Simple Summary
Around 25% of patients who undergo an ultrasound-guided thyroid biopsy end up with an indeterminate result based on cytology. This has propelled the use of other modalities, such as molecular testing, to further stratify these patients. The aim of our retrospective study was to report and compare two genetic mutations in our patient population. These mutations are RET/PTC and THADA/IGF2BP3 translocations, which have been hypothesized as oncogenic events in thyroid neoplasms. We confirm that our patient population exhibited these mutations, and all underwent a final histopathology analysis where surgery was the preferred treatment modality. We also report that the RET/PTC fusion exhibited more aggressive features than the THADA/IGF2BP3 fusion and was more likely to need post-surgical treatment.
Abstract
Background: Molecular testing has been used as an adjunct to morphological evaluation in the workup of thyroid nodules. This study investigated the impact of two gene fusions, RET/PTC and THADA/IGF2BP3, that have been described as oncogenic events in thyroid neoplasms. Methods: We performed a retrospective, single-centered study at a McGill University teaching hospital in Montreal, Canada, from January 2016 to August 2021. We included patients who underwent surgery for thyroid nodules that pre-operatively underwent molecular testing showing either RET/PTC or THADA/IGF2BP3 gene fusion. Results: This study included 697 consecutive operated thyroid nodules assessed using molecular testing, of which five had the RET/PTC fusion and seven had the THADA/IGF2BP3 fusion. Of the five nodules in the RET/PTC group, 100% were malignant and presented as Bethesda V/VI. Eighty percent (4/5) were found to have lymph node metastasis. Twenty percent (1/5) had extrathyroidal extensions. Sixty percent (3/5) were a diffuse sclerosing variant of papillary thyroid carcinoma, and the rest were the classical variant. Of the seven THADA/IGF2BP3 nodules, all presented as Bethesda III/IV and 71.4% (5/7) were malignant based on the final pathology analysis, and 28.6% (2/7) were NIFTP. All the THADA/IGF2BP3 fusion malignancies were a follicular variant of papillary thyroid carcinoma. None had lymph node metastasis or displayed extrathyroidal extensions. Conclusions: RET/PTC nodules presented as Bethesda V/VI and potentially had more aggressive features, whereas THADA/IGF2BP3 nodules presented as Bethesda III/IV and had more indolent behavior. This understanding may allow clinicians to develop more targeted treatment plans, such as the extent of surgery and adjuvant radioactive iodine treatment.
1. Introduction
Thyroid Cards
Cancer is the most common endocrine malignancy. Its incidence has been steadily increasing over the recent decades and can be attributed to the advancements in diagnostic modalities that have facilitated the detection of thyroid nodules earlier in the disease process [1,2]. The gold standard for the workup of suspicious thyroid lesions is the ultrasound-guided fine needle aspiration (USFNA) [3,4]. Although cytopathology classifies the majority of biopsies as benign or malignant, 20–25% of nodules are still classified as indeterminate, i.e., Bethesda III (atypia of undetermined significance) and Bethesda IV (follicular neoplasm or suspicious for follicular neoplasm) [5,6]. This has propelled the field of molecular testing as a concurrent modality to further diagnose and prognosticate thyroid nodules.
The latest iteration of the American Thyroid Association (ATA) guidelines acknowledges the use of molecular testing as an adjunct to USFNA in the workup of cytologically indeterminate nodules, provided patients are educated regarding the benefits and limitations of genetic testing. The ATA guidelines also discuss specific mutations in terms of their aggressive behavior to predict risk of recurrence and guide post-operative management [7]. It is crucial to identify the molecular markers not only to distinguish between benign and malignant tumors but also to predict aggressive phenotypes, prognosis, recurrence, and efficacy of treatment, including potential novel therapeutic targets [8].
The Bethesda scoring system is based on cytopathologic features of FNA biopsy. Score II represents potential benign thyroid nodule where monitoring using ultrasound and/or blood testing is recommended, and chance of significant growth remains low enough to not warrant surgical intervention [9]. Intermediate Bethesda scores (III/IV) represent a follicular lesion/atypia of undetermined significance. This entity of indeterminate thyroid nodules often presents a dogma to clinicians. Much of the literature has focused on this problematic entity, with the probability of having a malignant pathology often significantly fluctuating between different institutions and regions [10,11,12]. Bethesda score V includes lesions with features suspicious for, but not definitive for, a malignant thyroid pathology, and Bethesda VI lesions are presumed malignant and referred for surgical management [5].
