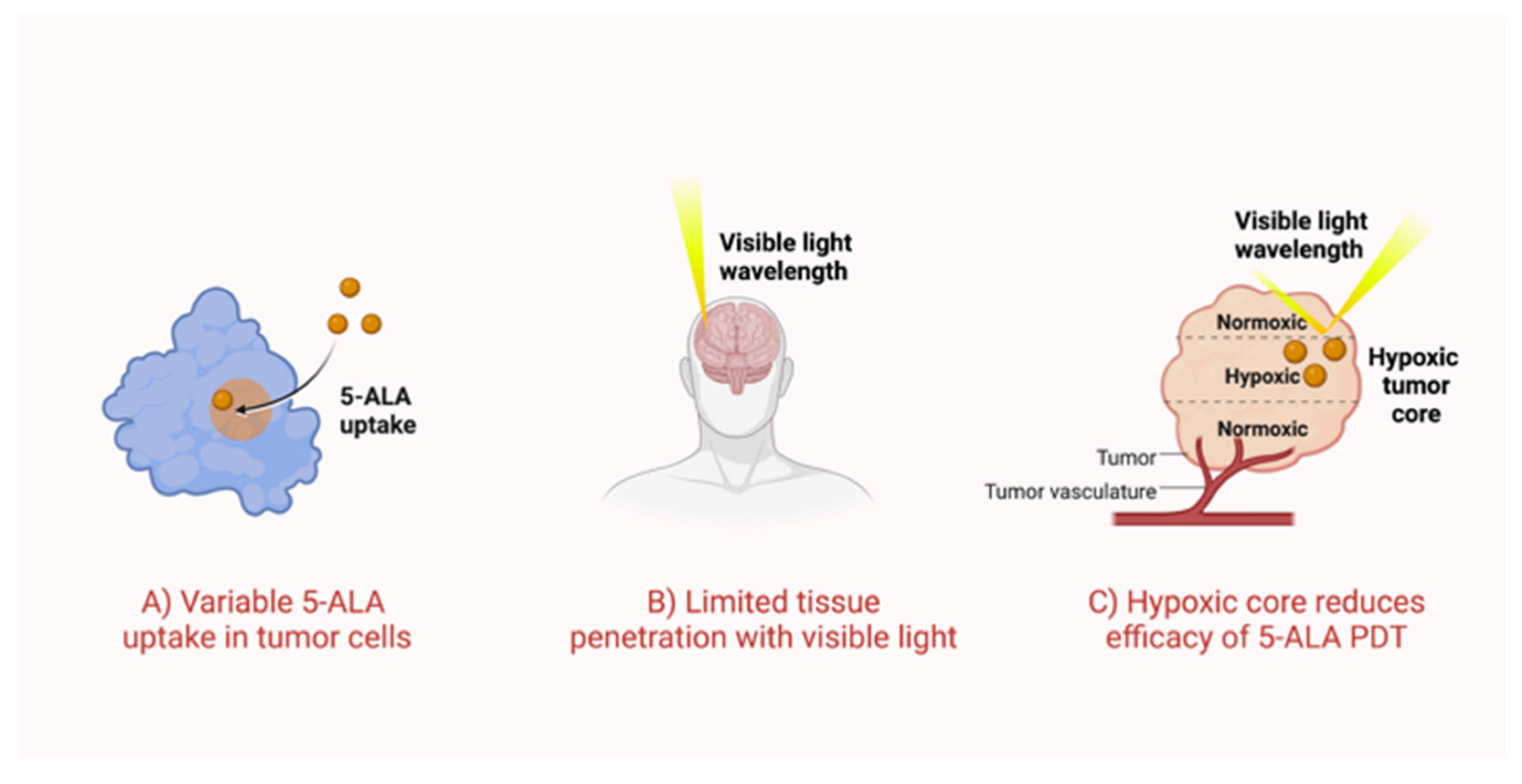
2.1. Variable Uptake of 5-ALA by Tumor Cells
2.2. Visible Light Has Weak Tissue Penetration
2.3. Poor Oxygen Recovery in 5-ALA-Based PDT
2.4. Low and Steady Wins the Race?
3. Discrepancies in In Vivo 5-ALA Research
4. The Path Ahead
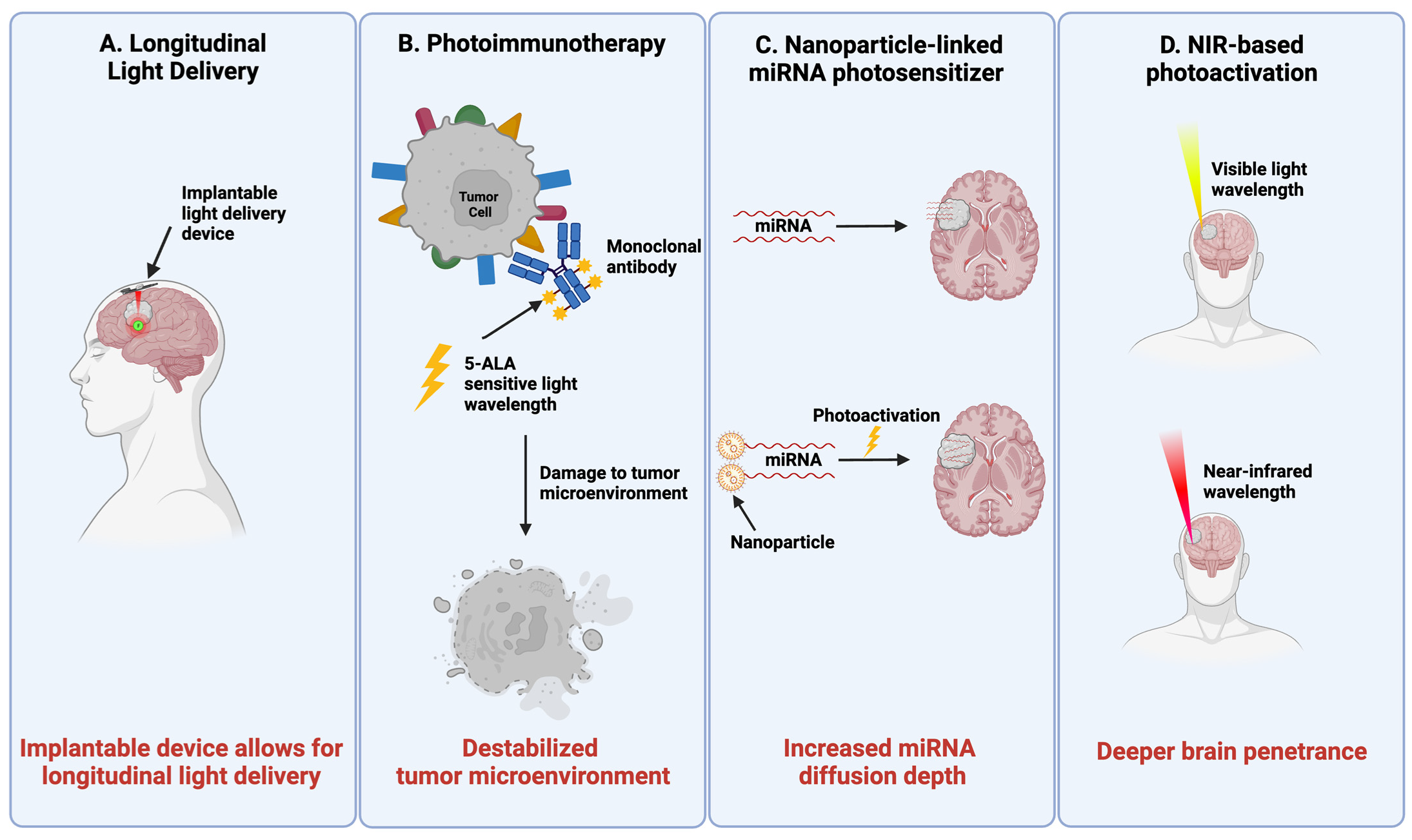
4.1. Longitudinal PDT Delivery
4.2. Harnessing Immunologic Response in PDT for GBM
4.3. Nanoparticle-Linked miRNA Photosensitizers
4.4. NIR Light Delivery
4.5. Other Nanoparticle-Linked Photosensitizers
4.6. Phytocompound Photosensitizers
5. Current Clinical Trials
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tan, A.C.; Ashley, D.M.; López, G.Y.; Malinzak, M.; Friedman, H.S.; Khasraw, M. Management of glioblastoma: State of the art and future directions. CA Cancer J. Clin. 2020, 70, 299–312. [Google Scholar] [CrossRef] [PubMed]
- Stupp, R.; Mason, W.P.; van den Bent, M.J.; Weller, M.; Fisher, B.; Taphoorn, M.J.B.; Belanger, K.; Brandes, A.A.; Marosi, C.; Bogdahn, U.; et al. Radiotherapy plus Concomitant and Adjuvant Temozolomide for Glioblastoma. N. Engl. J. Med. 2005, 352, 987–996. [Google Scholar] [CrossRef]
- Campos, B.; Olsen, L.R.; Urup, T.; Poulsen, H.S. A comprehensive profile of recurrent glioblastoma. Oncogene 2016, 35, 5819–5825. [Google Scholar] [CrossRef] [PubMed]
- Da Ros, M.; De Gregorio, V.; Iorio, A.L.; Giunti, L.; Guidi, M.; De Martino, M.; Genitori, L.; Sardi, I. Glioblastoma Chemoresistance: The Double Play by Microenvironment and Blood-Brain Barrier. Int. J. Mol. Sci. 2018, 19, 2879. [Google Scholar] [CrossRef]
- Stupp, R.; Hegi, M.E.; Mason, W.P.; van den Bent, M.J.; Taphoorn, M.J.B.; Janzer, R.C.; Ludwin, S.K.; Allgeier, A.; Fisher, B.; Belanger, K.; et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009, 10, 459–466. [Google Scholar] [CrossRef]
- Quirk, B.J.; Brandal, G.; Donlon, S.; Vera, J.C.; Mang, T.S.; Foy, A.B.; Lew, S.M.; Girotti, A.W.; Jogal, S.; LaViolette, P.S.; et al. Photodynamic therapy (PDT) for malignant brain tumors—Where do we stand? Photodiagnosis Photodyn. Ther. 2015, 12, 530–544. [Google Scholar] [CrossRef] [PubMed]
- Cramer, S.W.; Chen, C.C. Photodynamic Therapy for the Treatment of Glioblastoma. Front. Surg. 2020, 6, 81. [Google Scholar] [CrossRef] [PubMed]
- Mahmoudi, K.; Garvey, K.L.; Bouras, A.; Cramer, G.; Stepp, H.; Raj, J.G.J.; Bozec, D.; Busch, T.M.; Hadjipanayis, C.G. 5-aminolevulinic acid photodynamic therapy for the treatment of high-grade gliomas. J. Neuro-Oncol. 2019, 141, 595–607. [Google Scholar] [CrossRef]
- Withrow, S.J.; Poulson, J.M.; Lucroy, M.D. Withrow & MacEwen’s Small Animal Clinical Oncology. In Chapter 15—Miscellaneous Treatments for Solid Tumors, 4th ed.; Elsevier: Amsterdam, The Netherlands, 2007; pp. 275–290. [Google Scholar]
- O’Connor, A.E.; Gallagher, W.M.; Byrne, A.T. Porphyrin and Nonporphyrin Photosensitizers in Oncology: Preclinical and Clinical Advances in Photodynamic Therapy. Photochem. Photobiol. 2009, 85, 1053–1074. [Google Scholar] [CrossRef]
