Impact of Signet-Ring Cell Histology in the Management of Patients with Non-Metastatic Gastric Cancer: Results from a Retrospective Multicenter Analysis Comparing FLOT Perioperative Chemotherapy vs. Surgery Followed by Adjuvant Chemotherapy
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Methods
3. Results
3.1. Patients’ Characteristics
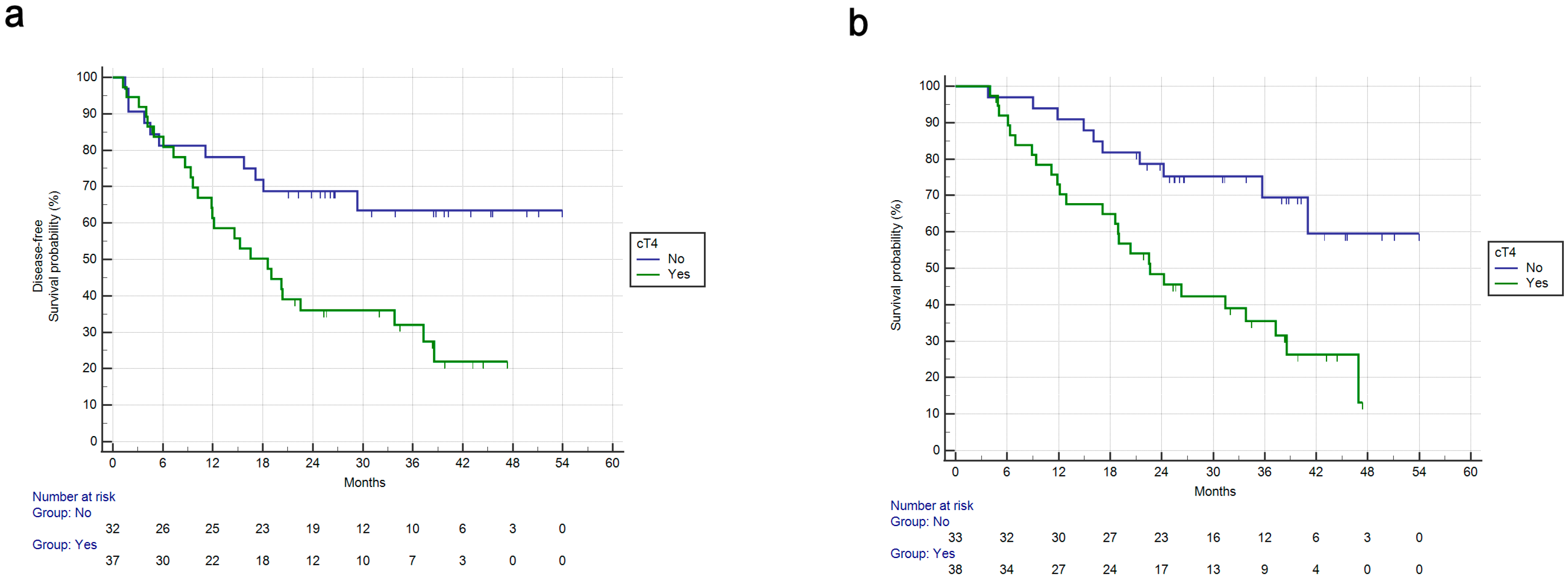
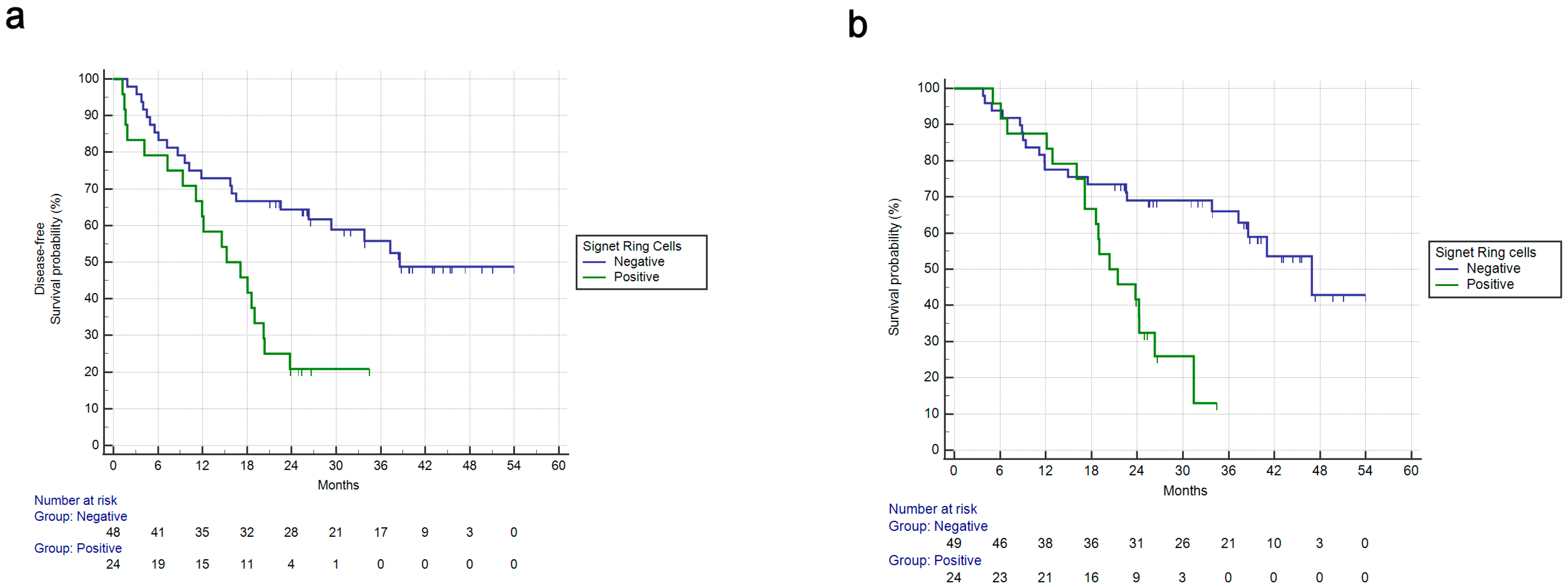
3.2. Univariate Survival Analysis
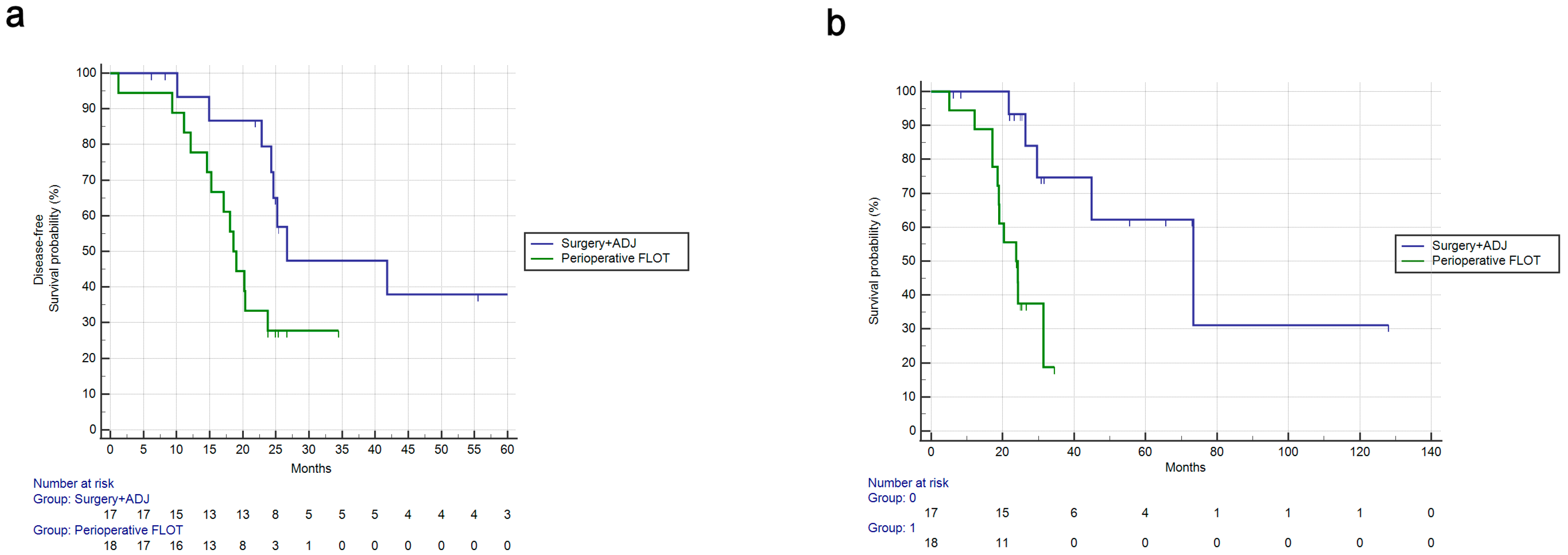
3.3. Multivariate Survival Analysis and Comparison to Adjuvant Treatment
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO. Globocan 2020. Available online: https://acsjournals.onlinelibrary.wiley.com/doi/10.3322/caac.21660 (accessed on 8 May 2023). [CrossRef]
- Boniface, M.; Wani, S.; Schefter, T.; Koo, P.; Meguid, C.; Leong, S.; Kaplan, J.; Wingrove, L.; McCarter, M. Multidisciplinary management for esophageal and gastric cancer. Cancer Manag. Res. 2016, 8, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Giampieri, R.; Del Prete, M.; Cantini, L.; Baleani, M.G.; Bittoni, A.; Maccaroni, E.; Berardi, R. Optimal management of resected gastric cancer. Cancer Manag. Res. 2018, 10, 1605–1618. [Google Scholar] [CrossRef] [PubMed]
- Al-Batran, S.E.; Homann, N.; Pauligk, C.; Goetze, T.O.; Meiler, J.; Kasper, S.; Kopp, H.G.; Mayer, F.; Haag, G.M.; Luley, K.; et al. Perioperative chemotherapy with fluorouracil plus leucovorin, oxaliplatin, and docetaxel versus fluorouracil or capecitabine plus cisplatin and epirubicin for locally advanced, resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4): A randomised, phase 2/3 trial. Lancet 2019, 393, 1948–1957. [Google Scholar]
- Al-Batran, S.E.; Hofheinz, R.D.; Schmalenberg, H.; Strumberg, D.; Goekkurt, E.; Angermeier, S.; Zander, T.; Potenberg, J.; Kopp, H.G.; Pink, D.; et al. Perioperative ramucirumab in combination with FLOT versus FLOT alone for resectable esophagogastric adenocarcinoma (RAMSES/FLOT7): Results of the phase II-portion—A multicenter, randomized phase II/III trial of the German AIO and Italian GOIM. J. Clin. Oncol. 2020, 38, 4501. [Google Scholar] [CrossRef]