There are several types of thyroid cancer, which differ in their presentation, aggressiveness, survival rates, and treatments. The most common types are papillary thyroid cancer (PTC) and follicular thyroid cancer (FTC), which together make roughly 90% of all cases [13]. Similarly, there are several variants of PTC that also differ in their prevalence, aggressiveness, and risk factors. One of the most aggressive variants that is relevant to this study is diffuse sclerosing variant (DSV), which is most frequently observed in younger patients [14]. It is very aggressive with a high risk of extrathyroidal extension, cervical lymph node metastasis, and recurrence [14,15].
A subtype of FTC is Hürthle cell carcinoma (HCC), which is more aggressive than FTC and makes up around 3% of all cases. Another subtype of FTC is noninvasive follicular thyroid neoplasm with papillary-like nuclear features (NIFTP), which was previously classified as a type of PTC.
Molecular studies have identified many oncogenic drivers in thyroid cancer. Amongst known drivers, 75% are described as chromosomal point mutations, 15% show nonoverlapping chromosomal rearrangements that generally lead to the activation of tyrosine kinases, and 10% where no driver is identified [16,17,18]. In addition to their diagnostic capabilities, molecular panels may also aid in the future in providing targeted therapies that can inhibit the activity of an aberrant driver pathway in thyroid tumorigenesis [19,20]. Indeed, the advent of molecular therapy is now considered gold standard in the treatment of certain anaplastic thyroid cancer (ATC), whereby the determination of the mutation status of BRAF gene is recommended in the ATA guidelines [21]. Specifically, treatment with dabrafenib has shown some efficacy in BRAF-mutated thyroid cancer, especially when combined with mitogen-activated protein kinase kinase (MEK) inhibitors, such as trametinib [22].
One gene rearrangement of interest is rearranged during transfection (RET). RET is a proto-oncogene that has been implicated in PTC and medullary thyroid carcinoma (MTC) [23]. The RET gene is located in the long arm of chromosome 10 and encodes a cell membrane tyrosine kinase whose activation stimulates mitogen-activated protein kinase (MAPK) and PI3K, which promote downstream cellular proliferation [24,25]. RET/PTC refers to the fusion of RET with one of the different heterologous genes to create a series of chimeric oncogenes [26]. This rearrangement, in turn, produces a protein that activates MAPK pathway [27]. It has been reported in up to 20–40% of adult sporadic papillary carcinomas; however, its prevalence is highly variable amongst studies [26]. The presence of RET/PTC translocation has also been implicated in tumor multifocality [28]. RET/PTC fusions have been shown to exist concomitantly with other mutations, namely BRAF(V600E) in malignant PTC [29]. RET/PTC fusions are also different from RET point mutations, which are single changes in the DNA sequence of the RET gene. These point mutations can be either inherited or acquired and have been implicated in MTC [27].
Another gene rearrangement of interest is referred to as thyroid adenoma associated (THADA) gene fusion, which is the second most common chromosomal rearrangement in thyroid cancer [26]. It is hypothesized to have a prevalence of around 5% of thyroid cancer that lack any other identifiable driver mutation [30]. Recent studies by Panebianco et al. have elucidated the mechanism by which THADA-gene fusion leads to increased cellular growth, migration, and invasion. Translocations between chromosomal bands 2p21 (THADA) and 7p15 lead to overexpression of IGF2, thereby activating downstream MAPK and PI3K signaling pathways [30,31,32,33]. It can also occur in other cancer types, such as breast, ovarian, lung, and colorectal cancers [30].
To date, several studies have assessed the prevalence and clinicopathological characteristics of these two genetic rearrangements. RET/PTC fusion prevalence has been reported in few studies with large variance [34]. THADA/IGF2BP3 fusion has been recently studied by Morariu et al. where a 2% prevalence was reported amongst cytologically indeterminate thyroid nodules [35]. The aim of the study was to establish the prevalence of RET/PTC and THADA/IGF2BP3 in our patient population as well as to study the histopathology, management, and surgical outcomes. Such insight will assist in the pre-operative/perioperative decision-making for patients with one of these molecular alterations.
2. Materials and Methods
2.1. Study Design
A retrospective chart review was performed on patients who were worked up for thyroid nodules from January 2016 to August 2021. All patients were treated at the Jewish General Hospital (JGH), Faculty of Medicine, McGill University in Montreal, Quebec. The study included patients that had undergone pre-operative molecular testing using one of the following commercially available tests: Afirma GSC, Thyroseq V3, or ThyGeNEXT/ThyraMIR. They later underwent surgery.
Patients were excluded if surgical pathology results were not available. None of study subjects had more than one driver mutation reported using molecular testing. Patients who had multiple nodules that were biopsied, with different cytopathology classifications, the higher Bethesda score was used to calculate the prevalence of different Bethesda scores and corresponding malignancy based on the final pathology analysis. Baseline characteristics, such as demographics, cytopathology, surgical pathology, and results of molecular/genetic findings, treatment, and outcome were collected for analysis (Table 1). In case of malignancy, the specific type (papillary thyroid carcinoma, follicular carcinoma, Hürthle cell carcinoma, and poorly differentiated thyroid carcinoma) and the variant (classical, follicular, oncocytic, tall cell, columnar cell, solid, and hobnail) were recorded (Table 1). Operating room reports were examined in detail for any complications or findings that were not seen on prior evaluation.

