Simple Summary
Hepatocellular carcinoma (HCC) is the fifth most common cancer worldwide and the second most common oncological reason for death. Liver resection and transplantation are considered the only potential cure options for HCC. The majority of patients, however, are late in presentation and, therefore, are considered non-suitable for surgery at the time of diagnosis. Locoregional therapies are becoming integral to its management along with systemic therapies. This review discusses the role and the advances of locoregional therapies in HCC management.
Abstract
Hepatocellular carcinoma (HCC) is responsible for 90% of primary hepatic cancer cases, and its incidence with associated morbidity and mortality is growing worldwide. In recent decades, there has been a revolution in HCC treatment. There are three main types of locoregional therapy: radiofrequency ablation, transarterial chemoembolisation, and transarterial radioembolisation. This article summarises recent advances in locoregional methods.
1. Introduction
Hepatocellular carcinoma (HCC) is responsible for 90% of primary hepatic cancer cases. Its incidence is growing; currently, it is the fifth most common cancer worldwide, accounting for over 800,000 new cases in 2018 [1]. According to Cancer Today by WHO, it is the second most common oncological reason for death, with 50% of cases occurring in East Asia [1]. Its incidence tripled between 1980 and 2020 in the United States [2]. In Asia and Africa, HCC is usually associated with hepatitis B, whereas, in Europe, Japan, and the US, it is more often associated with hepatitis C, non-alcoholic fatty liver disease (NAFLD) and chronic alcohol abuse [1,3,4]. Other factors associated with HCC are genetic haemochromatosis, tyrosinosis, alpha-one antitrypsin deficiency, and primary biliary cirrhosis [3,5].
The diagnosis of HCC Is based on a combination of clinical, laboratory, radiographic, and histopathologic features [5]. The imaging diagnosis is based on the detection of the lesion’s vascularity [6]. Advanced imaging techniques, such as contrast-enhanced ultrasound (CEUS) and magnetic resonance imaging (MRI), have shown promising results in detecting and characterising HCC [6]. CEUS utilises microbubble-based contrast agents to provide real-time imaging of the tumour vasculature, allowing for improved lesion detection and differentiation from non-malignant liver lesions. On the other hand, MRI offers multiparametric imaging capabilities, including dynamic contrast-enhanced and diffusion-weighted imaging, enabling better tumour characterisation and assessment of treatment response [6,7] (Figure 1). Moreover, molecular imaging techniques, such as positron emission tomography (PET) using tracers like fluorodeoxyglucose (FDG), have shown potential in assessing HCC metabolic activity and predicting prognosis [6]. Recent advancements in imaging technology have also facilitated the integration of artificial intelligence (AI) algorithms to aid in diagnosing and staging HCC. These AI-based approaches leverage machine learning techniques and large datasets to improve the accuracy and efficiency of HCC diagnosis, allowing for earlier detection and intervention [8].
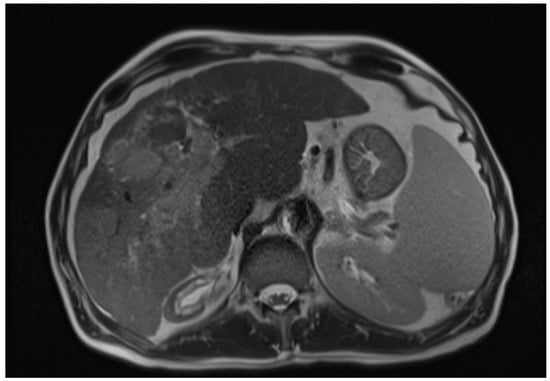
Figure 1.
Axial T2-weighted MRI image of hepatic cirrhosis with HCC [9].
The tumour typically starts as a small nodule and grows during the asymptomatic phase [3]. It doubles in a median of 6 months [3]. The 1 year survival is 50–90% among untreated patients with Child–Pugh A and only 20% with Child–Pugh C [3]. The 5 year survival is low, at less than 20% worldwide [1]. Treatment is challenging as it depends on the tumour burden and the level of associated liver cirrhosis [1]. Unfortunately, despite the availability of targeted screening for HCC among high-risk groups and improvements in the prevention and treatment of risk factors, such as hepatitis B/C or NAFLD, mortality rates continue to rise [3,5,10,11]. Only 10–30% of HCC patients are candidates for surgical treatment—a curative option—because most cancers are recognised at an intermediate or advanced stage [12,13,14]. However, adding biochemical markers—such as alpha fetoprotein—significantly increases the early detection of HCC in clinical practice [15].
Globally, multiple staging systems are used to select the best treatment option for patients. The first one is the Okuda staging system, which is based on three factors: liver functional status (albumin, ascites, and bilirubin) and tumor stage (more or less than 50% of liver area involved). It is used in Japan and other countries. The second is the Barcelona Clinic Liver Cancer (BCLC) system, comprising tumour stage, liver function, and physical status. This system has been widely adopted in Europe for HCC staging and treatment [5]. Thirdly, the mUICC staging system, adopted by Korea, is based on the number of tumours, the diameter of the largest tumour, and vascular or bile duct invasion [5].
The stages of HCC vary worldwide in their presentation. In the UK, patients usually present with advanced disease, which is most often detected among people with already abnormal liver function. In contrast, 80% of HCC cases in Japan are detected when asymptomatic due to widespread screening of all people with liver cirrhosis [3]. At the time of diagnosis, 75% of HCC nodules are inoperable [16,17]. When tumours have not expanded outside the liver, locoregional treatments are applied to downstage and increase the number of liver transplant candidates or improve outcomes of patients undergoing liver resection [18,19,20,21]. The potential increase in early-stage detection based on imaging and biochemical markers may lead to increased utilisation of locoregional therapies, which currently play a leading role in 50–60% of HCC treatments [4]. The choice of liver transplantation, resection, percutaneous ablation, transarterial chemoembolisation (TACE), and/or radioembolisation treatment largely depends on tumour burden and location, as well as comorbidities [5,22]. Systemic therapy is used in moderate and advanced diseases. Classical oncological treatments, such as cytotoxic chemotherapy and hormonal therapy, have not proven successful in hepatic cancer [2]. In recent years, multiple immunotherapy options and drugs have become available [2]. The first systemic treatment for HCC was sorafenib—a multi-kinase inhibitor [2,4,22]. Around 50–60% of HCCs are managed primarily by locoregional therapies, defined as imaging-guided liver tumour-directed procedures [4]. They can be based on local ablation or intraarterial technique. The primary aim is to prolong survival by decreasing or, if feasible, eliminating the burden of hepatic tumours [4]. Patients with advanced diseases and those in the terminal stage should receive the best supportive, palliative care [5].
The treatment algorithm for HCC is constantly changing, mainly driven by the expansion of criteria for hepatic resection, advancement of locoregional and radiation therapies, and novel systemic therapies [5].
Optimal management of liver cancers depends on a multidisciplinary approach, with input and collaboration from diagnostic radiology, pathology, hepatology, transplant surgery, surgical oncology, medical oncology, radiation oncology, and interventional radiology to achieve individualised and evidence-driven patient care. Patient preferences should also be taken into consideration [7,10].
Current guidelines recommend 6 monthly surveillance of high-risk patients with ultrasound [23,24]. Further research is ongoing to optimise follow-up pathways, especially regarding MRI-based imaging [6,7]. mRECIST has become a standard tool for measuring radiological endpoints that are added to the standard cancer overall survival rates [25].
This review aims to present the up-to-date status of locoregional therapies for HCC.
2. Radiofrequency Ablation (RFA)
Radiofrequency ablation was introduced in the 1990s as a treatment for osteoid osteomas [26]. It is now considered the standard treatment option among local ablative techniques for very-early-stage hepatic tumours (<2 cm) and for early-stage tumours that were disqualified from the surgical approach [4]. RFA has often been deemed a curative treatment modality, with a 5 year overall survival rate of around 40–70% [2,12,27]. It is also considered the most promising locoregional treatment [28,29,30,31,32]. The electrodes are inserted into pathological tissue, and, by delivering high-frequency alternating currents, they induce coagulative necrosis and tissue desiccation [28,29,30,31,32]. The major advantages of RFA are the potential for repeatability and safety for people with significant medical comorbidities due to the lack of a need for general anaesthesia [28,33]. There is also moderate evidence for using microwaves for ablation, and low evidence for using cryoablation and irreversible electroporation [4].
Local tumour progression post RFA is the Achilles heel of this well-established treatment modality [12,34]. The 5 year tumour recurrence has been reported to be as high as 80%. RFA also suffers from the following limitations: ablation volume up to 5 cm, limitations related to tumour localisation (i.e., hilar or subphrenic), heat-sink effect, spreading by intratumoral pressure during RFA, and tumour seeding [28,35,36,37,38].
