The Current State of Neoadjuvant Therapy in Resectable Advanced Stage Melanoma
Abstract
Simple Summary
Abstract
1. Introduction
2. Rationale
3. Neoadjuvant Trials
3.1. Trial Endpoints
3.2. Immunotherapy
3.2.1. Pembrolizumab in Resectable Stage III–IV Disease
3.2.2. Nivolumab vs. Ipilimumab + Nivolumab in Resectable Clinical Stage III or Oligometastatic Stage IV Disease
3.2.3. OpACIN Trial: Ipilimumab + Nivolumab in Resectable Stage III with Palpable Disease
3.2.4. OpACIN-Neo Trial: Ipilimumab + Nivolumab—Resectable Stage III
3.2.5. Survival and Biomarker Analysis of OpACIN and OpACIN-Neo Trials
3.2.6. PRADO Trial: Response Directed Therapy after Neoadjuvant Ipilimumab and Nivolumab in Stage III Melanoma
3.2.7. Relatlimab + Nivolumab in Stage III or Oligometastatic Melanoma
3.3. Neoadjuvant vs. Adjuvant Single-Agent Immunotherapy
3.4. Targeted Therapy
3.4.1. BRAF/MEK Inhibitor Combination
NeoCombi Trial: Dabrafenib + Trametinib—Resectable Clinical Stage IIIB-C Disease
Dabrafenib + Trametinib in Resectable Clinical Stage III or Oligometastatic Stage IV Disease
| Treatment Group | Trial (Registry Number) | Population | Design | Intervention | Primary Endpoint(s) | Response | Toxicity | Findings in Context |
|---|---|---|---|---|---|---|---|---|
| Mono-tx anti-PD1 | Huang 2019 [37] (NCT02434354) | Resectable stage III/IV | Phase Ib (N = 29) | Neoadj pembro 200 mg × 1, then adj pembro (1 year) | Safety | 8/27 (30%) with complete or major path response | No unexpected AEs | PD-1 blockade ↑ anti-tumor T cell response |
| Comb-tx anti-CTLA4 + anti-PD1 | Amaria 2018 [38] (NCT02519322) | Resectable stage III/IV | Phase II (N = 23) | [A] Neoadj nivo 3 mg/kg × 4 [B] Neoadj ipi 3 mg/kg + nivo 1 mg/kg × 3 | pCR | pCR in [A] 3/12 (25%), [B] 5/11 (45%) | Grade 3 AEs in [A] 8%, [B] 73%; no grade 4–5 AEs observed | Improved pCR in ipi + nivo comb tx but considerable toxicity |
| Comb-tx anti-CTLA4 + anti-PD1 | OpACIN, Blank 2018 [39] (NCT02437279) | Palpable stage III | Phase Ib (N = 20) | [A] Neoadj ipi 3 mg/kg + nivo 1 mg/kg × 2, then adj ipi + nivo × 2 [B] Adj-only ipi 3 mg/kg + nivo 1 mg/kg × 4 | Safety, immune response | Path response in [A] 7/9 (78%), favorable T cell response in [A] | Grade 3/4 AEs in 9/10 pts in each arm | Possible superiority of neoadj tx, but high toxicity |
| Comb-tx anti-CTLA4 + anti-PD1 | OpACIN-neo, Rozeman 2019 [40] (NCT02977052) | Resectable stage III | Phase II (N = 86) | [A] Neoadj ipi 3 mg/kg + nivo 1 mg/kg × 2 [B] Neoadj ipi 1 mg/kg + nivo 3 mg/kg × 2 [C] Neoadj ipi 3 mg/kg × 2 then nivo 3 mg/kg × 2 | Safety, rads/path response | Rads objective response—[A] 63%, [B] 57%, and [C] 35%; path response—[A] 80%, [B] 77%, and [C] 65% | AEs grade 3/4 in [A] 40%, [B] 20%, and [C] 50% | High path response rate in better tolerated dosing sch [B] of neoadj ipi + nivo |
| Comb-tx anti-CTLA4 + anti-PD1 | PRADO (OpACIN-neo expansion cohort), Reijers 2022 [45] (NCT02977052) | Nodal stage IIIB–D | Phase II (N = 99) | Neoadj ipi 1 mg/kg + nivo 3 mg/kg × 2, then assess ILN MPR in ILN -> TLND and adj tx omitted pPR in ILN -> TLND onlypNR in ILN -> TLND and adj chemo + radiotherapy | Safety, pRR, RFS | ILN resected 90/94 pts 1st attempt; MPR 61%, pPR 11%, pNR 21%; TLND omitted in 59/60 pts with MPR; 24-mo RFS 93% in MPR, 64% pPR, and 71% pNR | Grade 3/4 AEs in 22% | Supports response-driven personalization of tx after neoadj ipi+nivo |