Table 1.
Baseline Characteristics of Patients. FNA: fine needle aspiration.
2.2. Data Collection
Ethics approval for the purpose of this study was granted by the JGH Research Ethics Committee (2021-2617). Using patient identifiers in logs, the charts were accessed. This included baseline characteristics, such as age and USFNA results as per the Bethesda reporting system. Medical documents, such as reports, clinician notes, labs, and imaging of the patients were accessed.
2.3. Statistical Analysis
Statistical analysis was performed using SPSS to compare patient demographics and tumor characteristics between the genetic aberrations under study. Chi-squared analysis was used to calculate confidence intervals. Continuous variables, including Bethesda score, were compared using independent sample t-test or ANOVA. Statistical significance was set as p < 0.05.
3. Results
3.1. Prevalence
During the study period, 697 consecutive thyroid nodules were analyzed using molecular analysis in the pre-operative workup. Out of these thyroid nodules, 377 were Bethesda III or IV on USFNA and 312 were Bethesda V or VI on USFNA (see Figure 1). Amongst Bethesda III nodules, 105 out of a total of 165 (64%) were malignant or non-invasive follicular thyroid neoplasm with NIFTP in the final histopathology analysis; amongst Bethesda IV nodules, 159 out of a total of 212 (73%) were malignant or NIFTP in the final histopathology analysis.
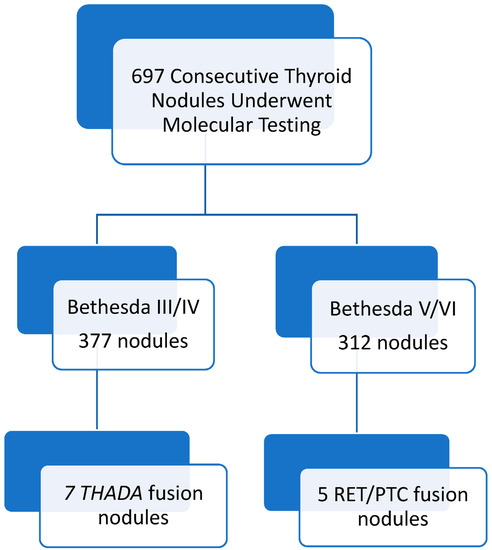
Figure 1.
Summary of log search results pertaining to both genetic translocations of interest.
All USFNA biopsies that were RET/PTC fusion positive were reported as Bethesda V or VI. All USFNA biopsies that were THADA/IGF2BP3 fusion positive were reported as Bethesda III or IV. Out of the 312 nodules with Bethesda V or VI on USFNA, five (1.60%) were positive for a RET/PTC fusion. Out of the 377 nodules with Bethesda III or IV on USFNA, seven (1.86%) were positive for a THADA/IGF2BP3 fusion. Overall, all nodules with RET/PTC or THADA/IGF2BP3 fusions were either malignant or NIFTP in the final histopathology analysis.
3.2. Baseline Characteristics
Baseline information was calculated for all 12 patients. Tumor size was reported as per the long-axis measurement in centimeters in the final pathology reports. The baseline characteristics are summarized in Table 1. No statistically significant differences were detected with regards to age, gender, or final pathologic tumor size between the RET/PTC and THADA/IGF2BP3 fusion cohorts (p = 0.064, p = 0.154, and p = 0.104, respectively). RET/PTC fusion translocations presented with a higher Bethesda Score Distribution than THADA/IGF2BP3 fusion translocations (p < 0.01). All patients underwent a sentinel lymph node (LN) biopsy intraoperatively in addition to a limited central neck dissection. If the sentinel lymph node is positive, then a more extensive central neck dissection is performed by the surgeon.
3.3. Cancer Type and Correlation with Clinicopathological Characteristics
All the RET/PTC fusion cases (5/5) presented as Bethesda V/VI. They were all positive for malignancy in the final histopathology analysis: 60% (3/5) were diffuse sclerosing variant (DSV) and the rest were classical variant of PTC. All DSV cases were positive for lymphovascular invasion, while the nodules with classical variant were all negative for lymphovascular invasion. None of the nodules were positive for perineural invasion. Only one case (1/5) was positive for extrathyroidal extension (minimal). The final pathology analysis reported 80% (4/5) of those patients had LN metastasis, with one of these cases presenting a lymph node with extranodal spread (ENS). All patients underwent one sentinel LN biopsy, which if positive, the surgeon proceeded to conduct a central compartment LN biopsy (see Table 2). All positive LNs had the shortest dimension of at least 0.2 cm.

