Risk of Treatment Failure and Death after Ablation in Hepatocellular Carcinoma Patients—A Multiparametric Prediction
Abstract
Simple Summary
Abstract
1. Introduction
2. Patients and Methods
2.1. Study Definitions
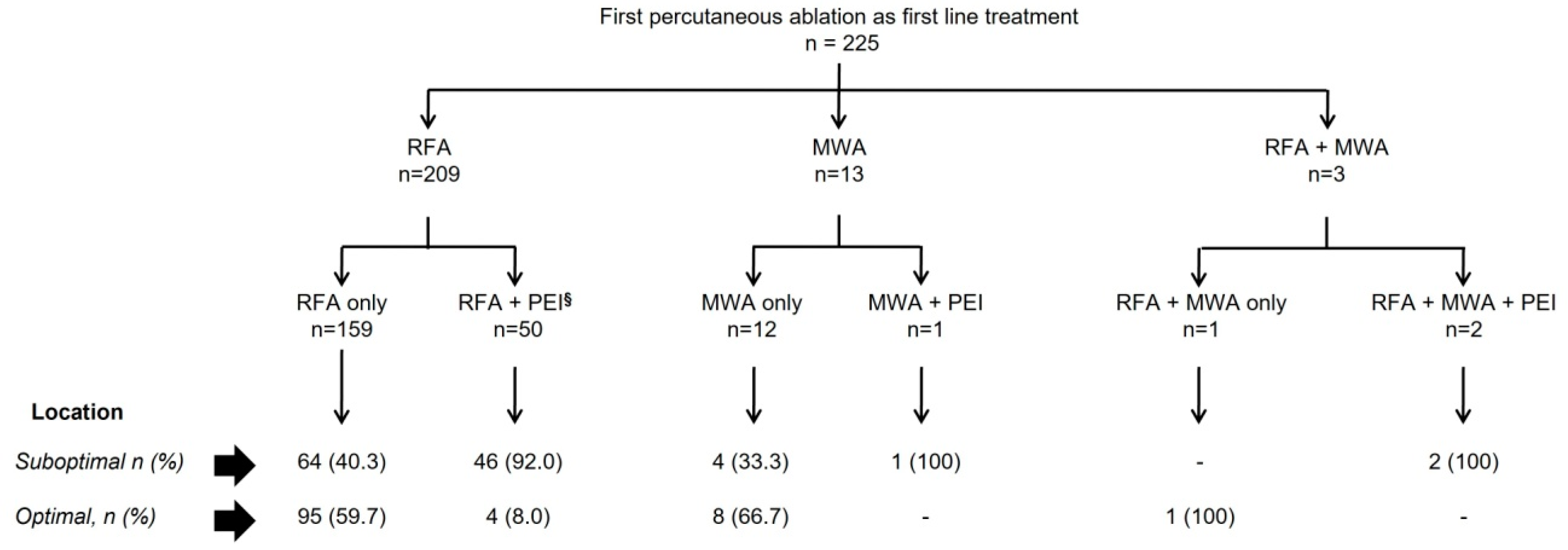
2.2. Ablation Techniques Used in the Study
2.3. Pattern of Recurrence/Progression after the First Percutaneous Ablation
2.4. Follow-Up Protocol
2.5. Statistical Analysis
3. Results
3.1. Baseline Characteristics and Percutaneous Ablation Procedure
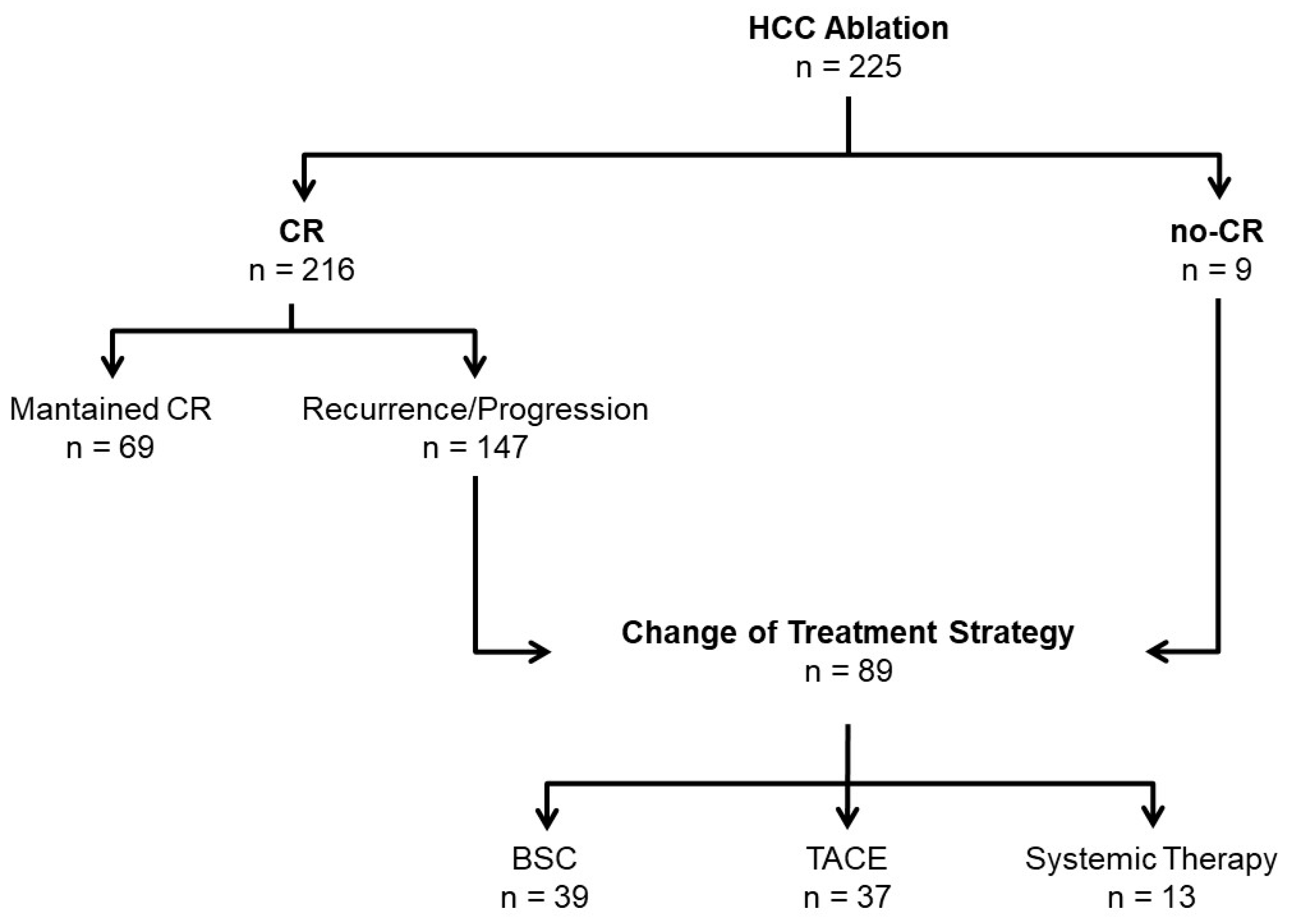
3.2. Failure of Ablation Strategy
3.3. Adverse Events
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Reig, M.; Forner, A.; Rimola, J.; Ferrer-Fàbrega, J.; Burrel, M.; Garcia-Criado, Á.; Kelley, R.K.; Galle, P.R.; Mazzaferro, V.; Salem, R.; et al. BCLC strategy for prognosis prediction and treatment recommendation: The 2022 update. J. Hepatol. 2021, 76, 681–693. [Google Scholar] [CrossRef] [PubMed]
- Vogel, A.; Meyer, T.; Sapisochin, G.; Salem, R.; Saborowski, A. Hepatocellular carcinoma. Lancet 2022, 400, 1345–1362. [Google Scholar] [CrossRef] [PubMed]
- Vogel, A.; Martinelli, E.; Cervantes, A.; Chau, I.; Daniele, B.; Llovet, J.; Meyer, T.; Nault, J.-C.; Neumann, U.; Ricke, J.; et al. Updated treatment recommendations for hepatocellular carcinoma (HCC) from the ESMO Clinical Practice Guidelines. Ann. Oncol. 2021, 32, 801–805. [Google Scholar] [CrossRef]
- Heimbach, J.K.; Kulik, L.M.; Finn, R.S.; Sirlin, C.B.; Abecassis, M.M.; Roberts, L.R.; Zhu, A.X.; Murad, M.H.; Marrero, J.A. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology 2018, 67, 358–380. [Google Scholar] [CrossRef] [PubMed]
- Singal, A.G.; Llovet, J.M.; Yarchoan, M.; Mehta, N.; Heimbach, J.K.; Dawson, L.A.; Jou, J.H.; Kulik, L.M.; Agopian, V.G.; Marrero, J.A.; et al. AASLD practice guidance on prevention, diagnosis, and treatment of hepatocellular carcinoma. Hepatology, 2023; online ahead of print. [Google Scholar]
- Sala, M.; Llovet, J.M.; Vilana, R.; Bianchi, L.; Solé, M.; Ayuso, C.; Brú, C.; Bruix, J. Barcelona Cly’nic Liver Cancer (BCLC) Group. Initial response to percutaneous ablation predicts survival in patients with hepatocellular carcinoma. Hepatology 2004, 40, 1352–1360. [Google Scholar] [CrossRef]