| Comb-tx anti-PD1 + anti-LAG3 | Amaria 2022 [47] (NCT02519322) | Resectable stage III/IV | Phase II (N = 30) | Neoadj nivo 480 mg + relatlimab 180 mg × 2, then adj nivo + relatlimab × 10 | Safety, pCR | pCR in 57%, near pCR 7%, pPR 7%, and pNR 27%; 2-year RFS 91% in pCR, 92% any path response, and 55% without path response | No grade 3–4 AEs in neoadj, 26% grade 3–4 AEs in adj | Comparable pCR and safety profile to other neoadj combination therapies |
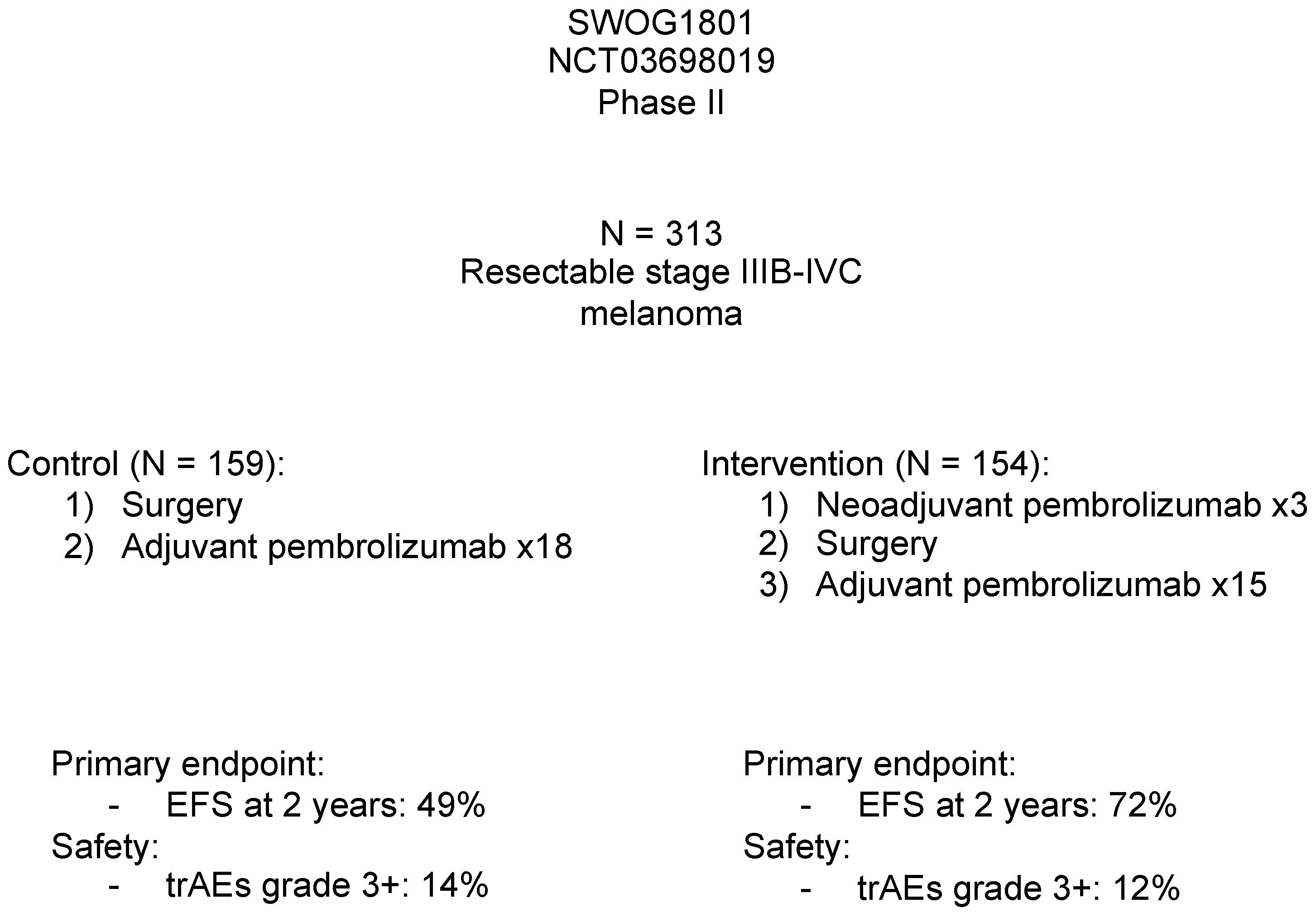
| Mono-tx anti-PD1 | SWOG 1801, Patel 2023 [48] (NCT03698019) | Resectable stage IIIB–IVC | Phase II (N = 313) | [A] Neoadj pembro 200 mg × 3, then adj pembro × 15 [B] Adj-only pembro × 18 | Event-free survival | EFS at 2 years in [A] 72%, and [B] 49% | AEs grade 3+ in [A] 12%, and [B] 14% | EFS significantly longer in neoadj arm |
| Comb-tx BRAFi + MEKi | NeoCombi, Long 2019 [49] (NCT01972347) | Resectable BRAF-mutated stage IIIB–C | Phase II (N = 35) | Neoadj dabrafenib 150 mg BID + trametinib 2 mg daily × 12 wks, then adj dabrafenib + trametinib × 40 wks | pCR, RECIST response at 12 wks | 35/35 had a path response, 17/35 (49%) had a pCR; RECIST response in 30/35 (86%), complete in 16/35 (46%), partial in 14/35 (40%) | Grade 3–4 AEs in 10/35 (29%) | Well tolerated and effective comb targ neoadj tx in resectable stage III BRAF-mutated melanoma |
| Comb-tx BRAFi + MEKi | Amaria 2018 [51] (NCT02231775) | Resectable BRAF-mutated stage III/IV | Phase II (N = 21) | [A] Neoadj dabrafenib 150 mg BID + trametinib 2 mg daily × 8 wks, then adj dabrafenib + trametinib × 44 wks [B] Adj standard-of-care | EFS at 12 mos | Median EFS in [A] 19.7 months, and [B] 2.9 months; pCR in [A] 7/12 (58%) | No grade 4 AEs in [A] | Support rationale for comb targ neoadj tx in resectable stage III/IV BRAF-mutated melanoma |
4. Discussion
5. Limitations and Challenges
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Saginala, K.; Barsouk, A.; Aluru, J.S.; Rawla, P.; Barsouk, A. Epidemiology of Melanoma. Med. Sci. 2021, 9, 63. [Google Scholar] [CrossRef]
- Farrow, N.E.; Turner, M.C.; Salama, A.K.S.; Beasley, G.M. Overall Survival Improved for Contemporary Patients with Melanoma: A 2004–2015 National Cancer Database Analysis. Oncol. Ther. 2020, 8, 261–275. [Google Scholar] [CrossRef]
- Dobry, A.S.; Zogg, C.K.; Hodi, F.S.; Smith, T.R.; Ott, P.A.; Iorgulescu, J.B. Management of metastatic melanoma: Improved survival in a national cohort following the approvals of checkpoint blockade immunotherapies and targeted therapies. Cancer Immunol. Immunother. 2018, 67, 1833–1844. [Google Scholar] [CrossRef]
- Weiss, S.A.; Wolchok, J.D.; Sznol, M. Immunotherapy of Melanoma: Facts and Hopes. Clin. Cancer Res. 2019, 25, 5191–5201. [Google Scholar] [CrossRef]
- Larkin, J.; Chiarion-Sileni, V.; Gonzalez, R.; Grob, J.J.; Rutkowski, P.; Lao, C.D.; Cowey, C.L.; Schadendorf, D.; Wagstaff, J.; Dummer, R.; et al. Five-Year Survival with Combined Nivolumab and Ipilimumab in Advanced Melanoma. N. Engl. J. Med. 2019, 381, 1535–1546. [Google Scholar] [CrossRef]
