Simple Summary
This study aims to find out which types of exercise can help improve the quality of life for people who have survived breast cancer. Researchers analyzed data from different studies to see how various exercises, such as aerobic and strength training, aerobic activity, yoga, and strength exercise, affected these individuals after 12 weeks. The results show that combining aerobic and strength training is the most effective way to improve their quality of life without causing more people to drop out of the exercise programs compared to regular care. This research may help doctors and patients make better decisions about exercise plans for breast cancer survivors.
Abstract
This study aimed to assess the effectiveness of various exercise interventions in enhancing the quality of life for breast cancer survivors. To achieve this, randomized controlled trials were identified from major electronic databases, focusing on the relationship between exercise and quality of life in breast cancer survivors. The primary outcome was the impact of exercise on quality of life 12 weeks after the intervention, with a secondary outcome comparing dropout rates between intervention groups and a regular care control group. The study protocol was registered with INPLASY (INPLASY202340007). A network meta-analysis of nine randomized controlled trials involving 725 participants was conducted, examining aerobic and strength training, aerobic activity, yoga, and strength exercise. Results showed that aerobic and strength training was the most effective intervention, significantly improving the quality of life of breast cancer survivors (1.31; 95% confidence interval: 0.49 to 2.12). Aerobic activity had a borderline effect (0.83; 0.03 to 1.63), while no exercise interventions were associated with an increased dropout risk compared to the control group (regular care). The study concluded that concurrent aerobic and strength training can improve breast cancer survivors’ quality of life after 12 weeks of intervention without increasing dropout risk compared to regular care.
1. Introduction
Breast cancer is the most common female malignancy worldwide and has the fifth highest mortality rate of all cancers [1]. Owing to progress in cancer screening and advancements in cancer treatments, the number of breast cancer survivors in the United States exceeds 3.8 million, and it is estimated to rise by more than 30% in the next ten years [2]. Even after completing treatment, long-lasting and severe treatment-related side effects, such as physical problems, psychological distress, and impaired social and work reintegration, can still cause a significant decline in the quality of life for breast cancer survivors. Therefore, evidence-based care to support this population is an important issue for overall social health [3].
Exercise has been demonstrated to provide a multitude of advantages for individuals who have survived breast cancer, including improvements in physical function [4], fatigue [5], depression [6], and overall quality of life [7]. Nonetheless, a vast array of physical activity options exists, including aerobic activity [8], strength training [9], yoga [10], and others [11]. Current meta-analyses provide evidence that physical activity in general positively impacts the quality of life, but they do not offer insights into the specific amount or kind of exercise needed [7]. As a result, we currently do not know what types of exercise are effective prescriptions for breast cancer survivors after completing treatment, nor how long the intervention should last in order to see an effect. Understanding which exercise interventions are most effective for improving quality of life in breast cancer survivors as well as the expected duration of intervention required to observe positive effects is critical for developing effective rehabilitation programs.
Network meta-analysis represents a statistical technique that enables the concurrent evaluation of numerous interventions, facilitating the identification of the most efficacious exercise approaches [12]. The research methodology involves first collecting and categorizing various common interventions or treatments. Then, a network model is constructed, which allows for comparisons between different interventions to rank their effects. When there are studies directly comparing interventions head-to-head, these are referred to as direct comparisons. In cases where direct head-to-head comparisons are lacking between different treatments, indirect comparisons are made through a common comparator. For example, let us consider a mathematics exam scenario where, on average, student A scores 10 points higher than student B, and student B scores 5 points higher than student C. These are direct comparisons. However, through indirect estimation, we can infer that student A would likely score approximately 15 points higher than student C. This is known as an indirect comparison. To ensure the reliability of these indirect comparisons, network meta-analysis examines whether there are statistically significant differences between comparisons that have both direct and indirect evidence in order to establish internal consistency [12,13]. By choosing research conducted within a particular time range, it is possible to predict the exercise interventions that may yield statistically significant outcomes after a specific period of implementation. The objective of this network meta-analysis is to establish a hierarchy of the efficacy of various exercise interventions in enhancing the quality of life for breast cancer survivors and estimating the necessary time frame for observing statistically significant results. Such insights can assist in determining the most suitable exercise approach for breast cancer survivors aiming to enhance their overall well-being.
2. Materials and Methods
We conducted this study in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) extension guidelines for network meta-analysis (PRISMA NMA) [14]. The study was registered in INPLASY with the registration number INPLASY202340007 [15], and the ethical review board approval or participant informed consent was not required.
2.1. Database Searches and Study Identification
Two authors (TCW and ICT) performed separate electronic searches in PubMed, Cochrane Reviews, Cochrane CENTRAL, Web of Science, and ClinicalTrials.gov databases using the following keywords: (‘breast cancer’) AND (‘quality of life’ OR ‘QoL’) AND (‘exercises’ OR ‘physical activity’ OR ‘yoga’ OR ‘aerobic’) AND (‘random’ OR ‘randomized’ OR ‘randomised’) AND (‘12 weeks’ OR ‘3 months’). The search approach for the systematic review and network meta-analysis spanned the duration from the first available entry in each database up to the most recent search date (7 April 2023).
In the initial stage, two authors were tasked with evaluating the titles and abstracts of identified studies for their eligibility using a consensus process. The search was conducted in the aforementioned databases to scrutinize eligible trials. Additionally, the reference lists of various review articles [5,7,16,17,18,19,20,21,22,23,24] were examined and manual searches were performed. In situations where the two initial reviewers were unable to reach a consensus, a third reviewer and study author (PLC) was consulted. No restrictions on language were imposed on this search.
2.2. Inclusion and Exclusion Criteria
The network meta-analysis employed the PICO model (population, intervention, comparison, outcome), featuring the subsequent criteria: (1) P: human participants with breast cancer and completed treatment, including surgery, chemotherapy, and/or radiotherapy; (2) I: exercise interventions; (3) C: control group without intervention; and (4) O: changes in quality of life. The definition of breast cancer survivor was based on the joint guideline provided by the American Cancer Society and the American Society of Clinical Oncology [25].
The study applied the following inclusion criteria: (1) randomized controlled trials that recruited breast cancer survivors who had completed treatments, including surgery, chemotherapy, and/or radiation therapy, (2) randomized controlled trials that investigated the quantitative assessment of quality of life after exercise intervention, (3) the control group that received no intervention or regular care, and (4) trials that had available data on quality of life pre- and post-intervention at 12 weeks.
The selection of the 12-week evaluation duration was based on the initial literature review, which indicated that it was the most commonly used assessment period in the included studies. Several previous large-scale literature analyses have also found that the onset of exercise effects occurred at 12 weeks for patients undergoing rehabilitation [26] after stroke or a transient ischemic attack [27]. In order to compare the effectiveness of various exercise interventions, a standardized time frame is necessary to establish a benchmark for comparison. Therefore, this study focuses specifically on a 12-week duration and excludes other time frames with fewer studies available.
Exclusion criteria for this review and network meta-analysis included: (1) non-randomized controlled trials, (2) studies without comparisons of exercise vs. exercise or exercise vs. regular care comparison, (3) studies lacking quantitative assessments of quality of life, (4) studies quantitatively assessed quality of life but only reported subscale data and did not provide a total score, (5) incomplete or unavailable data, even after attempts to contact the authors via email, and (6) studies enrolling participants overlapped with a published trial already enrolled in our analysis.
2.3. Modeling for Network Meta-Analysis
In the present network meta-analysis, we adhered to the following principles during the construction of the model. To prevent excessive heterogeneity, we restricted the paired comparisons to only exercise vs. exercise or exercise vs. regular care. Comparisons between exercise and cognitive behavioral therapy, eurythmy therapy, and various nutritional supplements were thus excluded. Inclusion of additional treatments might result in disparate network geometries, owing to the variation in the therapies being considered, leading to inconsistent outcomes in the network meta-analysis [28].
When categorizing the exercise type in our study, they were grouped based on the actual exercise prescription content discussion between two authors (TCW, ICT). If there is any disagreement in the categorization, consensus will be reached through discussion with the third author (PLC).