- Hofheinz, R.D.; Haag, G.M.; Ettrich, T.J.; Borchert, K.; Kretzschmar, A.; Teschendorf, C.; Siegler, G.M.; Ebert, M.P.; Goekkurt, E.; Welslau, M.; et al. Perioperative trastuzumab and pertuzumab in combination with FLOT versus FLOT alone for HER2-positive resectable esophagogastric adenocarcinoma: Final results of the PETRARCA multicenter randomized phase II trial of the AIO. J. Clin. Oncol. 2020, 38, 4502. [Google Scholar] [CrossRef]
- Al-Batran, S.-E.; Lorenzen, S.; Thuss-Patience, P.C.; Homann, N.; Schenk, M.; Lindig, U.; Heuer, V.; Kretzschmar, A.; Goekkurt, E.; Haag, G.M.; et al. Surgical and pathological outcome, and pathological regression, in patients receiving perioperative atezolizumab in combination with FLOT chemotherapy versus FLOT alone for resectable esophagogastric adenocarcinoma: Interim results from DANTE, a randomized, multicenter, phase IIb trial of the FLOT-AIO German Gastric Cancer Group and Swiss SAKK. J. Clin. Oncol. 2022, 40, 4003. [Google Scholar]
- Bittoni, A.; Scartozzi, M.; Giampieri, R.; Faloppi, L.; Bianconi, M.; Mandolesi, A.; Del Prete, M.; Pistelli, M.; Cecchini, L.; Bearzi, I.; et al. Clinical Evidence for Three Distinct Gastric Cancer Subtypes: Time for a New Approach. PLoS ONE 2013, 8, e78544. [Google Scholar] [CrossRef]
- Pernot, S.; Voron, T.; Perkins, G.; Lagorce-Pages, C.; Berger, A.; Taieb, J. Signet-ring cell carcinoma of the stomach: Impact on prognosis and specific therapeutic challenge. World J. Gastroenterol. 2015, 21, 11428–11438. [Google Scholar] [CrossRef]
- Lemoine, N.; Adenis, A.; Bouche, O.; Duhamel, A.; Heurgue, A.; Leteurtre, E.; Amela, E.; Salleron, J.; Hebbar, M. Signet Ring Cells and Efficacy of First-line Chemotherapy in Advanced Gastric or Oesogastric Junction Adenocarcinoma. Anticancer Res. 2016, 36, 5543–5549. [Google Scholar] [CrossRef]
- Yokota, T.; Kunii, Y.; Teshima, S.; Yamada, Y.; Saito, T.; Kikuchi, S.; Yamauchi, H. Signet Ring Cell Carcinoma of the Stomach: A Clinicopathological Comparison with the Other Histological Types. Tohoku J. Exp. Med. 1998, 186, 121–130. [Google Scholar] [CrossRef]