- Cabibbo, G.; Maida, M.F.; Genco, C.; Alessi, N.; Peralta, M.; Butera, G.; Galia, M.; Brancatelli, G.; Genova, C.; Raineri, M.; et al. Survival of Patients with Hepatocellular Carcinoma (HCC) Treated by Percutaneous Radio-Frequency Ablation (RFA) Is Affected by Complete Radiological Response. PLoS ONE 2013, 8, e70016. [Google Scholar] [CrossRef] [PubMed]
- De Zalán, C.M.C.S.-K.A.; Ruiter, S.J.S.; Berg, A.P.V.D.; Pennings, J.P.; de Jong, K.P. Outcomes after primary and repeat thermal ablation of hepatocellular carcinoma with or without liver transplantation. Eur. Radiol. 2022, 32, 4168–4176. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Chen, Y.; Ye, F.; Cao, X.; Xin, Y.; Wang, Y.; Lei, Y.; Li, X.; Feng, D.; Zhou, X.; et al. Late recurrence of hepatocellular carcinoma after radiofrequency ablation: A multicenter study of risk factors, patterns, and survival. Eur. Radiol. 2020, 31, 3053–3064. [Google Scholar] [CrossRef]
- Ryu, T.; Takami, Y.; Wada, Y.; Saitsu, H. Operative Microwave Ablation for Hepatocellular Carcinoma Within 3 cm and 3 Nodules: Experience in 559 Patients. J. Gastrointest. Surg. 2021, 26, 615–622. [Google Scholar] [CrossRef] [PubMed]
- Doyle, A.; Gorgen, A.; Muaddi, H.; Aravinthan, A.D.; Issachar, A.; Mironov, O.; Zhang, W.; Kachura, J.; Beecroft, R.; Cleary, S.P.; et al. Outcomes of radiofrequency ablation as first-line therapy for hepatocellular carcinoma less than 3 cm in potentially transplantable patients. J. Hepatol. 2019, 70, 866–873. [Google Scholar] [CrossRef]
- Yang, Y.; Chen, Y.; Zhang, X.; Xin, Y.; Wang, Y.; Li, X.; Fan, Q.; Zhou, X.; Ye, F. Predictors and patterns of recurrence after radiofrequency ablation for hepatocellular carcinoma within up-to-seven criteria: A multicenter retrospective study. Eur. J. Radiol. 2021, 138, 109623. [Google Scholar] [CrossRef]
- Sato, M.; Tateishi, R.; Moriyama, M.; Fukumoto, T.; Yamada, T.; Nakagomi, R.; Kinoshita, M.N.; Nakatsuka, T.; Minami, T.; Uchino, K.; et al. Machine Learning–Based Personalized Prediction of Hepatocellular Carcinoma Recurrence After Radiofrequency Ablation. Gastro Hep Adv. 2021, 1, 29–37. [Google Scholar] [CrossRef]