2.4. Methodological Quality Appraisal
To assess the methodological quality of the studies included in our analysis, we utilized the Cochrane risk of bias tool for randomized trials (version 2, RoB 2, London, UK) [29]. This tool appraises six principal components for assessing the quality of a study, including the randomization process, adherence to the intervention, missing outcome data, outcome measurement, selective reporting, and overall risk of bias.
2.5. Primary Outcome: Quality-of-Life Improvement, Standardized Mean Difference
The primary outcomes evaluated in this study were changes in quality of life measured by quantitative scales. If the study utilized a breast-cancer-specific quality-of-life scale, such as the Functional Assessment of Cancer Therapy-Breast [30,31] or the International Breast Cancer Study Group Quality of Life [32], data extraction was prioritized from these scales. If the study did not use a breast-cancer-specific quality-of-life scale, data extraction was prioritized in the following order: cancer-specific quality-of-life assessment tools, such as the European Organization for Research and Treatment of Cancer Quality-of-Life Questionnaire [33], followed by general quality-of-life assessment tools, such as the Functional Assessment of Cancer Therapy-General [34].
2.6. Secondary Outcome: Risk Difference of Dropout Rates
The secondary outcome measure was the risk difference of dropout rates at the 12th week, which provides an intuitive indicator. For example, if an individual chooses a specific exercise regimen to improve their quality of life and experiences a dropout rate of 12%, while the control group, which only receives regular care, has a dropout rate of 7% (which may result in some of them starting an exercise routine on their own), the risk difference in dropout rates would be 5%.
2.7. Data Extraction, Management and Conversion
Two authors (TCW and ICT) performed the data extraction process independently, including demographic information, study design, exercise protocol details, and primary and secondary outcomes from the evaluated studies. In situations where the necessary data were not available in the published articles, we reached out to the corresponding authors to obtain the primary data.
Data extraction, conversion, and result merging were conducted in accordance with the recommendations outlined in the Cochrane Handbook for Systematic Reviews of Interventions and relevant medical literature [12,35,36,37,38].
2.8. Statistical Analyses
Due to the inclusion of various exercise types, a random-effects model was utilized for the network meta-analysis [39]. The analysis was performed using MetaInsight (version 4.0.2, Complex Reviews Support Unit, National Institute for Health Research, London, UK) under a frequentist framework. MetaInsight represents a web-based platform for network meta-analysis that leverages the netmeta package in R software for conducting frequentist statistical calculations [40].
Initially, a forest plot and network plot were generated to display all pairwise comparisons from individual studies. Subsequently, forest plots were created for standardized mean differences in the change of quality of life at 12 weeks and the risk differences of dropout rates for each exercise type compared to the control group to provide an overall summary of the effects [41]. The effect sizes were presented as point estimates with a 95% confidence interval (95% CI) [41]. The exercise types were ranked, and numerical values for both direct and indirect comparisons were presented in tables. Inconsistency tests were conducted to detect any data disparities. Statistical significance was defined as a two-tailed p value of less than 0.05.
2.9. Sensitivity Analyses
Two sensitivity analyses were conducted to strengthen the robustness of the study findings. The first analysis employed a one-study removal method, which was performed to ensure that the effect estimates of individual studies did not excessively influence the overall results. Sequentially removing one study at a time from the analysis of quality-of-life changes at 12 weeks allowed us to determine whether the study conclusions and ranking remained consistent.
The second sensitivity analysis performed in this study involved the pre-post correlation coefficient. When transforming baseline and post-intervention quality-of-life measurements into mean and standard deviation of changes, it is necessary to assume a pre–post correlation coefficient. In this study, a coefficient of 0.8 was utilized, as recommended by the Cochrane handbook [35]. However, different scholars may hold varying opinions on this coefficient with commonly used values being 0.5, 0.7, and 0.8 [42]. To examine whether the selected coefficient would impact the study results, a sensitivity analysis was conducted by calculating the effect sizes of quality-of-life changes at 12 weeks with a coefficient of 0.5 [42]. The direction, size of the effect, statistical significance, and ranking of the results were assessed.
2.10. Publication Bias
Potential publication bias was assessed in accordance with the Cochrane Handbook for Systematic Reviews of Interventions [12]. The funnel plot was generated using Comprehensive Meta-Analysis software, version 4 (Biostat, Englewood, NJ, USA), based on the comparison with the control group. Additionally, an Egger’s regression test was conducted to quantify the presence of significant publication bias.
3. Results
3.1. Study Identification and Network Model Formation
The PRISMA flowchart detailing the literature search is presented in Figure 1. The PRISMA NMA extension’s checklist is provided in Table S1. The number of articles retrieved from various databases is presented in Table S2. After removing duplicate articles and excluding non-relevant articles by screening titles and abstracts, we ultimately included nine randomized controlled trials [6,8,9,10,11,43,44,45,46]. The articles excluded in the final stage [4,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97] along with their respective reasons for exclusion are listed in Table S3.
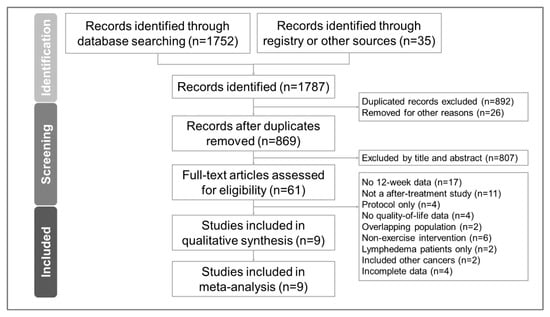
Figure 1.
Flow diagram for the study selection process based on the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.
Our analysis included a total of nine randomized controlled trials, involving 725 individuals. Based on the included studies, the exercise types were categorized as follows: aerobic and strength training (concurrent), aerobic activity, yoga, and strength exercise. The network model for the exercise interventions is displayed in Figure 2.
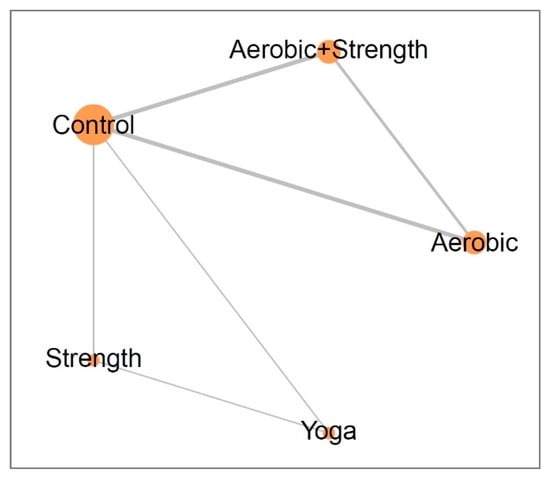
Figure 2.
Network plots illustrate the effects of different exercise interventions on the improvement of quality of life in breast cancer survivors after 12 weeks. The size of each node and thickness of each line represents the number of trials included in the analysis.
Among the nine studies included in our analysis, three studies exclusively recruited postmenopausal women [6,10,44], and two studies only enrolled patients with fatigue [11,45]. For further details on the inclusion criteria, the country where the study was conducted, the mean age and standard deviation of the participants, exercise intervention details, quality-of-life assessment scales, and dropout rates, please refer to Table 1.

Table 1.
Summary of the included trials investigating the effect of exercise to improve quality of life in breast cancer survivors.
3.2. Methodological Quality of the Included Studies
Regarding the overall methodological quality of the studies, we observed that 44.4% (4/9) of the studies had a low risk of bias, while 55.6% (5/9) had some risk of bias (refer to Figure S1). The studies with some risk of bias had differences in their protocols between study arms, which could potentially impact the adherence and outcomes of the interventions. The details of the risk of bias assessment are provided in Table S4.
3.3. Primary Outcome: Aerobic and Strength Concurrent Training Most Effective
After a 12-week intervention, aerobic and strength training showed a significant improvement in quality of life (effect size: 1.31; 95% CI: 0.49 to 2.12), while aerobic activity demonstrated a borderline effect (effect size: 0.83; 95% CI: 0.03 to 1.63). On the other hand, yoga (effect size: 0.63; 95% CI: −0.67 to 1.92) and strength training (effect size: 0.19; 95% CI: −1.08 to 1.46) did not show a significant difference compared to the control group (Figure 3). Please refer to Figure S2 for the detailed pair-wise comparisons between study arms as reported in individual studies.