- Efared, B.; Kadi, M.; Tahiri, L.; Lahmidani, N.; Hassani, K.M.; Bouhaddouti, H.E.; Benbrahim, Z.; Adil, I.S.; Chbani, L. Gastric Signet Ring Cell Carcinoma: A Comparative Analysis of Clinicopathologic Features. Cancer Control 2020, 27, 1073274820976596. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Wang, Q.; Tian, Q.; Bo, C.; Li, N.; Zhang, S.; Li, P. Clinicopathological Features and Prognostic-Related Risk Factors of Gastric Signet Ring Cell Carcinoma: A Meta-Analysis. Comput. Math. Methods Med. 2022, 2022, 3473445. [Google Scholar] [CrossRef] [PubMed]
- Mengardo, V.; Treppiedi, E.; Bencivenga, M.; Dal Cero, M.; Giacopuzzi, S. Tailored treatment for signet ring cell gastric cancer. Updates Surg. 2018, 70, 167–171. [Google Scholar] [CrossRef] [PubMed]
- Wang, J. To use or not to use propensity score matching? Pharm. Stat. 2021, 20, 15–24. [Google Scholar] [CrossRef]
- Ajani, J.A.; D’Amico, T.A.; Bentrem, D.J.; Chao, J.; Cooke, D.; Corvera, C.; Das, P.; Enzinger, P.C.; Enzler, T.; Fanta, P.; et al. Gastric Cancer, Version 2.2022, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2022, 20, 167–192. [Google Scholar] [CrossRef] [PubMed]
- Giampieri, R.; Cantini, L.; Del Prete, M.; Bittoni, A.; Giglio, E.; Mandolesi, A.; Maccaroni, E.; Lanese, A.; Meletani, T.; Baleani, M.G.; et al. An observational retrospective analysis of the main metastatic site and corresponding locoregional treatment as a prognostic factor in metastatic gastric cancer. Oncol. Lett. 2021, 21, 267. [Google Scholar] [CrossRef] [PubMed]
- Kinoshita, J.; Yamaguchi, T.; Moriyama, H.; Fushida, S. Current status of conversion surgery for stage IV gastric cancer. Surg. Today 2021, 51, 1736–1754. [Google Scholar] [CrossRef]
- Solaini, L.; Ministrini, S.; Bencivenga, M.; D’ignazio, A.; Marino, E.; Cipollari, C.; Molteni, B.; Mura, G.; Marrelli, D.; Graziosi, L.; et al. Conversion gastrectomy for stage IV unresectable gastric cancer: A GIRCG retrospective cohort study. Gastric Cancer 2019, 22, 1285–1293. [Google Scholar] [CrossRef]