- Kou, J.; Dou, D.; Yang, L. Porphyrin photosensitizers in photodynamic therapy and its applications. Oncotarget 2017, 8, 81591–81603. [Google Scholar] [CrossRef]
- Wachowska, M.; Muchowicz, A.; Firczuk, M.; Gabrysiak, M.; Winiarska, M.; Wańczyk, M.; Bojarczuk, K.; Golab, J. Aminolevulinic Acid (ALA) as a Prodrug in Photodynamic Therapy of Cancer. Molecules 2011, 16, 4140–4164. [Google Scholar] [CrossRef]
- Yang, X.; Palasuberniam, P.; Kraus, D.; Chen, B. Aminolevulinic Acid-Based Tumor Detection and Therapy: Molecular Mechanisms and Strategies for Enhancement. Int. J. Mol. Sci. 2015, 16, 25865–25880. [Google Scholar] [CrossRef] [PubMed]
- McNicholas, K.; MacGregor, M.; Gleadle, J. In order for the light to shine so brightly, the darkness must be present—Why do cancers fluoresce with 5-aminolaevulinic acid? Br. J. Cancer 2019, 121, 631–639. [Google Scholar] [CrossRef] [PubMed]
- Mazurek, M.; Szczepanek, D.; Orzyłowska, A.; Rola, R. Analysis of Factors Affecting 5-ALA Fluorescence Intensity in Visualizing Glial Tumor Cells—Literature Review. Int. J. Mol. Sci. 2022, 23, 926. [Google Scholar] [CrossRef]
- Wang, W.; Tabu, K.; Hagiya, Y.; Sugiyama, Y.; Kokubu, Y.; Murota, Y.; Ogura, S.-I.; Taga, T. Enhancement of 5-aminolevulinic acid-based fluorescence detection of side population-defined glioma stem cells by iron chelation. Sci. Rep. 2017, 7, srep42070. [Google Scholar] [CrossRef] [PubMed]
- Rampazzo, E.; Della Puppa, A.; Frasson, C.; Battilana, G.; Bianco, S.; Scienza, R.; Basso, G.; Persano, L. Phenotypic and functional characterization of Glioblastoma cancer stem cells identified trough 5-aminolevulinic acid-assisted surgery. J. Neuro-Oncol. 2014, 116, 505–513. [Google Scholar] [CrossRef]
- Piccirillo, S.G.M.; Dietz, S.; Madhu, B.; Griffiths, J.; Price, S.J.; Collins, V.P.; Watts, C. Fluorescence-guided surgical sampling of glioblastoma identifies phenotypically distinct tumour-initiating cell populations in the tumour mass and margin. Br. J. Cancer 2012, 107, 462–468. [Google Scholar] [CrossRef]
- Blake, E.; Curnow, A. The Hydroxypyridinone Iron Chelator CP94 Can Enhance PpIX-induced PDT of Cultured Human Glioma Cells. Photochem. Photobiol. 2010, 86, 1154–1160. [Google Scholar] [CrossRef]
- Ishikawa, T.; Kajimoto, Y.; Inoue, Y.; Ikegami, Y.; Kuroiwa, T. Critical Role of ABCG2 in ALA-Photodynamic Diagnosis and Therapy of Human Brain Tumor. Adv. Cancer Res. 2015, 125, 197–216. [Google Scholar] [CrossRef]
- Müller, P.; Gaber, S.A.A.; Zimmermann, W.; Wittig, R.; Stepp, H. ABCG2 influence on the efficiency of photodynamic therapy in glioblastoma cells. J. Photochem. Photobiol. B Biol. 2020, 210, 111963. [Google Scholar] [CrossRef]
- Zhao, Z.; Fairchild, P.W. Dependence of light transmission through human skin on incident beam diameter at different wavelengths. In Laser-Tissue Interaction IX; SPIE: Cergy, France, 1998; Volume 3254, pp. 354–360. [Google Scholar] [CrossRef]