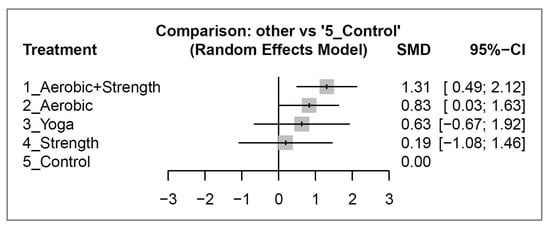
Figure 3.
Forest plots illustrating the standardized mean difference (SMD) in quality-of-life improvement between different exercise interventions and control groups among breast cancer survivors after 12 weeks of intervention.
The exercise interventions were ranked based on their effect sizes on quality of life, with aerobic and strength training (concurrent) being the most effective, followed by aerobic activity, yoga, and strength exercise in that order. Please see Table 2 for a detailed comparison and ranking of the exercise types.

Table 2.
Pairwise comparison and ranking of different exercise interventions for improving quality of life at 12 weeks in breast cancer survivors.
3.4. Secondary Outcome: Dropout Rates Statistically Similar
After 12 weeks of intervention, there was no significant difference in dropout rates between the various exercise types and the control group with all risk differences with their 95% CIs overlapped with 0 (see Figure 4). For a detailed analysis of the pair-wise comparisons between study arms as reported in individual studies, please consult Figure S3.
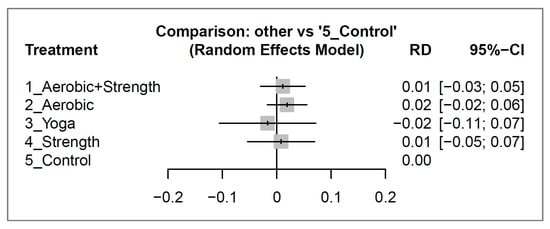
Figure 4.
Forest plots depicting the risk difference (RD) of dropout rates between different exercise interventions and control groups for breast cancer survivors after 12 weeks of intervention.
3.5. Inconsistency Test
The network was constructed by creating nodes and performing direct and indirect comparisons to determine consistency. The results of the quality-of-life inconsistency tests are presented in Table S5, while the dropout rate results are presented in Table S6. All available comparisons had p values greater than 0.05, indicating no evidence of inconsistency between direct and indirect comparisons.
3.6. Sensitivity Analyses
The results of the one-study removal analysis showed consistent rankings and clinical significance for all exercise types. The aerobic and strength-training intervention consistently demonstrated a significant improvement in the quality of life of breast cancer survivors, while the aerobic activity intervention remained at borderline significance. Yoga and strength exercise interventions consistently showed no significant effect on quality of life (See Figure S4a–i).
In the second sensitivity analysis, we adjusted the pre–post correlation coefficient from 0.8 to 0.5 and conducted a new network comparison (Figure S5). Our results showed that the direction of effect sizes, ranking, and interpretation of the results remained consistent with those obtained using a coefficient of 0.8 (Figure 3).
The above analyses indicate that the results of our study are consistent and not influenced by the inclusion or removal of individual studies as well as the adjustment of assumed values in the calculation process.
3.7. Publication Bias
Please see Figure S6 for the funnel plot. The Egger’s test yielded a p value of 0.25, indicating no significant publication bias.
4. Discussion
4.1. Main Findings and Clinical Implications
Our network meta-analysis revealed that among breast cancer survivors, aerobic and strength training was the most effective type of 12-week exercise intervention in improving quality of life (effect size: 1.31; 95% CI: 0.49 to 2.12). Aerobic activity had a borderline effect (effect size: 0.83; 95% CI: 0.03 to 1.63), while yoga and strength exercise showed no significant difference compared to the control group. In terms of dropout rates, there was no significant risk difference between the different types of exercise and the control group. For breast cancer survivors and caregivers, our network meta-analysis provides valuable information for exercise prescription. The data can be used to support the benefits of exercise and encourage patients to adhere to the exercise program for at least three months to achieve a significant improvement in quality of life.
4.2. Significance of the Findings Compared to Existing Literature
Aune et al. published a comprehensive pairwise meta-analysis in JNCI Cancer Spectrum in 2022 [7], which collected 79 randomized controlled trials and 14,554 breast cancer patients before 2019, including various exercise protocols and intervention durations. The study concluded that physical activity, compared to regular care, can effectively improve global health-related quality of life. However, the authors also stated that based on their analysis, the evidence regarding the dose and type of physical activity is still insufficient to draw conclusions.
Our study utilized network meta-analysis to compare various exercise interventions and concluded that within a 12-week timeframe, (concurrent) aerobic and strength training is the most effective type of exercise for improving quality of life in breast cancer survivors, followed by aerobic activity with a borderline effect. This study is the first in the literature to provide answers to questions regarding the effectiveness of different types of exercise, their comparison, and the ranking of exercise benefits.
Previously, studies often mentioned that yoga is beneficial for breast cancer survivors [98,99]. However, some of these studies relied on self-reported surveys and lacked prospective designs with specific intervention durations. They included patients with different frequencies and durations of yoga interventions [98]. Some systematic reviews also incorporated breast cancer patients during and after treatment without specifying the exact duration of yoga intervention [99]. In our study, we directly used a 12-week timeframe as the research benchmark and compared and ranked the effects of yoga on quality of life among various exercises. In other words, we are not answering whether yoga is effective for breast cancer survivors, but rather, within the 12-week timeframe, we assessed the varying impact on quality of life from different exercises performed by breast cancer survivors with yoga being part of the ranking results.
4.3. Possible Explanations for the Observed Results
Regarding the ranking of the effectiveness of different types of exercise in improving quality of life, we hypothesize that the intensity of the exercise may play a role. Ostman et al. found that the improvement in quality of life is more pronounced with increasing exercise intensity in patients with heart failure [100]. In the exercise protocols designed for breast cancer survivors in our included studies [6,8,11,43,44,46], aerobic exercise is easier to perform, can be sustained for longer durations, and is more likely to achieve moderate or even vigorous intensity. This may suggest that exercise interventions incorporating aerobic activity, such as concurrent aerobic and strength training and aerobic activity only, tend to result in better outcomes. In Dysart et al.’s study, yoga has been found to achieve moderate intensity only 32.75% of the time on average, and most of the time, it only achieves low intensity [101]. As for the strength-exercise-only protocols, our included studies consisted of a home-based exercise program without additional weight bearing [45] and a program based on 40% of one repetition maximum (1RM), gradually increasing to 70% 1RM based on the participant’s capacity with the help of a professional trainer [9]. Day et al.’s previous research on the correspondence between resistance training and exercise intensity suggests that 40%, 70%, and 90% 1RM correspond to low, moderate, and vigorous intensity, respectively [102]. Thus, a 40–70% 1RM training protocol [9] corresponds only to low-to-moderate intensity. Moreover, even at 70% 1RM, the actual exercise time of 12 lifts is shorter than that of aerobic exercise.
The lack of significant differences in dropout rates between the exercise interventions and regular care may be attributed to the design of the exercise protocols, which were easily followed. For instance, the yoga classes were led by professional instructors and provided a social component, lasting for 60–90 min [10,45]. The strength-training intervention was facilitated by professional trainers and included progressive overload, leading to a sense of accomplishment after each session [9]. Even the self-administered aerobic activities were completed within an hour, preventing excessive difficulty [44].
4.4. Limitations
Our study has limitations. Among the included studies, three studies enrolled only postmenopausal women and two studies enrolled breast cancer survivors with fatigue, which may violate the transitivity assumption due to the heterogeneous study population. However, based on the age distribution of the included participants, it was noted that the age range of participants in studies without specific menopause inclusion criteria was mostly in the postmenopausal phase (Table 1). Additionally, a previous study conducted by Álvarez-Bustos et al. investigated the prevalence of fatigue in breast cancer survivors and reported that only 9% of participants reported no fatigue at all [103]. These findings suggest that the actual participants included in these nine studies were not significantly different from each other, which supports the assumption of transitivity in network meta-analysis. As a confirmation, our study passed the inconsistency test and the sensitivity analysis of one-study removal, indicating that no specific study or study group caused inconsistency or instability in the results.