- Cunningham, D.; Allum, W.H.; Stenning, S.P.; Thompson, J.N.; Van de Velde, C.J.; Nicolson, M.; Scarffe, J.H.; Lofts, F.J.; Falk, S.J.; Iveson, T.J.; et al. Perioperative Chemotherapy versus Surgery Alone for Resectable Gastroesophageal Cancer. N. Engl. J. Med. 2006, 355, 11–20. [Google Scholar] [CrossRef]
- Reddavid, R.; Sofia, S.; Chiaro, P.; Colli, F.; Trapani, R.; Esposito, L.; Solej, M.; Degiuli, M. Neoadjuvant chemotherapy for gastric cancer. Is it a must or a fake? World J. Gastroenterol. 2018, 24, 274–289. [Google Scholar] [CrossRef]
- Yang, Y.; Yin, X.; Sheng, L.; Xu, S.; Dong, L.; Liu, L. Perioperative chemotherapy more of a benefit for overall survival than adjuvant chemotherapy for operable gastric cancer: An updated Meta-analysis. Sci. Rep. 2015, 5, 12850. [Google Scholar] [CrossRef]
- Zaafouri, H.; Jouini, R.; Khedhiri, N.; Khanchel, F.; Cherif, M.; Mesbahi, M.; Daghmouri, A.; Mahmoudi, W.; Akremi, S.; Sabbah, M.; et al. Comparison between signet-ring cell carcinoma and non-signet-ring cell carcinoma of the stomach: Clinicopathological parameters, epidemiological data, outcome, and prognosis-a cohort study of 123 patients from a non-endemic country. World J. Surg. Oncol. 2022, 20, 238. [Google Scholar] [CrossRef] [PubMed]
- Gronnier, C.; Messager, M.; Robb, W.B.; Thiebot, T.; Louis, D.; Luc, G.; Piessen, G.; Mariette, C. Is the negative prognostic impact of signet ring cell histology maintained in early gastric adenocarcinoma? Surgery 2013, 154, 1093–1099. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.-W.; Lu, J.; Xu, B.-B.; Zheng, C.-H.; Li, P.; Wang, J.-B.; Lin, J.-X.; Chen, Q.-Y.; Cao, L.-L.; Lin, M.; et al. Prognostic Value of Tumor Regression Grading in Patients Treated With Neoadjuvant Chemotherapy Plus Surgery for Gastric Cancer. Front. Oncol. 2021, 11, 587856. [Google Scholar] [CrossRef] [PubMed]
- Lombardi, P.M.; Mazzola, M.; Achilli, P.; Aquilano, M.C.; De Martini, P.; Curaba, A.; Gualtierotti, M.; Bertoglio, C.L.; Magistro, C.; Ferrari, G. Prognostic value of pathological tumor regression grade in locally advanced gastric cancer: New perspectives from a single-center experience. J. Surg. Oncol. 2021, 123, 923–931. [Google Scholar] [CrossRef]