Every medical procedure has inherent complication risks. RFA can be complicated by severe haemorrhage, RFA needle-track seeding, abscess formation, perforation of the gastrointestinal tract, liver failure, biloma, biliary stricture, portal vein thrombosis, and haemothorax or pneumothorax requiring drainage. It has been reported that complications affect 0.6–8.9% of procedures [28,39,40]. It is worth noting that the departments treating larger numbers of patients per month had a smaller number of complications and deaths [28,41].
There are conflicting reports in the literature comparing RFA to local surgical resection. Nevertheless, local surgical resection provides better long-term oncological outcomes [42,43].
Usually, RFA is performed under ultrasonographic guidance. Recently, six reported studies compared RFA using intraprocedural CT/MRI fusion imaging versus the standard of treatment. They suggested using fusion imaging to treat large tumours in difficult anatomical positions [44].
Advanced imaging with CT or MRI is typically used to assess treatment efficacy [28]. It is separated into the following categories:
- Grade A—absolutely curative with 5 mm ablative margin around the entire tumour.
- Grade B—relatively curative, mostly as grade A with some places with the lower margin.
- Grade C—an incomplete ablative margin around the tumour, although no residual tumour is apparent.
- Grade D—absolutely noncurative; the tumour was not completely ablated [28,45].
It was reported that liver ultrasound elastography with liver stiffness could be a reliable tool for predicting recurrence after RFA [46].
RFA is often compared with microwave frequency ablation, as they are primary types of percutaneous thermal ablation. Recent summaries of studies comparing those two techniques found little to no difference in their efficacy and safety [47,48,49,50,51,52,53].
Unanswered questions remain about combination techniques. A meta-analysis of 854 patients suggested that adding percutaneous ethanol injections improves overall survival; however, the evidence is heterogeneous [54]. A network meta-analysis of 3675 patients with advanced HCC revealed that the RAF with hepatic arterial infusion chemotherapy (HAIC) achieved the highest probability of 1 year overall survival and overall response rate [55]. TACE combined with RFA or MWA can provide significantly better overall survival (HR, 0.50, 95% confidence interval [CI]: 0.40–0.62), progression-free survival (HR, 0.47, 95% CI: 0.37–0.61), and local tumour control (OR, 0.36, 95% CI: 0.24–0.53) than TACE monotherapy for patients with intermediate-stage HCC, without increasing the risk of major complications (OR, 1.26, 95% CI: 0.74–2.16) [56]. Moreover, TACE + RFA offer comparable oncologic outcomes in patients with HCC compared to surgical resection and with the added benefit of lower morbidity [57].
There is continued effort to identify the best treatment technique for HCC. A study by Kwak et al. compared percutaneous and laparoscopic RAF for HCC in the subphrenic region. The laparoscopic approach resulted in fewer local tumour progressions and increased overall survival; therefore, it is proposed as a method of choice [58].
Within the last 3 years, we identified nine randomised controlled trials involving RFA for HCC. They are summarised in Table 1.

Table 1.
Summary of the recent randomised controlled trials on radiofrequency ablation (RAF).
3. TACE
TACE involves the injection of chemotherapy into liver tumours with a microembolus effect using iodised oil-based emulsion (lipiodol oil) to achieve arterial branch closure supplying the tumour in addition to medicinal suppression of tumour growth [21,67,68]. In 1972, the first surgical ligation of the hepatic artery with the consecutive injection of 5-fluorouracil to the portal vein was used to treat a liver tumour, which showed that the approach of blood interruption and local chemotherapy was safe. The development of an endovascular approach promptly followed it [69]. Today, an interventional radiologist enters the vascular system via the femoral approach, and then inserts the instruments to branch the hepatic artery supplying the tumour by navigating through the abdominal aorta, celiac trunk, and common hepatic artery.
TACE is the standard of care for intermediate-stage lesions (a multinodular liver-only disease in asymptomatic patients with compensated liver function). It usually contributes to the 2–2.5 year survival rate [3,4]. TACE can produce tumour necrosis and affects survival in selected patients with good liver reserve [3]. With preserved liver function, the risk of liver failure after c-TACE for HCC with portal vein invasion is acceptably low [1]. There is no consensus on optimal chemotherapeutic agents and no standardisation worldwide [1,70]. When used with lipiodol, there is an improvement in symptoms of pain and bleeding from HCC [3]. Neoadjuvant TACE can be used for patients with longer expected waiting list times for liver resection (specifically >6 months) or postoperatively in patients with a high risk of HCC recurrence [71,72,73,74].
A higher incidence of systemic adverse effects is connected with TACE due to the use of oil-based substances [68,75]. To mitigate this problem, TACE with drug-eluting beads (DEB-TACE) has been developed. It provides more selective and controlled drug delivery with microspheres [68,76,77,78]. Comparing those two treatment modalities for unresectable or recurrent HCC directly, there is no strong evidence of its increased efficacy, but it is associated with fewer side-effects [68].
There is ongoing research into clinical prognostication and patient selection for TACE. High pre-treatment albumin/bilirubin grade and aspartate aminotransferase-to-platelet index are associated with poorer outcomes [79,80]. Age, diabetes mellitus (DM), and the number of TACE sessions are risk factors for acute kidney injury—which increases mortality 4.74-fold—in patients with HCC after TACE [81]. Recently, an albumin-based algorithm was proposed [82].
There is a risk of incomplete treatment response after TACE, especially in large tumours, which are difficult to access. External beam radiotherapy provides favourable local control, but further systemic treatment could be required to improve overall survival [83]. Combining TACE with microwave ablation MWA improves 1, 2, and 3 year overall survival when compared to TACE alone for liver tumours greater than 5 cm [84,85].
There are no established imaging markers used for the prediction of TACE response. However, the delta of ADC values on MRI imaging higher than 20% facilitates early objective response to treatment [86,87]. To assess the presence of residual tumours, contrast-enhanced ultrasound can also be used. Its sensitivity is 0.85, specificity is 0.94, and accuracy is 93.5% [88]. There are many developments in post-procedure prognostication, and the optimal cut-off points in predicting the complete response of target lesions were a 52% ALT increase and a 46% AST increase after cTACE compared to the pre-treatment values [89].
The best intraarterial approach for unresectable HCC remains elusive. A network meta-analysis of 55 RCTs compared results of 5763 diverse patients among bland transarterial embolisation (TAE), cTACE, DEB-TACE, or transarterial radioembolisation (TARE), either alone or combined with adjuvant chemotherapy, local liver ablation, or external radiotherapy. All embolisation strategies improved survival, with TACE + external radiation/liver ablation achieving the highest [90]. Another study suggested the superiority of DEB-TACE over other treatment strategies [91].
Within the last 3 years, we identified 19 randomised controlled trials involving TACE for HCC. They are summarised in Table 2.

Table 2.
Summary of the recent randomised controlled trials on TAC.
4. Transarterial Radioembolisation (TARE), Also Known as Selective Internal Radiation Therapy (SIRT)
Liver tissue is very sensitive to radiation. The main problem with external beam radiotherapy was that it had to pass through the healthy tissue, causing its destruction. Intraarterial therapy became a solution to this problem [10]. TARE involves an injection of β-emitting yttrium-90 (Y90), holmium-166 (166Ho) integrated inside the glass matrix or on the surface of the resin microspheres, or metuximab-131 [21,111,112,113,114,115,116]. TARE can be performed with whole-liver treatment, as well as lobar or segmental approaches (the more distal catheter placement, the more localised the technique) [117].
TARE works by inducing necrosis and delaying tumour progression [118,119,120,121,122,123]. It is widely known that patients with HCC and portal vein thrombosis (PVT) are not amenable to TACE due to the high risk of ischemia and liver failure [5,24,124]. In particular, in this subset of patients, TARE provided competitive, if not more favourable, results compared to sorafenib [124,125,126]. Only limited HCC patients are responsive to immune checkpoint inhibitors, and a combination of these with RT may enhance the immune response; this phenomenon is named the systemic therapy augmented by radiotherapy (STAR) effect [12,127].
TARE appears to be a safe alternative treatment to TACE with a comparable complication profile and survival rates [21]. However, despite these undoubted advantages, a non-negligible proportion of advanced HCC patients still do not benefit from TARE, thus calling for more effective therapeutic regimens [124]. As combining systemic agents with locoregional treatments might represent a therapeutic tool in the armamentarium of hepato-oncology, there is no evidence that the addition of sorafenib prolongs survival or delay disease progression among HCC patients undergoing TARE [124].
TARE is well known to potentially lead to serious adverse events and suffers from a narrow safety profile, which limits its worldwide use despite favourable efficacy outcomes and cost-effective benefits [120,124]. It can lead to postradioembolisation syndrome (fatigue, nausea, vomiting, abdominal pain, and cachexia), radioembolisation-induced liver disease (jaundice, ascites, hyperbilirubinemia, and hypoalbuminemia 2–4 weeks post treatment), portal hypertension, and biliary complications (biliary strictures or cholecystitis), as well as radiation pneumonitis, gastrointestinal ulcers, and vascular injury [128,129,130]. However, in a meta-analysis of 1652 patients based on 11 studies, Y90-TARE not only improved 2 year overall survival and objective response among observational studies [130], but was also associated with fewer adverse events compared to TACE [90,130,131].