Furthermore, our study only investigated the effect of 12 weeks of exercise on quality of life, and it is unknown whether exercise types that did not show significant effects at 12 weeks might lead to improvements with longer duration of exercise (e.g., 24 or 48 weeks). Future network meta-analyses with longer follow-up periods are needed to investigate this question. However, we found that studies with longer intervention periods, such as 24-week ones [55,56,57,58,59,60,61], were less abundant in our literature review, and their results may not be directly comparable or applicable to our 12-week study.
5. Conclusions
In summary, for breast cancer survivors, aerobic and strength concurrent training for 12 weeks is the exercise of choice to improve quality of life, with dropout rates comparable to the control group.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/cancers15133380/s1, Table S1: PRISMA for network meta-analysis checklist; Table S2: Keywords and search results in different databases; Table S3: Excluded studies and reasons; Table S4: Detailed quality assessment of included studies; Table S5: Inconsistency test results of quality of life improvement for breast cancer survivors; Table S6: Inconsistency test results for risk difference of dropout rates; Figure S1: Summary of quality assessment for the studies included; Figure S2: The forest plot of pair-wise comparisons for quality of life improvement; Figure S3: The forest plot of pair-wise comparisons for the risk difference of dropout rates; Figure S4: Sensitivity analysis with the one-study removal method; Figure S5: Sensitivity analysis with pre-post correlation coefficient changed from 0.8 to 0.5; Figure S6: Funnel plot of all paired comparisons involving the common comparator, control group.
Author Contributions
Data curation: T.-C.W. and I.-C.T.; formal analysis: T.-C.W. and I.-C.T.; investigation: T.-C.W., P.-L.C., W.-C.L. and I.-C.T.; methodology: P.-L.C. and I.-C.T.; software: T.-C.W. and I.-C.T.; supervision: W.-C.L. and I.-C.T.; validation: W.-C.L. and I.-C.T.; writing the original draft: T.-C.W., P.-L.C., W.-C.L. and I.-C.T.; writing review and editing: T.-C.W., P.-L.C., W.-C.L. and I.-C.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Institutional Review Board Statement
This network meta-analysis did not intervene or interact with humans or collect identifiable private information and thus, does not require institutional review board approval.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article and Supplementary Files.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kern, R.; Correa, S.C.; Scandolara, T.B.; Carla da Silva, J.; Pires, B.R.; Panis, C. Current advances in the diagnosis and personalized treatment of breast cancer: Lessons from tumor biology. Per. Med. 2020, 17, 399–420. [Google Scholar] [CrossRef]
- Cathcart-Rake, E.J.; Ruddy, K.J. Evidence-based guidance for breast cancer survivorship. Hematol. Oncol. Clin. N. Am. 2023, 37, 225–243. [Google Scholar] [CrossRef]
- Soldato, D.; Arecco, L.; Agostinetto, E.; Franzoi, M.A.; Mariamidze, E.; Begijanashvili, S.; Brunetti, N.; Spinaci, S.; Solinas, C.; Vaz-Luis, I.; et al. The future of breast cancer research in the survivorship field. Oncol. Ther. 2023, 11, 199–229. [Google Scholar] [CrossRef]
- Wang, L.F.; Eaglehouse, Y.L.; Poppenberg, J.T.; Brufsky, J.W.; Geramita, E.M.; Zhai, S.; Davis, K.K.; Gibbs, B.B.; Metz, J.; van Londen, G.J. Effects of a personal trainer-led exercise intervention on physical activity, physical function, and quality of life of breast cancer survivors. Breast Cancer 2021, 28, 737–745. [Google Scholar] [CrossRef]
- Muñoz-Gómez, E.; Arnal-Gómez, A.; López Cascón, A.; Espí-López, G.V. Systematic review of aquatic therapeutic exercise efficacy in breast cancer survivors. Support. Care Cancer 2022, 31, 44. [Google Scholar] [CrossRef] [PubMed]
- Ergun, M.; Eyigor, S.; Karaca, B.; Kisim, A.; Uslu, R. Effects of exercise on angiogenesis and apoptosis-related molecules, quality of life, fatigue and depression in breast cancer patients. Eur. J. Cancer Care 2013, 22, 626–637. [Google Scholar] [CrossRef] [PubMed]
- Aune, D.; Markozannes, G.; Abar, L.; Balducci, K.; Cariolou, M.; Nanu, N.; Vieira, R.; Anifowoshe, Y.O.; Greenwood, D.C.; Clinton, S.K.; et al. Physical activity and health-related quality of life in women with breast cancer: A meta-analysis. JNCI Cancer Spectr. 2022, 6, pkac072. [Google Scholar] [CrossRef]
- Rogers, L.Q.; Courneya, K.S.; Anton, P.M.; Hopkins-Price, P.; Verhulst, S.; Vicari, S.K.; Robbs, R.S.; Mocharnuk, R.; McAuley, E. Effects of the BEAT Cancer physical activity behavior change intervention on physical activity, aerobic fitness, and quality of life in breast cancer survivors: A multicenter randomized controlled trial. Breast Cancer Res. Treat. 2015, 149, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Soriano-Maldonado, A.; Díez-Fernández, D.M.; Esteban-Simón, A.; Rodríguez-Pérez, M.A.; Artés-Rodríguez, E.; Casimiro-Artés, M.A.; Moreno-Martos, H.; Toro-de-Federico, A.; Hachem-Salas, N.; Bartholdy, C.; et al. Effects of a 12-week supervised resistance training program, combined with home-based physical activity, on physical fitness and quality of life in female breast cancer survivors: The EFICAN randomized controlled trial. J. Cancer Surviv. 2022; online ahead of print. [Google Scholar] [CrossRef]
- Cramer, H.; Rabsilber, S.; Lauche, R.; Kümmel, S.; Dobos, G. Yoga and meditation for menopausal symptoms in breast cancer survivors-A randomized controlled trial. Cancer 2015, 121, 2175–2184. [Google Scholar] [CrossRef]
- Kim, S.; Ko, Y.H.; Song, Y.; Kang, M.J.; Lee, H.; Kim, S.H.; Jeon, J.Y.; Cho, Y.U.; Yi, G.; Han, J. Pre-post analysis of a social capital-based exercise adherence intervention for breast cancer survivors with moderate fatigue: A randomized controlled trial. Support Care Cancer 2020, 28, 5281–5289. [Google Scholar] [CrossRef]
- Chaimani, A.; Caldwell, D.M.; Li, A.; Higgins, J.P.T.; Salanti, G. Chapter 11: Undertaking Network Meta-Analyses. Cochrane Handbook for Systematic Reviews of Interventions version 6.3 (updated August 2022). Available online: https://training.cochrane.org/handbook/current/chapter-11 (accessed on 25 February 2023).
- Su, X.; McDonough, D.J.; Chu, H.; Quan, M.; Gao, Z. Application of network meta-analysis in the field of physical activity and health promotion. J. Sport. Health Sci. 2020, 9, 511–520. [Google Scholar] [CrossRef]
- Hutton, B.; Salanti, G.; Caldwell, D.M.; Chaimani, A.; Schmid, C.H.; Cameron, C.; Ioannidis, J.P.A.; Straus, S.; Thorlund, K.; Jansen, J.P.; et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: Checklist and explanations. Ann. Intern. Med. 2015, 162, 777–784. [Google Scholar] [CrossRef]
- Tsai, I.C. INPLASY202340007 Comparative Effectiveness of Different Exercises for Improving Quality of Life in Breast Cancer Survivors: A Network Meta-Analysis of Randomized Controlled Trials. Available online: http://doi.org/10.37766/inplasy2023.4.0007 (accessed on 5 April 2023).