Table 2.
Baseline characteristics, procedure undergone, and final pathological history. NIFTP: noninvasive follicular thyroid neoplasm with papillary-like nuclear features; DSV: diffuse sclerosing variant.
Amongst the THADA/IGF2BP3 fusion group, all (7/7) presented as Bethesda III or IV. Furthermore, 71.4% (5/7) were malignant in the final pathology analysis, and 28.6% (2/7) were NIFTP [36]. Amongst malignant nodules, all presented as follicular variant of PTC. None of the nodules was positive for extrathyroidal extension. Only one nodule that presented as a follicular PTC was positive for focal lymphovascular invasion. None of the nodules had perineural invasion. In the final pathology analysis, none of the patients had LN metastasis or extranodal spread (see Table 2).
3.4. Patient Management
Of the five RET/PTC fusion cases, four underwent total thyroidectomy and one had undergone a hemi/subtotal thyroidectomy. The patient who underwent a hemi/subtotal thyroidectomy was the only patient whose intraoperative sentinel LN biopsy was negative for malignancy. Of the seven THADA/IGF2BP3 fusion cases, all were treated with hemi/subtotal thyroidectomy. None of these patients had a positive intraoperative sentinel LN biopsy. None underwent a completion thyroidectomy (see Table 2).
3.5. Patient Follow-Up
Five patients with THADA/IGF2BP3 fusion nodules had available data for follow-up. Four of these patients had malignant nodules and one with NIFTP. Mean follow-up period was 18 months (range 1–33 months). No recurrences were identified during that period.
Five patients with RET/PTC fusion nodules had follow-up data, where the mean follow-up period was 20.6 months (range 1–28 months). Three patients had thyroid scans consistent with remnant thyroid tissue and needed to undergo additional treatment. The patients who had positive post-surgical thyroid scans were the same patients that had diffuse sclerosing variant malignancies in surgical pathology.
4. Discussion
Over the last decade, the advent of molecular testing has been rapidly expanding in the pre-operative workup of thyroid nodules. This single-centered study was conducted in Montreal, Quebec, where molecular testing was offered to patients on a case-per-case and voluntary basis. Molecular testing panels have generally branded themselves as a strong rule-in or rule-out, reducing the need for diagnostic surgery in thyroid nodules with intermediate Bethesda scores [7].
The identification of various molecular markers during thyroid cell transformation and tumor progression is a critical step in understanding the underlying pathogenetic factors that can guide clinical management of thyroid cancer. Several genetic alterations have been reported thus far, which include passenger mutations and genetic drivers, such as BRAF, p53, NRAS, KRAS, and HRAS [37]. However, the RET/PTC and THADA/IGF2BP3 fusions are less well-studied as they comprise a small percentage of known genetic alterations. This retrospective study aimed to evaluate the clinical impact of these two genetic rearrangements as well as shed light on the pragmatic application of molecular testing in the workup and management of thyroid nodules.
Our retrospective study establishes an estimate of the prevalence of both mutations in our patient population, within their respective Bethesda buckets. All nodules harboring a RET/PTC fusion were Bethesda V/VI in the cytology analysis. In this context, the extent of surgery was based on available clinical information, patient preference as well as intraoperative findings. Most of the patients with a RET/PTC fusion in this study had lymph node metastases and/or extrathyroidal extension. Moreover, more than half the cases were a diffuse sclerosing variant. The knowledge that a thyroid nodule has a RET/PTC fusion with a high likelihood of being aggressive will guide thyroid specialists to making a more informed decision with respect to the extent of surgery, including the decision to perform a central neck dissection.
On the other hand, all cases of nodules with a THADA/IGF2BP3 fusion presented as indeterminate cytology in the USFNA assessment. All the nodules required surgery as the final pathology was malignant or NIFTP. While most of these nodules (5/7) were malignant in the final surgical pathology, none were considered as aggressive or at intermediate or higher risk for recurrence. As a result, a more conservative and limited surgical approach might be warranted in nodules with a THADA/IGF2BP3 fusion. These findings are in line with other reported findings by Panebianco et al. and Morariu et al.; however, the prevalence in the THADA/IGF2BP3 fusion nodules was lower in our patient population [30,35].