- Ascierto, P.A.; Del Vecchio, M.; Mackiewicz, A.; Robert, C.; Chiarion-Sileni, V.; Arance, A.; Lebbé, C.; Svane, I.M.; McNeil, C.; Rutkowski, P.; et al. Overall survival at 5 years of follow-up in a phase III trial comparing ipilimumab 10 mg/kg with 3 mg/kg in patients with advanced melanoma. J. Immunother. Cancer 2020, 8, e000391. [Google Scholar] [CrossRef]
- Sharon, C.E.; Karakousis, G.C. Educational Review: Neoadjuvant Approaches to Melanoma. Ann. Surg. Oncol. 2022, 29, 8492–8500. [Google Scholar] [CrossRef]
- Witt, R.G.; Erstad, D.J.; Wargo, J.A. Neoadjuvant therapy for melanoma: Rationale for neoadjuvant therapy and pivotal clinical trials. Ther. Adv. Med. Oncol. 2022, 14, 17588359221083052. [Google Scholar] [CrossRef]
- Seidel, J.A.; Otsuka, A.; Kabashima, K. Anti-PD-1 and Anti-CTLA-4 Therapies in Cancer: Mechanisms of Action, Efficacy, and Limitations. Front. Oncol. 2018, 8, 86. [Google Scholar] [CrossRef]
- Bushara, O.; Krogh, K.; Weinberg, S.E.; Finkelman, B.S.; Sun, L.; Liao, J.; Yang, G.Y. Human Immunodeficiency Virus Infection Promotes Human Papillomavirus-Mediated Anal Squamous Carcinogenesis: An Immunologic and Pathobiologic Review. Pathobiology 2022, 89, 1–12. [Google Scholar] [CrossRef]
- Vinay, D.S.; Ryan, E.P.; Pawelec, G.; Talib, W.H.; Stagg, J.; Elkord, E.; Lichtor, T.; Decker, W.K.; Whelan, R.L.; Kumara, H.; et al. Immune evasion in cancer: Mechanistic basis and therapeutic strategies. Semin. Cancer Biol. 2015, 35, S185–S198. [Google Scholar] [CrossRef]
- Ishida, Y.; Agata, Y.; Shibahara, K.; Honjo, T. Induced expression of PD-1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. EMBO J. 1992, 11, 3887–3895. [Google Scholar] [CrossRef]
- Salvi, S.; Fontana, V.; Boccardo, S.; Merlo, D.F.; Margallo, E.; Laurent, S.; Morabito, A.; Rijavec, E.; Dal Bello, M.G.; Mora, M.; et al. Evaluation of CTLA-4 expression and relevance as a novel prognostic factor in patients with non-small cell lung cancer. Cancer Immunol. Immunother. 2012, 61, 1463–1472. [Google Scholar] [CrossRef]
- Huang, P.Y.; Guo, S.S.; Zhang, Y.; Lu, J.B.; Chen, Q.Y.; Tang, L.Q.; Zhang, L.; Liu, L.T.; Zhang, L.; Mai, H.Q. Tumor CTLA-4 overexpression predicts poor survival in patients with nasopharyngeal carcinoma. Oncotarget 2016, 7, 13060–13068. [Google Scholar] [CrossRef]
- Prokopi, A.; Tripp, C.H.; Tummers, B.; Hornsteiner, F.; Spoeck, S.; Crawford, J.C.; Clements, D.R.; Efremova, M.; Hutter, K.; Bellmann, L.; et al. Skin dendritic cells in melanoma are key for successful checkpoint blockade therapy. J. Immunother. Cancer 2021, 9, e000832. [Google Scholar] [CrossRef]
- Peng, Q.; Qiu, X.; Zhang, Z.; Zhang, S.; Zhang, Y.; Liang, Y.; Guo, J.; Peng, H.; Chen, M.; Fu, Y.-X.; et al. PD-L1 on dendritic cells attenuates T cell activation and regulates response to immune checkpoint blockade. Nat. Commun. 2020, 11, 4835. [Google Scholar] [CrossRef]
- Phillips, A.L.; Reeves, D.J. Nivolumab/Relatlimab: A Novel Addition to Immune Checkpoint Inhibitor Therapy in Unresectable or Metastatic Melanoma. Ann. Pharmacother. 2022, 57, 738–745. [Google Scholar] [CrossRef]
- Thudium, K.; Selby, M.; Zorn, J.A.; Rak, G.; Wang, X.T.; Bunch, R.T.; Hogan, J.M.; Strop, P.; Korman, A.J. Preclinical Characterization of Relatlimab, a Human LAG-3-Blocking Antibody, Alone or in Combination with Nivolumab. Cancer Immunol. Res. 2022, 10, 1175–1189. [Google Scholar] [CrossRef]
