Simple Summary
Therapeutic advances in acute myeloid leukemia (AML) are dependent on identifying and targeting the molecular aberrations that drive disease. The European LeukemiaNet (ELN) 2022 guidelines have improved the categorization of AML into distinct molecular subgroups. One of the most notable recent inclusions is a group of mutations highly specific for secondary AML. This review examines how each of the secondary-type mutations contributes to the genesis of leukemia while spotlighting potential therapeutic avenues. While we highlight the limitations of the ELN 2022 revision, we also emphasize current progress, recent breakthroughs, and novel therapeutic options tailored to each molecular subset. This review provides a background and foundation for rational molecular-based therapeutic approaches and combination strategies to inspire future clinical trial designs.
Abstract
The characterization of the molecular landscape and the advent of targeted therapies have defined a new era in the prognostication and treatment of acute myeloid leukemia. Recent revisions in the European LeukemiaNet 2022 guidelines have refined the molecular, cytogenetic, and treatment-related boundaries between myelodysplastic neoplasms (MDS) and AML. This review details the molecular mechanisms and cellular pathways of myeloid maturation aberrancies contributing to dysplasia and leukemogenesis, focusing on recent molecular categories introduced in ELN 2022. We provide insights into novel and rational therapeutic combination strategies that exploit mechanisms of leukemogenesis, highlighting the underpinnings of splicing factors, the cohesin complex, and chromatin remodeling. Areas of interest for future research are summarized, and we emphasize approaches designed to advance existing treatment strategies.
1. Introduction
Next-generation sequencing has transformed the landscape of acute myeloid leukemia. Over the last several decades, the identification of recurrent genetic abnormalities in AML has guided modern clinical approaches toward a molecular era. The recent 2022 revision to the European LeukemiaNet guidelines builds upon its predecessor [1,2]; in addition to refinement of the favorable- and intermediate-risk categories, seven new mutations joined the adverse-risk category: SF3B1, SRSF2, U2AF1, ZRSR2, BCOR, EZH2, and STAG2. These seven mutations, along with several others, comprise a group known as the secondary-type mutations, aptly named for their ubiquity in secondary AML [3].
Secondary AML defines a subset of the disease with notoriously adverse outcomes. Based on preceding myelodysplastic neoplasms (MDS), myeloproliferative neoplasms (MPNs), or therapy-related clonal aberrations, secondary AML is associated with lower remission rates and overall survival compared with de novo AML [4,5,6]. While secondary-type mutations are frequently harbingers of dismal outcomes, the relationship between the presence of these mutations and prognosis is more complex. For example, the ELN 2022 revision classifies secondary-type mutations as adverse-risk AML but emphasizes that the risk should not be considered adverse if secondary-type signatures co-occur with favorable-risk mutations [2]. Indeed, real-world analyses from our center and others demonstrated that AML with secondary-type mutations and co-mutated NPM1 or rearrangements of the core-binding factor retain their favorable prognoses [7,8]. In contrast, the effects of mutational cooperation between secondary-type mutations and other mutations in the intermediate- and adverse-risk categories are less clearly defined. If our goal is to adopt a tailored approach to therapy for this heterogenous cohort of AML, what can be done for those with secondary-type mutations, and how impactful would such approaches be?
Spliceosome mutations, including SF3B1, SRSF2, U2AF1, and ZRSR2, are found in approximately 65% of patients with MDS [9]; they are among the earliest mutations to occur [10]. The acquisition of additional somatic lesions confers a subclonal survival advantage, driving the progression from MDS to AML [11]. In the VCU Massey Cancer Center Project ERIS database involving 600 patients with AML, 28.9% harbored a spliceosome mutation or a mutation in BCOR, EZH2, or STAG2; 36.8% harbored mutations in any of the seven previously mentioned genes or ASXL1 or RUNX1 (Figure 1). Alongside AML with mutated NPM1, the chromatin-spliceosome subgroup is one of the largest subgroups of AML [12]. Consequently, the impact of novel targeted therapies or additional treatment strategies unique to AML with secondary-type mutations would be substantial and would represent significant progress for many patients. Thus, this review highlights the mechanisms of leukemogenesis and potential targets of each of the seven secondary-type mutations introduced in the ELN 2022 revision—in addition to ASXL1 and RUNX1. First, we review the spliceosome to appreciate the contributions of each mutation to the genesis of AML.
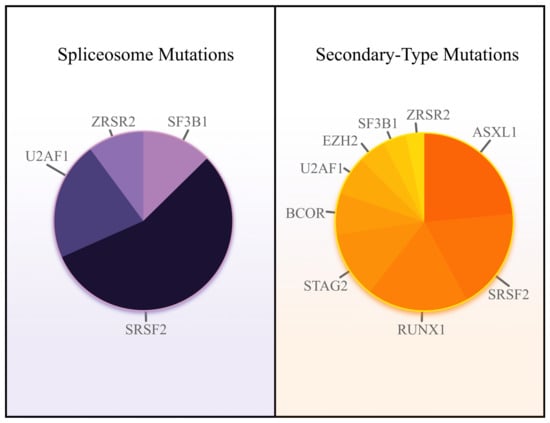
Figure 1.
Relative spliceosome mutation frequency (left) and secondary-type mutation frequency (right) in AML. Data source: VCU Massey Cancer Center Project ERIS database.
The Spliceosome and AML
The spliceosome coordinates mRNA splicing—the conversion of pre-mRNA into mature mRNA [13]. The spliceosome is a gargantuan complex composed of hundreds of proteins and small nuclear RNAs (snRNAs). When snRNAs bind to proteins, they form small nuclear ribonucleoproteins (snRNPs). These snRNPs comprise the major spliceosome—responsible for 99.5% of human splicing—and the minor spliceosome, which splices the remaining 0.5% [14]. Spliceosomes create mature mRNA from pre-mRNA by removing non-coding sequences called introns, named because they are intragenic regions. Introns are sandwiched between a proximal 5′ splice site and a distal 3′ splice site. To remove the introns, uridine-rich snRNPs introduce the 5′ splice site to the 3′ splice site, forming an intron loop called a lariat. The 5′ splice site attacks the 3′ splice site, excising the intron-containing lariat and leaving behind mature mRNA [15]. In summary, splicing produces a mature mRNA transcript—a template for protein translation (Figure 2).

Figure 2.
The spliceosome. After mRNA is transcribed from DNA, U1 binds to the 5′ intron region, and SF1 binds to the 3′ region. The U2AF1 complex is recruited near the 3′ region, which facilitates the binding of U2 and displaces SF1. Next, U4, U5, and U6 aggregate to form the activated spliceosome. The spliceosome creates a lariat from the intron loop, which is excised; the exons are ligated through two sequential transesterification reactions.
To appreciate the intricacies of the most common spliceosome mutations in AML, we will focus on the major spliceosome’s key players, the five uridine (U)-rich snRNPs: U1, U2, U4, U5, and U6. Starting from the proximal end of a target splice region, U1 binds to the 5′ splice site. Next, the budding spliceosome locates the distal end of a splice region through a two-step process. First, splicing factor 1 (SF1) binds to the branch site, an area near the 3′ splice site [16]. Second, the U2 axillary factors (U2AFs) form a complex that tethers to the 3′ splice site adjacent to SF1. After recruiting SF1 and the U2AF complex, U2 snRNPs displace SF1 from the branch site [16]. Finally, a triplet complex consisting of the remaining snRNPs—U4, U5, and U6—joins U1, U2, and the U2AF complex to create the activated spliceosome [17]. The spliceosome excises the intron-containing lariat and ligates the exons through two sequential transesterification reactions.
The diversity of proteins created through splicing is staggering; it is directly related to the selection of splice sites. The spliceosome always recognizes some splice sites, known as constitutive splice sites. In contrast, the recognition of other splice sites depends on the recruitment of additional factors; therefore, they are only occasionally spliced. These are alternative splice sites, which create combinatorial effects on protein diversity [11,17]. In multi-exon genes, 5′ splice sites can join different 3′ splice sites, and 3′ splice sites can join different 5′ splice sites, resulting in a large array of context-specific combinations achieved by alternative splicing. The serine/arginine-rich (SR) proteins and the heterogeneous nuclear ribonucleoproteins (hnRNPs) control alternative splicing by respectively promoting or repressing splicing events [18]; therefore, the regulatory SR proteins and hnRNPs can be oncoproteins or tumor suppressors. Consequently, mutations in spliceosome proteins or their regulators are frequent events in AML.