The findings of this study highlight the potential for optimizing patient care and management decisions based on molecular testing results. Prior to the release of the American Thyroid Association Guidelines in 2015 for Differentiated Thyroid Cancer, many thyroid malignancies were treated with total thyroidectomies and radioactive iodine. Following the recommendations in these guidelines, practice regimens changed, and less extensive surgeries (hemi-thyroidectomy/lobectomy) gained popularity. Whether to perform a total thyroidectomy or hemi-thyroidectomy for patients with thyroid malignancies became a management dilemma. Performing a hemi-thyroidectomy can lead to a completion thyroidectomy when the tumor is found to be aggressive in the final pathology analysis. As a result, the patient requires a second surgery. This gives rise to increased costs and resource allocation associated with this additional intervention. Additionally, the need for the patient to take out more time from their usual schedules (e.g., work, etc.) would increase. Accordingly, when a total thyroidectomy is performed for a thyroid malignancy that is not considered to be aggressive, the extent of this surgery may be considered as overtreatment. Moreover, total thyroidectomy is a longer intervention with more associated complications. In addition, patients are dependent on lifelong levothyroxine supplementation.
This study clearly demonstrates the value of molecular testing to help optimize the extent of surgery and avoid any unnecessary total thyroidectomies. Indeed, a patient with a THADA/IGF2BP3 fusion will likely benefit from a limited surgery, such as a hemi-thyroidectomy or lobectomy, as patients in this group either had low-grade malignancies or NIFTP tumors. These findings indicate that THADA/IGF2BP3 is generally a non-aggressive mutation. This knowledge can be implemented in patient care and the way tumors with these mutations are approached. Adversely, patients with a RET/PTC fusion had an 80% likelihood of exhibiting an aggressive disease, and thus, a total thyroidectomy is potentially the preferred surgery for this group of patients. Moreover, given that the likelihood of lymph node metastasis is elevated in this group, consideration for a central compartment neck dissection should be strongly considered.
There were several limitations in this study. The sample size of cases with RET/PTC or THADA/IGF2BP3 fusions is limited, restricting the power of our statistical inferences. The follow-up periods were limited, and the recurrence rates for both fusions are unknown. The study is also limited by the inherit weaknesses of retrospective studies. In addition, the surgeons and pathologists were not blinded to the results of the molecular test. As a result, the surgeon may have been more likely to perform a more comprehensive exploration of the central compartment for abnormal lymph nodes in the RET/PTC group than for patients found to have a THADA/IGF2BP3 fusion. When the mutation is a THADA/IGF2BP3 fusion, the surgeon might be less inclined to perform this lymph node exploration because they are aware that the likelihood of lymph node metastases is significantly less. The pathologist may have been biased towards the results of the molecular testing.
5. Conclusions
In this retrospective chart review analyzing RET/PTC and THADA/IGF2BP3 fusions, all were found to be malignant or NIFTP. The RET/PTC fusion group had more aggressive tendencies (extrathyroidal extension, lymph node metastasis, and diffuse sclerosing variant) and was classified as intermediate risk of recurrence as per the 2015 American Thyroid Association Guidelines for Differentiated Thyroid Cancer. Whereas the THADA/IGF2BP3 fusion nodules had a more indolent behavior, classified as either low-risk malignancy or NIFTP in the final pathology analysis. This understanding of genetic drivers may allow clinicians to develop more targeted treatment plans and supports the notion that molecular testing has significant clinical value in the pre-operative workup of cytologically indeterminate nodules. This study confirms that further research assessing RET/PTC and THADA/IGF2BP3 rearrangements in suspicious thyroid nodules is necessary to improve patient management, particularly for deciding if a targeted therapy plan can be developed. In resource-limited healthcare systems, performing the optimal surgery the first time is beneficial for the patient, the system, and the thyroid specialist. The knowledge of how to handle specific thyroid mutations, such as RET/PTC and THADA/IGF2BP3, can aid surgeons in making the most informed choices and maximize the efficiency of the surgical process. Similarly, understanding how to treat certain mutations can prevent their reoccurrence, which is advantageous for the patient and the physician. This study highlights the value of molecular testing not only as a rule out test to avoid unnecessary surgery but also as a tool to help determine the extent of surgery and need for adjuvant treatment.
Author Contributions
The study design and conception were done by V.-I.F. Surgical logs were provided by V.-I.F., M.P. and M.T. and reviewed by A.E.P., G.T. and T.J.H. were responsible for chart search, retrieval, and anonymization. Data analysis, interpretation and drafting of the original manuscript was done by G.T. and was reviewed and approved by all. T.J.H., A.E.P., S.D.d.S. and V.-I.F. made valuable contributions to the manuscript leading to its completion. Statistical analysis was reviewed by S.D.d.S. All authors have read and agreed to the published version of the manuscript.
Funding
No funding was received from any public, commercial, or not-for-profit entity. This research received no external funding.
Institutional Review Board Statement
The study was granted the final research ethics committee approval under project number 2021-2617, issued on 4 December 2020, and it was conducted in accordance with Centre Integré Universitaire de Santé et de Services Sociaux du Centre-Ouest-de-L’ile-de-Montréal.