- Bilusic, M.; Gulley, J.L. Neoadjuvant Immunotherapy: An Evolving Paradigm Shift? J. Natl. Cancer Inst. 2021, 113, 799–800. [Google Scholar] [CrossRef]
- Topalian, S.L.; Taube, J.M.; Pardoll, D.M. Neoadjuvant checkpoint blockade for cancer immunotherapy. Science 2020, 367, eaax0182. [Google Scholar] [CrossRef]
- Mittendorf, E.A.; Burgers, F.; Haanen, J.; Cascone, T. Neoadjuvant Immunotherapy: Leveraging the Immune System to Treat Early-Stage Disease. Am. Soc. Clin. Oncol. Educ. Book 2022, 42, 189–203. [Google Scholar] [CrossRef]
- Krishnamoorthy, M.; Lenehan, J.G.; Maleki Vareki, S. Neoadjuvant Immunotherapy for High-Risk, Resectable Malignancies: Scientific Rationale and Clinical Challenges. JNCI J. Natl. Cancer Inst. 2021, 113, 823–832. [Google Scholar] [CrossRef]
- Liu, J.; Blake, S.J.; Yong, M.C.R.; Harjunpää, H.; Ngiow, S.F.; Takeda, K.; Young, A.; O’Donnell, J.S.; Allen, S.; Smyth, M.J.; et al. Improved Efficacy of Neoadjuvant Compared to Adjuvant Immunotherapy to Eradicate Metastatic Disease. Cancer Discov. 2016, 6, 1382–1399. [Google Scholar] [CrossRef]
- Ascierto, P.A.; Kirkwood, J.M.; Grob, J.J.; Simeone, E.; Grimaldi, A.M.; Maio, M.; Palmieri, G.; Testori, A.; Marincola, F.M.; Mozzillo, N. The role of BRAF V600 mutation in melanoma. J. Transl. Med. 2012, 10, 85. [Google Scholar] [CrossRef]
- Sanchez, J.N.; Wang, T.; Cohen, M.S. BRAF and MEK Inhibitors: Use and Resistance in BRAF-Mutated Cancers. Drugs 2018, 78, 549–566. [Google Scholar] [CrossRef]
- Kuske, M.; Westphal, D.; Wehner, R.; Schmitz, M.; Beissert, S.; Praetorius, C.; Meier, F. Immunomodulatory effects of BRAF and MEK inhibitors: Implications for Melanoma therapy. Pharmacol. Res. 2018, 136, 151–159. [Google Scholar] [CrossRef]
- Dummer, R.; Ascierto, P.A.; Gogas, H.J.; Arance, A.; Mandala, M.; Liszkay, G.; Garbe, C.; Schadendorf, D.; Krajsova, I.; Gutzmer, R.; et al. Encorafenib plus binimetinib versus vemurafenib or encorafenib in patients with BRAF-mutant melanoma (COLUMBUS): A multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2018, 19, 603–615. [Google Scholar] [CrossRef]
- Eroglu, Z.; Ribas, A. Combination therapy with BRAF and MEK inhibitors for melanoma: Latest evidence and place in therapy. Ther. Adv. Med. Oncol. 2016, 8, 48–56. [Google Scholar] [CrossRef]
- Faut, M.; Jalving, M.; Diercks, G.F.; Hospers, G.A.; van Leeuwen, B.L.; Been, L.B. Preoperative BRAF inhibition in patients with irresectable locally advanced stage III melanoma. Melanoma Manag. 2018, 5, Mmt08. [Google Scholar] [CrossRef]
- Zippel, D.; Markel, G.; Shapira-Frommer, R.; Ben-Betzalel, G.; Goitein, D.; Ben-Ami, E.; Nissan, A.; Schachter, J.; Schneebaum, S. Perioperative BRAF inhibitors in locally advanced stage III melanoma. J. Surg. Oncol. 2017, 116, 856–861. [Google Scholar] [CrossRef]
- Chiocca, E.A.; Rabkin, S.D. Oncolytic viruses and their application to cancer immunotherapy. Cancer Immunol. Res. 2014, 2, 295–300. [Google Scholar] [CrossRef]
- Mondal, M.; Guo, J.; He, P.; Zhou, D. Recent advances of oncolytic virus in cancer therapy. Hum. Vaccin. Immunother. 2020, 16, 2389–2402. [Google Scholar] [CrossRef]
- Robinson, C.; Xu, M.M.; Nair, S.K.; Beasley, G.M.; Rhodin, K.E. Oncolytic viruses in melanoma. Front. Biosci. 2022, 27, 63. [Google Scholar] [CrossRef]
