Fluorometric Quantification of Total Cell-Free DNA as a Prognostic Biomarker in Non-Small-Cell Lung Cancer Patients Treated with Immune Checkpoint Blockade
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Processing and DNA Isolation and Quantification
2.2. Statistical Methods
3. Results
3.1. Cohorts Characteristics and Sample Collection
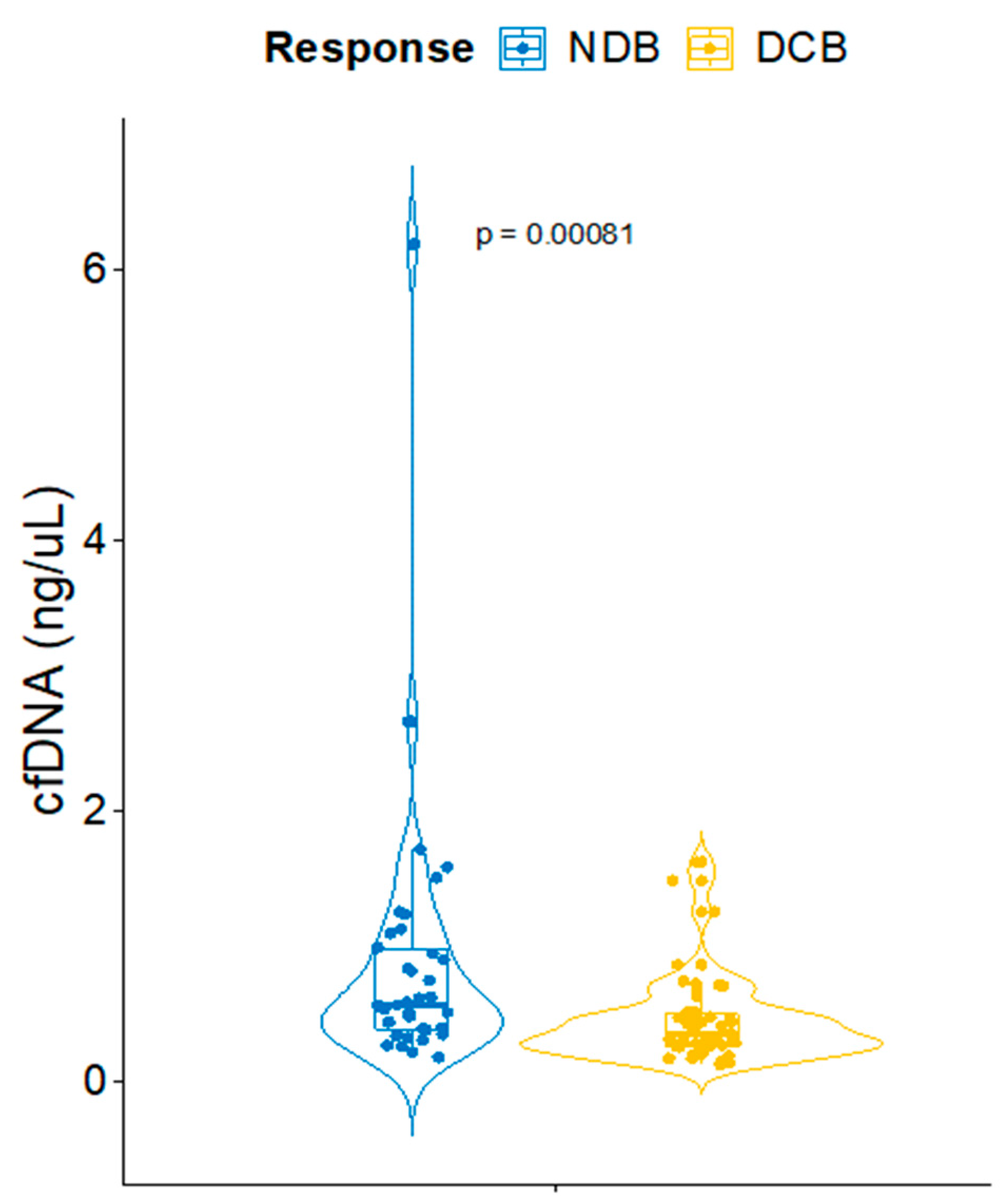
3.2. CfDNA Correlates with Response to ICB in NSCLC Patients
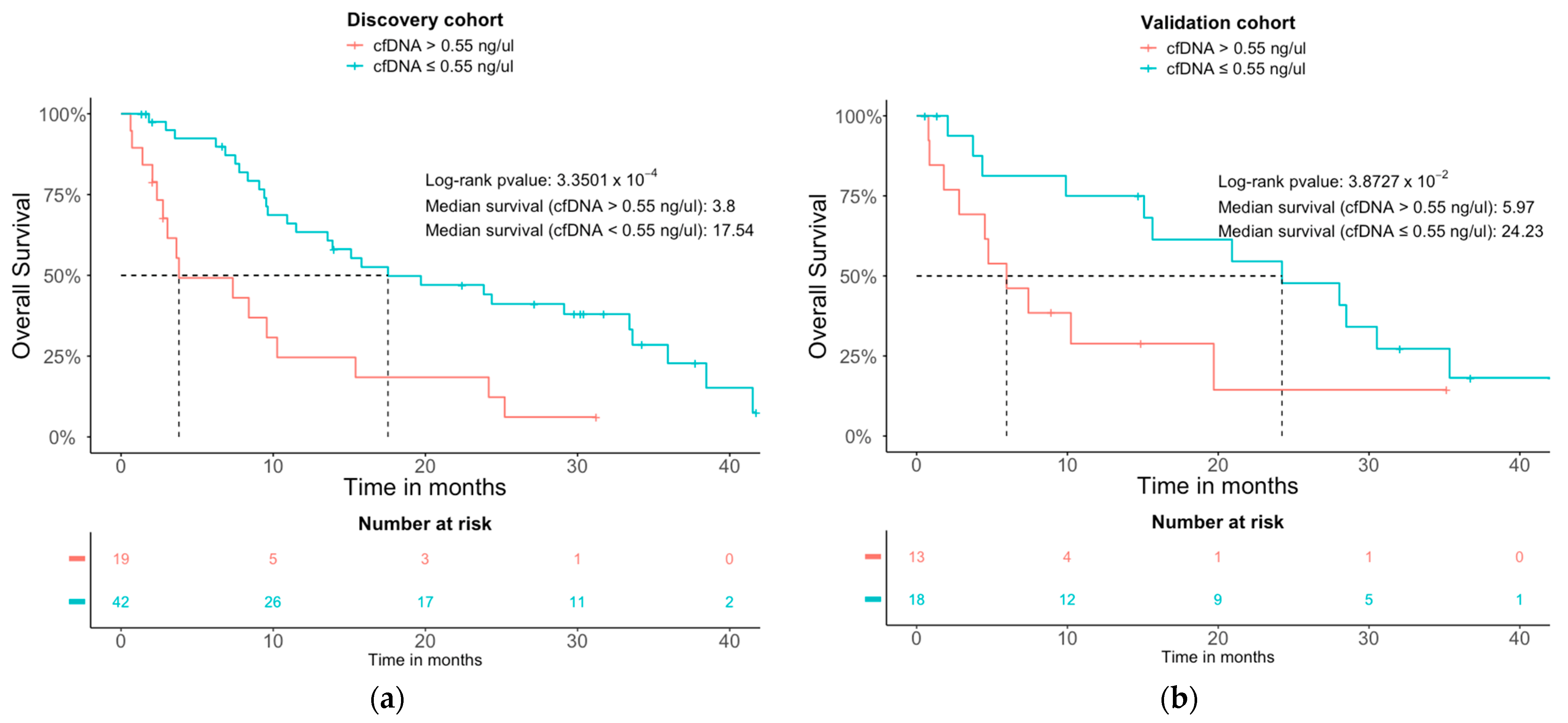
3.3. Prognostic Analysis and Models
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Mathers, C.; Parkin, D.M.; Piñeros, M.; Znaor, A.; Bray, F. Estimating the Global Cancer Incidence and Mortality in 2018: GLOBOCAN Sources and Methods. Int. J. Cancer 2019, 144, 1941–1953. [Google Scholar] [CrossRef] [PubMed]
- Special Section: Lung Cancer. Available online: https://www.cdc.gov/cancer/lung/basic_info/what-is-lung-cancer.htm (accessed on 29 May 2023).