The current evidence suggests that there is a dose–response relationship for HCC tumours, with the best current evidence for the target mean dose of 100–250 Gy [132]. There is a need for the development of reporting standards and dose-dependent guidelines [132].
The reported economics of TARE as an interventional modality of HCC is largely variable. Overall, it appears cost-effective as a short- and long-term treatment of intermediate-advanced HCC [133].
Within the last 3 years, we identified four randomised controlled trials involving TARE for HCC. They are summarised in Table 3.

Table 3.
Summary of the recent randomised controlled trials on TARE.
5. Conclusions
Locoregional therapies have established their place in the HCC management algorithm. RFA has the potential for repeatability and safety for patients with significant medical comorbidities. The primary concerns with this procedure remain local tumour progression post RFA, needle-track seeding, and abscess formation.
TACE is the standard of care for intermediate-stage lesions. It can produce tumour necrosis and improve survival in patients with good liver reserve. Neoadjuvant TACE can be used for patients with longer expected waiting list times for liver surgery (resection or transplant). TACE with drug-eluting beads (DEB-TACE) provides more selective and controlled drug delivery with microspheres than cTACE. Although DEB-TACE is associated with fewer side-effects, it has no strong evidence of increased efficacy compared to cTACE.
TARE provides a safe alternative treatment to TACE with a comparable complication profile and survival rates. TARE is well known to potentially lead to serious adverse events and suffers from a narrow safety profile, which limits its worldwide use despite favourable efficacy outcomes and cost-effective benefits. A dose–response relationship exists for HCC tumours with the best current evidence for the target mean dose of 100–250 Gy. However, there is a need to develop reporting standards and dose-dependent guidelines.
More research is needed to identify the optimal locoregional HCC treatment, better identify the early predictive factors, and develop an individualised treatment regimen. With the availability of the checkpoint immunotherapy modalities, the interest in combining locoregional and systemic therapies has resurfaced, and results of the ongoing trials of these combinations are eagerly awaited.
Author Contributions
Conceptualisation, K.B., D.B. and A.P.; methodology, K.B. and A.P.; software, N/A; validation, N/A.; formal analysis, N/A.; investigation, M.A. and A.P.; resources, A.P.; data curation, K.B. and A.P.; writing—original draft preparation, A.P.; writing—review and editing, A.P., M.A., D.B. and K.B.; visualisation, A.P.; supervision, D.B. and K.B.; project administration, A.P. and K.B.; funding acquisition, D.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Garcia-Monaco, R.D.; Chung, J.W.; Vilgrain, V.; Bouattour, M.; Covey, A.M. Summary of key guidelines for locoregional treatment of HCC in Asia, Europe, South and North America. Br. J. Radiol. 2022, 95, 20220179. [Google Scholar] [CrossRef] [PubMed]
- Su, G.L.; Altayar, O.; O’shea, R.; Shah, R.; Estfan, B.; Wenzell, C.; Sultan, S.; Falck-Ytter, Y. AGA Clinical Practice Guideline on Systemic Therapy for Hepatocellular Carcinoma. Gastroenterology 2022, 162, 920–934. [Google Scholar] [CrossRef] [PubMed]
- Ryder, S.D. Guidelines for the diagnosis and treatment of hepatocellular carcinoma (HCC) in adults. Gut 2003, 52, iii1–iii8. [Google Scholar] [CrossRef] [PubMed]
- Llovet, J.M.; De Baere, T.; Kulik, L.; Haber, P.K.; Greten, T.F.; Meyer, T.; Lencioni, R. Locoregional therapies in the era of molecular and immune treatments for hepatocellular carcinoma. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 293–313. [Google Scholar] [CrossRef]
- Wen, N.; Cai, Y.; Li, F.; Ye, H.; Tang, W.; Song, P.; Cheng, N. The clinical management of hepatocellular carcinoma worldwide: A concise review and comparison of current guidelines: 2022 update. Biosci. Trends 2022, 16, 20–30. [Google Scholar] [CrossRef]
- Renzulli, M.; Golfieri, R.; Bologna Liver Oncology Group (BLOG). Proposal of a new diagnostic algorithm for hepatocellular carcinoma based on the Japanese guidelines but adapted to the Western world for patients under surveillance for chronic liver disease. J. Gastroenterol. Hepatol. 2016, 31, 69–80. [Google Scholar] [CrossRef]
- Park, H.J.; Jang, H.Y.; Kim, S.Y.; Lee, S.J.; Won, H.J.; Byun, J.H.; Choi, S.H.; Lee, S.S.; An, J.; Lim, Y.-S. Non-enhanced magnetic resonance imaging as a surveillance tool for hepatocellular carcinoma: Comparison with ultrasound. J. Hepatol. 2020, 72, 718–724. [Google Scholar] [CrossRef]
- Zhang, Y.; Numata, K.; Du, Y.; Maeda, S. Contrast Agents for Hepatocellular Carcinoma Imaging: Value and Progression. Front. Oncol. 2022, 12, 921667. [Google Scholar] [CrossRef]
- El-Feky, M. Infiltrative HCC—Portal vein tumour thrombus. Radiopaedia 2020, 75063. [Google Scholar] [CrossRef]
- Apisarnthanarax, S.; Barry, A.; Cao, M.; Czito, B.; DeMatteo, R.; Drinane, M.; Hallemeier, C.L.; Koay, E.J.; Lasley, F.; Meyer, J.; et al. External Beam Radiation Therapy for Primary Liver Cancers: An ASTRO Clinical Practice Guideline. Pract. Radiat. Oncol. 2022, 12, 28–51. [Google Scholar] [CrossRef]
- Zhou, J.; Sun, H.; Wang, Z.; Cong, W.; Wang, J.; Zeng, M.; Zhou, W.; Bie, P.; Liu, L.; Wen, T.; et al. Guidelines for the Diagnosis and Treatment of Hepatocellular Carcinoma (2019 Edition). Liver Cancer 2020, 9, 682–720. [Google Scholar] [CrossRef]
- Chen, L.-C.; Lin, H.-Y.; Hung, S.-K.; Chiou, W.-Y.; Lee, M.-S. Role of modern radiotherapy in managing patients with hepatocellular carcinoma. World J. Gastroenterol. 2021, 27, 2434–2457. [Google Scholar] [CrossRef]
- Delis, S.-G.; Dervenis, C. Selection criteria for liver resection in patients with hepatocellular carcinoma and chronic liver disease. World J. Gastroenterol. 2008, 14, 3452–3460. [Google Scholar] [CrossRef] [PubMed]
- Bruix, J.; Sherman, M.; Llovet, J.M.; Beaugrand, M.; Lencioni, R.; Burroughs, A.K.; Christensen, E.; Pagliaro, L.; Colombo, M.; Rodés, J. Clinical Management of Hepatocellular Carcinoma. Conclusions of the Barcelona-2000 EASL Conference. J. Hepatol. 2001, 35, 421–430. [Google Scholar] [CrossRef] [PubMed]
- Tzartzeva, K.; Obi, J.; Rich, N.E.; Parikh, N.D.; Marrero, J.A.; Yopp, A.; Waljee, A.K.; Singal, A.G. Surveillance Imaging and Alpha Fetoprotein for Early Detection of Hepatocellular Carcinoma in Patients With Cirrhosis: A Meta-analysis. Gastroenterology 2018, 154, 1706–1718.e1. [Google Scholar] [CrossRef]
- Sparchez, Z.; Radu, P.; Bartos, A.; Nenu, I.; Craciun, R.; Mocan, T.; Horhat, A.; Spârchez, M.; Dufour, J.-F. Combined treatments in hepatocellular carcinoma: Time to put them in the guidelines? World J. Gastrointest. Oncol. 2021, 13, 1896–1918. [Google Scholar] [CrossRef]