- Huang, A.C.; Zappasodi, R. A decade of checkpoint blockade immunotherapy in melanoma: Understanding the molecular basis for immune sensitivity and resistance. Nat. Immunol. 2022, 23, 660–670. [Google Scholar] [CrossRef]
- Amaria, R.N.; Menzies, A.M.; Burton, E.M.; Scolyer, R.A.; Tetzlaff, M.T.; Antdbacka, R.; Ariyan, C.; Bassett, R.; Carter, B.; Daud, A.; et al. Neoadjuvant systemic therapy in melanoma: Recommendations of the International Neoadjuvant Melanoma Consortium. Lancet Oncol. 2019, 20, e378–e389. [Google Scholar] [CrossRef]
- Tetzlaff, M.T.; Messina, J.L.; Stein, J.E.; Xu, X.; Amaria, R.N.; Blank, C.U.; van de Wiel, B.A.; Ferguson, P.M.; Rawson, R.V.; Ross, M.I.; et al. Pathological assessment of resection specimens after neoadjuvant therapy for metastatic melanoma. Ann. Oncol. 2018, 29, 1861–1868. [Google Scholar] [CrossRef]
- Huang, A.C.; Orlowski, R.J.; Xu, X.; Mick, R.; George, S.M.; Yan, P.K.; Manne, S.; Kraya, A.A.; Wubbenhorst, B.; Dorfman, L.; et al. A single dose of neoadjuvant PD-1 blockade predicts clinical outcomes in resectable melanoma. Nat. Med. 2019, 25, 454–461. [Google Scholar] [CrossRef]
- Amaria, R.N.; Reddy, S.M.; Tawbi, H.A.; Davies, M.A.; Ross, M.I.; Glitza, I.C.; Cormier, J.N.; Lewis, C.; Hwu, W.J.; Hanna, E.; et al. Neoadjuvant immune checkpoint blockade in high-risk resectable melanoma. Nat. Med. 2018, 24, 1649–1654. [Google Scholar] [CrossRef]
- Blank, C.U.; Rozeman, E.A.; Fanchi, L.F.; Sikorska, K.; van de Wiel, B.; Kvistborg, P.; Krijgsman, O.; van den Braber, M.; Philips, D.; Broeks, A.; et al. Neoadjuvant versus adjuvant ipilimumab plus nivolumab in macroscopic stage III melanoma. Nat. Med. 2018, 24, 1655–1661. [Google Scholar] [CrossRef]
- Rozeman, E.A.; Menzies, A.M.; van Akkooi, A.C.J.; Adhikari, C.; Bierman, C.; van de Wiel, B.A.; Scolyer, R.A.; Krijgsman, O.; Sikorska, K.; Eriksson, H.; et al. Identification of the optimal combination dosing schedule of neoadjuvant ipilimumab plus nivolumab in macroscopic stage III melanoma (OpACIN-neo): A multicentre, phase 2, randomised, controlled trial. Lancet Oncol. 2019, 20, 948–960. [Google Scholar] [CrossRef]
- Ayers, M.; Lunceford, J.; Nebozhyn, M.; Murphy, E.; Loboda, A.; Kaufman, D.R.; Albright, A.; Cheng, J.D.; Kang, S.P.; Shankaran, V.; et al. IFN-γ–related mRNA profile predicts clinical response to PD-1 blockade. J. Clin. Investig. 2017, 127, 2930–2940. [Google Scholar] [CrossRef]
- Cui, C.; Xu, C.; Yang, W.; Chi, Z.; Sheng, X.; Si, L.; Xie, Y.; Yu, J.; Wang, S.; Yu, R.; et al. Ratio of the interferon-γ signature to the immunosuppression signature predicts anti-PD-1 therapy response in melanoma. NPJ Genom. Med. 2021, 6, 7. [Google Scholar] [CrossRef]
- Ning, B.; Liu, Y.; Wang, M.; Li, Y.; Xu, T.; Wei, Y. The Predictive Value of Tumor Mutation Burden on Clinical Efficacy of Immune Checkpoint Inhibitors in Melanoma: A Systematic Review and Meta-Analysis. Front. Pharmacol. 2022, 13, 748674. [Google Scholar] [CrossRef]
- Rozeman, E.A.; Hoefsmit, E.P.; Reijers, I.L.M.; Saw, R.P.M.; Versluis, J.M.; Krijgsman, O.; Dimitriadis, P.; Sikorska, K.; van de Wiel, B.A.; Eriksson, H.; et al. Survival and biomarker analyses from the OpACIN-neo and OpACIN neoadjuvant immunotherapy trials in stage III melanoma. Nat. Med. 2021, 27, 256–263. [Google Scholar] [CrossRef]