- Petty, W.J.; Paz-Ares, L. Emerging Strategies for the Treatment of Small Cell Lung Cancer: A Review. JAMA Oncol. 2023, 9, 419–429. [Google Scholar] [CrossRef] [PubMed]
- Aguilar, E.J.; Ricciuti, B.; Gainor, J.F.; Kehl, K.L.; Kravets, S.; Dahlberg, S.; Nishino, M.; Sholl, L.M.; Adeni, A.; Subegdjo, S.; et al. Outcomes to First-Line Pembrolizumab in Patients with Non-Small-Cell Lung Cancer and Very High PD-L1 Expression. Ann. Oncol. 2019, 30, 1653–1659. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Hu-Lieskovan, S.; Wargo, J.A.; Ribas, A. Primary, Adaptive, and Acquired Resistance to Cancer Immunotherapy. Cell 2017, 168, 707–723. [Google Scholar] [CrossRef]
- Verma, V.; Sprave, T.; Haque, W.; Simone, C.B.; Chang, J.Y.; Welsh, J.W.; Thomas, C.R. A Systematic Review of the Cost and Cost-Effectiveness Studies of Immune Checkpoint Inhibitors. J. Immunother. Cancer 2018, 6, 128. [Google Scholar] [CrossRef]
- Borghaei, H.; Paz-Ares, L.; Horn, L.; Spigel, D.R.; Steins, M.; Ready, N.E.; Chow, L.Q.; Vokes, E.E.; Felip, E.; Holgado, E.; et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2015, 373, 1627–1639. [Google Scholar] [CrossRef] [PubMed]
- Prelaj, A.; Tay, R.; Ferrara, R.; Chaput, N.; Besse, B.; Califano, R. Predictive Biomarkers of Response for Immune Checkpoint Inhibitors in Non–Small-Cell Lung Cancer. Eur. J. Cancer 2019, 106, 144–159. [Google Scholar] [CrossRef]
- Sacher, A.G.; Gandhi, L. Biomarkers for the Clinical Use of PD-1/PD-L1 Inhibitors in Non-Small-Cell Lung Cancer: A Review. JAMA Oncol. 2016, 2, 1217–1222. [Google Scholar] [CrossRef]
- Friedrich, M.J. Immunotherapy 2.0: Improving the Response to Checkpoint Inhibitors. JAMA J. Am. Med. Assoc. 2019, 321, 131–133. [Google Scholar] [CrossRef]
- Maio, M.; Ascierto, P.A.; Manzyuk, L.; Motola-Kuba, D.; Penel, N.; Cassier, P.A.; Bariani, G.M.; De Jesus Acosta, A.; Doi, T.; Longo, F.; et al. Pembrolizumab in Microsatellite Instability High or Mismatch Repair Deficient Cancers: Updated Analysis from the Phase II KEYNOTE-158 Study. Ann. Oncol. 2022, 33, 929–938. [Google Scholar] [CrossRef] [PubMed]
- Galvano, A.; Gristina, V.; Malapelle, U.; Pisapia, P.; Pepe, F.; Barraco, N.; Castiglia, M.; Perez, A.; Rolfo, C.; Troncone, G.; et al. The Prognostic Impact of Tumor Mutational Burden (TMB) in the First-Line Management of Advanced Non-Oncogene Addicted Non-Small-Cell Lung Cancer (NSCLC): A Systematic Review and Meta-Analysis of Randomized Controlled Trials. ESMO Open 2021, 6, 100124. [Google Scholar] [CrossRef] [PubMed]
- Krebs, M.G.; Malapelle, U.; André, F.; Paz-Ares, L.; Schuler, M.; Thomas, D.M.; Vainer, G.; Yoshino, T.; Rolfo, C. Practical Considerations for the Use of Circulating Tumor DNA in the Treatment of Patients with Cancer: A Narrative Review. JAMA Oncol. 2022, 8, 1830–1839. [Google Scholar] [CrossRef]