- Wang, H.-W.; Zhu, T.C.; Putt, M.E.; Solonenko, M.; Metz, J.; Dimofte, A.; Miles, J.; Fraker, D.L.; Glatstein, E.; Hahn, S.M.; et al. Broadband reflectance measurements of light penetration, blood oxygenation, hemoglobin concentration, and drug concentration in human intraperitoneal tissues before and after photodynamic therapy. J. Biomed. Opt. 2005, 10, 014004. [Google Scholar] [CrossRef]
- Hu, T.; Wang, Z.; Shen, W.; Liang, R.; Yan, D.; Wei, M. Recent advances in innovative strategies for enhanced cancer photodynamic therapy. Theranostics 2021, 11, 3278–3300. [Google Scholar] [CrossRef]
- Kim, M.M.; Darafsheh, A. Light Sources and Dosimetry Techniques for Photodynamic Therapy. Photochem. Photobiol. 2020, 96, 280–294. [Google Scholar] [CrossRef]
- Teh, D.B.L.; Bansal, A.; Chai, C.; Toh, T.B.; Tucker, R.A.J.; Gammad, G.G.L.; Yeo, Y.; Lei, Z.; Zheng, X.; Yang, F.; et al. A Flexi-PEGDA Upconversion Implant for Wireless Brain Photodynamic Therapy. Adv. Mater. 2020, 32, e2001459. [Google Scholar] [CrossRef]
- Ng, J.; Henriquez, N.; MacRobert, A.; Kitchen, N.; Williams, N.; Bown, S. Bioluminescence-activated photodynamic therapy for luciferase transfected, grade 4 astrocytoma cells in vitro. Photodiagnosis Photodyn. Ther. 2022, 38, 102856. [Google Scholar] [CrossRef]
- Wang, W.; Moriyama, L.T.; Bagnato, V.S. Photodynamic therapy induced vascular damage: An overview of experimental PDT. Laser Phys. Lett. 2012, 10, 3001. [Google Scholar] [CrossRef]
- Semyachkina-Glushkovskaya, O.; Kurths, J.; Borisova, E.; Sokolovski, S.; Mantareva, V.; Angelov, I.; Shirokov, A.; Navolokin, N.; Shushunova, N.; Khorovodov, A.; et al. Photodynamic opening of blood-brain barrier. Biomed. Opt. Express 2017, 8, 5040–5048. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Feng, L.; Liang, C.; Gao, M.; Song, G.; Liu, Z. Liposomes co-loaded with metformin and chlorin e6 modulate tumor hypoxia during enhanced photodynamic therapy. Nano Res. 2016, 10, 1200–1212. [Google Scholar] [CrossRef]
- Cheng, Y.; Cheng, H.; Jiang, C.; Qiu, X.; Wang, K.; Huan, W.; Yuan, A.; Wu, J.; Hu, Y. Perfluorocarbon nanoparticles enhance reactive oxygen levels and tumour growth inhibition in photodynamic therapy. Nat. Commun. 2015, 6, 8785. [Google Scholar] [CrossRef]
- Dysart, J.S.; Singh, G.; Patterson, M.S. Calculation of Singlet Oxygen Dose from Photosensitizer Fluorescence and Photobleaching During mTHPC Photodynamic Therapy of MLL Cells. Photochem. Photobiol. 2004, 81, 196–205. [Google Scholar] [CrossRef]
- Jarvi, M.T.; Patterson, M.S.; Wilson, B.C. Insights into Photodynamic Therapy Dosimetry: Simultaneous Singlet Oxygen Luminescence and Photosensitizer Photobleaching Measurements. Biophys. J. 2012, 102, 661–671. [Google Scholar] [CrossRef]