- Reijers, I.L.M.; Menzies, A.M.; van Akkooi, A.C.J.; Versluis, J.M.; van den Heuvel, N.M.J.; Saw, R.P.M.; Pennington, T.E.; Kapiteijn, E.; van der Veldt, A.A.M.; Suijkerbuijk, K.P.M.; et al. Personalized response-directed surgery and adjuvant therapy after neoadjuvant ipilimumab and nivolumab in high-risk stage III melanoma: The PRADO trial. Nat. Med. 2022, 28, 1178–1188. [Google Scholar] [CrossRef]
- Tawbi, H.A.; Schadendorf, D.; Lipson, E.J.; Ascierto, P.A.; Matamala, L.; Castillo Gutiérrez, E.; Rutkowski, P.; Gogas, H.J.; Lao, C.D.; De Menezes, J.J.; et al. Relatlimab and Nivolumab versus Nivolumab in Untreated Advanced Melanoma. N. Engl. J. Med. 2022, 386, 24–34. [Google Scholar] [CrossRef]
- Amaria, R.N.; Postow, M.; Burton, E.M.; Tetzlaff, M.T.; Ross, M.I.; Torres-Cabala, C.; Glitza, I.C.; Duan, F.; Milton, D.R.; Busam, K.; et al. Neoadjuvant relatlimab and nivolumab in resectable melanoma. Nature 2022, 611, 155–160. [Google Scholar] [CrossRef]
- Patel, S.P.; Othus, M.; Chen, Y.; Wright, G.P., Jr.; Yost, K.J.; Hyngstrom, J.R.; Hu-Lieskovan, S.; Lao, C.D.; Fecher, L.A.; Truong, T.G.; et al. Neoadjuvant-Adjuvant or Adjuvant-Only Pembrolizumab in Advanced Melanoma. N. Engl. J. Med. 2023, 388, 813–823. [Google Scholar] [CrossRef]
- Long, G.V.; Saw, R.P.M.; Lo, S.; Nieweg, O.E.; Shannon, K.F.; Gonzalez, M.; Guminski, A.; Lee, J.H.; Lee, H.; Ferguson, P.M.; et al. Neoadjuvant dabrafenib combined with trametinib for resectable, stage IIIB-C, BRAF(V600) mutation-positive melanoma (NeoCombi): A single-arm, open-label, single-centre, phase 2 trial. Lancet Oncol. 2019, 20, 961–971. [Google Scholar] [CrossRef]
- Robert, C.; Grob, J.J.; Stroyakovskiy, D.; Karaszewska, B.; Hauschild, A.; Levchenko, E.; Chiarion Sileni, V.; Schachter, J.; Garbe, C.; Bondarenko, I.; et al. Five-Year Outcomes with Dabrafenib plus Trametinib in Metastatic Melanoma. N. Engl. J. Med. 2019, 381, 626–636. [Google Scholar] [CrossRef]
- Amaria, R.N.; Prieto, P.A.; Tetzlaff, M.T.; Reuben, A.; Andrews, M.C.; Ross, M.I.; Glitza, I.C.; Cormier, J.; Hwu, W.J.; Tawbi, H.A.; et al. Neoadjuvant plus adjuvant dabrafenib and trametinib versus standard of care in patients with high-risk, surgically resectable melanoma: A single-centre, open-label, randomised, phase 2 trial. Lancet Oncol. 2018, 19, 181–193. [Google Scholar] [CrossRef]
- Adjuvant Treatment Determined by Pathological Response to Neoadjvuant Nivolumab. 2020. Available online: https://clinicaltrials.gov/ct2/show/NCT04013854 (accessed on 3 January 2023).
- Rohatgi, A.; Kirkwood, J.M. Beyond PD-1: The Next Frontier for Immunotherapy in Melanoma. Front. Oncol. 2021, 11, 640314. [Google Scholar] [CrossRef]
- Harjunpää, H.; Guillerey, C. TIGIT as an emerging immune checkpoint. Clin. Exp. Immunol. 2020, 200, 108–119. [Google Scholar] [CrossRef]
- Ge, Z.; Peppelenbosch, M.P.; Sprengers, D.; Kwekkeboom, J. TIGIT, the Next Step Towards Successful Combination Immune Checkpoint Therapy in Cancer. Front. Immunol. 2021, 12, 699895. [Google Scholar] [CrossRef]
- NeoACTIVATE: Neoadjuvant Therapy for Patients with High Risk Stage III Melanoma: A Pilot Clinical Trial. 2018. Available online: https://classic.clinicaltrials.gov/ct2/show/NCT03554083 (accessed on 3 January 2023).