- Mikkelsen, M.K.; Juhl, C.B.; Lund, C.M.; Jarden, M.; Vinther, A.; Nielsen, D.L. The effect of exercise-based interventions on health-related quality of life and physical function in older patients with cancer receiving medical antineoplastic treatments: A systematic review. Eur. Rev. Aging Phys. Act. 2020, 17, 18. [Google Scholar] [CrossRef] [PubMed]
- Bula, A.; Tatar, K.; Wysocka, R.; Chyrek, K.; Piejko, L.; Nawrat-Szołtysik, A.; Polak, A. Effect of physical activity on static and dynamic postural balance in women treated for breast cancer: A systematic review. Int. J. Environ. Res. Public. Health 2023, 20, 3722. [Google Scholar] [CrossRef]
- Luo, X.C.; Liu, J.; Fu, J.; Yin, H.Y.; Shen, L.; Liu, M.L.; Lan, L.; Ying, J.; Qiao, X.L.; Tang, C.Z.; et al. Effect of Tai Chi Chuan in breast cancer patients: A systematic review and meta-analysis. Front. Oncol. 2020, 10, 607. [Google Scholar] [CrossRef]
- O’Neill, M.; Samaroo, D.; Lopez, C.; Tomlinson, G.; Santa Mina, D.; Sabiston, C.; Culos-Reed, N.; Alibhai, S.M.H. The effect of yoga interventions on cancer-related fatigue and quality of life for women with breast cancer: A systematic review and meta-analysis of randomized controlled trials. Integr. Cancer Ther. 2020, 19, 1534735420959882. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Bilbao, T.; Alonso-Dueñas, M.; Peinado, A.B.; San Juan, A.F. Effects of combined interventions of exercise and diet or exercise and supplementation on breast cancer patients: A systematic review. Nutrients 2023, 15, 1013. [Google Scholar] [CrossRef]
- Toohey, K.; Chapman, M.; Rushby, A.M.; Urban, K.; Ingham, G.; Singh, B. The effects of physical exercise in the palliative care phase for people with advanced cancer: A systematic review with meta-analysis. J. Cancer Surviv. 2022, 17, 399–415. [Google Scholar] [CrossRef]
- Valenzuela, P.L.; Saco-Ledo, G.; Santos-Lozano, A.; Morales, J.S.; Castillo-García, A.; Simpson, R.J.; Lucia, A.; Fiuza-Luces, C. Exercise training and natural killer cells in cancer survivors: Current evidence and research gaps based on a systematic review and meta-analysis. Sports Med. Open. 2022, 8, 36. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, K.; Matsuoka, Y.J.; Ochi, E. High-intensity interval training in breast cancer survivors: A systematic review. BMC Cancer 2021, 21, 184. [Google Scholar] [CrossRef] [PubMed]
- Huizinga, F.; Westerink, N.L.; Berendsen, A.J.; Walenkamp, A.M.E.; De Greef, M.H.G.; Oude Nijeweeme, J.K.; De Bock, G.H.; Berger, M.Y.; Brandenbarg, D. Home-based physical activity to alleviate fatigue in cancer survivors: A systematic review and meta-analysis. Med. Sci. Sport. Exerc. 2021, 53, 2661–2674. [Google Scholar] [CrossRef]
- Runowicz, C.D.; Leach, C.R.; Henry, N.L.; Henry, K.S.; Mackey, H.T.; Cowens-Alvarado, R.L.; Cannady, R.S.; Pratt-Chapman, M.L.; Edge, S.B.; Jacobs, L.A.; et al. American Cancer Society/American Society of Clinical Oncology Breast Cancer Survivorship Care Guideline. CA Cancer J. Clin. 2016, 66, 43–73. [Google Scholar] [CrossRef] [PubMed]
- Gillison, F.B.; Skevington, S.M.; Sato, A.; Standage, M.; Evangelidou, S. The effects of exercise interventions on quality of life in clinical and healthy populations: A meta-analysis. Soc. Sci. Med. 2009, 68, 1700–1710. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Tabassum, D.; Baig, S.S.; Moyle, B.; Redgrave, J.; Nichols, S.; McGregor, G.; Evans, K.; Totton, N.; Cooper, C.; et al. Effect of exercise interventions on health-related quality of life after stroke and transient ischemic attack. Stroke 2021, 52, 2445–2455. [Google Scholar] [CrossRef]
- Zhang, J.; Yuan, Y.; Chu, H. The impact of excluding trials from network meta-analyses—An empirical study. PLoS ONE 2016, 11, e0165889. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Savovic, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef] [PubMed]
- Brady, M.J.; Cella, D.F.; Mo, F.; Bonomi, A.E.; Tulsky, D.S.; Lloyd, S.R.; Deasy, S.; Cobleigh, M.; Shiomoto, G. Reliability and validity of the Functional Assessment of Cancer Therapy-Breast quality-of-life instrument. J. Clin. Oncol. 1997, 15, 974–986. [Google Scholar] [CrossRef]
- Coster, S.; Poole, K.; Fallowfield, L.J. The validation of a quality of life scale to assess the impact of arm morbidity in breast cancer patients post-operatively. Breast Cancer Res. Treat. 2001, 68, 273–282. [Google Scholar] [CrossRef]
- Michels, F.A.S.; Latorre, M.R.D.O.; Maciel, M.S. Validation, reliability and comprehension of the IBCSG quality of life questionnaire specific to breast cancer. Appl. Cancer Res. 2010, 30, 348–352. [Google Scholar]
- Groenvold, M.; Klee, M.C.; Sprangers, M.A.; Aaronson, N.K. Validation of the EORTC QLQ-C30 quality of life questionnaire through combined qualitative and quantitative assessment of patient-observer agreement. J. Clin. Epidemiol. 1997, 50, 441–450. [Google Scholar] [CrossRef] [PubMed]
- Cella, D.F.; Tulsky, D.S.; Gray, G.; Sarafian, B.; Linn, E.; Bonomi, A.; Silberman, M.; Yellen, S.B.; Winicour, P.; Brannon, J. The Functional Assessment of Cancer Therapy scale: Development and validation of the general measure. J. Clin. Oncol. 1993, 11, 570–579. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Li, T.; Deeks, J.J. Chapter 6: Choosing Effect Measures and Computing Estimates of Effect. Cochrane Handbook for Systematic Reviews of Interventions. Version 6.3 (updated August 2022). Available online: https://training.cochrane.org/handbook/current/chapter-06 (accessed on 25 February 2023).
- Deeks, J.J.; Higgins, J.P.T.; Altman, D.G. Chapter 10: Analysing Data and Undertaking Meta-Analyses. Cochrane Handbook for Systematic Reviews of Interventions. Version 6.3 (updated August 2022). Available online: https://training.cochrane.org/handbook/current/chapter-10 (accessed on 25 February 2023).
- Page, M.J.; Higgins, J.P.T.; Sterne, J.A.C. Chapter 13: Assessing Risk of Bias Due to Missing Results in a Synthesis. Cochrane Handbook for Systematic Reviews of Interventions. Version 6.3 (updated August 2022). Available online: https://training.cochrane.org/handbook/current/chapter-13 (accessed on 25 February 2023).
- Higgins, J.P.T.; Eldridge, S.; Li, T. Chapter 23: Including Variants on Randomized Trials. Cochrane Handbook for Systematic Reviews of Interventions. Version 6.3 (updated August 2022). Available online: https://training.cochrane.org/handbook/current/chapter-23 (accessed on 25 February 2023).
- Borenstein, M.; Hedges, L.V.; Rothstein, H.R. Fixed-effect versus random-effects models. In Introduction to Meta-Analysis; Borenstein, M., Ed.; Wiley: Hoboken, NJ, USA, 2009; pp. 77–86. [Google Scholar]
- Owen, R.K.; Bradbury, N.; Xin, Y.; Cooper, N.; Sutton, A. MetaInsight: An interactive web-based tool for analyzing, interrogating, and visualizing network meta-analyses using R-shiny and netmeta. Res. Synth. Methods 2019, 10, 569–581. [Google Scholar] [CrossRef] [PubMed]
- Effect Size. Wikipedia. Available online: https://en.wikipedia.org/wiki/Effect_size (accessed on 17 May 2023).