The four most commonly mutated spliceosome mutations in AML are SF3B1, SRSF2, U2AF1, and ZRSR2. The first three are components of the major spliceosome; they exist in a heterozygous state and usually have altered or gain-of-function effects—consistent with their role as oncoproteins. In contrast, ZRSR2 is a component of the minor spliceosome and commonly exhibits a loss-of-function effect, acting as a tumor suppressor [17]. The spliceosome mutations are largely mutually exclusive and exhibit synthetic lethality [19]. This fundamental principle suggests that leukemic cells with spliceosome mutations may be uniquely sensitive to additional disruptions in splicing [20]. Thus, we will explore how the most common spliceosome mutations contribute to leukemogenesis and the avenues that may lead to more personalized therapeutic approaches.
2. Aberrations of Spliceosome Genes
2.1. SF3B1
Splicing factor 3B subunit 1, also known as SF3B1, is a protein component of the U2 snRNP; it binds to the branch site adjacent to the 3′ splice site. Therefore, the function of SF3B1 is to assist in correctly localizing the spliceosome to the 3′ splice site. In Project ERIS, mutations in SF3B1 occurred in 2.6% (95% CI, 1.4 to 4.9) of patients with AML, with a median upfront variant allele frequency (VAF) of 46.1%, consistent with its known heterozygous presentation; additional aggregate analyses have confirmed SF3B1mut frequencies of 2–5% in AML [21]. Mutations in SF3B1 frequently guide the spliceosome to an alternative 3′ splice site. Consequently, the introduction of a premature stop codon in target gene expression due to SF3B1mut is a common occurrence, resulting in reduced protein expression due to aberrant splicing [22].
The impact of SF3B1mut in hematological malignancies can be best appreciated in MDS with ringed sideroblasts. The abnormal splice site degrades a mitochondrial iron exporter, ABCB7 [23]. Reduction in functional ABCB7 leads to mitochondrial iron retention in erythroblasts, characteristically resulting in ringed sideroblasts, aberrant erythropoiesis, and a predilection for anemia [21]. Other mutations in SF3B1 create terminal blocks in erythroid maturation but without ringed sideroblasts [9,24]. In AML, SF3B1 mutations are frequently found with AML-driving genes: RUNX1mut or rearrangements of MECOM [25]. As SF3B1 mutations are often early events, this finding suggests that AML with SF3B1mut arises following the acquisition of additional adverse-risk mutations—highlighting the need for more effective therapeutic approaches.
The numerous inhibitors of SF3b are the most well-studied spliceosome inhibitors. Pladienolide analogs were the first splicing modulators to enter clinical trials. One of the earliest splicing modulators was E7107, which disrupts splicing by targeting SF3b and inhibiting U2 [26,27]. Attributable to its activity in splicing interference, E7107 promotes apoptosis and cell cycle arrest [28,29]. While E7107 showed promising activity in pre-clinical studies, it was associated with vision loss and little efficacy in humans, resulting in trial termination [30]. This led to the investigation of other spliceosome inhibitors and the discovery of H3B-8800, a synthetic pladienolide derivative with a similar mechanism. A multi-center phase I trial evaluated H3B-8800 in MDS, CMML, and AML; while the drug was associated with decreased transfusion requirements and splicing modulation, it did not produce clinical responses [31,32].
Newer pre-clinical studies suggest that AML with internal tandem duplications in FMS-like tyrosine kinase (FLT3-ITD) may be more sensitive to the pladienolide analogs. In AML with FLT3-ITD, investigators noted increased induction of cell cycle arrest and a shift in the splicing patterns toward a pro-apoptotic state due to reduced MCL1 following pladienolide treatment [33]. These findings suggest a biological rationale for combining splicing modulators with FLT3 inhibitors or inducers of apoptosis, such as venetoclax.
2.2. SRSF2
Serine/arginine-rich splicing factor 2 (SRSF2) is a regulatory SR protein that promotes splicing events by binding to splicing enhancer segments [34]. Mutated SRSF2 has reduced binding to splicing enhancers, culminating in global splicing changes [16]. Mutations in SRSF2 result in the mis-splicing of EZH2 and BCOR, explaining the observed mutual exclusivity between these genes [35,36]. Due to SRSF2mut-induced splicing, alterations in splicing factors block hematopoietic differentiation, which impairs maturation and drives leukemogenesis [37].
Mutations in SRSF2 ubiquitously occur at proline 95, identified in 90.9% (95% CI, 76.4 to 96.9) of AML in Project ERIS—the reminder commonly appear at proline 94 or 96. A single allele mutation in SRSF2 at proline 95 disrupts RNA-binding specificity and is sufficient to induce myelodysplasia [35]. Consequently, a single mutation at proline 95 produced the phenotypic characteristics of MDS in mice: anemia, leukopenia, and dysplasia. These observations led investigators to assess the efficacy of spliceosome inhibitors in SRSF2mut AML.
Both investigational drugs that interfere with SF3b were evaluated in SRSF2mut AML. Investigators administered E7107 to mice with SRSF2P95H. Untreated mice developed bone marrow failure; treated mice had a significantly reduced leukemic burden [20]. Moreover, splicing inhibition was greater in SRSF2mut AML than in wild-type genotypes [20]. Similarly, H3B-8800—a synthetic pladienolide derivative—also showed decreased leukemic burden in SRSF2P95H mice compared with wild-type mice [38]. Neither drug, however, produced clinically significant activity in trials, leading to the investigation of alternative approaches.
The discovery that spliceosome proteins require phosphorylation for nuclear transport—a process regulated by the CDC-like kinase (CLK) family—paved the way for innovation [39]. CTX-712, an orally available CLK inhibitor, strongly inhibits the phosphorylation of SR proteins that bind SRSF2. An SRSF2mut PDX model showed a significant and dose-dependent response to CTX-712; additionally, the drug demonstrated anti-leukemic efficacy in PDX AML models without spliceosome mutations [40]. In a phase I trial, CTX-712 was associated with a composite complete remission rate of 60.0% in AML, with further safety assessment ongoing [41]. A phase I/II trial assessing CTX-712 in AML and MDS is currently recruiting at MD Anderson Cancer Center (NCT05732103).
2.3. U2AF1
U2 small nuclear RNA axillary factor 1 (U2AF1) recognizes the 3′ splice site and recruits the U2 snRNP during splicing [42]. Mutations in U2AF1 induce splicing alterations in genes critical for hematopoietic stem cell function [43], predisposing stem cells to DNA damage. Therefore, a crucial role of U2AF1 is to maintain the function and regenerative potential of stem cells in hematopoiesis. Indeed, mouse models with U2AF1 knockout show pancytopenia, a reduction in hematopoietic stem cells, and decreased survival [43].
U2AF1 has additional roles aside from splicing. U2AF1 and its binding partner, U2AF2, bind to cytoplasmic RNA and act as translational repressors [44]. U2AF1S34F predisposes to increased translation of interleukin 8, upregulating the inflammatory response and correlating with a higher incidence of relapsed or refractory AML in humans; blocking interleukin 8 led to tumor burden reduction in mice [44]. Consistent with these early observations of an upregulated inflammatory state, the focus has shifted to interleukin-1-associated kinase 4 (IRAK4), the predominant isoform that arises from alternative splicing in MDS and AML (Figure 3). U2AF1mut is responsible for the alternative splicing of IRAK4, which results in the retention of an exon and creates a long isoform: IRAK4-long (IRAK4-L).
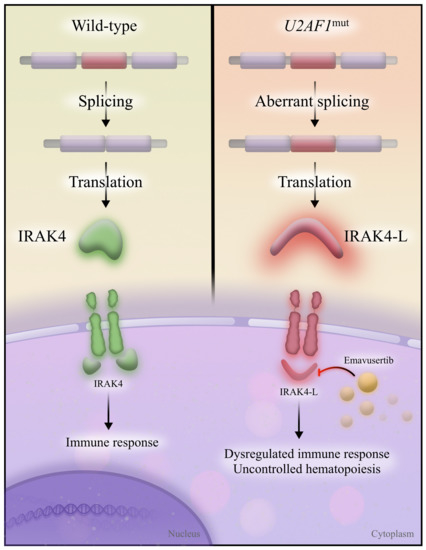
Figure 3.
IRAK4 and IRAK4-L. Wild-type spliceosome proteins allow for the correct splicing of IRAK4 (left), which removes an exon (red) and creates a functional IRAK4 protein. IRAK4 then appropriately activates the immune response. In the presence of U2AF1 mutations (right), the exon is inappropriately included in the final transcript, resulting in a longer IRAK4 isoform (IRAK4-L). IRAK4-L results in dysregulated immune response and uncontrolled hematopoiesis; it is inhibited by emavusertib.