- Qiu, J.; Chen, S.; Wu, H. Long-term outcomes after hepatic resection combined with radiofrequency ablation for initially unresectable multiple and bilobar liver malignancies. J. Surg. Res. 2014, 188, 14–20. [Google Scholar] [CrossRef]
- Di Martino, M.; Vitale, A.; Ferraro, D.; Maniscalco, M.; Pisaniello, D.; Arenga, G.; Falaschi, F.; Terrone, A.; Iacomino, A.; Lanza, A.G.; et al. Downstaging Therapies for Patients with Hepatocellular Carcinoma Awaiting Liver Transplantation: A Systematic Review and Meta-Analysis on Intention-to-Treat Outcomes. Cancers 2022, 14, 5102. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Lan, T.; Zhong, H.; Zhang, Z.; Xie, H.; Li, Y.; Huang, W. To Systematically Evaluate and Analyze the Efficacy and Safety of Transcatheter Arterial Chemoembolization (TACE) in the Treatment of Primary Liver Cancer. J. Health Eng. 2022, 2022, 8223336. [Google Scholar] [CrossRef]
- Chen, X.; Lai, L.; Ye, J.; Li, L. Downstaging Therapies for Unresectable Hepatocellular Carcinoma Prior to Hepatic Resection: A Systematic Review and Meta-Analysis. Front. Oncol. 2021, 11, 740762. [Google Scholar] [CrossRef]
- Lobo, L.; Yakoub, D.; Picado, O.; Ripat, C.; Pendola, F.; Sharma, R.; ElTawil, R.; Kwon, D.; Venkat, S.; Portelance, L.; et al. Unresectable Hepatocellular Carcinoma: Radioembolization Versus Chemoembolization: A Systematic Review and Meta-analysis. Cardiovasc. Interv. Radiol. 2016, 39, 1580–1588. [Google Scholar] [CrossRef]
- Sangro, B.; Sarobe, P.; Hervás-Stubbs, S.; Melero, I. Advances in immunotherapy for hepatocellular carcinoma. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 525–543. [Google Scholar] [CrossRef] [PubMed]
- Marrero, J.A.; Kulik, L.M.; Sirlin, C.B.; Zhu, A.X.; Finn, R.S.; Abecassis, M.M.; Roberts, L.R.; Heimbach, J.K. Diagnosis, Staging, and Management of Hepatocellular Carcinoma: 2018 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology 2018, 68, 723–750. [Google Scholar] [CrossRef] [PubMed]
- European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J. Hepatol. 2018, 69, 182–236. [Google Scholar] [CrossRef] [PubMed]
- Llovet, J.M.; Lencioni, R. mRECIST for HCC: Performance and novel refinements. J. Hepatol. 2020, 72, 288–306. [Google Scholar] [CrossRef]
- Rosenthal, D.I.; Alexander, A.; Rosenberg, A.A.; Springfield, D. Ablation of osteoid osteomas with a percutaneously placed electrode: A new procedure. Radiology 1992, 183, 29–33. [Google Scholar] [CrossRef]
- Omata, M.; Lesmana, L.A.; Tateishi, R.; Chen, P.-J.; Lin, S.-M.; Yoshida, H.; Kudo, M.; Lee, J.M.; Choi, B.I.; Poon, R.T.P.; et al. Asian Pacific Association for the Study of the Liver consensus recommendations on hepatocellular carcinoma. Hepatol. Int. 2010, 4, 439–474. [Google Scholar] [CrossRef]
- Nishikawa, H.; Kimura, T.; Kita, R.; Osaki, Y. Radiofrequency ablation for hepatocellular carcinoma. Int. J. Hyperth. 2013, 29, 558–568. [Google Scholar] [CrossRef]
- Curley, S.A.; Izzo, F.; Ellis, L.M.; Vauthey, J.N.; Vallone, P. Radiofrequency Ablation of Hepatocellular Cancer in 110 Patients with Cirrhosis. Ann. Surg. 2000, 232, 381–391. [Google Scholar] [CrossRef]
- Ishikawa, T.; Kohno, T.; Shibayama, T.; Fukushima, Y.; Obi, S.; Teratani, T.; Shiina, S.; Shiratori, Y.; Omata, M. Thoracoscopic Thermal Ablation Therapy for Hepatocellular Carcinoma Located Beneath the Diaphragm. Endoscopy 2001, 33, 697–702. [Google Scholar] [CrossRef]
- Tiong, L.; Maddern, G.J. Systematic review and meta-analysis of survival and disease recurrence after radiofrequency ablation for hepatocellular carcinoma. Br. J. Surg. 2011, 98, 1210–1224. [Google Scholar] [CrossRef] [PubMed]
- Kudo, M. Radiofrequency Ablation for Hepatocellular Carcinoma: Updated Review in 2010. Oncology 2010, 78, 113–124. [Google Scholar] [CrossRef] [PubMed]
- HirokiNishikawa, H.; Inuzuka, T.; Takeda, H.; Nakajima, J.; Matsuda, F.; Sakamoto, A.; Henmi, S.; Hatamaru, K.; Ishikawa, T.; Saito, S.; et al. Comparison of percutaneous radiofrequency thermal ablation and surgical resection for small hepatocellular carcinoma. BMC Gastroenterol. 2011, 11, 143. [Google Scholar] [CrossRef]
- Chan, A.C.Y.; Chan, S.C.; Chok, K.S.H.; Cheung, T.T.; Chiu, D.W.; Poon, R.T.P.; Fan, S.T.; Lo, C.M. Treatment strategy for recurrent hepatocellular carcinoma: Salvage transplantation, repeated resection, or radiofrequency ablation? Liver Transplant. 2013, 19, 411–419. [Google Scholar] [CrossRef]
- Lau, W.Y.; Lai, E.C.H. The Current Role of Radiofrequency Ablation in the Management of Hepatocellular Carcinoma. Ann. Surg. 2009, 249, 20–25. [Google Scholar] [CrossRef]
- Lencioni, R. Loco-regional treatment of hepatocellular carcinoma. Hepatology 2010, 52, 762–773. [Google Scholar] [CrossRef]
- Sheiman, R.G.; Mullan, C.; Ahmed, M. In vivo determination of a modified heat capacity of small hepatocellular carcinomas prior to radiofrequency ablation: Correlation with adjacent vasculature and tumour recurrence. Int. J. Hyperth. 2012, 28, 122–131. [Google Scholar] [CrossRef]
- Rossi, S.; Di Stasi, M.; Buscarini, E.; Quaretti, P.; Garbagnati, F.; Squassante, L.; Paties, C.T.; E Silverman, D. Percutaneous RF interstitial thermal ablation in the treatment of hepatic cancer. Am. J. Roentgenol. 1996, 167, 759–768. [Google Scholar] [CrossRef]
- Germani, G.; Pleguezuelo, M.; Gurusamy, K.; Meyer, T.; Isgrò, G.; Burroughs, A.K. Clinical outcomes of radiofrequency ablation, percutaneous alcohol and acetic acid injection for hepatocelullar carcinoma: A meta-analysis. J. Hepatol. 2010, 52, 380–388. [Google Scholar] [CrossRef]
- Kudo, M. Local ablation therapy for hepatocellular carcinoma: Current status and future perspectives. J. Gastroenterol. 2004, 39, 205–214. [Google Scholar] [CrossRef]
- Kasugai, H.; Osaki, Y.; Oka, H.; Kudo, M.; Seki, T. Severe Complications of Radiofrequency Ablation Therapy for Hepatocellular Carcinoma: An Analysis of 3891 Ablations in 2614 Patients. Oncology 2007, 72, 72–75. [Google Scholar] [CrossRef] [PubMed]
- Xuan, D.; Wen, W.; Xu, D.; Jin, T. Survival comparison between radiofrequency ablation and surgical resection for patients with small hepatocellular carcinoma: A systematic review and meta-analysis. Medicine 2021, 100, e24585. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Wang, R.; Liu, S.; Peng, G.; Yu, H.; Wang, X. Comparison of the safety and efficacy of hepatic resection and radiofrequency ablation in the treatment of single small hepatocellular carcinoma: Systematic review and meta-analysis. Transl. Cancer Res. 2022, 11, 580–590. [Google Scholar] [CrossRef] [PubMed]
- Jie, T.; Guoying, F.; Gang, T.; Zhengrong, S.; Maoping, L. Efficacy and Safety of Fusion Imaging in Radiofrequency Ablation of Hepatocellular Carcinoma Compared to Ultrasound: A Meta-Analysis. Front. Surg. 2021, 8, 728098. [Google Scholar] [CrossRef] [PubMed]
- Nishikawa, H.; Inuzuka, T.; Takeda, H.; Nakajima, J.; Sakamoto, A.; Henmi, S.; Matsuda, F.; Eso, Y.; Ishikawa, T.; Saito, S.; et al. Percutaneous radiofrequency ablation therapy for hepatocellular carcinoma: A proposed new grading system for the ablative margin and prediction of local tumor progression and its validation. J. Gastroenterol. 2011, 46, 1418–1426. [Google Scholar] [CrossRef] [PubMed]