- Preel, A.; Hermida, M.; Allimant, C.; Assenat, E.; Guillot, C.; Gozzo, C.; Aho-Glele, S.; Pageaux, G.-P.; Cassinotto, C.; Guiu, B. Uni-, Bi- or Trifocal Hepatocellular Carcinoma in Western Patients: Recurrence and Survival after Percutaneous Thermal Ablation. Cancers 2021, 13, 2700. [Google Scholar] [CrossRef]
- Chen, H.-Y.; Lu, S.-N.; Hung, C.-H.; Wang, J.-H.; Chen, C.-H.; Yen, Y.-H.; Kuo, Y.-H.; Kee, K.-M. Predicting outcomes for recurrent hepatocellular carcinoma within Milan criteria after complete radiofrequency ablation. PLoS ONE 2020, 15, e0242113. [Google Scholar] [CrossRef]
- Roche’s Tecentriq plus Avastin Is the First Treatment Combination to Reduce the Risk of Cancer Returning in People with Certain Types of Early-Stage Liver Cancer in a Phase III Trial n.d. Available online: https://www.roche.com/investors/updates/inv-update-2023-01-19 (accessed on 22 March 2023).
- Murray, K.F.; Carithers, R.L. AASLD practice guidelines: Evaluation of the patient for liver transplantation. Hepatology 2005, 41, 1407–1432. [Google Scholar] [CrossRef]
- Bruix, J.; Sherman, M. Management of hepatocellular carcinoma: An update. Hepatology 2011, 53, 1020–1022. [Google Scholar] [CrossRef] [PubMed]
- Marrero, J.A.; Kulik, L.M.; Sirlin, C.B.; Zhu, A.X.; Finn, R.S.; Abecassis, M.M.; Roberts, L.R.; Heimbach, J.K. Diagnosis, Staging, and Management of Hepatocellular Carcinoma: 2018 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology 2018, 68, 723–750. [Google Scholar] [CrossRef] [PubMed]
- United Nations, Department of Economic and Social Affairs, Population Division (2020). World Population Ageing 2019 (ST/ESA/SER.A/444). Available online: https://www.un.org/en/development/desa/population/publications/pdf/ageing/WorldPopulationAgeing2019-Report.pdf (accessed on 4 October 2020).
- Ageing Europe—2019 Edition—Products Statistical Books—Eurostat n.d. Available online: https://ec.europa.eu/eurostat/web/products-statistical-books/-/ks-02-19-681 (accessed on 22 September 2022).
- Demography—Elderly Population—OECD Data. Available online: http://data.oecd.org/pop/elderly-population.htm (accessed on 22 September 2022).