- Gautschi, O.; Bigosch, C.; Huegli, B.; Jermann, M.; Marx, A.; Chassé, E.; Ratschiller, D.; Weder, W.; Joerger, M.; Betticher, D.C.; et al. Circulating Deoxyribonucleic Acid as Prognostic Marker in Non-Small-Cell Lung Cancer Patients Undergoing Chemotherapy. J. Clin. Oncol. 2004, 22, 4157–4164. [Google Scholar] [CrossRef] [PubMed]
- McShane, L.M.; Altman, D.G.; Sauerbrei, W.; Taube, S.E.; Gion, M.; Clark, G.M. REporting Recommendations for Tumour MARKer Prognostic Studies (REMARK). Eur. J. Cancer 2005, 41, 1690–1696. [Google Scholar] [CrossRef] [PubMed]
- Simon, R.M.; Paik, S.; Hayes, D.F. Use of Archived Specimens in Evaluation of Prognostic and Predictive Biomarkers. JNCI J. Natl. Cancer Inst. 2009, 101, 1446–1452. [Google Scholar] [CrossRef]
- Hothorn, T.; Lausen, B. On the Exact Distribution of Maximally Selected Rank Statistics. Comput. Stat. Data Anal. 2003, 43, 121–137. [Google Scholar] [CrossRef]
- Uno, H.; Cai, T.; Tian, L.; Wei, L.J. Evaluating Prediction Rules for T-Year Survivors with Censored Regression Models. J. Am. Stat. Assoc. 2012, 102, 527–537. [Google Scholar] [CrossRef]
- Remon, J.; Besse, B.; Aix, S.P.; Callejo, A.; Al-Rabi, K.; Bernabe, R.; Greillier, L.; Majem, M.; Reguart, N.; Monnet, I.; et al. Osimertinib Treatment Based on Plasma T790M Monitoring in Patients with EGFR-Mutant Non-Small Cell Lung Cancer (NSCLC): EORTC Lung Cancer Group 1613 APPLE Phase II Randomized Clinical Trial. Ann. Oncol. 2023, 34, 468–476. [Google Scholar] [CrossRef]
- Al-Showbaki, L.; Wilson, B.; Tamimi, F.; Molto, C.; Mittal, A.; Cescon, D.W.; Amir, E. Changes in Circulating Tumor DNA and Outcomes in Solid Tumors Treated with Immune Checkpoint Inhibitors: A Systematic Review. J. Immunother. Cancer 2023, 11, e005854. [Google Scholar] [CrossRef]
- Valpione, S.; Gremel, G.; Mundra, P.; Middlehurst, P.; Galvani, E.; Girotti, M.R.; Lee, R.J.; Garner, G.; Dhomen, N.; Lorigan, P.C.; et al. Plasma Total Cell-Free DNA (CfDNA) Is a Surrogate Biomarker for Tumour Burden and a Prognostic Biomarker for Survival in Metastatic Melanoma Patients. Eur. J. Cancer 2018, 88, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Chen, E.; Cario, C.L.; Leong, L.; Lopez, K.; Márquez, C.P.; Chu, C.; Li, P.S.; Oropeza, E.; Tenggara, I.; Cowan, J.; et al. Cell-Free DNA Concentration and Fragment Size as a Biomarker for Prostate Cancer. Sci. Rep. 2021, 11, 5040. [Google Scholar] [CrossRef] [PubMed]
- Matsumae, T.; Kodama, T.; Myojin, Y.; Maesaka, K.; Sakamori, R.; Takuwa, A.; Oku, K.; Motooka, D.; Sawai, Y.; Oshita, M.; et al. Circulating Cell-Free DNA Profiling Predicts the Therapeutic Outcome in Advanced Hepatocellular Carcinoma Patients Treated with Combination Immunotherapy. Cancers 2022, 14, 3367. [Google Scholar] [CrossRef] [PubMed]
- Alama, A.; Coco, S.; Genova, C.; Rossi, G.; Fontana, V.; Tagliamento, M.; Dal Bello, M.G.; Rosa, A.; Boccardo, S.; Rijavec, E.; et al. Prognostic Relevance of Circulating Tumor Cells and Circulating Cell-Free DNA Association in Metastatic Non-Small Cell Lung Cancer Treated with Nivolumab. J. Clin. Med. 2019, 8, 1011. [Google Scholar] [CrossRef]