- Vestito, A.; Dajti, E.; Cortellini, F.; Montagnani, M.; Bazzoli, F.; Zagari, R.M. Can Liver Ultrasound Elastography Predict the Risk of Hepatocellular Carcinoma Recurrence After Radiofrequency Ablation? A Systematic Review and Meta-Analysis. Ultraschall Med. 2021, 44, e139–e147. [Google Scholar] [CrossRef]
- Yu, Q.; Liu, C.; Navuluri, R.; Ahmed, O. Percutaneous microwave ablation versus radiofrequency ablation of hepatocellular carcinoma: A meta-analysis of randomized controlled trials. Abdom. Radiol. 2021, 46, 4467–4475. [Google Scholar] [CrossRef]
- Spiliotis, A.E.; Gäbelein, G.; Holländer, S.; Scherber, P.-R.; Glanemann, M.; Patel, B. Microwave ablation compared with radiofrequency ablation for the treatment of liver cancer: A systematic review and meta-analysis. Radiol. Oncol. 2021, 55, 247–258. [Google Scholar] [CrossRef]
- Khan, A.; Mostowy, M.; Owusu, M.; Mutambanengwe, M.; Habimana, S.; Bence, S.; Facciorusso, A. Microwave ablation determines similar survival outcomes as compared to radiofrequency ablation for the treatment of hepatocellular carcinoma. Expert Rev. Gastroenterol. Hepatol. 2020, 15, 829–833. [Google Scholar] [CrossRef]
- Ricci, A.D.; Rizzo, A.; Bonucci, C.; Tavolari, S.; Palloni, A.; Frega, G.; Mollica, V.; Tober, N.; Mazzotta, E.; Felicani, C.; et al. The (Eternal) Debate on Microwave Ablation Versus Radiofrequency Ablation in BCLC-A Hepatocellular Carcinoma. Vivo 2020, 34, 3421–3429. [Google Scholar] [CrossRef]
- Han, J.; Fan, Y.-C.; Wang, K. Radiofrequency ablation versus microwave ablation for early stage hepatocellular carcinoma. Medicine 2020, 99, e22703. [Google Scholar] [CrossRef] [PubMed]
- Cui, R.; Yu, J.; Kuang, M.; Duan, F.; Liang, P. Microwave ablation versus other interventions for hepatocellular carcinoma: A systematic review and meta-analysis. J. Cancer Res. Ther. 2020, 16, 379–386. [Google Scholar] [CrossRef]
- Facciorusso, A.; El Aziz, M.A.A.; Tartaglia, N.; Ramai, D.; Mohan, B.P.; Cotsoglou, C.; Pusceddu, S.; Giacomelli, L.; Ambrosi, A.; Sacco, R. Microwave Ablation Versus Radiofrequency Ablation for Treatment of Hepatocellular Carcinoma: A Meta-Analysis of Randomized Controlled Trials. Cancers 2020, 12, 3796. [Google Scholar] [CrossRef]
- Lu, D.-E.; Cheng, S.-W.; Lin, Y.-S.; Tu, M.-W.; Lee, C.-H.; Chen, C.; Chen, K.-H. Radiofrequency ablation and percutaneous ethanol injection versus radiofrequency ablation alone for hepatocellular carcinoma: A systematic review and meta-analysis. Ann. Hepatol. 2022, 27, 100729. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Tong, Y.; Yang, L.; He, X.; Bao, G.; Du, X. Identifying optimal therapies in patients with advanced hepatocellular carcinoma: A systematic review and network meta-analysis. Transl. Gastroenterol. Hepatol. 2022, 7, 38. [Google Scholar] [CrossRef]
- Yang, Y.; Yu, H.; Qi, L.; Liu, C.; Feng, Y.; Qi, J.; Li, J.; Zhu, Q. Combined radiofrequency ablation or microwave ablation with transarterial chemoembolization can increase efficiency in intermediate-stage hepatocellular carcinoma without more complication: A systematic review and meta-analysis. Int. J. Hyperth. 2022, 39, 455–465. [Google Scholar] [CrossRef]
- Gui, C.H.; Baey, S.; D’Cruz, R.T.; Shelat, V.G. Trans-arterial chemoembolization + radiofrequency ablation versus surgical resection in hepatocellular carcinoma—A meta-analysis. Eur. J. Surg. Oncol. 2020, 46, 763–771. [Google Scholar] [CrossRef]
- Kwak, M.H.; Lee, M.W.; Ko, S.E.; Rhim, H.; Kang, T.W.; Song, K.D.; Kim, J.M.; Choi, G.-S. Laparoscopic radiofrequency ablation versus percutaneous radiofrequency ablation for subphrenic hepatocellular carcinoma. Ultrasonography 2022, 41, 543–552. [Google Scholar] [CrossRef] [PubMed]
- Hendriks, P.; Rietbergen, D.D.D.; van Erkel, A.R.; Coenraad, M.J.; Arntz, M.J.; Bennink, R.J.; Braat, A.E.; Crobach, A.S.L.P.; van Delden, O.M.; van der Hulle, T.; et al. Study Protocol: Adjuvant Holmium-166 Radioembolization After Radiofrequency Ablation in Early-Stage Hepatocellular Carcinoma Patients—A Dose-Finding Study (HORA EST HCC Trial). Cardiovasc. Interv. Radiol. 2022, 45, 1057–1063. [Google Scholar] [CrossRef]
- Radosevic, A.; Quesada, R.; Serlavos, C.; Sánchez, J.; Zugazaga, A.; Sierra, A.; Coll, S.; Busto, M.; Aguilar, G.; Flores, D.; et al. Microwave versus radiofrequency ablation for the treatment of liver malignancies: A randomized controlled phase 2 trial. Sci. Rep. 2022, 12, 1–10. [Google Scholar] [CrossRef]
- Suh, Y.S.; Choi, J.W.; Yoon, J.H.; Lee, D.H.; Kim, Y.J.; Lee, J.H.; Yu, S.J.; Cho, E.J.; Yoon, J.H. No-Touch vs. Conventional Radiofrequency Ablation Using Twin Internally Cooled Wet Electrodes for Small Hepatocellular Carcinomas: A Randomized Prospective Comparative Study. Korean J. Radiol. 2022, 22, 1974–1984. [Google Scholar] [CrossRef] [PubMed]
- Bockorny, B.; Bullock, A.J.; Abrams, T.A.; Faintuch, S.; Alsop, D.C.; Goldberg, S.N.; Ahmed, M.; Miksad, R.A. Priming of Sorafenib Prior to Radiofrequency Ablation Does Not Increase Treatment Effect in Hepatocellular Carcinoma. Dig. Dis. Sci. 2022, 67, 3455–3463. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.W.; Lee, J.M.; Lee, D.H.; Yoon, J.-H.; Kim, Y.J.; Yu, S.J.; Cho, E.J. Radiofrequency Ablation Using a Separable Clustered Electrode for the Treatment of Hepatocellular Carcinomas: A Randomized Controlled Trial of a Dual-Switching Monopolar Mode Versus a Single-Switching Monopolar Mode. Korean J. Radiol. 2021, 22, 179–188. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.W.; Lee, J.-H.; Lee, D.H.; Yoon, J.-H.; Kim, Y.J.; Yu, S.J.; Cho, E.J. Radiofrequency ablation using internally cooled wet electrodes in bipolar mode for the treatment of recurrent hepatocellular carcinoma after locoregional treatment: A randomized prospective comparative study. PLoS ONE 2020, 15, e0239733. [Google Scholar] [CrossRef] [PubMed]
- Chong, C.C.; Lee, K.F.; Cheung, S.Y.; Chu, C.C.; Fong, A.K.; Wong, J.; Hui, J.W.; Fung, A.K.; Lok, H.T.; Lo, E.Y.; et al. Prospective double-blinded randomized controlled trial of Microwave versus RadioFrequency Ablation for hepatocellular carcinoma (McRFA trial). HPB 2020, 22, 1121–1127. [Google Scholar] [CrossRef]
- Paul, S.; Acharya, S.; Gamanagatti, S.; Sreenivas, V.; Shalimar, S.; Gulati, M. Acetic acid versus radiofrequency ablation for the treatment of hepatocellular carcinoma: A randomized controlled trial. Diagn. Interv. Imaging 2020, 101, 101–110. [Google Scholar] [CrossRef]
- Virmani, S.; Rhee, T.K.; Ryu, R.K.; Sato, K.T.; Lewandowski, R.J.; Mulcahy, M.F.; Kulik, L.M.; Szolc-Kowalska, B.; Woloschak, G.E.; Yang, G.-Y.; et al. Comparison of Hypoxia-inducible Factor-1α Expression before and after Transcatheter Arterial Embolization in Rabbit VX2 Liver Tumors. J. Vasc. Interv. Radiol. 2008, 19, 1483–1489. [Google Scholar] [CrossRef]