- Pietrantonio, F.; Miceli, R.; Raimondi, A.; Kim, Y.W.; Kang, W.K.; Langley, R.E.; Choi, Y.Y.; Kim, K.M.; Nankivell, M.G.; Morano, F.; et al. Individual Patient Data Meta-Analysis of the Value of Microsatellite Instability As a Biomarker in Gastric Cancer. J. Clin. Oncol. 2019, 37, 3392–3400. [Google Scholar] [CrossRef] [PubMed]
- Messager, M.; Lefevre, J.H.; Pichot-Delahaye, V.; Souadka, A.; Piessen, G.; Mariette, C.; Arnaud, J.P.; Balon, J.M.; Bonnetain, F.; Borie, F.; et al. The Impact of Perioperative Chemotherapy on Survival in Patients With Gastric Signet Ring Cell Adenocarcinoma. Ann. Surg. 2011, 254, 684–693. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Ma, F.H.; Xue, L.Y.; Tian, Y.T. Neoadjuvant chemotherapy vs. upfront surgery for gastric signet ring cell carcinoma: A retrospective, propensity score-matched study. World J. Gastroenterol. 2020, 26, 818. [Google Scholar] [CrossRef]
- Villanueva, L.; Anabalón, J.; Butte, J.M.; Salman, P.; Panay, S.; Milla, E.; Gallardo, C.; Hoefler, S.; Charles, R.; Reyes, F.; et al. Total neoadjuvant chemotherapy with FLOT scheme in resectable adenocarcinoma of the gastro-oesophageal junction or gastric adenocarcinoma: Impact on pathological complete response and safety. Ecancermedicalscience 2021, 15, 1168. [Google Scholar] [CrossRef]
- Schulz, C.; Kullmann, F.; Kunzmann, V.; Fuchs, M.; Geissler, M.; Vehling-Kaiser, U.; Stauder, H.; Wein, A.; Al-Batren, S.-E.; Kubin, T.; et al. NeoFLOT: Multicenter phase II study of perioperative chemotherapy in resectable adenocarcinoma of the gastroesophageal junction or gastric adenocarcinoma—Very goodresponse predominantly in patients with intestinal type tumors. Int. J. Cancer 2015, 137, 678–685. [Google Scholar] [CrossRef]
| Characteristic | N (%) Tot = 76 |
|---|---|
| Sex | |
| male | 53 (70%) |
| female | 23 (30%) |
| Age | |
| ≥75 | 4 (5%) |
| 45–75 | 64 (85%) |
| ≤45 | 8 (10%) |
| Primary tumor location | |
| GEJ | 21 (28%) |
| fundus | 5 (6%) |
| body | 44 (57%) |
| pylorus | 6 (9%) |
| cT before treatment start | |
| cTx | 7 (9%) |
| cT1 | 1 (1%) |
| cT2 | 4 (5%) |
| cT3 | 26 (34%) |