Investigators discovered that inhibition of IRAK4-L disrupts leukemic growth [45]. Thus, the presence of U2AF1mut in AML induces the expression of a novel therapeutic target, IRAK4-L, which is inhibited with emavusertib. Single-agent emavusertib demonstrated a composite complete remission rate of 40.0% in AML with spliceosome mutations, and 57.1% of high-risk MDS patients had <5% marrow blasts [46]. Emavusertib continues to be evaluated in multicenter clinical trials as monotherapy and in combination with azacitidine and venetoclax (NCT04278768).
2.4. ZRSR2
The zinc finger (CCCH type), RNA-binding motif, and serine/arginine rich 2 (ZRSR2) gene encodes a key member of the minor spliceosome that recognizes 3′-intron splice sites [47]. ZRSR2 mutations differ from the remaining spliceosome mutations in two critical ways. First, ZRSR2 mutations primarily affect the minor spliceosome; SF3B1, SRSF2, and U2AF1 mutations tend to affect the major spliceosome. Second, mutations in ZRSR2 are scattered throughout the length of the gene, in contrast to the other three spliceosome mutations, which occur at hotspots. As ZRSR2 is located on the X chromosome, mutations are linked to male-predominant leukemia—particularly in blastic plasmacytoid dendritic cell neoplasm [48]. In the Project ERIS database, 75% (95% CI, 40.9 to 95.6) of patients with ZRSR2mut were male, suggesting a similar male-dominant presentation in AML.
ZRSR2 is required for splice site recognition of U12-type introns, which are removed by the minor spliceosome. Knockdown of ZRSR2 results in reduced differentiation of hematopoietic precursors due to the aberrant retention of U12-type introns, suggesting that a competent minor spliceosome is necessary for myeloid differentiation [47]. Loss of functional ZRSR2 leads to increased self-renewal of hematopoietic stem cells [49]. Additionally, leukemia cells with ZRSR2 knockdown exhibited slower growth compared with wild-type controls, a pattern not exclusive to loss of ZRSR2; U2AF1mut AML demonstrated similar findings of reduced hematopoietic reconstitution [47]. Therefore, mutations in ZRSR2 appear to lead to constitutive stem cell self-renewal but do not appear to contribute to proliferative phenotypes.
There is relatively limited data on adopting a targeted approach to ZRSR2mut AML. Novel agents that reverse the retention of U12-type introns and restore the function of the minor spliceosome have yet to be widely identified or studied, representing an area of unmet clinical need and a growing area of research interest. However, several mis-spliced targets may be of interest—ZRSR2 mutations frequently result in the aberrant splicing of several E2F and MAP kinase signaling regulators, including the RAS pathway [47]. Breakthroughs in the treatment of ZRSR2mut AML will likely require the identification of compounds that restore the function of the minor spliceosome, induce synthetic lethality through inhibition of other targets, or modify downstream mediators dysregulated by aberrant U12-type intron splicing.
3. Aberrations of Chromatin Modifiers
3.1. BCOR
The BCL6 corepressor (BCOR) is a transcription factor that regulates stem cell function and hematopoiesis. To understand the role of BCOR in AML, we will first review the polycomb group proteins. The polycomb group proteins are responsible for remodeling chromatin; they are recognized for silencing HOX genes through chromatin structure alteration, a critical process for hematopoiesis. Two key complexes in the polycomb group family are polycomb repressive complex 1 (PRC1) and polycomb repressive complex 2 (PRC2). PRC1 catalyzes the ubiquitination of histones (a histone ubiquitin ligase), repressing target genes that control stem cell pluripotency [50]. PRC2 is a histone methyltransferase that inhibits transcription through chromatin compaction [51]. In the context of BCORmut AML, we will shift our focus to the interaction between PRC1 and PCR2.
BCOR is a key component of PRC1.1, a noncanonical PRC1 [52]. BCOR recruits PRC1.1 to specific chromatin sites, resulting in histone ubiquitination and subsequent recruitment of PRC2, culminating in transcriptional repression [52]. Loss of BCOR, therefore, results in decreased histone ubiquitination at the HOX and CEBPA promoters, which aberrantly activates these myeloid-related genes [53]. BCOR mutations consequently lead to the expansion of myeloid precursors and produce phenotypes characteristic of MDS, driving ineffective hematopoiesis and dysplasia [53]. Furthermore, in BCORmut MDS, the acquisition of proliferative mutations through clonal evolution promotes the progression of MDS to AML, commonly through NRAS, KRAS, or FLT3-ITD [3].
BCOR mutations are associated with primary refractory AML [54], frequently cooperating with oncogenic KRAS mutations—particularly KRASG12D [55]. In Project ERIS, BCORmut AML also commonly cooperated with NRAS mutations, most frequently at glycine 12, with a median VAF of 25.03% (range, 19.59 to 37.22). In striking accordance with these observations, BCORmut AML appears to be sensitive to tyrosine kinase inhibition targeting pathways involved in stem cell pluripotency [56]. Ongoing studies now focus on modulating signal transduction to overcome BCORmut-mediated disease refractoriness in AML [56].
3.2. EZH2
As we have reviewed the role of chromatin compaction with BCOR in PRC1, we will similarly unpack the function of enhancer of zeste homologue 2 (EZH2) in PRC2. EZH2 is the catalytic component of PRC2, a transcriptional corepressor with lysine methyltransferase activity that controls gene expression through histone modification [51]. The addition of a methyl group to lysines on histones induces a repressive chromatin state. Mutations in EZH2 are a double-edged sword: as its target genes are required for stem cell maintenance, both gain- and loss-of-function mutations result in characteristic hematopoietic perturbations. A detailed review of epigenetics in leukemogenesis can be found here [51].
EZH2 acts as an oncoprotein in gain-of-function mutations. Well-studied in the context of lymphomagenesis, EZH2 overexpression results in transcriptional suppression of genes responsible for differentiation [57]. During disease maintenance in AML, EZH2 adopts an oncogenic role, suggesting its function can be therapeutically targeted [51,58]. In this context, gain-of-function mutations in EZH2 can be targeted by EZH2 inhibitors such as valemetostat, tazemetostat, GSK126, or 3-deazaneplanocin. Of recent interest, valemetostat, a dual inhibitor of EZH1 and EZH2, recruits leukemic stem cells into the cell cycle while potentiating apoptosis following treatment with a hypomethylating agent and venetoclax [59]. Another combination approach appears viable with intensive chemotherapy: inhibition of EZH2 rendered AML cells more susceptible to anthracycline-based therapy, with relatively lower anthracycline doses producing marked leukemia suppression [60].
In contrast, loss-of-function EZH2 mutations are more challenging to approach. As gain-of-function mutations act as oncoproteins, loss-of-function EZH2 mutations, conversely, act as tumor suppressors. In mouse models, loss-of-function EZH2 mutations activate bivalent promoters that accelerate AML induction [58]. Therefore, the approach to loss-of-function EZH2mut will require more innovative approaches compared with its gain-of-function counterpart.
Similar to BCORmut AML, loss-of-function of EZH2 is associated with resistance to cytarabine [61]; however, the mechanisms of resistance are complex—driven by apoptosis evasion, increased proliferation, and alteration of transporter function. We speculate that therapies targeting the control of apoptosis and proliferation may provide insights into overcoming EZH2mut-mediated resistance to treatment. An additional therapeutic avenue involves potentially targeting a mutually exclusive mutation. For example, SRSF2mut and EZH2 loss-of-function mutations are mutually exclusive in MDS [9]. Thus, in the context of loss-of-function EZH2 mutations, SRSF2 inhibition theoretically induces synthetic lethality. Further research is needed to characterize the dynamic landscape associated with EZH2 loss-of-function in AML.
3.3. ASXL1
Additional sex combs-like 1 (ASXL1) is jointly responsible for deubiquitinating histones—a function it performs with its histone deubiquitinase partner, BRCA1-associated protein 1 (BAP1). BAP1 is part of the polycomb repressive deubiquitinase (PR-DUB) complex, which reverses the repressive ubiquitination signatures of polycomb repressive complex 1. Therefore, the PR-DUB complex promotes gene expression [50].
ASXL1 activates BAP1 [62]. In turn, the PR-DUB complex is recruited to chromatin and regulates the gene expression of critical mediators of hematopoietic development, including the HOX genes. Mutations in ASXL1 result in BAP1 hyperactivation. BAP1 gain-of-function creates a state of histone deubiquitination at HOX gene regions, leading to leukemic transformation [63]. Under normal conditions, PRC1 maintains the inactive state of HOX genes through histone ubiquitination at these regions; however, hyperactive BAP1 reverses PRC1-mediated ubiquitination signatures and creates constitutive HOX gene expression, resulting in myeloid leukemogenesis [64]. Therefore, BAP1 is a tumor promoter in ASXL1mut AML [63].