- Bzeizi, K.I.; Arabi, M.; Jamshidi, N.; Albenmousa, A.; Sanai, F.M.; Al-Hamoudi, W.; Alghamdi, S.; Broering, D.; Alqahtani, S.A. Conventional Transarterial Chemoembolization Versus Drug-Eluting Beads in Patients with Hepatocellular Carcinoma: A Systematic Review and Meta-Analysis. Cancers 2021, 13, 6172. [Google Scholar] [CrossRef]
- Guan, Y.-S.; He, Q.; Wang, M.-Q. Transcatheter Arterial Chemoembolization: History for More than 30 Years. ISRN Gastroenterol. 2012, 2012, 480650. [Google Scholar] [CrossRef]
- Renzulli, M.; Peta, G.; Vasuri, F.; Marasco, G.; Caretti, D.; Bartalena, L.; Spinelli, D.; Giampalma, E.; D’errico, A.; Golfieri, R. Standardization of conventional chemoembolization for hepatocellular carcinoma. Ann. Hepatol. 2020, 22, 100278. [Google Scholar] [CrossRef]
- Butcher, D.A.; Brandis, K.J.; Wang, H.; Spannenburg, L.; Bridle, K.R.; Crawford, D.H.; Liang, X. Long-term survival and postoperative complications of pre-liver transplantation transarterial chemoembolisation in hepatocellular carcinoma: A systematic review and meta-analysis. Eur. J. Surg. Oncol. 2021, 48, 621–631. [Google Scholar] [CrossRef] [PubMed]
- Liang, L.; Li, C.; Diao, Y.-K.; Jia, H.-D.; Xing, H.; Pawlik, T.M.; Lau, W.Y.; Shen, F.; Huang, D.-S.; Zhang, C.-W.; et al. Survival benefits from adjuvant transcatheter arterial chemoembolization in patients undergoing liver resection for hepatocellular carcinoma: A systematic review and meta-analysis. Ther. Adv. Gastroenterol. 2020, 13, 1756284820977693. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Ma, T.; Zhang, J.; Zhang, X.; Chen, W.; Shen, Y.; Bai, X.; Liang, T. A systematic review and meta-analysis of adjuvant transarterial chemoembolization after curative resection for patients with hepatocellular carcinoma. HPB 2020, 22, 795–808. [Google Scholar] [CrossRef] [PubMed]
- Huo, Y.R.; Chan, M.V.; Chan, C. Resection Plus Post-operative Adjuvant Transcatheter Arterial Chemoembolization (TACE) Compared with Resection Alone for Hepatocellular Carcinoma: A Systematic Review and Meta-analysis. Cardiovasc. Interv. Radiol. 2020, 43, 572–586. [Google Scholar] [CrossRef] [PubMed]
- De Baere, T.; Arai, Y.; Lencioni, R.; Geschwind, J.-F.; Rilling, W.; Salem, R.; Matsui, O.; Soulen, M.C. Treatment of Liver Tumors with Lipiodol TACE: Technical Recommendations from Experts Opinion. Cardiovasc. Interv. Radiol. 2016, 39, 334–343. [Google Scholar] [CrossRef]
- Li, H.; Wu, F.; Duan, M.; Zhang, G. Drug-eluting bead transarterial chemoembolization (TACE) vs conventional TACE in treating hepatocellular carcinoma patients with multiple conventional TACE treatments history. Medicine 2019, 98, e15314. [Google Scholar] [CrossRef]
- Varela, M.; Real, M.I.; Burrel, M.; Forner, A.; Sala, M.; Brunet, M.; Ayuso, C.; Castells, L.; Montañá, X.; Llovet, J.M.; et al. Chemoembolization of hepatocellular carcinoma with drug eluting beads: Efficacy and doxorubicin pharmacokinetics. J. Hepatol. 2007, 46, 474–481. [Google Scholar] [CrossRef]
- Nouri, Y.M.; Kim, J.H.; Yoon, H.-K.; Ko, H.-K.; Shin, J.H.; Gwon, D.I. Update on Transarterial Chemoembolization with Drug-Eluting Microspheres for Hepatocellular Carcinoma. Korean J. Radiol. 2019, 20, 34–49. [Google Scholar] [CrossRef]
- Mishra, G.; Majeed, A.; Dev, A.; Eslick, G.D.; Pinato, D.J.; Izumoto, H.; Hiraoka, A.; Huo, T.-I.; Liu, P.-H.; Johnson, P.J.; et al. Clinical Utility of Albumin Bilirubin Grade as a Prognostic Marker in Patients with Hepatocellular Carcinoma Undergoing Transarterial Chemoembolization: A Systematic Review and Meta-analysis. J. Gastrointest. Cancer 2022, 1–13. [Google Scholar] [CrossRef]
- Zhang, X.; Svn, Z.; Liv, M.; Liu, M.; Zhang, Y.; Sun, Q. Assessment of Prognostic Value of Aspartate Aminotransferase-to-Platelet Ratio Index in Patients with Hepatocellular Carcinoma: Meta-Analysis of 28 Cohort Studies. Front. Med. 2021, 8, 756210. [Google Scholar] [CrossRef]
- Mou, Z.; Guan, T.; Chen, L. Acute Kidney Injury in Adult Patients With Hepatocellular Carcinoma After TACE or Hepatectomy Treatment. Front. Oncol. 2022, 12, 627895. [Google Scholar] [CrossRef] [PubMed]
- Ho, S.-Y.; Liu, P.-H.; Hsu, C.-Y.; Huang, Y.-H.; Liao, J.-I.; Su, C.-W.; Hou, M.-C.; Huo, T.-I. Comparison of Four Albumin-Based Liver Reserve Models (ALBI/EZ-ALBI/PALBI/PAL) against MELD for Patients with Hepatocellular Carcinoma Undergoing Transarterial Chemoembolization. Cancers 2023, 15, 1925. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.S.; Park, S.; Rim, C.H.; Yoon, W.S.; Shin, I.-S.; Lee, H.A. Salvage External Beam Radiotherapy after Incomplete Transarterial Chemoembolization for Hepatocellular Carcinoma: A Meta-Analysis and Systematic Review. Medicina 2021, 57, 1000. [Google Scholar] [CrossRef]
- Shen, A.; Liu, M.; Zheng, D.; Chen, Q.; Wu, Z. Adjuvant transarterial chemoembolization after curative hepatectomy for hepatocellular carcinoma with microvascular invasion: A systematic review and meta-analysis. Clin. Res. Hepatol. Gastroenterol. 2020, 44, 142–154. [Google Scholar] [CrossRef]
- Wang, L.; Ke, Q.; Lin, N.; Huang, Q.; Zeng, Y.; Liu, J. The efficacy of transarterial chemoembolization combined with microwave ablation for unresectable hepatocellular carcinoma: A systematic review and meta-analysis. Int. J. Hyperth. 2019, 36, 1287–1295. [Google Scholar] [CrossRef]
- Drewes, R.; Heinze, C.; Pech, M.; Powerski, M.; Woidacki, K.; Wienke, A.; Surov, A.; Omari, J. Apparent Diffusion Coefficient Can Predict Therapy Response of Hepatocellular Carcinoma to Transcatheter Arterial Chemoembolization. Dig. Dis. 2022, 40, 596–606. [Google Scholar] [CrossRef]
- Liu, Z.; Fan, J.-M.; He, C.; Li, Z.-F.; Xu, Y.-S.; Liu, H.-F.; Lei, J.-Q.; Li, Z. Utility of diffusion weighted imaging with the quantitative apparent diffusion coefficient in diagnosing residual or recurrent hepatocellular carcinoma after transarterial chemoembolization: A meta-analysis. Cancer Imaging 2020, 20, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Hai, Y.; Savsani, E.; Chong, W.; Eisenbrey, J.; Lyshchik, A. Meta-analysis and systematic review of contrast-enhanced ultrasound in evaluating the treatment response after locoregional therapy of hepatocellular carcinoma. Abdom. Imaging 2021, 46, 5162–5179. [Google Scholar] [CrossRef]
- Granito, A.; Facciorusso, A.; Sacco, R.; Bartalena, L.; Mosconi, C.; Cea, U.V.; Cappelli, A.; Antonino, M.; Modestino, F.; Brandi, N.; et al. TRANS-TACE: Prognostic Role of the Transient Hypertransaminasemia after Conventional Chemoembolization for Hepatocellular Carcinoma. J. Pers. Med. 2021, 11, 1041. [Google Scholar] [CrossRef]
- Katsanos, K.; Kitrou, P.; Spiliopoulos, S.; Maroulis, I.; Petsas, T.; Karnabatidis, D. Comparative effectiveness of different transarterial embolization therapies alone or in combination with local ablative or adjuvant systemic treatments for unresectable hepatocellular carcinoma: A network meta-analysis of randomized controlled trials. PLoS ONE 2017, 12, e0184597. [Google Scholar] [CrossRef]