- Hori, T.; Nagata, K.; Hasuike, S.; Onaga, M.; Motoda, M.; Moriuchi, A.; Iwakiri, H.; Uto, H.; Kato, J.; Ido, A.; et al. Risk factors for the local recurrence of hepatocellular carcinoma after a single session of percutaneous radiofrequency ablation. J. Gastroenterol. 2003, 38, 977–981. [Google Scholar] [CrossRef]
- Yang, B.; Zou, J.; Xia, J.; Ren, Z.; Gan, Y.; Wang, Y.; Zhang, B.; Ge, N.; Wang, D.; Chen, Y.; et al. Risk factors for recurrence of small hepatocellular carcinoma after long-term follow-up of percutaneous radiofrequency ablation. Eur. J. Radiol. 2011, 79, 196–200. [Google Scholar] [CrossRef]
- Komorizono, Y.; Oketani, M.; Sako, K.; Yamasaki, N.; Shibatou, T.; Maeda, M.; Kohara, K.; Shigenobu, S.; Ishibashi, K.; Arima, T. Risk factors for local recurrence of small hepatocellular carcinoma tumors after a single session, single application of percutaneous radiofrequency ablation. Cancer 2003, 97, 1253–1262. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-S.; Rhim, H.; Cho, O.K.; Koh, B.H.; Kim, Y. Intrahepatic recurrence after percutaneous radiofrequency ablation of hepatocellular carcinoma: Analysis of the pattern and risk factors. Eur. J. Radiol. 2006, 59, 432–441. [Google Scholar] [CrossRef] [PubMed]
- Zytoon, A.A.; Ishii, H.; Murakami, K.; El-Kholy, M.R.; Furuse, J.; El-Dorry, A.; El-Malah, A. Recurrence-free Survival after Radiofrequency Ablation of Hepatocellular Carcinoma. A Registry Report of the Impact of Risk Factors on Outcome. Jpn. J. Clin. Oncol. 2007, 37, 658–672. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.-Y.; Choi, M.S.; Lee, G.S.; Sohn, W.; Ahn, J.; Sinn, D.-H.; Gwak, G.-Y.; Paik, Y.-H.; Lee, J.H.; Koh, K.C.; et al. Clinical significance and predictive factors of early massive recurrence after radiofrequency ablation in patients with a single small hepatocellular carcinoma. Clin. Mol. Hepatol. 2016, 22, 477–486. [Google Scholar] [CrossRef]
- Bruix, J.; Sherman, M.; Llovet, J.M.; Beaugrand, M.; Lencioni, R.; Burroughs, A.K.; Christensen, E.; Pagliaro, L.; Colombo, M.; Rodés, J. Clinical Management of Hepatocellular Carcinoma. Conclusions of the Barcelona-2000 EASL Conference. European Association for the Study of the Liver. J. Hepatol. 2001, 35, 421–430. [Google Scholar] [CrossRef] [PubMed]
- Rimola, J.; Forner, A.; Sapena, V.; Llarch, N.; Darnell, A.; Díaz, A.; García-Criado, A.; Bianchi, L.; Vilana, R.; Díaz-González, Á.; et al. Performance of gadoxetic acid MRI and diffusion-weighted imaging for the diagnosis of early recurrence of hepatocellular carcinoma. Eur. Radiol. 2019, 30, 186–194. [Google Scholar] [CrossRef]
- Dindo, D.; Demartines, N.; Clavien, P.-A. Classification of Surgical Complications: A new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann. Surg. 2004, 240, 205–213. [Google Scholar] [CrossRef]
- Khalilzadeh, O.; Baerlocher, M.O.; Shyn, P.B.; Connolly, B.L.; Devane, A.M.; Morris, C.S.; Cohen, A.M.; Midia, M.; Thornton, R.H.; Gross, K.; et al. Proposal of a New Adverse Event Classification by the Society of Interventional Radiology Standards of Practice Committee. J. Vasc. Interv. Radiol. 2017, 28, 1432–1437. [Google Scholar] [CrossRef]
- Baerlocher, M.O.; Nikolic, B.; Sze, D.Y. Adverse Event Classification: Clarification and Validation of the Society of Interventional Radiology Specialty–Specific System. J. Vasc. Interv. Radiol. 2022, 34, 1–3. [Google Scholar] [CrossRef]
- Austin, P.C. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat. Med. 2009, 28, 3083–3107. [Google Scholar] [CrossRef]
- Okuwaki, Y.; Nakazawa, T.; Kokubu, S.; Hidaka, H.; Tanaka, Y.; Takada, J.; Watanabe, M.; Shibuya, A.; Minamino, T.; Saigenji, K. Repeat Radiofrequency Ablation Provides Survival Benefit in Patients with Intrahepatic Distant Recurrence of Hepatocellular Carcinoma. Am. J. Gastroenterol. 2009, 104, 2747–2753. [Google Scholar] [CrossRef]
- N’Kontchou, G.; Mahamoudi, A.; Aout, M.; Ganne-Carrié, N.; Grando, V.; Coderc, E.; Vicaut, E.; Trinchet, J.C.; Sellier, N.; Beaugrand, M.; et al. Radiofrequency ablation of hepatocellular carcinoma: Long-term results and prognostic factors in 235 Western patients with cirrhosis. Hepatology 2009, 50, 1475–1483. [Google Scholar] [CrossRef]