- Mcllroy, B.; Curnow, A.; Buonaccorsi, G.; Scott, M.; Bown, S.; MacRobert, A. Spatial measurement of oxygen levels during photodynamic therapy using time-resolved optical spectroscopy. J. Photochem. Photobiol. B Biol. 1998, 43, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Ubbink, R.; Prens, E.; Mik, E. Quantitative intracellular oxygen availability before and after 5-aminolevulinic acid skin photodynamic therapy. Photodiagnosis Photodyn. Ther. 2021, 36, 102599. [Google Scholar] [CrossRef]
- Busch, T.M.; Xing, X.; Yu, G.; Yodh, A.; Wileyto, E.P.; Wang, H.-W.; Durduran, T.; Zhu, T.C.; Wang, K.K.-H. Fluence rate-dependent intratumor heterogeneity in physiologic and cytotoxic responses to Photofrin photodynamic therapy. Photochem. Photobiol. Sci. 2009, 8, 1683–1693. [Google Scholar] [CrossRef] [PubMed]
- Madsen, S.J.; Sun, C.-H.; Tromberg, B.J.; Hirschberg, H. Development of a novel indwelling balloon applicator for optimizing light delivery in photodynamic therapy. Lasers Surg. Med. 2001, 29, 406–412. [Google Scholar] [CrossRef]
- Belykh, E.; Miller, E.J.; Patel, A.A.; Bozkurt, B.; Yağmurlu, K.; Robinson, T.R.; Nakaji, P.; Spetzler, R.F.; Lawton, M.T.; Nelson, L.Y.; et al. Optical Characterization of Neurosurgical Operating Microscopes: Quantitative Fluorescence and Assessment of PpIX Photobleaching. Sci. Rep. 2018, 8, 12543. [Google Scholar] [CrossRef]
- Chen, Q.; Chopp, M.; Madigan, L.; Dereski, M.O.; Hetzel, F.W. Damage Threshold of Normal Rat Brain in Photodynamic Therapy. Photochem. Photobiol. 1996, 64, 163–167. [Google Scholar] [CrossRef] [PubMed]
- Lilge, L.; Portnoy, M.; Wilson, B.C. Apoptosis induced in vivo by photodynamic therapy in normal brain and intracranial tumour tissue. Br. J. Cancer 2000, 83, 1110–1117. [Google Scholar] [CrossRef]
- Davies, N.; Wilson, B.C. Interstitial in vivo ALA-PpIX mediated metronomic photodynamic therapy (mPDT) using the CNS-1 astrocytoma with bioluminescence monitoring. Photodiagnosis Photodyn. Ther. 2007, 4, 202–212. [Google Scholar] [CrossRef]
- Hirschberg, H.; Sorensen, D.R.; Angell-Petersen, E.; Peng, Q.; Tromberg, B.; Sun, C.-H.; Spetalen, S.; Madsen, S.J. Repetitive Photodynamic Therapy of Malignant Brain Tumors. J. Environ. Pathol. Toxicol. Oncol. 2006, 25, 261–280. [Google Scholar] [CrossRef]
- Olzowy, B.; Hundt, C.S.; Stocker, S.; Bise, K.; Reulen, H.J.; Stummer, W. Photoirradiation therapy of experimental malignant glioma with 5-aminolevulinic acid. J. Neurosurg. 2002, 97, 970–976. [Google Scholar] [CrossRef]
- Tetard, M.-C.; Vermandel, M.; Leroy, H.-A.; Leroux, B.; Maurage, C.-A.; Lejeune, J.-P.; Mordon, S.; Reyns, N.; Information, P.E.K.F.C. Interstitial 5-ALA photodynamic therapy and glioblastoma: Preclinical model development and preliminary results. Photodiagnosis Photodyn. Ther. 2016, 13, 218–224. [Google Scholar] [CrossRef] [PubMed]
- Yi, W.; Xu, H.-T.; Tian, D.-F.; Wu, L.-Q.; Zhang, S.-Q.; Wang, L.; Ji, B.-W.; Zhu, X.-N.; Okechi, H.; Liu, G.; et al. Photodynamic therapy mediated by 5-aminolevulinic acid suppresses gliomas growth by decreasing the microvessels. J. Huazhong Univ. Sci. Technol. 2015, 35, 259–264. [Google Scholar] [CrossRef] [PubMed]