Informed Consent Statement
Patient consent was waived as this study is a retrospective chart review, without any contact made to patients, and without any identifiers included in the study or analysis.
Data Availability Statement
The data presented in this study are available in this article.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| BRAF | v-raf murine sarcoma viral oncogene homolog B1 |
| DSV | diffuse sclerosing variant |
| FTC | follicular cell carcinoma |
| HCC | Hürthle cell carcinoma |
| IGF2BP3 | insulin-like growth factor 2 mRNA-binding protein 3 |
| MAPK | mitogen-activated protein kinase |
| MEK | mitogen-activated protein kinase kinase |
| NIFTP | noninvasive follicular thyroid neoplasm with papillary-like nuclear features |
| PI3K | phosphatidylinositol 3-kinase |
| PTC | papillary thyroid cancer |
| RET | rearranged during transfection |
| THADA | thyroid adenoma associated |
| USFNA | ultrasound-guided fine needle aspiration |
References
- Furuya-Kanamori, L.; Bell, K.J.L.; Clark, J.; Glasziou, P.; Doi, S.A.R. Prevalence of Differentiated Thyroid Cancer in Autopsy Studies Over Six Decades: A Meta-Analysis. J. Clin. Oncol. 2016, 34, 3672–3679. [Google Scholar] [CrossRef] [PubMed]
- Nikiforov, Y.E.; Ohori, N.P.; Hodak, S.P.; Carty, S.E.; LeBeau, S.O.; Ferris, R.L.; Yip, L.; Seethala, R.R.; Tublin, M.E.; Stang, M.T.; et al. Impact of mutational testing on the diagnosis and management of patients with cytologically indeterminate thyroid nodules: A prospective analysis of 1056 FNA samples. J. Clin. Endocrinol. Metab. 2011, 96, 3390–3397. [Google Scholar] [CrossRef]
- Danese, D.; Sciacchitano, S.; Farsetti, A.; Andreoli, M.; Pentecorvi, A. Diagnostic Accuracy of Conventional Versus Sonography-Guided Fine-Needle Aspiration Biopsy of Thyroid Nodules. Thyroid 1998, 8, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Mahar, S.A.; Husain, A.; Islam, N. Fine needle aspiration cytology of thyroid nodule: Diagnostic accuracy and pitfalls. J. Ayub Med. Coll. 2006, 18, 26–29. [Google Scholar]
- Cibas, E.S.; Ali, S.Z. The 2017 Bethesda System for Reporting Thyroid Cytopathology. Thyroid 2017, 27, 1341–1346. [Google Scholar] [CrossRef] [PubMed]
- Vargas-Salas, S.; Martínez, J.R.; Urra, S.; Domínguez, J.M.; Mena, N.; Uslar, T.; Lagos, M.; Henríquez, M.; González, H.E. Genetic testing for indeterminate thyroid cytology: Review and meta-analysis. Endocr. Relat. Cancer 2018, 25, R163–R177. [Google Scholar] [CrossRef]
- Haugen, B.R.; Alexander, E.K.; Bible, K.C.; Doherty, G.M.; Mandel, S.J.; Nikiforov, Y.E.; Pacini, F.; Randolph, G.W.; Sawka, A.M.; Schlumberger, M.; et al. 2015 American Thyroid Association Management Guidelines for Adult Patient with Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 2016, 26, 1–133. [Google Scholar] [CrossRef]
- Nylén, C.; Mechera, R.; Maréchal-Ross, I.; Tsang, V.; Chou, A.; Gill, A.J.; Clifton-Bligh, R.J.; Robinson, B.G.; Sywak, M.S.; Sidhu, S.B.; et al. Molecular Markers Guiding Thyroid Cancer Management. Cancers 2020, 12, 2164. [Google Scholar] [CrossRef]
- Durante, C.; Costante, G.; Lucisano, G.; Bruno, R.; Meringolo, D.; Paciaroni, A.; Puxeddu, E.; Torlontano, M.; Tumino, S.; Attard, M.; et al. The natural history of benign thyroid nodules. JAMA 2015, 313, 926–935. [Google Scholar] [CrossRef]