- Zhang, W.; Luo, E.; Gan, J.; Song, X.; Bao, Z.; Zhang, H.; Chen, M. Long-term survival of hepatocellular carcinoma after percutaneous radiofrequency ablation guided by ultrasound. World J. Surg. Oncol. 2017, 15, 122. [Google Scholar] [CrossRef]
- Takaura, K.; Kurosaki, M.; Inada, K.; Kirino, S.; Yamashita, K.; Muto, T.; Osawa, L.; Sekiguchi, S.; Hayakawa, Y.; Higuchi, M.; et al. The impact of background liver disease on the long-term prognosis of very-early-stage HCC after ablation therapy. PLoS ONE 2022, 17, e0264075. [Google Scholar] [CrossRef]
- Nguyen, N.; Rode, A.; Trillaud, H.; Aubé, C.; Manichon, A.F.; Hocquelet, A.; Paisant, A.; Dao, T.; Nahon, P.; Ganne-Carrié, N.; et al. Percutaneous radiofrequency ablation for hepatocellular carcinoma developed on non-alcoholic fatty liver disease. Liver Int. 2021, 42, 905–917. [Google Scholar] [CrossRef]
- Yokoyama, K.; Anan, A.; Iwata, K.; Nishizawa, S.; Morihara, D.; Ueda, S.-I.; Sakurai, K.; Iwashita, H.; Hirano, G.; Sakamoto, M.; et al. Limitation of repeated radiofrequency ablation in hepatocellular carcinoma: Proposal of a three (times) × 3 (years) index. J. Gastroenterol. Hepatol. 2012, 27, 1044–1050. [Google Scholar] [CrossRef]
- Tao, Q.; Zeng, Q.; Liu, W.; Liu, J.; Jiang, L.; Tu, X.; Li, K.; Zhao, P.; Tang, X.; Liu, Z.; et al. A novel prognostic nomogram for hepatocellular carcinoma after thermal ablation. Am. J. Cancer Res. 2021, 11, 5126–5140. [Google Scholar]
- Chow, P.; Chen, M.; Cheng, A.-L.; Kaseb, A.O.; Kudo, M.; Lee, H.C.; Yopp, A.; Zhou, J.; Wang, L.; Wen, X.; et al. IMbrave050: Phase 3 study of adjuvant atezolizumab + bevacizumab versus active surveillance in patients with hepatocellular carcinoma at high risk of disease recurrence following resection or ablation. In Proceedings of the American Association for Cancer Research Annual Meeting 2023, Orlando, FL, USA, 14–19 April 2023. [Google Scholar]
- Modest, D.P.; von Weikersthal, L.F.; Decker, T.; Vehling-Kaiser, U.; Uhlig, J.; Schenk, M.; Freiberg-Richter, J.; Peuser, B.; Denzlinger, C.; Reddemann, C.P.G.; et al. Sequential Versus Combination Therapy of Metastatic Colorectal Cancer Using Fluoropyrimidines, Irinotecan, and Bevacizumab: A Randomized, Controlled Study—XELAVIRI (AIO KRK0110). J. Clin. Oncol. 2019, 37, 22–32. [Google Scholar] [CrossRef] [PubMed]
- Yun, B.-Y.; Lee, H.W.; Min, I.K.; Kim, S.U.; Park, J.Y.; Kim, D.Y.; Ahn, S.H.; Kim, B.K. Prognosis of Early-Stage Hepatocellular Carcinoma: Comparison between Trans-Arterial Chemoembolization and Radiofrequency Ablation. Cancers 2020, 12, 2527. [Google Scholar] [CrossRef]
- Finn, R.S.; Ryoo, B.-Y.; Merle, P.; Kudo, M.; Bouattour, M.; Lim, H.Y.; Breder, V.; Edeline, J.; Chao, Y.; Ogasawara, S.; et al. Pembrolizumab as Second-Line Therapy in Patients With Advanced Hepatocellular Carcinoma in KEYNOTE-240: A Randomized, Double-Blind, Phase III Trial. J. Clin. Oncol. 2020, 38, 193–202. [Google Scholar] [CrossRef] [PubMed]
- Yau, T.; Kang, Y.-K.; Kim, T.-Y.; El-Khoueiry, A.B.; Santoro, A.; Sangro, B.; Melero, I.; Kudo, M.; Hou, M.-M.; Matilla, A.; et al. Efficacy and Safety of Nivolumab Plus Ipilimumab in Patients with Advanced Hepatocellular Carcinoma Previously Treated With Sorafenib: The CheckMate 040 Randomized Clinical Trial. JAMA Oncol. 2020, 6, e204564. [Google Scholar] [CrossRef] [PubMed]
- Yau, T.; Park, J.-W.; Finn, R.S.; Cheng, A.-L.; Mathurin, P.; Edeline, J.; Kudo, M.; Harding, J.J.; Merle, P.; Rosmorduc, O.; et al. Nivolumab versus sorafenib in advanced hepatocellular carcinoma (CheckMate 459): A randomised, multicentre, open-label, phase 3 trial. Lancet Oncol. 2021, 23, 77–90. [Google Scholar] [CrossRef] [PubMed]
- Final analysis of RATIONALE-301: Randomized, phase III study of tislelizumab versus sorafenib as first-line treatment for unresectable hepatocellul...|OncologyPRO n.d. Available online: https://oncologypro.esmo.org/meeting-resources/esmo-congress/final-analysis-of-rationale-301-randomized-phase-iii-study-of-tislelizumab-versus-sorafenib-as-first-line-treatment-for-unresectable-hepatocellul (accessed on 6 October 2022).