- Yang, B.; Liang, J.; Qu, Z.; Yang, F.; Liao, Z.; Gou, H. Transarterial strategies for the treatment of unresectable hepatocellular carcinoma: A systematic review. PLoS ONE 2020, 15, e0227475. [Google Scholar] [CrossRef]
- Zhang, X.; Luo, Y.; Tsauo, J.; Zhao, H.; Gong, T.; Li, J.; Li, Y.; Zeng, H.; Sun, W.; Li, X. Transradial versus transfemoral access without closure device for transarterial chemoembolization in patients with hepatocellular carcinoma: A randomized trial. Eur. Radiol. 2022, 32, 6812–6819. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Chen, Y.; Liao, H.; Gu, Y.; Meng, X.; Dong, W. Operator radiation dose during trans-hepatic arterial chemoembolization: Different patients’ positions via transradial or transfemoral access. Diagn. Interv. Radiol. 2022, 28, 376–382. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.-D.; Li, X.; Ji, J.-S.; Huang, M.; Shao, G.-L.; Lu, J.; Zhao, X.-Y.; Li, H.-L.; Yang, Z.-Q.; Tu, J.-F.; et al. TACE with dicycloplatin in patients with unresectable hepatocellular carcinoma: A multicenter randomized phase II trial. Eur. Radiol. 2022, 32, 7335–7343. [Google Scholar] [CrossRef]
- Dhondt, E.; Lambert, B.; Hermie, L.; Huyck, L.; Vanlangenhove, P.; Geerts, A.; Verhelst, X.; Aerts, M.; Vanlander, A.; Berrevoet, F.; et al. 90Y Radioembolization versus Drug-eluting Bead Chemoembolization for Unresectable Hepatocellular Carcinoma: Results from the TRACE Phase II Randomized Controlled Trial. Radiology 2022, 303, 699–710. [Google Scholar] [CrossRef]
- Llovet, J.M.; Vogel, A.; Madoff, D.C.; Finn, R.S.; Ogasawara, S.; Ren, Z.; Mody, K.; Li, J.J.; Siegel, A.B.; Dubrovsky, L.; et al. Randomized Phase 3 LEAP-012 Study: Transarterial Chemoembolization with or Without Lenvatinib Plus Pembrolizumab for Intermediate-Stage Hepatocellular Carcinoma Not Amenable to Curative Treatment. Cardiovasc. Interv. Radiol. 2022, 45, 405–412. [Google Scholar] [CrossRef]
- Chen, W.-T.; Lin, S.-M.; Lee, W.-C.; Wu, T.-J.; Lin, C.-C.; Shen, C.-H.; Chang, M.-L.; Lin, C.-L.; Yeh, C.-T. GALNT14 genotype-guided chemoembolization plus sorafenib therapy in hepatocellular carcinoma: A randomized trial. Hepatol. Int. 2022, 16, 148–158. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Zhao, Y.; He, L.; Bo, C.; An, Y.; Li, N.; Ma, W.; Guo, Y.; Guo, Y.; Zhang, C. Effect of camrelizumab plus transarterial chemoembolization on massive hepatocellular carcinoma. Clin. Res. Hepatol. Gastroenterol. 2022, 46, 101851. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wu, J.-L.; Li, L.-Q. Efficacy comparison of optimal treatments for hepatocellular carcinoma patients with portal vein tumor thrombus. Ann. Hepatol. 2021, 27, 100552. [Google Scholar] [CrossRef]
- Aramaki, O.; Takayama, T.; Moriguchi, M.; Sakamoto, H.; Yodono, H.; Kokudo, N.; Yamanaka, N.; Kawasaki, S.; Sasaki, Y.; Kubota, K.; et al. Arterial chemoembolisation with cisplatin versus epirubicin for hepatocellular carcinoma (ACE 500 study): A multicentre, randomised controlled phase 2/3 trial. Eur. J. Cancer 2021, 157, 373–382. [Google Scholar] [CrossRef]
- Fu, J.; Wang, Y.; Zhang, J.; Yuan, K.; Yan, J.; Yuan, B.; Guan, Y.; Wang, M. The safety and efficacy of transarterial chemoembolisation with bleomycin for hepatocellular carcinoma unresponsive to doxorubicin: A prospective single-centre study. Clin. Radiol. 2021, 76, 864.e7–864.e12. [Google Scholar] [CrossRef]
- Ding, X.; Sun, W.; Li, W.; Shen, Y.; Guo, X.; Teng, Y.; Liu, X.; Zheng, L.; Li, W.; Chen, J. Transarterial chemoembolization plus lenvatinib versus transarterial chemoembolization plus sorafenib as first-line treatment for hepatocellular carcinoma with portal vein tumor thrombus: A prospective randomized study. Cancer 2021, 127, 3782–3793. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Feng, Y.; Liu, Y.; Ye, X.; Ji, X.; Sun, L.; Gao, F.; Zhang, Q.; Li, Y.; Zhu, B.; et al. Fuzheng Jiedu Xiaoji formulation inhibits hepatocellular carcinoma progression in patients by targeting the AKT/CyclinD1/p21/p27 pathway. Phytomedicine 2021, 87, 153575. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Chang, R.; He, Z.; Hong, M. How to prophylactically alleviate postembolization syndrome following transarterial chemoembolization? Medicine 2021, 100, e25360. [Google Scholar] [CrossRef]
- Bessar, A.A.; Farag, A.; Monem, S.M.A.; Wadea, F.M.; Shaker, S.E.; Ebada, M.A.; Bessar, M.A. Transarterial chemoembolisation in patients with hepatocellular carcinoma: Low-dose doxorubicin reduces post-embolisation syndrome without affecting survival—Prospective interventional study. Eur. Radiol. Exp. 2021, 5, 1–10. [Google Scholar] [CrossRef]
- Zaitoun, M.M.A.; Elsayed, S.B.; Zaitoun, N.A.; Soliman, R.K.; Elmokadem, A.H.; Farag, A.A.; Amer, M.; Hendi, A.M.; Mahmoud, N.E.M.; El Deen, D.S.; et al. Combined therapy with conventional trans-arterial chemoembolization (cTACE) and microwave ablation (MWA) for hepatocellular carcinoma >3–<5 cm. Int. J. Hyperth. 2021, 38, 248–256. [Google Scholar] [CrossRef]
- Gjoreski, A.; Jovanoska, I.; Risteski, F.; Veljanova, B.P.; Nedelkovski, D.; Dimov, V.; Jovanovska, R.P.; Angelovska, B.G.; Mitrevski, N.; Dimova, B. Single-center randomized trial comparing conventional chemoembolization versus doxorubicin-loaded polyethylene glycol microspheres for early- and intermediate-stage hepatocellular carcinoma. Eur. J. Cancer Prev. 2021, 30, 258–266. [Google Scholar] [CrossRef]
- Guo, J.-H.; Liu, S.-X.; Gao, S.; Kou, F.-X.; Zhang, X.; Wu, D.; Li, X.-T.; Chen, H.; Wang, X.-D.; Liu, P.; et al. Transarterial chemoembolization with hepatic arterial infusion chemotherapy plus S-1 for hepatocellular carcinoma. World J. Gastroenterol. 2020, 26, 3975–3988. [Google Scholar] [CrossRef] [PubMed]
- Turpin, A.; de Baere, T.; Heurgué, A.; Le Malicot, K.; Ollivier-Hourmand, I.; Lecomte, T.; Perrier, H.; Vergniol, J.; Sefrioui, D.; Rinaldi, Y.; et al. Liver transarterial chemoembolization and sunitinib for unresectable hepatocellular carcinoma: Results of the PRODIGE 16 study. Clin. Res. Hepatol. Gastroenterol. 2021, 45, 101464. [Google Scholar] [CrossRef]
- Kudo, M.; Ueshima, K.; Ikeda, M.; Torimura, T.; Tanabe, N.; Aikata, H.; Izumi, N.; Yamasaki, T.; Nojiri, S.; Hino, K.; et al. Randomised, multicentre prospective trial of transarterial chemoembolisation (TACE) plus sorafenib as compared with TACE alone in patients with hepatocellular carcinoma: TACTICS trial. Gut 2020, 69, 1492–1501. [Google Scholar] [CrossRef]
- Sato, K.; Lewandowski, R.J.; Bui, J.T.; Omary, R.; Hunter, R.D.; Kulik, L.; Mulcahy, M.; Liu, D.; Chrisman, H.; Resnick, S.; et al. Treatment of Unresectable Primary and Metastatic Liver Cancer with Yttrium-90 Microspheres (TheraSphere®): Assessment of Hepatic Arterial Embolization. Cardiovasc. Interv. Radiol. 2006, 29, 522–529. [Google Scholar] [CrossRef] [PubMed]
- Murthy, R.; Kamat, P.; Nuñez, R.; Salem, R. Radioembolization of Yttrium-90 Microspheres for Hepatic Malignancy. Semin. Interv. Radiol. 2008, 25, 048–057. [Google Scholar] [CrossRef] [PubMed]