Additionally, mutated ASXL1 acquires a novel binding partner: bromodomain-containing protein 4 (BRD4) [65]. BRD4 acetylates histones and activates gene transcription via RNA polymerase II, resulting in stem cell self-renewal [65]. ASXL1 mutations that bind to BRD4 have higher expression of genes responsible for leukemogenesis, suggesting that inhibition of the ASXL1 and BRD4 interaction potentiates leukemic cell death [65].
Indeed, small-molecule inhibitors that target bromodomain and extraterminal (BET) domain proteins—including BRD4—demonstrate antileukemic activity [66]. BRD4 inhibition reduced the expression of self-renewal genes in ASXL1mut AML by inhibiting the interaction between mutated ASXL1 and BRD4, suggesting BET inhibitors may have a role in ASXL1mut AML [65]. Furthermore, BET inhibitors synergize with venetoclax in PDX models and may even help overcome venetoclax resistance, paving the way for combination approaches [67,68]. Other approaches include BAP1 inhibitors for ASXL1mut AML, which were shown to inhibit HOX gene expression [63].
3.4. STAG2
The cohesin subunit SA-2 is encoded by STAG2, one of the four core units of the cohesin complex. The cohesin complex regulates chromatin structure; it is named because it mediates the cohesion of sister chromatids during replication and facilitates the transition from metaphase to anaphase during cell division [69,70]. While the role of the cohesin complex proteins in regulating chromatin has been well studied, the role of STAG2 in myeloid leukemogenesis has been more challenging to elucidate.
STAG2, alongside the remaining three core cohesin partners—RAD21, SMC1A, and SMC3—promotes hematopoietic stem cell differentiation [71]. Inactivation of any of the four core cohesin complex proteins creates stem cell self-renewal [72,73]; knockout mouse models showed changes strikingly consistent with early leukemogenesis, with induction of acute leukemia following the acquisition of FLT3-ITD [74]. Efforts to target STAG2 mutations in AML, however, have not focused on indirectly targeting common cooperating mutations but on leukemic clone elimination directly through synthetic lethality.
STAG2 knockout cell lines had homologous recombination deficiencies [75]. Talazoparib, a poly (ADP-ribose) polymerase (PARP) inhibitor, resulted in a significantly increased response in SRSF2mut cells compared with wild-type cells [75]. Therefore, DNA damage deficiencies appear to sensitize STAG2 knockout cells to PARP inhibition. Talazoparib showed moderate anti-leukemic activity as monotherapy in a molecularly unselected, heavily pretreated AML population [76]; combination approaches with decitabine or gemtuzumab ozogamicin suggested combination approaches were safe and tolerable [77,78].
STAG1, a protein paralogous to STAG2, is an additional target for the induction of synthetic lethality in STAG2mut AML. Cancer cells depend on the overlapping function of STAG1 in STAG2 knockout cell lines to maintain the integrity of chromatin cohesion; double knockout of STAG1 and STAG2 induces synthetic lethality [79]. Recently, investigators performed a proof-of-concept demonstration in primary human leukemic cells: disrupting STAG1 eliminated STAG2mut AML cells and was well tolerated by wild-type hematopoietic stem cells [80]. These findings pave the way for the evaluation of STAG1 inhibition for the treatment of STAG2mut AML.
3.5. RUNX1
Runt-related transcription factor 1 (RUNX1) mutations do not directly alter chromatin structure. However, they affect the binding of transcription factors to chromatin and indirectly influence gene expression through altered chromatin interactions [81]. RUNX1 encodes the α subunit of the core-binding factor and is therefore responsible for binding transcription complexes to DNA [82]. There are two broad categories of RUNX1 aberrations in AML: RUNX1-related chromosomal rearrangements and RUNX1 somatic mutations. In the most well-known RUNX1-related rearrangement in AML, RUNX1 fuses to a transcriptional corepressor (RUNX1T1), creating the t(8;21) fusion protein, RUNX1::RUNX1T1, which we have reviewed elsewhere [51]. While this RUNX1-related rearrangement in AML confers a favorable prognosis, somatic mutations do not [2]. Therefore, we will now focus on the role of the somatic RUNX1mut in AML.
Truncated RUNX1 mutations lack DNA binding activity; missense mutations similarly lack DNA binding activity and down-regulate wild-type RUNX1, creating a dominant-negative effect [81]. Other dominant-negative RUNX1 mutations occupy the target site on DNA and block functional RUNX1 from occupying the same sites [83]. These alterations have profound downstream effects: mutated RUNX1 disrupts a transcription complex that regulates critical targets of hematopoiesis [84]. Although wild-type RUNX1 is necessary for the genesis of core-binding factor leukemia and rearrangements of mixed lineage leukemia AML, functional RUNX1 is also essential for hematopoietic differentiation [84]. In addition to its role in hematopoietic differentiation, RUNX1 also controls cell cycle regulation, the p53 pathway, and ribosome synthesis [84].
The effects of RUNX1 mutations in AML suggest sensitivity to ribosome disruptions (Figure 4). Investigators are now targeting RUNX1mut AML after discovering that the knockdown of wild-type RUNX1 induces more potent cell death with ribosome inhibitors [85]. Homoharringtonine is an antileukemic ribosome inhibitor derived from a plant alkaloid that shares structural properties with its synthetic form, omacetaxine mepesuccinate. Treatment with homoharringtonine induces a similar gene expression signature as RUNX1 knockdown [85]. AML blasts with RUNX1mut demonstrated synthetic lethality in a combination approach with omacetaxine mepesuccinate and venetoclax, perhaps partly owing to the omacetaxine-related repression of MCL1, an antiapoptotic protein commonly implicated in venetoclax resistance [85,86]. A clinical trial evaluating the combination of omacetaxine and venetoclax in RUNX1mut AML is recruiting at MD Anderson Cancer Center (NCT04874194).

Figure 4.
Synthetic lethality in RUNX1mut AML. Omacetaxine mepesuccinate is a ribosome inhibitor—it induces RUNX1 knockdown-like gene expression (left). Venetoclax is a BCL2 inhibitor that binds to BCL2 and allows the BAX pore to form on the surface of mitochondria. BAX pore formation facilitates apoptosis. Omacetaxine and venetoclax result in synthetic lethality in RUNX1mut AML.
4. Conclusions and Future Directions
The addition of these seven recurrent genetic aberrations to the ELN 2022 adverse risk category has refined the prognostication of AML. This group of newly added mutations plays a dominant role in splicing and epigenetic regulation, with unique opportunities for targeted therapies in each molecular cohort. In addition, alternative therapeutic approaches likely exist in larger subgroups by exploiting the nature of the underlying epigenetic or splicing aberrations.
For example, spliceosome mutations lead to alternative splicing of other splicing factors [37]. This results in an aberrant splicing cascade, creating a diverse array of neoantigens. Investigators have recently proposed that the accumulation of neoantigens could make AML with spliceosome mutations more responsive to antibodies against programmed cell death 1 (PD-1) or its ligand (PD-L1) [37]. Limited sample sizes have precluded definitive analyses of outcomes with immunotherapy; however, investigators reported outcomes in a more general cohort of secondary AML.
A phase II study of pembrolizumab and cytarabine in relapsed or refractory AML resulted in a composite complete remission rate of 25.0% in secondary AML [87]; similarly, the combination of nivolumab and azacitidine produced a composite complete remission rate of 22.0% and superior overall survival compared with historical controls treated with azacitidine [88]. In the nivolumab and azacitidine cohort, 44% of patients in the immunotherapy arm had secondary AML. In Project ERIS, we identified one patient (AML-230) with ZRSR2mut AML that underwent 19 cycles of consolidation with nivolumab following conventional 7 + 3; the patient achieved a complete response, and the overall survival was 26.3 months. Nevertheless, reports of immunotherapy-associated outcomes in AML with spliceosome mutations are limited.
Other approaches indirectly target splicing. RBM39, an RNA splicing factor, prolongs the survival of AML cells, suggesting a role in AML maintenance [89]. E7820 is an aryl sulfonamide that degrades RBM39 [90]. It induces aberrant splicing and disrupts downstream targets responsible for AML maintenance, such as HOXA9, resulting in the preferential lethality of spliceosome-mutated AML [89]. In a phase II trial, investigators administered a similar aryl sulfonamide, indisulam, in combination with idarubicin and cytarabine as salvage therapy in MDS and AML. The cohort was molecularly unselected; the response rate was 35%, and the median duration of response was 5.3 months [91]. Other targeted approaches in spliceosome-mutated AML include disrupting post-translational splicing factors or areas of replication stress, known as R-loops [90,92]. The effectiveness of indirect approaches remains an open question: can we target spliceosome mutations as a general class?