- Gan, Y.; Li, X.; Han, S.; Liang, Q.; Ma, X.; Rong, P.; Wang, W.; Li, W. The cGAS/STING Pathway: A Novel Target for Cancer Therapy. Front. Immunol. 2022, 12, 795401. [Google Scholar] [CrossRef]
- Garland, K.M.; Rosch, J.C.; Carson, C.S.; Wang-Bishop, L.; Hanna, A.; Sevimli, S.; Van Kaer, C.; Balko, J.M.; Ascano, M.; Wilson, J.T. Pharmacological Activation of cGAS for Cancer Immunotherapy. Front. Immunol. 2021, 12, 753472. [Google Scholar] [CrossRef]
- Chen, S.; Wainwright, D.A.; Wu, J.D.; Wan, Y.; Matei, D.E.; Zhang, Y.; Zhang, B. CD73: An emerging checkpoint for cancer immunotherapy. Immunotherapy 2019, 11, 983–997. [Google Scholar] [CrossRef]
- Roh, M.; Wainwright, D.A.; Wu, J.D.; Wan, Y.; Zhang, B. Targeting CD73 to augment cancer immunotherapy. Curr. Opin. Pharmacol. 2020, 53, 66–76. [Google Scholar] [CrossRef]
- Vernon, M.; Wilski, N.A.; Kotas, D.; Cai, W.; Pomante, D.; Tiago, M.; Alnemri, E.S.; Aplin, A.E. Raptinal Induces Gasdermin E-Dependent Pyroptosis in Naïve and Therapy-Resistant Melanoma. Mol. Cancer Res. 2022, 20, 1811–1821. [Google Scholar] [CrossRef]
- Li, Z.; Mo, F.; Wang, Y.; Li, W.; Chen, Y.; Liu, J.; Chen-Mayfield, T.-J.; Hu, Q. Enhancing Gasdermin-induced tumor pyroptosis through preventing ESCRT-dependent cell membrane repair augments antitumor immune response. Nat. Commun. 2022, 13, 6321. [Google Scholar] [CrossRef]
- Jiang, Y.; Yang, Y.; Hu, Y.; Yang, R.; Huang, J.; Liu, Y.; Wu, Y.; Li, S.; Ma, C.; Humphries, F.; et al. Gasdermin D restricts anti-tumor immunity during PD-L1 checkpoint blockade. Cell Rep. 2022, 41, 111553. [Google Scholar] [CrossRef]
- Menzies, A.M.; Amaria, R.N.; Rozeman, E.A.; Huang, A.C.; Tetzlaff, M.T.; van de Wiel, B.A.; Lo, S.; Tarhini, A.A.; Burton, E.M.; Pennington, T.E.; et al. Pathological response and survival with neoadjuvant therapy in melanoma: A pooled analysis from the International Neoadjuvant Melanoma Consortium (INMC). Nat. Med. 2021, 27, 301–309. [Google Scholar] [CrossRef]
- Atkins, M.B.; Lee, S.J.; Chmielowski, B.; Tarhini, A.A.; Cohen, G.I.; Truong, T.G.; Moon, H.H.; Davar, D.; O’Rourke, M.; Stephenson, J.J.; et al. Combination Dabrafenib and Trametinib Versus Combination Nivolumab and Ipilimumab for Patients With Advanced BRAF-Mutant Melanoma: The DREAMseq Trial-ECOG-ACRIN EA6134. J. Clin. Oncol. 2023, 41, 186–197. [Google Scholar] [CrossRef]

| Treatment Group | Trial (Registry Number) | Population | Design | Intervention | Primary Endpoint(s) |
|---|---|---|---|---|---|
| Comb-tx Anti-CTLA4 + anti-PD1 | NCT04013854 | Resectable stage III melanoma | Phase II (N = 60) | [A] One dose of nivo IV; Surgery; If pCR, nivo IV for up to 1 year [B] One dose of nivo IV; Surgery; If <near pCR, nivo IV for up to 1 year [C] One dose of nivo IV; Surgery; If <near pCR, ipi IV + nivo IV for 4 doses, then nivo IV alone for a total of 1 yr | RFS |
| Comb-tx Anti-CTLA4 + anti-PD1 | NADINA NCT04949113 | Macroscopic stage III melanoma | Phase III (N = 420) | [A] Ipi IV + nivo IV q3 wks, 2 cycles; TLND; Nivo IV q4 wks, up to 11 cycles IF pPR or pNR If BRAF V600E/K mutant, dabrafenib + trametinib for 46 wks instead of nivo [B] TLND; Nivo q4 wks, 12 cycles | EFS |
| Comb-tx T-VEC + anti-PD1 | NCT04330430 | Stage III–IV melanoma | Phase II (N = 24) | T-VEC intra-lesional inj × 4 q2 wks after 3 wks of 1st dose; nivo IV × 3 q2 wks starting after 2nd T-VEC course | pCR |
| Comb-tx T-VEC + anti-PD1 | NCT03842943 | Resectable stage III cutaneous melanoma | Phase II (N = 28) | T-VEC intra-lesional inj q3 wks, up to 6 mos; pembro IV q3 wks, up to 6 mos, then q3 wks for 1 year in adj setting | pCR |