- Abou-Alfa, G.K.; Lau, G.; Kudo, M.; Chan, S.L.; Kelley, R.K.; Furuse, J.; Sukeepaisarnjaroen, W.; Kang, Y.-K.; Van Dao, T.; De Toni, E.N.; et al. Tremelimumab plus Durvalumab in Unresectable Hepatocellular Carcinoma. NEJM Évid. 2022, 1, 8. [Google Scholar] [CrossRef]
| Patient Profile | n = 225 |
|---|---|
| Age at diagnosis (years), median [IQR] | 66 [57–74.6] |
| Gender (male), n (%) | 144 (64) |
| Etiology, n (%) | |
| HCV a | 154 (68.4) |
| Enol | 42 (18.7) |
| Others b | 11 (4.9) |
| HBV c | 11 (4.9) |
| Metabolic syndrome | 7 (3.1) |
| Arterial hypertension, n (%) | 104 (46.2) |
| Diabetes, n (%) | 60 (26.7) |
| Patients treated with DAA, n (%) | 55 (35.7) d |
| Sustained Viral Response, n (%) | 53 (96.4) |
| Child-Pugh, n (%) | |
| A | 180 (81.8) |
| B | 39 (17.7) |
| C | 1 (0.5) |
| Non-cirrhotic | 5 |
| ALBI score, n (%) | |
| ALBI 1 | 192 (85.3) |
| ALBI 2 | 31 (13.8) |
| ALBI 3 | 2 (0.9) |
| BCLC, n (%) | |
| 0 | 88 (39.1) |
| A | 137 (60.9) |
| Nodule diameter (mm), median [IQR] (min–max) | 22 [17–28] (10–50) |
| Median number of nodules, [IQR] (min–max) | 1 [1,1] (1–3) d |
| Number of nodules, n (%) | |
| 1 | 190 (84.4) |
| >1 | 35 (15.6) |
| Unifocal HCC, n (%) e | |
| ≤2 cm | 88 (46.3) |
| 2–≤3 cm | 69 (36.3) |
| >3 cm | 33 (17.4) |
| Total bilirubin (mg/dL), median [IQR] | 1.1 [0.8–1.6] |
| Albumin (g/L), median [IQR] | 38 [35–41] |
| AFP (ng/mL), median [IQR] (min–max) | 7 [3,4,6–19] (1–2928) |
| AFP (ng/mL), n (%) | |
| <10 | 135 (60) |
| ≥10 | 90 (40) |
| INR, median [IQR] | 1.2 [1.1–1.3] |
| Platelet count (×109/L), median [IQR] | 107 [70–150] |
| Patients Profile | All Cohort (n = 225) | Optimal Location HCC (n = 108) | Suboptimal Location HCC (n = 117) | STD (%) |
|---|---|---|---|---|
| Age at diagnosis (Years), median [IQR] | 66 [57–74.6] | 66 [56.6–76.1] | 66.6 [57.2–74] | 2.0 |
| Gender (male), n (%) | 144 (64) | 70 (64.8) | 74 (63.2) | 3.3 |
| Etiology, n (%) | ||||
| HCV | 154 (68.4) | 75 (69.4) a | 79 (67.5) b | 31.3 |
| Enol | 42 (18.7) | 21 (19.4) | 21 (18.0) | |
| Others | 11 (4.9) | 2 (1.9) c | 9 (7.7) d | |
| HBV | 11 (4.9) | 7 (6.5) e | 4 (3.4) f | |
| Metabolic syndrome | 7 (3.1) | 3 (2.8) | 4 (3.4) | |
| Patients treated with DAA, n (%) | 55 (35.7) | 24 (32) | 31 (39.2) | 15.2 |
| Child-Pugh, n (%) | ||||
| A | 180 (81.8) | 90 (84.9) | 90 (78.9) | 23.0 |
| B | 39 (17.7) | 15 (14.2) | 24 (21.1) | |
| C | 1 (0.5) | 1 (0.9) | 0 (0) | |
| Non-cirrhotic | 5 | 2 | 3 | |
| ALBI score, n (%) | ||||
| ALBI 1 | 192 (85.3) | 97 (89.8) | 95 (81.2) | 38.0 |
| ALBI 2 | 31 (13.8) | 9 (8.3) | 22 (18.8) | |
| ALBI 3 | 2 (0.9) | 2 (1.9) | 0 (0) | |
| Arterial hypertension, n (%) | 104 (46.2) | 52 (48.1) | 52 (44.8) | 6.7 |
| Diabetes, n (%) | 60 (26.7) | 27 (25) | 33 (28.4) | 7.8 |
| BCLC, n (%) | ||||