- Mondelo-Macía, P.; García-González, J.; León-Mateos, L.; Anido, U.; Aguín, S.; Abdulkader, I.; Sánchez-Ares, M.; Abalo, A.; Rodríguez-Casanova, A.; Díaz-Lagares, Á.; et al. Clinical Potential of Circulating Free DNA and Circulating Tumour Cells in Patients with Metastatic Non-Small-Cell Lung Cancer Treated with Pembrolizumab. Mol. Oncol. 2021, 15, 2923–2940. [Google Scholar] [CrossRef]
- Kustanovich, A.; Schwartz, R.; Peretz, T.; Grinshpun, A. Life and Death of Circulating Cell-Free DNA. Cancer Biol. Ther. 2019, 20, 1057–1067. [Google Scholar] [CrossRef]
- Moss, J.; Magenheim, J.; Neiman, D.; Zemmour, H.; Loyfer, N.; Korach, A.; Samet, Y.; Maoz, M.; Druid, H.; Arner, P.; et al. Comprehensive Human Cell-Type Methylation Atlas Reveals Origins of Circulating Cell-Free DNA in Health and Disease. Nat. Commun. 2018, 9, 5068. [Google Scholar] [CrossRef] [PubMed]
- Thakur, B.K.; Zhang, H.; Becker, A.; Matei, I.; Huang, Y.; Costa-Silva, B.; Zheng, Y.; Hoshino, A.; Brazier, H.; Xiang, J.; et al. Double-Stranded DNA in Exosomes: A Novel Biomarker in Cancer Detection. Cell Res. 2014, 24, 766–769. [Google Scholar] [CrossRef]
- Thierry, A.R.; El Messaoudi, S.; Gahan, P.B.; Anker, P.; Stroun, M. Origins, Structures, and Functions of Circulating DNA in Oncology. Cancer Metastasis Rev. 2016, 35, 347–376. [Google Scholar] [CrossRef]
- Schwarzenbach, H.; Hoon, D.S.B.; Pantel, K. Cell-Free Nucleic Acids as Biomarkers in Cancer Patients. Nat. Rev. Cancer 2011, 11, 426–437. [Google Scholar] [CrossRef]
- Morbelli, S.; Alama, A.; Ferrarazzo, G.; Coco, S.; Genova, C.; Rijavec, E.; Bongioanni, F.; Biello, F.; Bello, M.G.D.; Barletta, G.; et al. Circulating Tumor DNA Reflects Tumor Metabolism Rather Than Tumor Burden in Chemotherapy-Naive Patients with Advanced Non–Small Cell Lung Cancer: 18 F-FDG PET/CT Study. J. Nucl. Med. 2017, 58, 1764–1769. [Google Scholar] [CrossRef] [PubMed]
- Spector, B.L.; Harrell, L.; Sante, D.; Wyckoff, G.J.; Willig, L. The Methylome and Cell-Free DNA: Current Applications in Medicine and Pediatric Disease. Pediatr. Res. 2023, 16, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Nicolás-Ávila, J.Á.; Adrover, J.M.; Hidalgo, A. Neutrophils in Homeostasis, Immunity, and Cancer. Immunity 2017, 46, 15–28. [Google Scholar] [CrossRef]
- Branzk, N.; Lubojemska, A.; Hardison, S.E.; Wang, Q.; Gutierrez, M.G.; Brown, G.D.; Papayannopoulos, V. Neutrophils Sense Microbe Size and Selectively Release Neutrophil Extracellular Traps in Response to Large Pathogens. Nat. Immunol. 2014, 15, 1017–1025. [Google Scholar] [CrossRef] [PubMed]