- Reinders, M.T.M.; van Erpecum, K.J.; Smits, M.L.J.; Braat, A.J.A.T.; de Bruijne, J.; Bruijnen, R.C.; Sprengers, D.; de Man, R.A.; Vegt, E.; Ijzermans, J.N.; et al. Safety and Efficacy of166Ho Radioembolization in Hepatocellular Carcinoma: The HEPAR Primary Study. J. Nucl. Med. 2022, 63, 1891–1898. [Google Scholar] [CrossRef] [PubMed]
- Radosa, C.G.; Radosa, J.C.; Grosche-Schlee, S.; Zöphel, K.; Plodeck, V.; Kühn, J.P.; Kotzerke, J.; Hoffmann, R.-T. Holmium-166 Radioembolization in Hepatocellular Carcinoma: Feasibility and Safety of a New Treatment Option in Clinical Practice. Cardiovasc. Interv. Radiol. 2019, 42, 405–412. [Google Scholar] [CrossRef]
- Bult, W.; Vente, M.A.; Vandermeulen, E.; Gielen, I.; Seevinck, P.R.; Saunders, J.; Schip, A.D.V.H.; Bakker, C.J.; Krijger, G.C.; Peremans, K.; et al. Microbrachytherapy using holmium-166 acetylacetonate microspheres: A pilot study in a spontaneous cancer animal model. Brachytherapy 2013, 12, 171–177. [Google Scholar] [CrossRef]
- Chen, H.; Nan, G.; Wei, D.; Zhai, R.-Y.; Huang, M.; Yang, W.-W.; Xing, B.-C.; Zhu, X.; Xu, H.-F.; Wang, X.-D.; et al. Hepatic Artery Injection of 131I-Metuximab Combined with Transcatheter Arterial Chemoembolization for Unresectable Hepatocellular Carcinoma: A Prospective Nonrandomized, Multicenter Clinical Trial. J. Nucl. Med. 2022, 63, 556–559. [Google Scholar] [CrossRef]
- Riaz, A.; Gates, V.L.; Atassi, B.; Lewandowski, R.J.; Mulcahy, M.F.; Ryu, R.K.; Sato, K.T.; Baker, T.; Kulik, L.; Gupta, R.; et al. Radiation Segmentectomy: A Novel Approach to Increase Safety and Efficacy of Radioembolization. Int. J. Radiat. Oncol. Biol. Phys. 2011, 79, 163–171. [Google Scholar] [CrossRef]
- Salem, R.; Lewandowski, R.J.; Mulcahy, M.F.; Riaz, A.; Ryu, R.K.; Ibrahim, S.; Atassi, B.; Baker, T.; Gates, V.; Miller, F.H.; et al. Radioembolization for Hepatocellular Carcinoma Using Yttrium-90 Microspheres: A Comprehensive Report of Long-term Outcomes. Gastroenterology 2010, 138, 52–64. [Google Scholar] [CrossRef]
- Mazzaferro, V.; Sposito, C.; Bhoori, S.; Romito, R.; Chiesa, C.; Morosi, C.; Maccauro, M.; Marchianò, A.; Bongini, M.; Lanocita, R.; et al. Yttrium-90 radioembolization for intermediate-advanced hepatocellular carcinoma: A phase 2 study. Hepatology 2013, 57, 1826–1837. [Google Scholar] [CrossRef]
- Rognoni, C.; Ciani, O.; Sommariva, S.; Bargellini, I.; Bhoori, S.; Cioni, R.; Facciorusso, A.; Golfieri, R.; Gramenzi, A.; Mazzaferro, V.; et al. Trans-arterial radioembolization for intermediate-advanced hepatocellular carcinoma: A budget impact analysis. BMC Cancer 2018, 18, 1–9. [Google Scholar] [CrossRef]
- Facciorusso, A.; Chierici, A.; Cincione, I.; Sacco, R.; Ramai, D.; Mohan, B.P.; Chandan, S.; Ofosu, A.; Cotsoglou, C. Stereotactic body radiotherapy vs. radiofrequency ablation for the treatment of hepatocellular carcinoma: A meta-analysis. Expert Rev. Anticancer. Ther. 2021, 21, 681–688. [Google Scholar] [CrossRef]
- Salem, R.; Gordon, A.C.; Mouli, S.; Hickey, R.; Kallini, J.; Gabr, A.; Mulcahy, M.F.; Baker, T.; Abecassis, M.; Miller, F.H.; et al. Y90 Radioembolization Significantly Prolongs Time to Progression Compared with Chemoembolization in Patients with Hepatocellular Carcinoma. Gastroenterology 2016, 151, 1155–1163.e2. [Google Scholar] [CrossRef]
- Facciorusso, A.; Serviddio, G.; Muscatiello, N. Transarterial radioembolization vs. chemoembolization for hepatocarcinoma patients: A systematic review and meta-analysis. World J. Hepatol. 2016, 8, 770–778. [Google Scholar] [CrossRef]
- Facciorusso, A.; Paolillo, R.; Tartaglia, N.; Ramai, D.; Mohan, B.P.; Cotsoglou, C.; Chandan, S.; Ambrosi, A.; Bargellini, I.; Renzulli, M.; et al. Efficacy of combined transarterial radioembolization and sorafenib in the treatment of hepatocarcinoma: A meta-analysis. Dig. Liver Dis. 2022, 54, 316–323. [Google Scholar] [CrossRef]
- Chow, P.K.; Gandhi, M.; Tan, S.-B.; Khin, M.W.; Khasbazar, A.; Ong, J.; Choo, S.P.; Cheow, P.C.; Chotipanich, C.; Lim, K.; et al. SIRveNIB: Selective Internal Radiation Therapy Versus Sorafenib in Asia-Pacific Patients with Hepatocellular Carcinoma. J. Clin. Oncol. 2018, 36, 1913–1921. [Google Scholar] [CrossRef]
- Zou, J.; Zhu, W.; Meng, H.; Luo, P.; Zhang, J. Efficacy and safety of selective internal radiotherapy versus sorafenib for intermediate-locally advanced hepatocellular carcinoma: A systematic review and meta-analysis. Expert Rev. Gastroenterol. Hepatol. 2019, 13, 271–279. [Google Scholar] [CrossRef]
- Torok, J.A.; Salama, J.K. Combining immunotherapy and radiotherapy for the STAR treatment. Nat. Rev. Clin. Oncol. 2019, 16, 666–667. [Google Scholar] [CrossRef]
- Braat, M.N.; Van Erpecum, K.J.; Zonnenberg, B.A.; Bosch, M.A.V.D.; Lam, M.G. Radioembolization-induced liver disease. Eur. J. Gastroenterol. Hepatol. 2017, 29, 144–152. [Google Scholar] [CrossRef]
- Riaz, A.; Awais, R.; Salem, R. Side Effects of Yttrium-90 Radioembolization. Front. Oncol. 2014, 4, 198. [Google Scholar] [CrossRef]
- Yi, Y.; Tongguo, S. Yttrium-90 transarterial radioembolization versus conventional transarterial chemoembolization for patients with hepatocellular carcinoma: A systematic review and meta-analysis. Cancer Biol. Med. 2018, 15, 299–310. [Google Scholar] [CrossRef]
- Lemieux, S.; Buies, A.; Turgeon, A.F.; Hallet, J.; Daigle, G.; Côté, F.; Provencher, S. Effect of Yttrium-90 transarterial radioembolization in patients with non-surgical hepatocellular carcinoma: A systematic review and meta-analysis. PLoS ONE 2021, 16, e0247958. [Google Scholar] [CrossRef]
- Roosen, J.; Klaassen, N.J.M.; Gotby, L.E.L.W.; Overduin, C.G.; Verheij, M.; Konijnenberg, M.W.; Nijsen, J.F.W. To 1000 Gy and back again: A systematic review on dose-response evaluation in selective internal radiation therapy for primary and secondary liver cancer. Eur. J. Nucl. Med. 2021, 48, 3776–3790. [Google Scholar] [CrossRef]
- Alonso, J.C.; Casans, I.; González, F.M.; Fuster, D.; Rodríguez, A.; Sánchez, N.; Oyagüez, I.; Burgos, R.; Williams, A.O.; Espinoza, N. Economic evaluations of radioembolization with Itrium-90 microspheres in hepatocellular carcinoma: A systematic review. BMC Gastroenterol. 2022, 22, 1–23. [Google Scholar] [CrossRef]
- Pereira, H.; Bouattour, M.; Burgio, M.D.; Assenat, E.; Grégory, J.; Bronowicki, J.-P.; Chatellier, G.; Vilgrain, V.; Delhom-Christol, E.; Fourcade, M.; et al. Health-related quality of life in locally advanced hepatocellular carcinoma treated by either radioembolisation or sorafenib (SARAH trial). Eur. J. Cancer 2021, 154, 46–56. [Google Scholar] [CrossRef]
- Eisenbrey, J.R.; Forsberg, F.; Wessner, C.E.; Delaney, L.J.; Bradigan, K.; Gummadi, S.; Tantawi, M.; Lyshchik, A.; O’kane, P.; Liu, J.-B.; et al. US-triggered Microbubble Destruction for Augmenting Hepatocellular Carcinoma Response to Transarterial Radioembolization: A Randomized Pilot Clinical Trial. Radiology 2021, 298, 450–457. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