| cT4a | 35 (46%) |
| cT4b | 3 (4%) |
| cN before treatment start | |
| cNx | 2 (3%) |
| cN0 | 15 (20%) |
| cN+ | 59 (77%) |
| ypT after surgery | |
| ypT0 | 6 (8%) |
| ypTis | 1 (1%) |
| ypT1a/b | 6 (8%) |
| ypT2 | 3 (4%) |
| ypT3 | 28 (37%) |
| ypT4a | 17 (22%) |
| ypT4b | 5 (6%) |
| not resected | 10 (13%) |
| ypN after surgery | |
| ypN0 | 20 (26%) |
| ypN1 | 11 (14%) |
| ypN2 | 15 (20%) |
| ypN3 | 20 (26%) |
| not resected | 10 (13%) |
| Surgical radicality | |
| R0 | 54 (71%) |
| R1 | 9 (12%) |
| R2 | 3 (4%) |
| not resected | 10 (13%) |
| Adjuvant therapy started | |
| yes | 57 (75%) |
| no | 9 (12%) |
| not resected | 10 (13%) |
| ECOG PS upon neoadjuvant start | |
| 0 | 57 (75%) |
| 1 | 15 (20%) |
| 2 | 3 (4%) |
| unknown | 1 (1%) |
| Histotype by Lauren | |
| intestinal-type | 23 (30%) |
| diffuse-type | 24 (32%) |
| mixed | 4 (5%) |
| indetermined-type | 25 (33%) |
| Signet-ring cells | |
| yes | 24 (32%) |
| no | 52 (68%) |
| Univariate | Multivariate | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Factor | OS(HR) | 95%CI | p | DFS(HR) | 95%CI | p | OS(HR) | 95%CI | p | DFS(HR) | 95%CI | p |
| cN+ vs. cN0 | 1.76 | 0.83–3.73 | 0.14 | 1.68 | 0.82–3.44 | 0.16 | / | / | / | / | / | / |
| cT4 vs. not | 2.86 | 1.48–5.52 | 0.0017 | 2.38 | 1.24–4.54 | 0.0087 | 2.33 | 1.03–5.25 | 0.0409 | |||
| Diffuse vs. not | 1.49 | 0.74–3.01 | 0.26 | 1.27 | 0.65–2.47 | 0.475 | / | / | / | / | / | / |
| G3 vs. not | 2.19 | 1.08–4.45 | 0.029 | 1.86 | 0.96–3.63 | 0.06 | 1.07 | 0.48–2.38 | 0.86 | / | / | / |
| Signet ring vs. not | 3.30 | 1.56–7.00 | 0.0018 | 3.31 | 1.6–6.83 | 0.0012 | 2.59 | 1.09–6.15 | 0.309 | 3.21 | 1.37–7.56 | 0.0072 |
| ATOM vs. not | 2.74 | 0.85–8.85 | 0.092 | 2.30 | 0.75–7.10 | 0.1448 | / | / | / | / | / | / |
| ECOG PS 0 vs. 12 | 0.62 | 0.29–1.33 | 0.22 | 0.72 | 0.36–1.46 | 0.37 | / | / | / | / | / | / |
| Radicality (R0 vs. R1 vs. R2) | / | / | <0.0001 | / | / | <0.0001 | 24.01 (R0–R1 vs. R2) | 3.72–154.92 | 0.0008 | 62.64 (R0–R1 vs. R2) | 8.78–447.07 | <0.0001 |
| CR vs. not | 0.41 | 0.13–1.29 | 0.128 | 0.39 | 0.14–1.10 | 0.0762 | / | / | / | / | / | / |