- Melero, I.; Villalba-Esparza, M.; Recalde-Zamacona, B.; Jiménez-Sánchez, D.; Teijeira, Á.; Argueta, A.; García-Tobar, L.; Álvarez-Gigli, L.; Sainz, C.; Garcia-Ros, D.; et al. Neutrophil Extracellular Traps, Local IL-8 Expression, and Cytotoxic T-Lymphocyte Response in the Lungs of Patients With Fatal COVID-19. Chest 2022, 162, 1006–1016. [Google Scholar] [CrossRef]
- Teijeira, Á.; Garasa, S.; Gato, M.; Alfaro, C.; Migueliz, I.; Cirella, A.; de Andrea, C.; Ochoa, M.C.; Otano, I.; Etxeberria, I.; et al. CXCR1 and CXCR2 Chemokine Receptor Agonists Produced by Tumors Induce Neutrophil Extracellular Traps That Interfere with Immune Cytotoxicity. Immunity 2020, 52, 856–871.e8. [Google Scholar] [CrossRef] [PubMed]
- Dang, D.K.; Park, B.H. Circulating Tumor DNA: Current Challenges for Clinical Utility. J. Clin. Investig. 2022, 132, e154941. [Google Scholar] [CrossRef]

| Variable | Group | Discovery Cohort | Validation Cohort | Combined Cohort | p-Value Discovery vs. Validation | Pct NA—Complete | Pct NA—Discovery | Pct NA—Validation |
|---|---|---|---|---|---|---|---|---|
| Sex | 0.45 | 0 (0%) | 0 (0%) | 0 (0%) | ||||
| Female | 12 (19.67%) | 9 (29.03%) | 21 (22.83%) | |||||
| Male | 49 (80.33%) | 22 (70.97%) | 71 (77.17%) | |||||
| Age IT Start | 64.56 [43.38–84.9] | 66.38 [53.29–79.12] | 65.97 [43.38–84.9] | 0.75 | 0 (0%) | 0 (0%) | 0 (0%) | |
| Histology | 0.79 | 1 (1.09%) | 1 (1.64%) | 0 (0%) | ||||
| LUAD | 39 (65.00%) | 20 (64.52%) | 59 (64.83%) | |||||
| Others | 2 (3.33%) | 0 (0%) | 2 (2.20%) | |||||
| SCC | 19 (31.67%) | 11 (35.48%) | 30 (32.97%) | |||||
| Immunotherapy type | 0.30 | 0 (0%) | 0 (0%) | 0 (0%) | ||||
| ICB-mono | 23 (37.7%) | 11 (35.48%) | 34 (36.96%) | |||||
| ICB + chemo | 8 (13.11%) | 8 (25.81%) | 16 (17.39%) | |||||
| ICB + chemo + Others | 30 (49.18%) | 12 (38.71%) | 42 (45.65%) | |||||
| Brain metastasis | 0.32 | 5 (5.43%) | 3 (4.92%) | 2 (6.45%) | ||||
| No | 48 (78.69%) | 27 (87.1%) | 75 (81.52%) | |||||
| Yes | 10 (16.39%) | 2 (6.45%) | 12 (13.04%) | |||||
| Lymph node metastasis | 1.00 | 5 (5.43%) | 3 (4.92%) | 2 (6.45%) | ||||
| No | 25 (40.98%) | 13 (41.94%) | 38 (41.3%) | |||||
| Yes | 33 (54.1%) | 16 (51.61%) | 49 (53.26%) | |||||
| Liver metastasis | 1.00 | 5 (5.43%) | 3 (4.92%) | 2 (6.45%) | ||||
| No | 51 (83.61%) | 25 (80.65%) | 76 (82.61%) | |||||
| Yes | 7 (11.48%) | 4 (12.9%) | 11 (11.96%) | |||||
| Lung metastasis | 0.82 | 5 (5.43%) | 3 (4.92%) | 2 (6.45%) | ||||
| No | 33 (54.1%) | 18 (58.06%) | 51 (55.43%) | |||||
| Yes | 25 (40.98%) | 11 (35.48%) | 36 (39.13%) | |||||
| Toxicity to IT (all grades) | 0.97 | 7 (7.61%) | 6 (9.84%) | 1 (3.23%) | ||||
| No | 24 (39.34%) | 14 (45.16%) | 38 (41.3%) | |||||
| Yes | 31 (50.82%) | 16 (51.61%) | 47 (51.09%) | |||||
| Maximum toxicity grade | 0.18 | 14 (15.22%) | 10 (16.39%) | 4 (12.9%) | ||||
| 0 | 17 (27.87%) | 10 (32.26%) | 27 (29.35%) | |||||
| 1 | 23 (37.7%) | 6 (19.35%) | 29 (31.52%) | |||||
| 2 | 4 (6.56%) | 6 (19.35%) | 10 (10.87%) | |||||
| 3 | 5 (8.2%) | 4 (12.91%) | 9 (9.80%) | |||||
| 4 | 1 (1.64%) | 1 (3.23%) | 2 (2.17%) | |||||