Mutational cooperation invariably complicates targeted approaches. Combination therapies with multiple targeted agents hold promise in the treatment of AML, and clinical trials should be designed to encourage the recruitment of specific molecular cohorts. Innovations in AML require mutation-driven treatment paradigms to bring new hope to the field as we begin a new chapter of the molecular era.
Author Contributions
I.M.B. prepared the manuscript and created the figures. K.R.M. revised the manuscript. S.G. oversaw the project and revised the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This project is part of Virginia Commonwealth University’s Massey Cancer Center’s Project ERIS: Expanding Research in Induction and Salvage. This research was supported by Virginia Commonwealth University’s institutional REDCap grant: UL1TR002649; P30 CA16059; and UM1 CA186644.
Acknowledgments
We thank the patients and their caregivers for their contribution to the efforts of Project ERIS.
Conflicts of Interest
K.R. Maher is a consultant for Sobi and Bristol-Myers Squibb. The remaining authors have no conflict of interest to disclose.
References
- Dohner, H.; Estey, E.; Grimwade, D.; Amadori, S.; Appelbaum, F.R.; Buchner, T.; Dombret, H.; Ebert, B.L.; Fenaux, P.; Larson, R.A.; et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood 2017, 129, 424–447. [Google Scholar] [CrossRef] [PubMed]
- Döhner, H.; Wei, A.H.; Appelbaum, F.R.; Craddock, C.; DiNardo, C.D.; Dombret, H.; Ebert, B.L.; Fenaux, P.; Godley, L.A.; Hasserjian, R.P.; et al. Diagnosis and Management of AML in Adults: 2022 ELN Recommendations from an International Expert Panel. Blood 2022, 140, 1345–1377. [Google Scholar] [CrossRef] [PubMed]
- Lindsley, R.C.; Mar, B.G.; Mazzola, E.; Grauman, P.V.; Shareef, S.; Allen, S.L.; Pigneux, A.; Wetzler, M.; Stuart, R.K.; Erba, H.P.; et al. Acute myeloid leukemia ontogeny is defined by distinct somatic mutations. Blood 2015, 125, 1367–1376. [Google Scholar] [CrossRef] [PubMed]
- Granfeldt Ostgard, L.S.; Medeiros, B.C.; Sengelov, H.; Norgaard, M.; Andersen, M.K.; Dufva, I.H.; Friis, L.S.; Kjeldsen, E.; Marcher, C.W.; Preiss, B.; et al. Epidemiology and Clinical Significance of Secondary and Therapy-Related Acute Myeloid Leukemia: A National Population-Based Cohort Study. J. Clin. Oncol. 2015, 33, 3641–3649. [Google Scholar] [CrossRef]
- Hulegardh, E.; Nilsson, C.; Lazarevic, V.; Garelius, H.; Antunovic, P.; Rangert Derolf, A.; Mollgard, L.; Uggla, B.; Wennstrom, L.; Wahlin, A.; et al. Characterization and prognostic features of secondary acute myeloid leukemia in a population-based setting: A report from the Swedish Acute Leukemia Registry. Am. J. Hematol. 2015, 90, 208–214. [Google Scholar] [CrossRef]
- Short, N.J.; Venugopal, S.; Qiao, W.; Kadia, T.M.; Ravandi, F.; Macaron, W.; Dinardo, C.D.; Daver, N.; Konopleva, M.; Borthakur, G.; et al. Impact of frontline treatment approach on outcomes in patients with secondary AML with prior hypomethylating agent exposure. J. Hematol. Oncol. 2022, 15, 12. [Google Scholar] [CrossRef]
- Wright, M.F.; Pozdnyakova, O.; Hasserjian, R.P.; Aggarwal, N.; Shaver, A.C.; Weinberg, O.K.; Irlmeier, R.; Koyama, T.; Seegmiller, A.C.; Strickland, S.A.; et al. Secondary-type mutations do not impact prognosis in acute myelogenous leukemia AML with mutated NPM1. Am. J. Hematol. 2022, 97, E462–E465. [Google Scholar] [CrossRef]
- Hang, Y.; Bouligny, I.M.; Murray, G.; Patel, T.; Doyel, M.; Boron, J.; Tran, V.; Gor, J.; Ho, T.; Zacholski, K.; et al. Clinical outcomes of core binding factor acute myeloid leukemia in the modern era. J. Clin. Oncol. 2023, 41, e19054. [Google Scholar] [CrossRef]
- Nagata, Y.; Maciejewski, J.P. The functional mechanisms of mutations in myelodysplastic syndrome. Leukemia 2019, 33, 2779–2794. [Google Scholar] [CrossRef]
- Bersanelli, M.; Travaglino, E.; Meggendorfer, M.; Matteuzzi, T.; Sala, C.; Mosca, E.; Chiereghin, C.; Di Nanni, N.; Gnocchi, M.; Zampini, M.; et al. Classification and Personalized Prognostic Assessment on the Basis of Clinical and Genomic Features in Myelodysplastic Syndromes. J. Clin. Oncol. 2021, 39, 1223–1233. [Google Scholar] [CrossRef]
- Cazzola, M. Myelodysplastic Syndromes. N. Engl. J. Med. 2020, 383, 1358–1374. [Google Scholar] [CrossRef]
- Papaemmanuil, E.; Gerstung, M.; Bullinger, L.; Gaidzik, V.I.; Paschka, P.; Roberts, N.D.; Potter, N.E.; Heuser, M.; Thol, F.; Bolli, N.; et al. Genomic Classification and Prognosis in Acute Myeloid Leukemia. N. Engl. J. Med. 2016, 374, 2209–2221. [Google Scholar] [CrossRef]
- Lee, Y.; Rio, D.C. Mechanisms and Regulation of Alternative Pre-mRNA Splicing. Annu. Rev. Biochem. 2015, 84, 291–323. [Google Scholar] [CrossRef]
- Chen, W.; Moore, M.J. Spliceosomes. Curr. Biol. 2015, 25, R181–R183. [Google Scholar] [CrossRef]
- Will, C.L.; Luhrmann, R. Spliceosome structure and function. Cold Spring Harb. Perspect. Biol. 2011, 3, a003707. [Google Scholar] [CrossRef]
- Taylor, J.; Lee, S.C. Mutations in spliceosome genes and therapeutic opportunities in myeloid malignancies. Genes Chromosomes Cancer 2019, 58, 889–902. [Google Scholar] [CrossRef]
- Dvinge, H.; Kim, E.; Abdel-Wahab, O.; Bradley, R.K. RNA splicing factors as oncoproteins and tumour suppressors. Nat. Rev. Cancer 2016, 16, 413–430. [Google Scholar] [CrossRef]
- Singh, R.; Valcarcel, J. Building specificity with nonspecific RNA-binding proteins. Nat. Struct. Mol. Biol. 2005, 12, 645–653. [Google Scholar] [CrossRef]
- Lee, S.C.; North, K.; Kim, E.; Jang, E.; Obeng, E.; Lu, S.X.; Liu, B.; Inoue, D.; Yoshimi, A.; Ki, M.; et al. Synthetic Lethal and Convergent Biological Effects of Cancer-Associated Spliceosomal Gene Mutations. Cancer Cell 2018, 34, 225–241.e228. [Google Scholar] [CrossRef]
- Lee, S.C.; Dvinge, H.; Kim, E.; Cho, H.; Micol, J.B.; Chung, Y.R.; Durham, B.H.; Yoshimi, A.; Kim, Y.J.; Thomas, M.; et al. Modulation of splicing catalysis for therapeutic targeting of leukemia with mutations in genes encoding spliceosomal proteins. Nat. Med. 2016, 22, 672–678. [Google Scholar] [CrossRef]
- Cilloni, D.; Itri, F.; Bonuomo, V.; Petiti, J. SF3B1 Mutations in Hematological Malignancies. Cancers 2022, 14, 4927. [Google Scholar] [CrossRef] [PubMed]
- Dolatshad, H.; Pellagatti, A.; Liberante, F.G.; Llorian, M.; Repapi, E.; Steeples, V.; Roy, S.; Scifo, L.; Armstrong, R.N.; Shaw, J.; et al. Cryptic splicing events in the iron transporter ABCB7 and other key target genes in SF3B1-mutant myelodysplastic syndromes. Leukemia 2016, 30, 2322–2331. [Google Scholar] [CrossRef] [PubMed]