| Comb-tx Anti-PD1 + hGM-CSF HSV | NCT04197882 | Resectable stage III and IV (M1a) melanoma | Phase Ib (N = 33) | Toripalimab IV q2 wks × 6, OrienX010 intratumoral inj q2 wks × 6; Surgery; Toripalimab IV q3 wks for up to 1 yr | pCR, RECIST response |
| Comb-tx Anti-PD1 + BRAFi + MEKi | Neo Trio NCT02858921 | Resectable BRAF V600 mutant stage IIIB/IIIC melanoma | Phase II (N = 60) | [SEQ] Dabrafenib PO BID + trametinib PO QD × 1 wk, then followed by pembro IV at wks 1, 3, and 6, then q3 wks from wks 6–36 [CON] Dabrafenib PO BID + trametinib PO QD + pembro IV q3 wks for 6 wks; then pembro alone for 46 wks [ALONE] Pembro IV q3 wks for 52 wks | pCR |
| Comb-tx Anti-PDL1 + BRAFi + MEKi, Anti-PDL1 + anti-TIGIT | NeoACTIVATE NCT03554083 | Stage III cutaneous melanoma | Phase II (N = 30) | [A] Vemurafenib PO BID on days 1–28, cobimetinib PO QD on days 1–21, atezolizumab IV on days 1 and 15 of cycles 2 and 3, up to 3 cycles; Surgery; Atezolizumab IV on day 1, repeats q3 wks, up to 8 cycles [B] Cobimetinib as in arm [A], atezolizumab IV on days 1 and 15, repeats q4 wks up to 3 cycles; Surgery; Atezolizumab on day 1, repeats q3 wks, up to 8 cycles [C] Atezolizumab IV on day 1, tiragolumab IV on day 1, repeats q3 wks, up to 4 cycles | pCR, RFS |
| Comb-tx Anti-CTLA4 + anti-PD1, Anti-PD1/LAG3, Anti-PDL1 + anti-TIGIT, Anti-PD1/LAG3 + anti-TIGIT | Morpheus-Melanoma NCT05116202 | Resectable stage III (cohort 1) and stage IV (cohort 2) melanoma | Phase Ib/II (N = 191) | [A1] nivo IV on day 1, ipi IV on day 1, repeat q3 wks, 2 cycles [B1] RO7247669 IV on day 1, repeat q3 wks, 2 cycles [C1] Atezolizumab IV on day 1, tiragolumab on day 1, repeat q3 wks, 2 cycles [D1] RO7247669 IV on day 1, tiragolumab on day 1, repeat q3 wks, 2 cycles [A2] RO7247669 IV on day 1, tiragolumab on day 1, repeat q3 wks, until unacceptable toxicity or loss of clinical benefit | pCR, RECIST response |
| Comb-tx Anti-PD1 + multiple RTKi | Neo PeLe NCT04207086 | Resectable stage III melanoma | Phase II (N = 40) | Pembro + lenvatinib for 6 wks; Surgery; Pembro for 46 wks | pCR, immune response |
| Comb-tx Anti-PD1 + multiple RTKi | Neo PeLeMM NCT05545969 | Resectable mucosal melanoma | Phase II (N = 44) | Pembro + lenvatinib for 6 wks, Surgery, Pembro alone for 46 wks | pCR, immune response |
| Comb-tx Anti-PD1 + multiple RTKi | NCT04622566 | Resectable mucosal melanoma | Phase II (N = 26) | Pembro IV on day 1, repeat q3 wks, for 6 wks, lenvatinib PO QD for 6 wks; Surgery; Pembro IV on day 1, repeat q3 wks, up to 15 cycles | pCR |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bushara, O.; Tidwell, J.; Wester, J.R.; Miura, J. The Current State of Neoadjuvant Therapy in Resectable Advanced Stage Melanoma. Cancers 2023, 15, 3344. https://doi.org/10.3390/cancers15133344
Bushara O, Tidwell J, Wester JR, Miura J. The Current State of Neoadjuvant Therapy in Resectable Advanced Stage Melanoma. Cancers. 2023; 15(13):3344. https://doi.org/10.3390/cancers15133344
Chicago/Turabian StyleBushara, Omar, Jerica Tidwell, James R. Wester, and John Miura. 2023. "The Current State of Neoadjuvant Therapy in Resectable Advanced Stage Melanoma" Cancers 15, no. 13: 3344. https://doi.org/10.3390/cancers15133344
APA StyleBushara, O., Tidwell, J., Wester, J. R., & Miura, J. (2023). The Current State of Neoadjuvant Therapy in Resectable Advanced Stage Melanoma. Cancers, 15(13), 3344. https://doi.org/10.3390/cancers15133344