- Pearson, M.J.; Smart, N.A. Reported methods for handling missing change standard deviations in meta-analyses of exercise therapy interventions in patients with heart failure: A systematic review. PLoS ONE 2018, 13, e0205952. [Google Scholar] [CrossRef] [PubMed]
- Milne, H.M.; Wallman, K.E.; Gordon, S.; Courneya, K.S. Effects of a combined aerobic and resistance exercise program in breast cancer survivors: A randomized controlled trial. Breast Cancer Res. Treat. 2008, 108, 279–288. [Google Scholar] [CrossRef] [PubMed]
- Baruth, M.; Wilcox, S.; Der Ananian, C.; Heiney, S. Effects of home-based walking on quality of life and fatigue outcomes in early stage breast cancer survivors: A 12-week pilot study. J. Phys. Act. Health 2015, 12, S110–S118. [Google Scholar] [CrossRef] [PubMed]
- Stan, D.L.; Croghan, K.A.; Croghan, I.T.; Jenkins, S.M.; Sutherland, S.J.; Cheville, A.L.; Pruthi, S. Randomized pilot trial of yoga versus strengthening exercises in breast cancer survivors with cancer-related fatigue. Support. Care Cancer 2016, 24, 4005–4015. [Google Scholar] [CrossRef]
- Lin, Y.; Wu, C.; He, C.; Yan, J.; Chen, Y.; Gao, L.; Liu, R.; Cao, B. Effectiveness of three exercise programs and intensive follow-up in improving quality of life, pain, and lymphedema among breast cancer survivors: A randomized, controlled 6-month trial. Support. Care Cancer 2023, 31, 9. [Google Scholar] [CrossRef] [PubMed]
- Heiman, J.; Onerup, A.; Bock, D.; Haglind, E.; Olofsson Bagge, R. The effect of nonsupervised physical activity before and after breast cancer surgery on quality of life: Results from a randomized controlled trial (PhysSURG-B). Scand. J. Surg. 2022, 111, 75–82. [Google Scholar] [CrossRef]
- Mostafaei, F.; Azizi, M.; Jalali, A.; Salari, N.; Abbasi, P. Effect of exercise on depression and fatigue in breast cancer women undergoing chemotherapy: A randomized controlled trial. Heliyon 2021, 7, e07657. [Google Scholar] [CrossRef]
- Naczk, A.; Huzarski, T.; Doś, J.; Górska-Doś, M.; Gramza, P.; Gajewska, E.; Naczk, M. Impact of inertial training on muscle strength and quality of life in breast cancer survivors. Int. J. Environ. Res. Public. Health 2022, 19, 3278. [Google Scholar] [CrossRef]
- Schröder, M.L.; Stöckigt, B.; Binting, S.; Tissen-Diabaté, T.; Bangemann, N.; Goerling, U.; Kröz, M.; Blohmer, J.U.; Ortiz, M.; Brinkhaus, B. Feasibility and possible effects of mindful walking and moderate walking in breast cancer survivors: A randomized controlled pilot study with a nested qualitative study part. Integr. Cancer Ther. 2022, 21, 15347354211066067. [Google Scholar] [CrossRef]
- Boing, L.; Fretta, T.B.; Lynch, B.M.; Dias, M.; Rosa, L.M.D.; Baptista, F.; Bergmann, A.; Fausto, D.Y.; Bocchi Martins, J.B.; Guimarães, A.C.A. Mat Pilates and belly dance: Effects on patient-reported outcomes among breast cancer survivors receiving hormone therapy and adherence to exercise. Complement Ther. Clin. Pract. 2023, 50, 101683. [Google Scholar] [CrossRef]
- Dieli-Conwright, C.M.; Courneya, K.S.; Demark-Wahnefried, W.; Sami, N.; Lee, K.; Sweeney, F.C.; Stewart, C.; Buchanan, T.A.; Spicer, D.; Tripathy, D.; et al. Aerobic and resistance exercise improves physical fitness, bone health, and quality of life in overweight and obese breast cancer survivors: A randomized controlled trial. Breast Cancer Res. 2018, 20, 124. [Google Scholar] [CrossRef]
- Inbaraj, G.; Sathyaprabha, T.N.; Udupa, K.; Ram, A.; Patil, S.; Rajeswaran, J.; Nandakumar, K.K.; Belur, S.; Singh, A.D.; Prathyusha, P.V.; et al. Impact of integrated yoga therapy on cognitive impairment and cardiac dysfunction in relation to quality of life in breast cancer patients undergoing chemotherapy: Study protocol for a two-arm randomized controlled trial. Front. Oncol. 2022, 12, 955184. [Google Scholar] [CrossRef]
- Ammitzbøll, G.; Kristina Kjær, T.; Johansen, C.; Lanng, C.; Wreford Andersen, E.; Kroman, N.; Zerahn, B.; Hyldegaard, O.; Envold Bidstrup, P.; Oksbjerg Dalton, S. Effect of progressive resistance training on health-related quality of life in the first year after breast cancer surgery—Results from a randomized controlled trial. Acta Oncol. 2019, 58, 665–672. [Google Scholar] [CrossRef] [PubMed]
- Gal, R.; Monninkhof, E.M.; van Gils, C.H.; Groenwold, R.H.H.; Elias, S.G.; van den Bongard, D.; Peeters, P.H.M.; Verkooijen, H.M.; May, A.M. Effects of exercise in breast cancer patients: Implications of the trials within cohorts (TwiCs) design in the UMBRELLA Fit trial. Breast Cancer Res. Treat. 2021, 190, 89–101. [Google Scholar] [CrossRef]
- Koevoets, E.W.; Schagen, S.B.; de Ruiter, M.B.; Geerlings, M.I.; Witlox, L.; van der Wall, E.; Stuiver, M.M.; Sonke, G.S.; Velthuis, M.J.; Jobsen, J.J.; et al. Effect of physical exercise on cognitive function after chemotherapy in patients with breast cancer: A randomized controlled trial (PAM study). Breast Cancer Res. 2022, 24, 36. [Google Scholar] [CrossRef] [PubMed]
- van de Wiel, H.J.; Stuiver, M.M.; May, A.M.; van Grinsven, S.; Aaronson, N.K.; Oldenburg, H.S.A.; van der Poel, H.G.; Koole, S.N.; Retèl, V.P.; van Harten, W.H.; et al. Effects of and lessons learned from an internet-based physical activity support program (with and without physiotherapist telephone counselling) on physical activity levels of breast and prostate cancer survivors: The PABLO randomized controlled trial. Cancers 2021, 13, 3665. [Google Scholar] [CrossRef] [PubMed]
- Bruce, J.; Mazuquin, B.; Canaway, A.; Hossain, A.; Williamson, E.; Mistry, P.; Lall, R.; Petrou, S.; Lamb, S.E.; Rees, S.; et al. Exercise versus usual care after non-reconstructive breast cancer surgery (UK PROSPER): Multicentre randomised controlled trial and economic evaluation. BMJ 2021, 375, e066542. [Google Scholar] [CrossRef] [PubMed]
- Odynets, T.; Briskin, Y.; Todorova, V. Effects of different exercise interventions on quality of life in breast cancer patients: A randomized controlled trial. Integr. Cancer Ther. 2019, 18, 1534735419880598. [Google Scholar] [CrossRef]
- Reeves, M.M.; Terranova, C.O.; Winkler, E.A.H.; McCarthy, N.; Hickman, I.J.; Ware, R.S.; Lawler, S.P.; Eakin, E.G.; Demark-Wahnefried, W. Effect of a remotely delivered weight loss intervention in early-stage breast cancer: Randomized controlled trial. Nutrients 2021, 13, 4091. [Google Scholar] [CrossRef] [PubMed]
- Vincent, F.; Deluche, E.; Bonis, J.; Leobon, S.; Antonini, M.T.; Laval, C.; Favard, F.; Dobbels, E.; Lavau-Denes, S.; Labrunie, A.; et al. Home-based physical activity in patients with breast cancer: During and/or after chemotherapy? Impact on cardiorespiratory fitness. A 3-arm randomized controlled trial (APAC). Integr. Cancer Ther. 2020, 19, 1534735420969818. [Google Scholar] [CrossRef] [PubMed]