- Clough, C.A.; Pangallo, J.; Sarchi, M.; Ilagan, J.O.; North, K.; Bergantinos, R.; Stolla, M.C.; Naru, J.; Nugent, P.; Kim, E.; et al. Coordinated missplicing of TMEM14C and ABCB7 causes ring sideroblast formation in SF3B1-mutant myelodysplastic syndrome. Blood 2022, 139, 2038–2049. [Google Scholar] [CrossRef] [PubMed]
- Mupo, A.; Seiler, M.; Sathiaseelan, V.; Pance, A.; Yang, Y.; Agrawal, A.A.; Iorio, F.; Bautista, R.; Pacharne, S.; Tzelepis, K.; et al. Hemopoietic-specific Sf3b1-K700E knock-in mice display the splicing defect seen in human MDS but develop anemia without ring sideroblasts. Leukemia 2017, 31, 720–727. [Google Scholar] [CrossRef] [PubMed]
- Huber, S.; Haferlach, T.; Meggendorfer, M.; Hutter, S.; Hoermann, G.; Baer, C.; Kern, W.; Haferlach, C. SF3B1 mutations in AML are strongly associated with MECOM rearrangements and may be indicative of an MDS pre-phase. Leukemia 2022, 36, 2927–2930. [Google Scholar] [CrossRef]
- Kotake, Y.; Sagane, K.; Owa, T.; Mimori-Kiyosue, Y.; Shimizu, H.; Uesugi, M.; Ishihama, Y.; Iwata, M.; Mizui, Y. Splicing factor SF3b as a target of the antitumor natural product pladienolide. Nat. Chem. Biol. 2007, 3, 570–575. [Google Scholar] [CrossRef]
- Yokoi, A.; Kotake, Y.; Takahashi, K.; Kadowaki, T.; Matsumoto, Y.; Minoshima, Y.; Sugi, N.H.; Sagane, K.; Hamaguchi, M.; Iwata, M.; et al. Biological validation that SF3b is a target of the antitumor macrolide pladienolide. FEBS J. 2011, 278, 4870–4880. [Google Scholar] [CrossRef]
- Zhang, Q.; Di, C.; Yan, J.; Wang, F.; Qu, T.; Wang, Y.; Chen, Y.; Zhang, X.; Liu, Y.; Yang, H.; et al. Inhibition of SF3b1 by pladienolide B evokes cycle arrest, apoptosis induction and p73 splicing in human cervical carcinoma cells. Artif. Cells Nanomed. Biotechnol. 2019, 47, 1273–1280. [Google Scholar] [CrossRef]
- Jorge, J.; Petronilho, S.; Alves, R.; Coucelo, M.; Goncalves, A.C.; Nascimento Costa, J.M.; Sarmento-Ribeiro, A.B. Apoptosis induction and cell cycle arrest of pladienolide B in erythroleukemia cell lines. Investig. New Drugs 2020, 38, 369–377. [Google Scholar] [CrossRef]
- Hong, D.S.; Kurzrock, R.; Naing, A.; Wheler, J.J.; Falchook, G.S.; Schiffman, J.S.; Faulkner, N.; Pilat, M.J.; O’Brien, J.; LoRusso, P. A phase I, open-label, single-arm, dose-escalation study of E7107, a precursor messenger ribonucleic acid (pre-mRNA) splicesome inhibitor administered intravenously on days 1 and 8 every 21 days to patients with solid tumors. Investig. New Drugs 2014, 32, 436–444. [Google Scholar] [CrossRef]
- Steensma, D.P.; Wermke, M.; Klimek, V.M.; Greenberg, P.L.; Font, P.; Komrokji, R.S.; Yang, J.; Brunner, A.M.; Carraway, H.E.; Ades, L.; et al. Results of a Clinical Trial of H3B-8800, a Splicing Modulator, in Patients with Myelodysplastic Syndromes (MDS), Acute Myeloid Leukemia (AML) or Chronic Myelomonocytic Leukemia (CMML). Blood 2019, 134, 673. [Google Scholar] [CrossRef]
- Steensma, D.P.; Wermke, M.; Klimek, V.M.; Greenberg, P.L.; Font, P.; Komrokji, R.S.; Yang, J.; Brunner, A.M.; Carraway, H.E.; Ades, L.; et al. Phase I First-in-Human Dose Escalation Study of the oral SF3B1 modulator H3B-8800 in myeloid neoplasms. Leukemia 2021, 35, 3542–3550. [Google Scholar] [CrossRef]
- van der Werf, I.; Wojtuszkiewicz, A.; Yao, H.; Sciarrillo, R.; Meggendorfer, M.; Hutter, S.; Walter, W.; Janssen, J.; Kern, W.; Haferlach, C.; et al. SF3B1 as therapeutic target in FLT3/ITD positive acute myeloid leukemia. Leukemia 2021, 35, 2698–2702. [Google Scholar] [CrossRef]
- Zhou, Z.; Fu, X.D. Regulation of splicing by SR proteins and SR protein-specific kinases. Chromosoma 2013, 122, 191–207. [Google Scholar] [CrossRef]
- Kim, E.; Ilagan, J.O.; Liang, Y.; Daubner, G.M.; Lee, S.C.; Ramakrishnan, A.; Li, Y.; Chung, Y.R.; Micol, J.B.; Murphy, M.E.; et al. SRSF2 Mutations Contribute to Myelodysplasia by Mutant-Specific Effects on Exon Recognition. Cancer Cell 2015, 27, 617–630. [Google Scholar] [CrossRef]
- Zhang, J.; Lieu, Y.K.; Ali, A.M.; Penson, A.; Reggio, K.S.; Rabadan, R.; Raza, A.; Mukherjee, S.; Manley, J.L. Disease-associated mutation in SRSF2 misregulates splicing by altering RNA-binding affinities. Proc. Natl. Acad. Sci. USA 2015, 112, E4726–E4734. [Google Scholar] [CrossRef]
- Liang, Y.; Tebaldi, T.; Rejeski, K.; Joshi, P.; Stefani, G.; Taylor, A.; Song, Y.; Vasic, R.; Maziarz, J.; Balasubramanian, K.; et al. SRSF2 mutations drive oncogenesis by activating a global program of aberrant alternative splicing in hematopoietic cells. Leukemia 2018, 32, 2659–2671. [Google Scholar] [CrossRef]
- Seiler, M.; Yoshimi, A.; Darman, R.; Chan, B.; Keaney, G.; Thomas, M.; Agrawal, A.A.; Caleb, B.; Csibi, A.; Sean, E.; et al. H3B-8800, an orally available small-molecule splicing modulator, induces lethality in spliceosome-mutant cancers. Nat. Med. 2018, 24, 497–504. [Google Scholar] [CrossRef]
- Naro, C.; Sette, C. Phosphorylation-mediated regulation of alternative splicing in cancer. Int. J. Cell Biol. 2013, 2013, 151839. [Google Scholar] [CrossRef]
- Yoda, A.; Morishita, D.; Mizutani, A.; Satoh, Y.; Ochi, Y.; Nannya, Y.; Makishima, H.; Miyake, H.; Ogawa, S. CTX-712, a Novel Clk Inhibitor Targeting Myeloid Neoplasms with SRSF2 Mutation. Blood 2019, 134, 404. [Google Scholar] [CrossRef]
- Yokoyama, H.; Ando, K.; Fukuhara, N.; Iida, H.; Fukuhara, S.; Miyake, H.; Tanoue, Y.; Yamamoto, M.; Tozaki, H.; Mizutani, A.; et al. A First-in-Human Phase I Study of CTX-712 in Patients with Advanced, Relapsed or Refractory Malignant Tumors—Hematologic Malignancies Dose Escalation Cohort. Blood 2022, 140, 6211–6212. [Google Scholar] [CrossRef]
- Graubert, T.A.; Shen, D.; Ding, L.; Okeyo-Owuor, T.; Lunn, C.L.; Shao, J.; Krysiak, K.; Harris, C.C.; Koboldt, D.C.; Larson, D.E.; et al. Recurrent mutations in the U2AF1 splicing factor in myelodysplastic syndromes. Nat. Genet. 2011, 44, 53–57. [Google Scholar] [CrossRef] [PubMed]
- Dutta, A.; Yang, Y.; Le, B.T.; Zhang, Y.; Abdel-Wahab, O.; Zang, C.; Mohi, G. U2af1 is required for survival and function of hematopoietic stem/progenitor cells. Leukemia 2021, 35, 2382–2398. [Google Scholar] [CrossRef] [PubMed]
- Palangat, M.; Anastasakis, D.G.; Fei, D.L.; Lindblad, K.E.; Bradley, R.; Hourigan, C.S.; Hafner, M.; Larson, D.R. The splicing factor U2AF1 contributes to cancer progression through a noncanonical role in translation regulation. Genes Dev. 2019, 33, 482–497. [Google Scholar] [CrossRef]