| 0 | 88 (39.1) | 47 (43.5) | 41 (35) | 17.4 |
| A | 137 (60.9) | 61 (56.5) | 76 (65) | |
| AFP (ng/mL), median [IQR] (min-max) | 7 [3,4,6–19] (1–2928) | 7 [4–14.5] (2–286) | 7 [3,4,6–19] (1–2928) | 7.8 |
| AFP (ng/mL), n (%) | ||||
| <10 | 135 (60) | 70 (64.8) | 65 (55.6) | 19.0 |
| ≥10 | 90 (40) | 38 (35.2) | 52 (44.4) | |
| Number of nodules, n (%) | ||||
| 1 nodule | 190 (84.4) | 101 (93.5) | 89 (76.1) | 50.1 |
| >1 nodule | 35 (15.6) | 7 (6.5) | 28 (23.9) | |
| Type of treatment, n (%) | ||||
| RFA | 209 (92.9) | 99 (91.7) | 110 (94) | 42.6 |
| MWA | 13 (5.8) | 8 (7.4) | 5 (4.3) | |
| Combined RFA/MWA g | 3 (1.3) | 1 (0.9) | 2 (1.7) | |
| Number of insertions, median [IQR] (min–max) | 0/1 [1] (1–3) | 0/1 [1] (1–3) | 0/1 [1] (1–3) | 48.2 |
| PEI performed as additional treatment, n (%) | 53 (23.6) | 4 (3.7) h | 49 (41.9) | 100 |
| Change of Treatment Strategy (n = 89) | Optimal Location HCC (n = 42, 47.2%) | Suboptimal Location HCC (n = 47, 52.8%) |
|---|---|---|
| Best Support Care, n (%) | 20 (47.6) | 19 (40.4) |
| HCC treatment, n (%) | 22 (52.4) | 28 (59.6) |
| • TACE, n (%) | 17 (77.3) | 20 (71.4) |
| • Systemic treatment, n (%) | 5 (22.7) | 8 (28.6) |
| Event | Parameter | Categories (Cat vs. Ref.) | Events/ Patients at Risk | Hazard Ratio (95%CI) | p-Value (Category) | p-Value (Parameter) |
|---|---|---|---|---|---|---|
| All patients (n = 225) | ||||||
| Death | Age | ≥65 vs. <65 | 67/119 vs. 39/106 | 0.97 (0.65–1.44) | 0.8716 | 0.8716 |
| Age (cont.) | Increase of 1 year | 106/225 | 1.01 (0.99–1.03) | 0.3524 | 0.3524 | |
| AFP (baseline) | Increase of 100 units | 106/225 | 0.99 (0.86–1.12) | 0.8273 | 0.8273 | |
| BCLC | A vs. 0 | 67/137 vs. 39/88 | 1.43 (0.96–2.13) | 0.0760 | 0.0760 | |
| Child-Pugh | B or C vs. non-cirrhotic or A | 22/40 vs. 84/185 | 2.39 (1.48–3.84) | 0.0003 | 0.0003 | |
| ALBI score | ALBI 2 vs. ALBI 1 | 18/31 vs. 87/192 | 3.22 (1.92–5.4) | <0.0001 | <0.0001 | |
| ALBI 3 vs. ALBI 1 | 1/2 vs. 87/192 | 2.43 (0.34–17.58) | 0.3796 | |||
| Number of nodules | >1 nodule vs. 1 nodule | 17/35 vs. 89/190 | 3.1 (1.8–5.33) | <0.0001 | <0.0001 | |
| Suboptimal location | Yes vs. No | 57/117 vs. 49/108 | 1.28 (0.87–1.87) | 0.2111 | 0.2111 | |
| Nodule diameter | >20–≤30 mm vs. 10–≤20 mm | 48/92 vs. 43/99 | 1.4 (0.92–2.11) | 0.1145 | 0.2791 | |
| >30 mm vs. 10–≤20 mm | 15/34 vs. 43/99 | 1.11 (0.62–2.01) | 0.7265 | |||
| Change of treatment strategy | Age | ≥ 65 vs. <65 | 55/119 vs. 34/106 | 0.98 (0.64–1.51) | 0.9290 | 0.9290 |
| Age (cont.) | Increase of 1 year | 89/225 | 1.00 (0.98–1.02) | 0.7098 | 0.7098 | |
| AFP | Increase of 100 units | 89/225 | 1.10 (1.04–1.16) | 0.0012 | 0.0012 | |
| BCLC | A vs. 0 | 54/137 vs. 35/88 | 1.34 (0.87–2.06) | 0.1823 | 0.1823 | |