- Bruce, J.; Mazuquin, B.; Mistry, P.; Rees, S.; Canaway, A.; Hossain, A.; Williamson, E.; Padfield, E.J.; Lall, R.; Richmond, H.; et al. Exercise to prevent shoulder problems after breast cancer surgery: The PROSPER RCT. Health Technol. Assess. 2022, 26, 1–124. [Google Scholar] [CrossRef]
- Knoerl, R.; Giobbie-Hurder, A.; Sannes, T.S.; Chagpar, A.B.; Dillon, D.; Dominici, L.S.; Frank, E.S.; Golshan, M.; McTiernan, A.; Rhei, E.; et al. Exploring the impact of exercise and mind-body prehabilitation interventions on physical and psychological outcomes in women undergoing breast cancer surgery. Support. Care Cancer 2022, 30, 2027–2036. [Google Scholar] [CrossRef]
- An, K.Y.; Morielli, A.R.; Kang, D.W.; Friedenreich, C.M.; McKenzie, D.C.; Gelmon, K.; Mackey, J.R.; Reid, R.D.; Courneya, K.S. Effects of exercise dose and type during breast cancer chemotherapy on longer-term patient-reported outcomes and health-related fitness: A randomized controlled trial. Int. J. Cancer 2020, 146, 150–160. [Google Scholar] [CrossRef]
- Bloomquist, K.; Adamsen, L.; Hayes, S.C.; Lillelund, C.; Andersen, C.; Christensen, K.B.; Oturai, P.; Ejlertsen, B.; Tuxen, M.K.; Møller, T. Heavy-load resistance exercise during chemotherapy in The impact of maximal strength at risk for lymphedema: A randomized trial. Acta Oncol. 2019, 58, 1667–1675. [Google Scholar] [CrossRef]
- Cešeiko, R.; Eglītis, J.; Srebnijs, A.; Timofejevs, M.; Purmalis, E.; Erts, R.; Vētra, A.; Tomsone, S. The impact of maximal strength training on quality of life among women with breast cancer undergoing treatment. Exp. Oncol. 2019, 41, 166–172. [Google Scholar] [CrossRef]
- Jacot, W.; Arnaud, A.; Jarlier, M.; Lefeuvre-Plesse, C.; Dalivoust, P.; Senesse, P.; Azzedine, A.; Tredan, O.; Sadot-Lebouvier, S.; Mas, S.; et al. Brief hospital supervision of exercise and diet during adjuvant breast cancer therapy is not enough to relieve fatigue: A multicenter randomized controlled trial. Nutrients 2020, 12, 3081. [Google Scholar] [CrossRef] [PubMed]
- Mavropalias, G.; Cormie, P.; Peddle-McIntyre, C.J.; Galvão, D.A.; Taaffe, D.R.; Schofield, C.; Ray, S.; Zissiadis, Y.; Newton, R.U. The effects of home-based exercise therapy for breast cancer-related fatigue induced by radical radiotherapy. Breast Cancer 2023, 30, 139–150. [Google Scholar] [CrossRef]
- Rogers, L.Q.; Hopkins-Price, P.; Vicari, S.; Pamenter, R.; Courneya, K.S.; Markwell, S.; Verhulst, S.; Hoelzer, K.; Naritoku, C.; Jones, L.; et al. A randomized trial to increase physical activity in breast cancer survivors. Med. Sci. Sport. Exerc. 2009, 41, 935–946. [Google Scholar] [CrossRef] [PubMed]
- Wonders, K.Y.; Schmitz, K.; Wise, R.; Hale, R. Cost-savings analysis of an individualized exercise oncology program in early-stage breast cancer survivors: A randomized clinical control trial. JCO Oncol. Pract. 2022, 18, e1170–e1180. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Yuan, R.; Yang, J.; Zheng, W.; Jin, Y.; Wang, M.; Jiang, J.; Wu, C.; Li, K. Effects of Baduanjin exercise on cognitive function and cancer-related symptoms in women with breast cancer receiving chemotherapy: A randomized controlled trial. Support. Care Cancer 2022, 30, 6079–6091. [Google Scholar] [CrossRef] [PubMed]
- Sheean, P.; Matthews, L.; Visotcky, A.; Banerjee, A.; Moosreiner, A.; Kelley, K.; Chitambar, C.R.; Papanek, P.E.; Stolley, M. Every Day Counts: A randomized pilot lifestyle intervention for women with metastatic breast cancer. Breast Cancer Res. Treat. 2021, 187, 729–741. [Google Scholar] [CrossRef]
- Rogers, L.Q.; Hopkins-Price, P.; Vicari, S.; Markwell, S.; Pamenter, R.; Courneya, K.S.; Hoelzer, K.; Naritoku, C.; Edson, B.; Jones, L.; et al. Physical activity and health outcomes three months after completing a physical activity behavior change intervention: Persistent and delayed effects. Cancer Epidemiol. Biomark. Prev. 2009, 18, 1410–1418. [Google Scholar] [CrossRef]
- Patel, D.I.; Gonzalez, A.; Moon, C.; Serra, M.; Bridges, P.B.; Hughes, D.; Clarke, G.; Kilpela, L.; Jiwani, R.; Musi, N. Exercise and creatine supplementation to augment the adaptation of exercise training among breast cancer survivors completing chemotherapy: Protocol for an open-label randomized controlled trial (the THRIVE Study). JMIR Res. Protoc. 2022, 11, e26827. [Google Scholar] [CrossRef]
- Smith-Turchyn, J.; McCowan, M.E.; O’Loughlin, E.; Fong, A.J.; McDonough, M.H.; Santa Mina, D.; Arbour-Nicitopoulos, K.P.; Trinh, L.; Jones, J.M.; Bender, J.L.; et al. Connecting breast cancer survivors for exercise: Protocol for a two-arm randomized controlled trial. BMC Sports Sci. Med. Rehabil. 2021, 13, 128. [Google Scholar] [CrossRef]
- Wang, C.C.; Geraghty, S.; Fox-Harding, C.; Wang, C. Effects of a nurse-led Tai Chi programme on improving quality of life, mental wellbeing, and physical function of women with breast cancer: Protocol for a randomized controlled trial. Womens Health 2022, 18, 17455057221127813. [Google Scholar] [CrossRef]
- Lynch, B.M.; Nguyen, N.H.; Reeves, M.M.; Moore, M.M.; Rosenberg, D.E.; Wheeler, M.J.; Boyle, T.; Vallance, J.K.; Friedenreich, C.M.; English, D.R. Study design and methods for the ACTIVity And TEchnology (ACTIVATE) trial. Contemp. Clin. Trials 2018, 64, 112–117. [Google Scholar] [CrossRef]
- Salchow, J.L.; Strunk, M.A.; Niels, T.; Steck, J.; Minto, C.A.; Baumann, F.T. A randomized controlled pilot trial about the influence of Kyusho Jitsu exercise on self-efficacy, fear, depression, and distress of breast cancer patients within follow-up care. Integr. Cancer Ther. 2021, 20, 15347354211037955. [Google Scholar] [CrossRef]
- Lin, H.P.; Kuo, Y.H.; Tai, W.Y.; Liu, H.E. Exercise effects on fatigue in breast cancer survivors after treatments: A systematic review and meta-analysis. Int. J. Nurs. Pract. 2022, 28, e12989. [Google Scholar] [CrossRef] [PubMed]
- Bertoli, J.; Bezerra, E.S.; Winters-Stone, K.M.; Alberto Gobbo, L.; Freitas, I.F.J. Mat Pilates improves lower and upper body strength and flexibility in breast cancer survivors undergoing hormone therapy: A randomized controlled trial (HAPiMat study). Disabil. Rehabil. 2023, 45, 494–503. [Google Scholar] [CrossRef] [PubMed]
- Jacquinot, Q.; Meneveau, N.; Falcoz, A.; Bouhaddi, M.; Roux, P.; Degano, B.; Chatot, M.; Curtit, E.; Mansi, L.; Paillard, M.J.; et al. Cardiotoxicity is mitigated after a supervised exercise program in HER2-positive breast cancer undergoing adjuvant trastuzumab. Front. Cardiovasc. Med. 2022, 9, 1000846. [Google Scholar] [CrossRef] [PubMed]