| 5 | 1 (1.64%) | 0 (0%) | 1 (1.09%) | |||||
| PDL1 | 7 (7.61%) | 4 (6.56%) | 3 (9.68%) | |||||
| <50% | 44 (72.13%) | 23 (74.19%) | 67 (72.83%) | |||||
| >50% | 13 (21.31%) | 5 (16.13%) | 18 (19.57%) | |||||
| Previous treatment | 0.73 | 0 (0%) | 0 (0%) | 0 (0%) | ||||
| No | 43 (70.49%) | 20 (64.52%) | 63 (68.48%) | |||||
| Yes | 18 (29.51%) | 11 (35.48%) | 29 (31.52%) | |||||
| Progression | 1.00 | 0 (0%) | 0 (0%) | 0 (0%) | ||||
| No | 18 (29.51%) | 9 (29.03%) | 27 (29.35%) | |||||
| Yes | 43 (70.49%) | 22 (70.97%) | 65 (70.65%) | |||||
| State last evaluation | 1.00 | 0 (0%) | 0 (0%) | 0 (0%) | ||||
| Dead | 44 (72.13%) | 22 (70.97%) | 66 (71.74%) | |||||
| Alive | 17 (27.87%) | 9 (29.03%) | 26 (28.26%) | |||||
| T1 ECOG | 0.79 | 4 (4.35%) | 1 (1.64%) | 3 (9.68%) | ||||
| 0 | 15 (24.59%) | 6 (19.35%) | 21 (22.83%) | |||||
| 1 | 43 (70.49%) | 22 (70.97%) | 65 (70.65%) | |||||
| 2 | 2 (3.28%) | 0 (0%) | 2 (2.17%) | |||||
| T1 LDH | 0.89 | 22 (23.91%) | 13 (21.31%) | 9 (29.03%) | ||||
| <=2x | 8 (13.11%) | 5 (16.13%) | 13 (14.13%) | |||||
| >2x | 3 (4.92%) | 1 (3.23%) | 4 (4.35%) | |||||
| Normal | 37 (60.66%) | 16 (51.61%) | 53 (57.61%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oliver, J.; Onieva, J.L.; Garrido-Barros, M.; Cobo-Dols, M.; Martínez-Gálvez, B.; García-Pelícano, A.I.; Dubbelman, J.; Benítez, J.C.; Martín, J.Z.; Cantero, A.; et al. Fluorometric Quantification of Total Cell-Free DNA as a Prognostic Biomarker in Non-Small-Cell Lung Cancer Patients Treated with Immune Checkpoint Blockade. Cancers 2023, 15, 3357. https://doi.org/10.3390/cancers15133357
Oliver J, Onieva JL, Garrido-Barros M, Cobo-Dols M, Martínez-Gálvez B, García-Pelícano AI, Dubbelman J, Benítez JC, Martín JZ, Cantero A, et al. Fluorometric Quantification of Total Cell-Free DNA as a Prognostic Biomarker in Non-Small-Cell Lung Cancer Patients Treated with Immune Checkpoint Blockade. Cancers. 2023; 15(13):3357. https://doi.org/10.3390/cancers15133357
Chicago/Turabian StyleOliver, Javier, Juan Luis Onieva, María Garrido-Barros, Manuel Cobo-Dols, Beatriz Martínez-Gálvez, Ana Isabel García-Pelícano, Jaime Dubbelman, José Carlos Benítez, Juan Zafra Martín, Alejandra Cantero, and et al. 2023. "Fluorometric Quantification of Total Cell-Free DNA as a Prognostic Biomarker in Non-Small-Cell Lung Cancer Patients Treated with Immune Checkpoint Blockade" Cancers 15, no. 13: 3357. https://doi.org/10.3390/cancers15133357
APA StyleOliver, J., Onieva, J. L., Garrido-Barros, M., Cobo-Dols, M., Martínez-Gálvez, B., García-Pelícano, A. I., Dubbelman, J., Benítez, J. C., Martín, J. Z., Cantero, A., Pérez-Ruiz, E., Rueda-Domínguez, A., & Barragán, I. (2023). Fluorometric Quantification of Total Cell-Free DNA as a Prognostic Biomarker in Non-Small-Cell Lung Cancer Patients Treated with Immune Checkpoint Blockade. Cancers, 15(13), 3357. https://doi.org/10.3390/cancers15133357