- Al-Kurd, A.; Maree, A.; Mizrahi, I.; Kaganov, K.; Weinberger, J.M.; Mali, B.; Mazeh, H.; Hirshoren, N. An Institutional Analysis of Malignancy Rate in Bethesda III and IV Nodules of the Thyroid. Am. J. Otolaryngol. Head Neck Surg. 2019, 2, 1034. [Google Scholar]
- Ho, A.S.; Sarti, E.E.; Jain, K.S.; Wang, H.; Nixon, I.J.; Shaha, A.R.; Shah, J.P.; Kraus, D.H.; Ghossein, R.; Fish, S.A.; et al. Malignancy Rate in Thyroid Nodules Classified as Bethesda Category III (AUS/FLUS). Thyroid 2014, 24, 832–839. [Google Scholar] [CrossRef]
- Bayrak, B.Y.; Eruyar, A.T. Malignancy rates for Bethesda III and IV thyroid nodules: A retrospective study of the correlation between fine-needle aspiration cytology and histopathology. BMC Endocr. Disord. 2020, 20, 48. [Google Scholar] [CrossRef]
- Stephen, J.K.; Chitale, D.; Narra, V.; Chen, K.M.; Sawhney, R.; Worsham, M.J. DNA methylation in thyroid tumorigenesis. Cancers 2011, 3, 1732–1743. [Google Scholar] [CrossRef]
- Cavaco, D.; Martin, A.F.; Cabrera, R.; Vilar, H.; Leite, V. Diffuse sclerosing variant of papillary thyroid carcinoma: Outcomes of 33 cases. Eur. Thyroid J. 2022, 11, e210020. [Google Scholar] [CrossRef]
- Vuong, H.G.; Kondo, T.; Phan, T.Q.; Oishi, N.; Mochizuki, K.; Nakazawa, T.; Hassell, L.; Katoh, R. Prognostic significance of diffuse sclerosing variant papillary thyroid carcinoma: A systematic review and meta-analysis. Eur. J. Endocrinol. 2017, 176, 433–441. [Google Scholar] [CrossRef]
- Stransky, N.; Cerami, E.; Schalm, S.; Kim, J.L.; Lengauer, C. The landscape of kinase fusions in cancer. Nat. Commun. 2014, 5, 4846. [Google Scholar] [CrossRef]
- Nikiforov, Y.E.; Carty, S.E.; Chiosea, S.I.; Coyne, C.; Duvvuri, U.; Ferris, R.L.; Gooding, W.E.; Hodak, S.P.; LeBeau, S.O.; Ohori, N.P.; et al. Highly accurate diagnosis of cancer in thyroid nodules with follicular neoplasm/suspicious for a follicular neoplasm cytology by ThyroSeq v2 next-generation sequencing assay. Cancer 2014, 120, 3627–3634. [Google Scholar] [CrossRef]
- Agrawal, N.; Akbani, R.; Aksoy, B.A.; Ally, A.; Arachchi, H.; Asa, S.L.; Auman, J.T.; Balasundaram, M.; Balu, S.; Baylin, S.B.; et al. The Cancer Genome Atlas Research Network, Integrated Genomic Characterization of Papillary Thyroid Carcinoma. Cell 2014, 159, 676–690. [Google Scholar] [CrossRef]
- Giordano, T.J.; Haugen, B.R.; Sherman, S.I.; Shah, M.H.; Caoili, E.M.; Koenig, R.J. Pioglitazone Therapy of PAX8-PPARγ Fusion Protein Thyroid Carcinoma. J. Clin. Endocrinol. Metab. 2018, 103, 1277–1281. [Google Scholar] [CrossRef]
- Xing, M. Molecular pathogenesis and mechanisms of thyroid cancer. Nat. Rev. Cancer 2013, 13, 184–199. [Google Scholar] [CrossRef]
- Bible, K.C.; Kebebew, E.; Brierley, J.; Brito, J.P.; Cabanillas, M.E.; Clark, T.J., Jr.; Di Cristofano, A.; Foote, R.; Giordano, T.; Kasperbauer, J.; et al. 2021 American Thyroid Association Guidelines for Management of Patients with Anaplastic Thyroid Cancer. Thyroid 2021, 31, 337–386. [Google Scholar] [CrossRef] [PubMed]
- Ratajczak, M.; Gawel, D.; Godlewska, M. Novel Inhibitor-Based Therapies for Thyroid Cancer—An Update. Int. J. Mol. Sci. 2021, 22, 11829. [Google Scholar] [CrossRef] [PubMed]
- Romei, C.; Ciampi, R.; Elisei, R. A Comprehensive Overview of the Role of the RET Proto-Oncogene in Thyroid Carcinoma. Nat. Rev. Endocrinol. 2016, 12, 192–202. [Google Scholar] [CrossRef] [PubMed]
- Rangel-Pozzo, A.; Sisdelli, L.; Cordioli, M.I.V.; Vaisman, F.; Caria, P.; Mai, S.; Cerutti, J.M. Genetic Landscape of Papillary Thyroid Carcinoma and Nuclear Architecture: An Overview Comparing Pediatric and Adult Populations. Cancers 2020, 12, 3146. [Google Scholar] [CrossRef]