- Munegowda, M.A.; Fisher, C.; Molehuis, D.; Foltz, W.; Roufaiel, M.; Bassan, J.; Nitz, M.; Mandel, A.; Lilge, L. Efficacy of ruthenium coordination complex–based Rutherrin in a preclinical rat glioblastoma model. Neuro-Oncol. Adv. 2019, 1, vdz006. [Google Scholar] [CrossRef] [PubMed]
- Fisher, C.J.; Niu, C.; Foltz, W.; Chen, Y.; Sidorova-Darmos, E.; Eubanks, J.H.; Lilge, L. ALA-PpIX mediated photodynamic therapy of malignant gliomas augmented by hypothermia. PLoS ONE 2017, 12, e0181654. [Google Scholar] [CrossRef]
- Fisher, C.; Obaid, G.; Niu, C.; Foltz, W.; Goldstein, A.; Hasan, T.; Lilge, L. Liposomal Lapatinib in Combination with Low-Dose Photodynamic Therapy for the Treatment of Glioma. J. Clin. Med. 2019, 8, 2214. [Google Scholar] [CrossRef]
- Chen, Z.; Hambardzumyan, D. Immune Microenvironment in Glioblastoma Subtypes. Front. Immunol. 2018, 9, 1004. [Google Scholar] [CrossRef]
- Castano, A.P.; Mroz, P.; Hamblin, M.R. Photodynamic therapy and anti-tumour immunity. Nat. Rev. Cancer 2006, 6, 535–545. [Google Scholar] [CrossRef]
- Garg, A.D.; Nowis, D.; Golab, J.; Agostinis, P. Photodynamic therapy: Illuminating the road from cell death towards anti-tumour immunity. Apoptosis 2010, 15, 1050–1071. [Google Scholar] [CrossRef]
- Letchuman, V.; Ampie, L.; Shah, A.H.; Brown, D.A.; Heiss, J.D.; Chittiboina, P. Syngeneic murine glioblastoma models: Reactionary immune changes and immunotherapy intervention outcomes. Neurosurg. Focus 2022, 52, E5. [Google Scholar] [CrossRef]
- Rajendrakumar, S.K.; Uthaman, S.; Cho, C.-S.; Park, I.-K. Nanoparticle-Based Phototriggered Cancer Immunotherapy and Its Domino Effect in the Tumor Microenvironment. Biomacromolecules 2018, 19, 1869–1887. [Google Scholar] [CrossRef]
- Mączyńska, J.; Raes, F.; Da Pieve, C.; Turnock, S.; Boult, J.K.R.; Hoebart, J.; Niedbala, M.; Robinson, S.P.; Harrington, K.J.; Kaspera, W.; et al. Triggering anti-GBM immune response with EGFR-mediated photoimmunotherapy. BMC Med. 2022, 20, 16. [Google Scholar] [CrossRef]
- Ahir, B.K.; Ozer, H.; Engelhard, H.H.; Lakka, S.S. MicroRNAs in glioblastoma pathogenesis and therapy: A comprehensive review. Crit. Rev. Oncol. 2017, 120, 22–33. [Google Scholar] [CrossRef]
- Kumal, R.R.; Abu-Laban, M.; Hamal, P.; Kruger, B.; Smith, H.T.; Hayes, D.J.; Haber, L.H. Near-Infrared Photothermal Release of siRNA from the Surface of Colloidal Gold–Silver–Gold Core–Shell–Shell Nanoparticles Studied with Second-Harmonic Generation. J. Phys. Chem. C 2018, 122, 19699–19704. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Bailey, J.T.; Abu-Laban, M.; Li, S.; Chen, C.; Glick, A.B.; Hayes, D.J. Photocontrolled miR-148b nanoparticles cause apoptosis, inflammation and regression of Ras induced epidermal squamous cell carcinomas in mice. Biomaterials 2020, 256, 120212. [Google Scholar] [CrossRef] [PubMed]
- Henderson, T.A.; Morries, L. Near-infrared photonic energy penetration: Can infrared phototherapy effectively reach the human brain? Neuropsychiatr. Dis. Treat. 2015, 11, 2191–2208. [Google Scholar] [CrossRef] [PubMed]