- Smith, M.A.; Choudhary, G.S.; Pellagatti, A.; Choi, K.; Bolanos, L.C.; Bhagat, T.D.; Gordon-Mitchell, S.; Von Ahrens, D.; Pradhan, K.; Steeples, V.; et al. U2AF1 mutations induce oncogenic IRAK4 isoforms and activate innate immune pathways in myeloid malignancies. Nat. Cell Biol. 2019, 21, 640–650. [Google Scholar] [CrossRef]
- Garcia-Manero, G.; Winer, E.S.; DeAngelo, D.J.; Tarantolo, S.; Sallman, D.A.; Dugan, J.; Groepper, S.; Giagounidis, A.; Götze, K.; Metzeler, K.H.; et al. S129: Takeaim Leukemia- a Phase 1/2a Study of the Irak4 Inhibitor Emavusertib (Ca-4948) as Monotherapy or in Combination with Azacitidine or Venetoclax in Relapsed/Refractory Aml or Mds. HemaSphere 2022, 6, 30–31. [Google Scholar] [CrossRef]
- Madan, V.; Kanojia, D.; Li, J.; Okamoto, R.; Sato-Otsubo, A.; Kohlmann, A.; Sanada, M.; Grossmann, V.; Sundaresan, J.; Shiraishi, Y.; et al. Aberrant splicing of U12-type introns is the hallmark of ZRSR2 mutant myelodysplastic syndrome. Nat. Commun. 2015, 6, 6042. [Google Scholar] [CrossRef]
- Togami, K.; Chung, S.S.; Madan, V.; Booth, C.A.G.; Kenyon, C.M.; Cabal-Hierro, L.; Taylor, J.; Kim, S.S.; Griffin, G.K.; Ghandi, M.; et al. Sex-Biased ZRSR2 Mutations in Myeloid Malignancies Impair Plasmacytoid Dendritic Cell Activation and Apoptosis. Cancer Discov. 2022, 12, 522–541. [Google Scholar] [CrossRef]
- Inoue, D.; Polaski, J.T.; Taylor, J.; Castel, P.; Chen, S.; Kobayashi, S.; Hogg, S.J.; Hayashi, Y.; Pineda, J.M.B.; El Marabti, E.; et al. Minor intron retention drives clonal hematopoietic disorders and diverse cancer predisposition. Nat. Genet. 2021, 53, 707–718. [Google Scholar] [CrossRef]
- Scheuermann, J.C.; de Ayala Alonso, A.G.; Oktaba, K.; Ly-Hartig, N.; McGinty, R.K.; Fraterman, S.; Wilm, M.; Muir, T.W.; Muller, J. Histone H2A deubiquitinase activity of the Polycomb repressive complex PR-DUB. Nature 2010, 465, 243–247. [Google Scholar] [CrossRef]
- Bouligny, I.M.; Maher, K.R.; Grant, S. Mechanisms of myeloid leukemogenesis: Current perspectives and therapeutic objectives. Blood Rev. 2023, 57, 100996. [Google Scholar] [CrossRef]
- Sportoletti, P.; Sorcini, D.; Falini, B. BCOR gene alterations in hematologic diseases. Blood 2021, 138, 2455–2468. [Google Scholar] [CrossRef]
- Tara, S.; Isshiki, Y.; Nakajima-Takagi, Y.; Oshima, M.; Aoyama, K.; Tanaka, T.; Shinoda, D.; Koide, S.; Saraya, A.; Miyagi, S.; et al. Bcor insufficiency promotes initiation and progression of myelodysplastic syndrome. Blood 2018, 132, 2470–2483. [Google Scholar] [CrossRef]
- Honda, A.; Koya, J.; Yoshimi, A.; Miyauchi, M.; Taoka, K.; Kataoka, K.; Arai, S.; Kurokawa, M. Loss-of-function mutations in BCOR contribute to chemotherapy resistance in acute myeloid leukemia. Exp. Hematol. 2021, 101–102, 42–48.e11. [Google Scholar] [CrossRef]
- Kelly, M.J.; So, J.; Rogers, A.J.; Gregory, G.; Li, J.; Zethoven, M.; Gearhart, M.D.; Bardwell, V.J.; Johnstone, R.W.; Vervoort, S.J.; et al. Bcor loss perturbs myeloid differentiation and promotes leukaemogenesis. Nat. Commun. 2019, 10, 1347. [Google Scholar] [CrossRef]
- Schaefer, E.J.; Wang, H.C.; Karp, H.Q.; Meyer, C.A.; Cejas, P.; Gearhart, M.D.; Adelman, E.R.; Fares, I.; Apffel, A.; Lim, K.; et al. BCOR and BCORL1 Mutations Drive Epigenetic Reprogramming and Oncogenic Signaling by Unlinking PRC1.1 from Target Genes. Blood Cancer Discov. 2022, 3, 116–135. [Google Scholar] [CrossRef]
- Kim, K.H.; Roberts, C.W. Targeting EZH2 in cancer. Nat. Med. 2016, 22, 128–134. [Google Scholar] [CrossRef]
- Basheer, F.; Giotopoulos, G.; Meduri, E.; Yun, H.; Mazan, M.; Sasca, D.; Gallipoli, P.; Marando, L.; Gozdecka, M.; Asby, R.; et al. Contrasting requirements during disease evolution identify EZH2 as a therapeutic target in AML. J. Exp. Med. 2019, 216, 966–981. [Google Scholar] [CrossRef]
- Chang, K.H.; Alaniz, Z.; Nishida, Y.; Dos Santos, C.E.; Slosberg, E.; Daver, N.; Andreeff, M. Inhibition of EZH1 and EZH2 Restores Chemosensitivity of Leukemia Stem Cells in Acute Myeloid Leukemia By Recruitment of Quiescent AML Stem/Progenitor Cells. Blood 2020, 136, 27–28. [Google Scholar] [CrossRef]
- Porazzi, P.; Petruk, S.; Pagliaroli, L.; De Dominici, M.; Deming, D., II; Puccetti, M.V.; Kushinsky, S.; Kumar, G.; Minieri, V.; Barbieri, E.; et al. Targeting Chemotherapy to Decondensed H3K27me3-Marked Chromatin of AML Cells Enhances Leukemia Suppression. Cancer Res. 2022, 82, 458–471. [Google Scholar] [CrossRef]
- Kempf, J.M.; Weser, S.; Bartoschek, M.D.; Metzeler, K.H.; Vick, B.; Herold, T.; Volse, K.; Mattes, R.; Scholz, M.; Wange, L.E.; et al. Loss-of-function mutations in the histone methyltransferase EZH2 promote chemotherapy resistance in AML. Sci. Rep. 2021, 11, 5838. [Google Scholar] [CrossRef] [PubMed]
- Sahtoe, D.D.; van Dijk, W.J.; Ekkebus, R.; Ovaa, H.; Sixma, T.K. BAP1/ASXL1 recruitment and activation for H2A deubiquitination. Nat. Commun. 2016, 7, 10292. [Google Scholar] [CrossRef]
- Asada, S.; Goyama, S.; Inoue, D.; Shikata, S.; Takeda, R.; Fukushima, T.; Yonezawa, T.; Fujino, T.; Hayashi, Y.; Kawabata, K.C.; et al. Mutant ASXL1 cooperates with BAP1 to promote myeloid leukaemogenesis. Nat. Commun. 2018, 9, 2733. [Google Scholar] [CrossRef]
- Medina, E.A.; Delma, C.R.; Yang, F.C. ASXL1/2 mutations and myeloid malignancies. J. Hematol. Oncol. 2022, 15, 127. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Kurtenbach, S.; Guo, Y.; Lohse, I.; Durante, M.A.; Li, J.; Li, Z.; Al-Ali, H.; Li, L.; Chen, Z.; et al. Gain of function of ASXL1 truncating protein in the pathogenesis of myeloid malignancies. Blood 2018, 131, 328–341. [Google Scholar] [CrossRef] [PubMed]
- Abedin, S.M.; Boddy, C.S.; Munshi, H.G. BET inhibitors in the treatment of hematologic malignancies: Current insights and future prospects. Onco. Targets Ther. 2016, 9, 5943–5953. [Google Scholar] [CrossRef]
- Karjalainen, R.; Liu, M.; Kumar, A.; He, L.; Malani, D.; Parsons, A.; Kontro, M.; Kallioniemi, O.; Porkka, K.; Heckman, C.A. Elevated expression of S100A8 and S100A9 correlates with resistance to the BCL-2 inhibitor venetoclax in AML. Leukemia 2019, 33, 2548–2553. [Google Scholar] [CrossRef]
- Ramsey, H.E.; Greenwood, D.; Zhang, S.; Childress, M.; Arrate, M.P.; Gorska, A.E.; Fuller, L.; Zhao, Y.; Stengel, K.; Fischer, M.A.; et al. BET Inhibition Enhances the Antileukemic Activity of Low-dose Venetoclax in Acute Myeloid Leukemia. Clin. Cancer Res. 2021, 27, 598–607. [Google Scholar] [CrossRef]