- Rogers, L.Q.; Courneya, K.S.; Carter, S.J.; Anton, P.M.; Verhulst, S.; Vicari, S.K.; Robbs, R.S.; McAuley, E. Effects of a multicomponent physical activity behavior change intervention on breast cancer survivor health status outcomes in a randomized controlled trial. Breast Cancer Res. Treat. 2016, 159, 283–291. [Google Scholar] [CrossRef] [PubMed]
- Schleicher, E.; McAuley, E.; Courneya, K.S.; Anton, P.; Ehlers, D.K.; Phillips, S.M.; Oster, R.A.; Pekmezi, D.; Rogers, L.Q. Moderators of physical activity and quality of life response to a physical activity intervention for breast cancer survivors. Support. Care Cancer 2022, 31, 53. [Google Scholar] [CrossRef]
- Holtdirk, F.; Mehnert, A.; Weiss, M.; Mayer, J.; Meyer, B.; Bröde, P.; Claus, M.; Watzl, C. Results of the Optimune trial: A randomized controlled trial evaluating a novel Internet intervention for breast cancer survivors. PLoS ONE 2021, 16, e0251276. [Google Scholar] [CrossRef]
- Duijts, S.F.; van Beurden, M.; Oldenburg, H.S.; Hunter, M.S.; Kieffer, J.M.; Stuiver, M.M.; Gerritsma, M.A.; Menke-Pluymers, M.B.; Plaisier, P.W.; Rijna, H.; et al. Efficacy of cognitive behavioral therapy and physical exercise in alleviating treatment-induced menopausal symptoms in patients with breast cancer: Results of a randomized, controlled, multicenter trial. J. Clin. Oncol. 2012, 30, 4124–4133. [Google Scholar] [CrossRef]
- Poier, D.; Büssing, A.; Rodrigues Recchia, D.; Beerenbrock, Y.; Reif, M.; Nikolaou, A.; Zerm, R.; Gutenbrunner, C.; Kröz, M. Influence of a multimodal and multimodal-aerobic therapy concept on health-related quality of life in breast cancer survivors. Integr. Cancer Ther. 2019, 18, 1534735418820447. [Google Scholar] [CrossRef]
- Vallance, J.K.; Nguyen, N.H.; Moore, M.M.; Reeves, M.M.; Rosenberg, D.E.; Boyle, T.; Milton, S.; Friedenreich, C.M.; English, D.R.; Lynch, B.M. Effects of the ACTIVity And TEchnology (ACTIVATE) intervention on health-related quality of life and fatigue outcomes in breast cancer survivors. Psychooncology 2020, 29, 204–211. [Google Scholar] [CrossRef]
- Chan, D.N.S.; Chow, K.M.; Anderson, D.J.; Porter-Steele, J.; Laing, B.; Ling, W.M.; Lam, C.C.H.; Choi, K.C.; Chan, C.W.H.; So, W.K.W.; et al. Cultural adaptation of the younger women’s wellness after cancer program for younger Chinese women with breast cancer: A pilot randomized controlled trial. Cancer Nurs. 2023. [Google Scholar] [CrossRef]
- Naderi, M.; Kordestani, H.; Sahebi, Z.; Khedmati Zare, V.; Amani-Shalamzari, S.; Kaviani, M.; Wiskemann, J.; Molanouri Shamsi, M. Serum and gene expression profile of cytokines following combination of yoga training and vitamin D supplementation in breast cancer survivors: A randomized controlled trial. BMC Womens Health 2022, 22, 90. [Google Scholar] [CrossRef]
- Cormie, P.; Pumpa, K.; Galvão, D.A.; Turner, E.; Spry, N.; Saunders, C.; Zissiadis, Y.; Newton, R.U. Is it safe and efficacious for women with lymphedema secondary to breast cancer to lift heavy weights during exercise: A randomised controlled trial. J. Cancer Surviv. 2013, 7, 413–424. [Google Scholar] [CrossRef]
- Buchan, J.; Janda, M.; Box, R.; Schmitz, K.; Hayes, S. A randomized trial on the effect of exercise mode on breast cancer-related lymphedema. Med. Sci. Sport. Exerc. 2016, 48, 1866–1874. [Google Scholar] [CrossRef]
- Zhi, W.I.; Baser, R.E.; Zhi, L.M.; Talukder, D.; Li, Q.S.; Paul, T.; Patterson, C.; Piulson, L.; Seluzicki, C.; Galantino, M.L.; et al. Yoga for cancer survivors with chemotherapy-induced peripheral neuropathy: Health-related quality of life outcomes. Cancer Med. 2021, 10, 5456–5465. [Google Scholar] [CrossRef] [PubMed]
- Ax, A.K.; Johansson, B.; Lyth, J.; Nordin, K.; Börjeson, S. Short- and long-term effect of high versus low-to-moderate intensity exercise to optimise health-related quality of life after oncological treatment-results from the Phys-Can project. Support. Care Cancer 2022, 30, 5949–5963. [Google Scholar] [CrossRef]
- Koch, A.K.; Rabsilber, S.; Lauche, R.; Kümmel, S.; Dobos, G.; Langhorst, J.; Cramer, H. The effects of yoga and self-esteem on menopausal symptoms and quality of life in breast cancer survivors-A secondary analysis of a randomized controlled trial. Maturitas 2017, 105, 95–99. [Google Scholar] [CrossRef]
- McNeil, J.; Fahim, M.; Stone, C.R.; O’Reilly, R.; Courneya, K.S.; Friedenreich, C.M. Adherence to a lower versus higher intensity physical activity intervention in the Breast Cancer & Physical Activity Level (BC-PAL) Trial. J. Cancer Surviv. 2022, 16, 353–365. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Yi, X.; Gao, D.; Gao, Z.; Huang, S.; Chao, M.; Chen, W.; Ding, M. The effects of the combined exercise intervention based on internet and social media software (CEIBISMS) on quality of life, muscle strength and cardiorespiratory capacity in Chinese postoperative breast cancer patients: A randomized controlled trial. Health Qual. Life Outcomes 2019, 17, 109. [Google Scholar] [CrossRef] [PubMed]
- Strunk, M.A.; Zopf, E.M.; Steck, J.; Hamacher, S.; Hallek, M.; Baumann, F.T. Effects of Kyusho Jitsu on physical activity-levels and quality of life in breast cancer patients. In Vivo 2018, 32, 819–824. [Google Scholar] [CrossRef]
- Patel, S.R.; Zayas, J.; Medina-Inojosa, J.R.; Loprinzi, C.; Cathcart-Rake, E.J.; Bhagra, A.; Olson, J.E.; Couch, F.J.; Ruddy, K.J. Real-World Experiences With Yoga on Cancer-Related Symptoms in Women With Breast Cancer. Glob. Adv. Health Med. 2021, 10, 2164956120984140. [Google Scholar] [CrossRef]
- Danhauer, S.C.; Addington, E.L.; Cohen, L.; Sohl, S.J.; Van Puymbroeck, M.; Albinati, N.K.; Culos-Reed, S.N. Yoga for symptom management in oncology: A review of the evidence base and future directions for research. Cancer 2019, 125, 1979–1989. [Google Scholar] [CrossRef] [PubMed]
- Ostman, C.; Jewiss, D.; Smart, N.A. The effect of exercise training intensity on quality of life in heart failure patients: A systematic review and meta-analysis. Cardiology 2017, 136, 79–89. [Google Scholar] [CrossRef]
- Dysart, A.; Harden, S.M. Effects of temperature and tempo: Evaluating how much time in a typical community-based yoga class is moderate-intensity aerobic activity. Int. J. Environ. Res. Public. Health 2023, 20, 2349. [Google Scholar] [CrossRef] [PubMed]
- Day, M.L.; McGuigan, M.R.; Brice, G.; Foster, C. Monitoring exercise intensity during resistance training using the session RPE scale. J. Strength. Cond. Res. 2004, 18, 353–358. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Bustos, A.; de Pedro, C.G.; Romero-Elías, M.; Ramos, J.; Osorio, P.; Cantos, B.; Maximiano, C.; Méndez, M.; Fiuza-Luces, C.; Méndez-Otero, M.; et al. Prevalence and correlates of cancer-related fatigue in breast cancer survivors. Support. Care Cancer 2021, 29, 6523–6534. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).