- Dupont, C.; Mordon, S.; Deleporte, P.; Reyns, N.; Vermandel, M. A novel device for intraoperative photodynamic therapy dedicated to glioblastoma treatment. Futur. Oncol. 2017, 13, 2441–2454. [Google Scholar] [CrossRef]
- Dupont, C.; Vermandel, M.; Leroy, H.-A.; Quidet, M.; Lecomte, F.; Delhem, N.; Mordon, S.; Reyns, N. INtraoperative photoDYnamic Therapy for GliOblastomas (INDYGO): Study Protocol for a Phase I Clinical Trial. Neurosurgery 2018, 84, E414–E419. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Weitemier, A.Z.; Zeng, X.; He, L.; Wang, X.; Tao, Y.; Huang, A.J.Y.; Hashimotodani, Y.; Kano, M.; Iwasaki, H.; et al. Near-infrared deep brain stimulation via upconversion nanoparticle–mediated optogenetics. Science 2018, 359, 679–684. [Google Scholar] [CrossRef]
- Lee, S.Y.; Lee, R.; Kim, E.; Lee, S.; Park, Y.I. Near-Infrared Light-Triggered Photodynamic Therapy and Apoptosis Using Upconversion Nanoparticles With Dual Photosensitizers. Front. Bioeng. Biotechnol. 2020, 8, 275. [Google Scholar] [CrossRef]
- Abu-Laban, M.; Hamal, P.; Arrizabalaga, J.H.; Forghani, A.; Dikkumbura, A.S.; Kumal, R.R.; Haber, L.H.; Hayes, D.J.; Abu-Laban, M.; Hamal, P.; et al. Combinatorial Delivery of miRNA-Nanoparticle Conjugates in Human Adipose Stem Cells for Amplified Osteogenesis. Small 2019, 15, e1902864. [Google Scholar] [CrossRef] [PubMed]
- Lerouge, L.; Gries, M.; Chateau, A.; Daouk, J.; Lux, F.; Rocchi, P.; Cedervall, J.; Olsson, A.-K.; Tillement, O.; Frochot, C.; et al. Targeting Glioblastoma-Associated Macrophages for Photodynamic Therapy Using AGuIX®-Design Nanoparticles. Pharmaceutics 2023, 15, 997. [Google Scholar] [CrossRef] [PubMed]
- Caverzán, M.D.; Oliveda, P.M.; Beaugé, L.; Palacios, R.E.; Chesta, C.A.; Ibarra, L.E. Metronomic Photodynamic Therapy with Conjugated Polymer Nanoparticles in Glioblastoma Tumor Microenvironment. Cells 2023, 12, 1541. [Google Scholar] [CrossRef] [PubMed]
- Pellosi, D.S.; de Paula, L.B.; de Melo, M.T.; Tedesco, A.C. Targeted and Synergic Glioblastoma Treatment: Multifunctional Nanoparticles Delivering Verteporfin as Adjuvant Therapy for Temozolomide Chemotherapy. Mol. Pharm. 2019, 16, 1009–1024. [Google Scholar] [CrossRef]
- Pistollato, F.; Bremer-Hoffmann, S.; Basso, G.; Cano, S.S.; Elio, I.; Vergara, M.M.; Giampieri, F.; Battino, M. Targeting Glioblastoma with the Use of Phytocompounds and Nanoparticles. Target. Oncol. 2015, 11, 1–16. [Google Scholar] [CrossRef]
- Du, W.-Z.; Feng, Y.; Wang, X.-F.; Piao, X.-Y.; Cui, Y.-Q.; Chen, L.-C.; Lei, X.-H.; Sun, X.; Liu, X.; Wang, H.-B.; et al. Curcumin Suppresses Malignant Glioma Cells Growth and Induces Apoptosis by Inhibition of SHH/GLI1 Signaling Pathway in Vitro and Vivo. CNS Neurosci. Ther. 2013, 19, 926–936. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Zhou, Q.; Liu, C.; Tao, M.-L. Drug screening study using glioma stem-like cells. Mol. Med. Rep. 2012, 6, 1117–1120. [Google Scholar] [CrossRef]