- Peters, J.M.; Tedeschi, A.; Schmitz, J. The cohesin complex and its roles in chromosome biology. Genes Dev. 2008, 22, 3089–3114. [Google Scholar] [CrossRef]
- Waldman, T. Emerging themes in cohesin cancer biology. Nat. Rev. Cancer 2020, 20, 504–515. [Google Scholar] [CrossRef]
- Eckardt, J.N.; Stasik, S.; Rollig, C.; Sauer, T.; Scholl, S.; Hochhaus, A.; Crysandt, M.; Brummendorf, T.H.; Naumann, R.; Steffen, B.; et al. Alterations of cohesin complex genes in acute myeloid leukemia: Differential co-mutations, clinical presentation and impact on outcome. Blood Cancer J. 2023, 13, 18. [Google Scholar] [CrossRef]
- Mazumdar, C.; Shen, Y.; Xavy, S.; Zhao, F.; Reinisch, A.; Li, R.; Corces, M.R.; Flynn, R.A.; Buenrostro, J.D.; Chan, S.M.; et al. Leukemia-Associated Cohesin Mutants Dominantly Enforce Stem Cell Programs and Impair Human Hematopoietic Progenitor Differentiation. Cell Stem Cell 2015, 17, 675–688. [Google Scholar] [CrossRef]
- Mullenders, J.; Aranda-Orgilles, B.; Lhoumaud, P.; Keller, M.; Pae, J.; Wang, K.; Kayembe, C.; Rocha, P.P.; Raviram, R.; Gong, Y.; et al. Cohesin loss alters adult hematopoietic stem cell homeostasis, leading to myeloproliferative neoplasms. J. Exp. Med. 2015, 212, 1833–1850. [Google Scholar] [CrossRef]
- Viny, A.D.; Ott, C.J.; Spitzer, B.; Rivas, M.; Meydan, C.; Papalexi, E.; Yelin, D.; Shank, K.; Reyes, J.; Chiu, A.; et al. Dose-dependent role of the cohesin complex in normal and malignant hematopoiesis. J. Exp. Med. 2015, 212, 1819–1832. [Google Scholar] [CrossRef]
- Black, H.E.; Jhujh, S.; Stewart, G.S.; Savage, K.I.; Mills, K.I. STAG2 Loss Gives Rise to Therapeutically Targetable DNA Damage Repair Defects and Altered Replication Fork Dynamics in Acute Myeloid Leukaemia. Blood 2019, 134, 1255. [Google Scholar] [CrossRef]
- Gopal, A.K.; Popat, R.; Mattison, R.J.; Menne, T.; Bloor, A.; Gaymes, T.; Khwaja, A.; Juckett, M.; Chen, Y.; Cotter, M.J.; et al. A Phase I trial of talazoparib in patients with advanced hematologic malignancies. Int. J. Hematol. Oncol. 2021, 10, IJH35. [Google Scholar]
- Swaminathan, M.; Przespolewski, A.; Griffiths, E.A.; Thompson, J.E.; Elshoury, A.; Walinski, W.; Said, M.; Halliwell, S.; Puff, C.; Attwood, K.; et al. Phase 1b Trial of Talazoparib and Gemtuzumab Ozogamicin in Adult Patients with CD33+ Relapsed or Refractory Acute Myeloid Leukemia. Blood 2021, 138, 4435. [Google Scholar] [CrossRef]
- Baer, M.R.; Kogan, A.A.; Bentzen, S.M.; Mi, T.; Lapidus, R.G.; Duong, V.H.; Emadi, A.; Niyongere, S.; O’Connell, C.L.; Youngblood, B.A.; et al. Phase I Clinical Trial of DNA Methyltransferase Inhibitor Decitabine and PARP Inhibitor Talazoparib Combination Therapy in Relapsed/Refractory Acute Myeloid Leukemia. Clin. Cancer Res. 2022, 28, 1313–1322. [Google Scholar] [CrossRef]
- Benedetti, L.; Cereda, M.; Monteverde, L.; Desai, N.; Ciccarelli, F.D. Synthetic lethal interaction between the tumour suppressor STAG2 and its paralog STAG1. Oncotarget 2017, 8, 37619–37632. [Google Scholar] [CrossRef]
- Subramaniam, A.; Sanden, C.; Moura-Castro, L.; Zemaitis, K.; Schmiderer, L.; Baudet, A.; Backstrom, A.; Arvidsson, E.; Hultmark, S.; Miharada, N.; et al. Inducing synthetic lethality for selective targeting of acute myeloid leukemia cells harboring STAG2 mutations. Haematologica 2022, 107, 2271–2275. [Google Scholar] [CrossRef]
- Harada, H.; Harada, Y.; Tanaka, H.; Kimura, A.; Inaba, T. Implications of somatic mutations in the AML1 gene in radiation-associated and therapy-related myelodysplastic syndrome/acute myeloid leukemia. Blood 2003, 101, 673–680. [Google Scholar] [CrossRef] [PubMed]
- Bowers, S.R.; Calero-Nieto, F.J.; Valeaux, S.; Fernandez-Fuentes, N.; Cockerill, P.N. Runx1 binds as a dimeric complex to overlapping Runx1 sites within a palindromic element in the human GM-CSF enhancer. Nucleic Acids Res. 2010, 38, 6124–6134. [Google Scholar] [CrossRef] [PubMed]
- Bellissimo, D.C.; Speck, N.A. RUNX1 Mutations in Inherited and Sporadic Leukemia. Front. Cell Dev. Biol. 2017, 5, 111. [Google Scholar] [CrossRef] [PubMed]
- Sood, R.; Kamikubo, Y.; Liu, P. Role of RUNX1 in hematological malignancies. Blood 2017, 129, 2070–2082. [Google Scholar] [CrossRef]
- Mill, C.P.; Fiskus, W.; DiNardo, C.D.; Birdwell, C.; Davis, J.A.; Kadia, T.M.; Takahashi, K.; Short, N.; Daver, N.; Ohanian, M.; et al. Effective therapy for AML with RUNX1 mutation by cotreatment with inhibitors of protein translation and BCL2. Blood 2022, 139, 907–921. [Google Scholar] [CrossRef]
- Bouligny, I.M.; Maher, K.R.; Grant, S. Augmenting Venetoclax Activity Through Signal Transduction in AML. J. Cell. Signal. 2023, 4, 1–12. [Google Scholar] [CrossRef]
- Zeidner, J.F.; Vincent, B.G.; Ivanova, A.; Moore, D.; McKinnon, K.P.; Wilkinson, A.D.; Mukhopadhyay, R.; Mazziotta, F.; Knaus, H.A.; Foster, M.C.; et al. Phase II Trial of Pembrolizumab after High-Dose Cytarabine in Relapsed/Refractory Acute Myeloid Leukemia. Blood Cancer Discov. 2021, 2, 616–629. [Google Scholar] [CrossRef]
- Daver, N.; Garcia-Manero, G.; Basu, S.; Boddu, P.C.; Alfayez, M.; Cortes, J.E.; Konopleva, M.; Ravandi-Kashani, F.; Jabbour, E.; Kadia, T.; et al. Efficacy, Safety, and Biomarkers of Response to Azacitidine and Nivolumab in Relapsed/Refractory Acute Myeloid Leukemia: A Nonrandomized, Open-Label, Phase II Study. Cancer Discov. 2019, 9, 370–383. [Google Scholar] [CrossRef]
- Wang, E.; Lu, S.X.; Pastore, A.; Chen, X.; Imig, J.; Chun-Wei Lee, S.; Hockemeyer, K.; Ghebrechristos, Y.E.; Yoshimi, A.; Inoue, D.; et al. Targeting an RNA-Binding Protein Network in Acute Myeloid Leukemia. Cancer Cell 2019, 35, 369–384.e367. [Google Scholar] [CrossRef]
- Chen, S.; Benbarche, S.; Abdel-Wahab, O. Splicing factor mutations in hematologic malignancies. Blood 2021, 138, 599–612. [Google Scholar] [CrossRef]
- Assi, R.; Kantarjian, H.M.; Kadia, T.M.; Pemmaraju, N.; Jabbour, E.; Jain, N.; Daver, N.; Estrov, Z.; Uehara, T.; Owa, T.; et al. Final results of a phase 2, open-label study of indisulam, idarubicin, and cytarabine in patients with relapsed or refractory acute myeloid leukemia and high-risk myelodysplastic syndrome. Cancer 2018, 124, 2758–2765. [Google Scholar] [CrossRef]
- Santos-Pereira, J.M.; Aguilera, A. R loops: New modulators of genome dynamics and function. Nat. Rev. Genet. 2015, 16, 583–597. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).