- Ciampi, R.; Nikiforov, Y.E. RET/PTC Rearrangements and BRAF Mutations in Thyroid Tumorigenesis. Endocrinology 2007, 148, 936–941. [Google Scholar] [CrossRef]
- Khan, M.S.; Qadri, Q.; Makhdoomi, M.J.; Wani, M.A.; Malik, A.A.; Nihaz, M.; Masoodi, S.R.; Andrabi, K.I.; Ahmad, R.; Mufassar, S. RET/PTC Gene Rearrangements in Thyroid Carcinogenesis: Assessment and Clinico-Pathological Correlations. Pathol. Oncol. Res. 2020, 26, 507–513. [Google Scholar] [CrossRef]
- Pacini, F.; Elisei, R.; Romei, C.; Pinchera, A. RET proto-oncogene mutations in thyroid carcinomas: Clinical relevance. J. Endocrinol. Investig. 2000, 23, 328–338. [Google Scholar] [CrossRef]
- Zhang, X.; Su, X.; Chen, W.C.; Li, Y.; Yang, Z.Y.; Deng, W.Z.; Deng, T.C.; Yang, A.K. RET/ PTC rearrangement affects multifocal formation of papillary thyroid carcinoma. Chin. J. Otorhinolaryngol. Head Neck Surg. 2016, 52, 435–439. [Google Scholar]
- Guerra, A.; Zeppa, P.; Bifulco, M.; Vitale, M. Concomitant BRAF(V600E) mutation and RET/PTC rearrangement is a frequent occurrence in papillary thyroid carcinoma. Thyroid 2014, 24, 254–259. [Google Scholar] [CrossRef]
- Panebiano, F.; Kelly, L.M.; Liu, P.; Zhong, S.; Dacic, S.; Wang, X.; Singhi, A.D.; Dhir, R.; Chiosea, S.I.; Kuan, S.-F.; et al. Thada Fusion is a Mechanism of IGF2BP3 Activation and IGF1R Signaling in Thyroid Cancer. Proc. Natl. Acad. Sci. USA 2017, 114, 2307–2312. [Google Scholar] [CrossRef]
- Flanigan, S.A.; Pitts, T.M.; Newton, T.P.; Kulikowski, G.N.; Tn, A.C.; McManus, M.C.; Spreafico, A.; Kachaeva, M.I. Overcoming IGF1R/IR resistance through inhibition of MEK signaling in colorectal cancer models. Clin. Cancer Res. 2013, 19, 6219–6229. [Google Scholar] [CrossRef]
- Rippe, V.; Drieschner, N.; Meiboom, M.; Escobar, H.M.; Bonk, U.; Belge, G.; Bullerdiek, J. Identification of a gene rearranged by 2p21 aberrations in thyroid adenomas. Oncogene 2003, 22, 6111–6114. [Google Scholar] [CrossRef]
- Kloth, L.; Belge, G.; Burchardt, K.; Loeschke, S.; Wosniok, W.; Fu, X.; Nimzyk, R.; Mohamed, S.A.; Drieschner, N.; Rippe, V.; et al. Decrease in thyroid adenoma associated (THADA) expression is a marker of dedifferentiation of thyroid tissue. BMC Clin. Pathol. 2011, 11, 13. [Google Scholar] [CrossRef]
- Marotta, V.; Guerra, A.; Sapio, M.R.; Vitale, M. RET/PTC Rearrangement in Benign and Malignant Thyroid Diseases: A clinical standpoint. Eur. J. Endocrinol. 2011, 165, 499–507. [Google Scholar] [CrossRef]
- Morariu, E.M.; McCoy, K.L.; Chiosea, S.I.; Nikitski, A.V.; Manroa, P.; Nikiforova, M.N.; Nikiforov, Y.E. Clinicopathologic Characteristics of Thyroid Nodules Positive for the THADA-IGF2BP3 Fusion on Preoperative Molecular Analysis. Thyroid 2021, 31, 1212–1218. [Google Scholar] [CrossRef]
- Nikiforov, Y.E.; Seethala, R.R.; Tallini, G.; Balock, Z.W.; Basolo, F.; Thompson, L.D.R.; Barletta, J.A.; Wenig, B.M.; Al Ghuzlan, A.; Kakudo, K.; et al. Nomenclature Revision for Encapsulated Follicular Variant of Papillary Thyroid Carcinoma: A Paradigm Shift to Reduce Overtreatment of Indolent Tumors. JAMA Oncol. 2016, 2, 1023–1029. [Google Scholar] [CrossRef]
- Kopczyńska, E.; Kwapisz, J.; Junik, R.; Tyrakowski, T. Cellular tumor markers in thyroid cancer. Pol. Merkur. Lekarski. 2007, 22, 295–299. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).