| GEJ vs. other | 0.82 | 0.41–1.64 | 0.58 | 0.90 | 0.44–1.84 | 0.79 | / | / | / | / | / | / |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giampieri, R.; Baleani, M.G.; Bittoni, A.; Rastelli, F.; Catalano, V.; Del Prete, M.; Chiorrini, S.; Pinterpe, G.; Graziano, F.; Giorgi, F.C.; et al. Impact of Signet-Ring Cell Histology in the Management of Patients with Non-Metastatic Gastric Cancer: Results from a Retrospective Multicenter Analysis Comparing FLOT Perioperative Chemotherapy vs. Surgery Followed by Adjuvant Chemotherapy. Cancers 2023, 15, 3342. https://doi.org/10.3390/cancers15133342
Giampieri R, Baleani MG, Bittoni A, Rastelli F, Catalano V, Del Prete M, Chiorrini S, Pinterpe G, Graziano F, Giorgi FC, et al. Impact of Signet-Ring Cell Histology in the Management of Patients with Non-Metastatic Gastric Cancer: Results from a Retrospective Multicenter Analysis Comparing FLOT Perioperative Chemotherapy vs. Surgery Followed by Adjuvant Chemotherapy. Cancers. 2023; 15(13):3342. https://doi.org/10.3390/cancers15133342
Chicago/Turabian StyleGiampieri, Riccardo, Maria Giuditta Baleani, Alessandro Bittoni, Francesca Rastelli, Vincenzo Catalano, Michela Del Prete, Silvia Chiorrini, Giada Pinterpe, Francesco Graziano, Francesca Chiara Giorgi, and et al. 2023. "Impact of Signet-Ring Cell Histology in the Management of Patients with Non-Metastatic Gastric Cancer: Results from a Retrospective Multicenter Analysis Comparing FLOT Perioperative Chemotherapy vs. Surgery Followed by Adjuvant Chemotherapy" Cancers 15, no. 13: 3342. https://doi.org/10.3390/cancers15133342
APA StyleGiampieri, R., Baleani, M. G., Bittoni, A., Rastelli, F., Catalano, V., Del Prete, M., Chiorrini, S., Pinterpe, G., Graziano, F., Giorgi, F. C., Bisonni, R., Silva, R., Alessandroni, P., Mencarini, L., & Berardi, R. (2023). Impact of Signet-Ring Cell Histology in the Management of Patients with Non-Metastatic Gastric Cancer: Results from a Retrospective Multicenter Analysis Comparing FLOT Perioperative Chemotherapy vs. Surgery Followed by Adjuvant Chemotherapy. Cancers, 15(13), 3342. https://doi.org/10.3390/cancers15133342