- Zhuang, W.; Long, L.; Zheng, B.; Ji, W.; Yang, N.; Zhang, Q.; Liang, Z. Curcumin promotes differentiation of glioma-initiating cells by inducing autophagy. Cancer Sci. 2011, 103, 684–690. [Google Scholar] [CrossRef]
- Kielbik, A.; Wawryka, P.; Przystupski, D.; Rossowska, J.; Szewczyk, A.; Saczko, J.; Kulbacka, J.; Chwiłkowska, A. Effects of Photosensitization of Curcumin in Human Glioblastoma Multiforme Cells. Vivo 2019, 33, 1857–1864. [Google Scholar] [CrossRef]
- Carriero, F.; Martinelli, C.; Gabriele, F.; Barbieri, G.; Zanoletti, L.; Milanesi, G.; Casali, C.; Azzalin, A.; Manai, F.; Paolillo, M.; et al. Berberine Photo-Activation Potentiates Cytotoxicity in Human Astrocytoma Cells through Apoptosis Induction. J. Pers. Med. 2021, 11, 942. [Google Scholar] [CrossRef]
- Vermandel, M.; Dupont, C.; Lecomte, F.; Leroy, H.-A.; Tuleasca, C.; Mordon, S.; Hadjipanayis, C.G.; Reyns, N. Standardized intraoperative 5-ALA photodynamic therapy for newly diagnosed glioblastoma patients: A preliminary analysis of the INDYGO clinical trial. J. Neuro-Oncol. 2021, 152, 501–514. [Google Scholar] [CrossRef] [PubMed]
| Author, Year | Animal Model (# of Animals) | PDT Parameters | Outcome Variables | Main Results | Shortcomings |
|---|---|---|---|---|---|
| Hirschberg, 2006 [42] | BDIX rats, BT4C HGG spheroids (15) | Longitudinal; 7–30 mW; 10–30 min/week (×3) | -PpIX biodistribution -Overall survival -Necrosis | 1. ↑ Overall survival in repetitive PDT compared to single session. 2. ↑ Necrosis in low fluence groups compared to high fluence. | -Did not control for probe or heat damage -Repeat surgeries and anesthesia for each PDT delivery = stress on animal |
| Davies, 2007 [41] | Fischer Rats, CNS-1 Astrocytoma (37) | Longitudinal; 0.5 mW/cm2; 24–96 h | -Tumor volume -Tumor regrowth -Necrosis -Apoptosis | Tumor volume reduction greater with 96 h vs. 24 h. | -Did not control for probe or heat-induced damage -Astrocytoma model, not GBM or high-grade -Duration not translatable |
| Tetard, 2016 [44] | Fox1 rnu/rnu rats, U87 GBM (22) | Fractionated and continuous; 4.8–30 mW; 120 s between 5 J and 21 J of delivery | -Necrosis -ICP -Hemorrhage | 1. ↑ Necrosis in fractionated group compared to continuous. 2. ↑ ICP and hemorrhage in high fluence group. | -Single session PDT -Histological images provided do not compare normal brain to PDT treated area |
| Yi, 2015 [45] | Wistar rats, C6 glioma cells (24) | Single session; 100 mW/cm2; 60 min | -Tumor size -Tumor volume -Necrosis -Micro-vessel density (MVD) -Apoptosis | 1. ↓ Tumor volume in PDT group compared to controls. 2. ↑ Necrosis in PDT group compared to controls. 3. ↓ MVD in PDT group compared to controls. 4. No difference in apoptosis between groups. | -Single session -Not an intracranial model (graft implanted in abdomen) |
| Munegowda, 2019 [46] | CDF Fischer rats, RG-2 cells (46) | Single session; 18 mW; 22 min | -Survival -Tumor volume -Intratumor edema -CD8 T-cell stain | Compared 5-ALA to Rutherrin photosensitizer: 1. ↑ Overall survival in R-ALA- and Rutherrin-treated rats compared to controls. Rutherrin increased survival more than 5-ALA. 2. ↓ Edema in Rutherrin group compared to 5-ALA. 3. ↑ CD8+ T-cell infiltration in Rutherrin compared to 5-ALA groups. | -Single session PDT -Did not compare 5-ALA to controls for edema and CD8+ T-cell infiltration -Small control cohort (n = 4) -Small PDT cohort (n = 6) |
| Olzowy, 2002 [43] | Wistar, C6 glioma cells (30) | Single session; 100 mW/cm2 | -Cortical damage -Tumor size -Hemorrhage | 1. No difference in cortical damage between PDT group and no irradiation group. | -Single session PDT -Histological images provided did not compare normal brain to PDT treated areas |
| Fisher, 2017 [47] | CDF Fischer rats, RG-2 cells (12) | Single session; 18 mW for 22 min | -Intratumor edema -Reactive gliosis -Survival | Compared 5-ALA PDT in hypothermic and normothermic conditions: 1. ↓ Edema in hypothermic conditions. 2. ↑ PpIX fluorescence in hypothermia conditions. 3. ↑ median overall survival in hypothermic conditions. 4. Increased cellular protection to normal brain structures in hypothermic conditions. | -Single session PDT -High variation in edema data |
| Fisher, 2019 [48] | GSC30 Rag2 -/- SCID rats, U87 cells (20) | Single session; 22.2 mW/cm2 | -Hypoxia -Tumor blood flow -Survival -Hemorrhage | Compared 5-ALA PDT with and without lapatinib and lapatinib along: 1. ↑ Overall survival in PDT + lapatinib group. 2. No difference in hypoxia between groups. 3. ↓ Tumor blood flow in PDT + lapatinib group. 4. ↑ Edema in PDT + lapatinib group | -Single session PDT -results only applicable to EGFR sensitive tumors |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bhanja, D.; Wilding, H.; Baroz, A.; Trifoi, M.; Shenoy, G.; Slagle-Webb, B.; Hayes, D.; Soudagar, Y.; Connor, J.; Mansouri, A. Photodynamic Therapy for Glioblastoma: Illuminating the Path toward Clinical Applicability. Cancers 2023, 15, 3427. https://doi.org/10.3390/cancers15133427
Bhanja D, Wilding H, Baroz A, Trifoi M, Shenoy G, Slagle-Webb B, Hayes D, Soudagar Y, Connor J, Mansouri A. Photodynamic Therapy for Glioblastoma: Illuminating the Path toward Clinical Applicability. Cancers. 2023; 15(13):3427. https://doi.org/10.3390/cancers15133427
Chicago/Turabian StyleBhanja, Debarati, Hannah Wilding, Angel Baroz, Mara Trifoi, Ganesh Shenoy, Becky Slagle-Webb, Daniel Hayes, Yasaman Soudagar, James Connor, and Alireza Mansouri. 2023. "Photodynamic Therapy for Glioblastoma: Illuminating the Path toward Clinical Applicability" Cancers 15, no. 13: 3427. https://doi.org/10.3390/cancers15133427
APA StyleBhanja, D., Wilding, H., Baroz, A., Trifoi, M., Shenoy, G., Slagle-Webb, B., Hayes, D., Soudagar, Y., Connor, J., & Mansouri, A. (2023). Photodynamic Therapy for Glioblastoma: Illuminating the Path toward Clinical Applicability. Cancers, 15(13), 3427. https://doi.org/10.3390/cancers15133427