| Child-Pugh | B or C vs. non-cirrhotic or A | 10/40 vs. 79/185 | 0.9 (0.46–1.74) | 0.7440 | 0.7440 | |
| ALBI score | ALBI 2 vs. ALBI 1 | 9/31 vs. 80/192 | 1.4 (0.7–2.83) | 0.3433 | 0.6381 | |
| ALBI 3 vs. ALBI 1 | 0/2 vs. 80/192 | NE | - | |||
| Number of nodules | >1 nodule vs. 1 nodule | 13/35 vs. 76/190 | 2.34 (1.27–4.32) | 0.0066 | 0.0066 | |
| Suboptimal location | Yes vs. No | 47/117 vs. 42/108 | 1.36 (0.9–2.07) | 0.1462 | 0.1462 | |
| Nodule diameter | >20–≤30 mm vs. 10–≤20 mm | 35/92 vs. 39/99 | 1.1 (0.7–1.74) | 0.6824 | 0.3339 | |
| >30 mm vs. 10–≤20 mm | 15/34 vs. 39/99 | 1.57 (0.86–2.87) | 0.1404 | |||
| Patients with 1 nodule (n = 190) | ||||||
| Death | Age | ≥65 vs. <65 | 59/106 vs. 30/84 | 1.08 (0.70–1.69) | 0.7236 | 0.7236 |
| Age (cont.) | Increase of 1 year | 89/190 | 1.01 (0.99–1.03) | 0.2287 | 0.2287 | |
| AFP | Increase of 100 units | 89/190 | 0.99 (0.86–1.14) | 0.8493 | 0.8493 | |
| BCLC | A vs. 0 | 50/102 vs. 39/88 | 1.19 (0.78–1.82) | 0.4142 | 0.4142 | |
| Child-Pugh | B or C vs. non-cirrhotic or A | 18/33 vs. 71/157 | 2.32 (1.37–3.91) | 0.0016 | 0.0016 | |
| ALBI score | ALBI 2 vs. ALBI 1 | 13/23 vs. 75/165 | 2.89 (1.6–5.25) | 0.0005 | 0.0015 | |
| ALBI 3 vs. ALBI 1 | 1/2 vs. 75/165 | 2.76 (0.38–20.1) | 0.3150 | |||
| Suboptimal location | Yes vs. No | 44/89 vs. 45/101 | 1.13 (0.74–1.71) | 0.5798 | 0.5798 | |
| Nodule diameter | >20–≤30 mm vs. 10–≤20 mm | 36/69 vs. 39/88 | 1.24 (0.78–1.96) | 0.3599 | 0.6566 | |
| >30 mm vs. 10–≤20 mm | 14/33 vs. 39/88 | 1.09 (0.59–2.01) | 0.7831 | |||
| Change of treatment strategy | Age | ≥65 vs. <65 | 48/106 vs. 28/84 | 1.00 (0.62–1.60) | 0.9910 | 0.9910 |
| Age (cont.) | Increase of 1 year | 76/190 | 1.00 (0.98–1.02) | 0.8141 | 0.8141 | |
| AFP | Increase of 100 units | 76/190 | 1.10 (1.04–1.16) | 0.0013 | 0.0013 | |
| BCLC | A vs. 0 | 41/102 vs. 35/88 | 1.17 (0.74–1.84) | 0.5102 | 0.5103 | |
| Child-Pugh | B or C vs. non-cirrhotic or A | 8/33 vs. 68/157 | 0.85 (0.4–1.77) | 0.6544 | 0.6544 | |
| ALBI score | ALBI 2 vs. ALBI 1 | 6/23 vs. 70/165 | 1.22 (0.52–2.84) | 0.6469 | 0.9003 | |
| ALBI 3 vs. ALBI 1 | 0/2 vs. 70/165 | NE | - | |||
| Suboptimal location | Yes vs. No | 39/89 vs. 37/101 | 1.42 (0.9–2.23) | 0.1297 | 0.1297 | |
| Nodule diameter | >20–≤30 mm vs. 10–≤20 mm | 26/69 vs. 35/88 | 1.00 (0.6–1.66) | 0.9912 | 0.2148 | |
| >30 mm vs. 10–≤20 mm | 15/33 vs. 35/88 | 1.66 (0.9–3.07) | 0.1032 | |||
| Patients with 1 nodule and nodule ≤3 cm (n = 149) | ||||||
| Death | Age | ≥65 vs. <65 | 45/86 vs. 26/63 | 0.98 (0.60–1.59) | 0.9295 | 0.9295 |
| Age (cont.) | Increase of 1 year | 71/149 | 1.01 (0.99–1.04) | 0.2694 | 0.2694 | |
| AFP | Increase of 100 units | 71/149 | 1.62 (1.01–2.58) | 0.0438 | 0.0438 | |
| BCLC | A vs. 0 | 32/61 vs. 39/88 | 1.27 (0.79–2.04) | 0.3164 | 0.3164 | |
| Child-Pugh | B or C vs. non-cirrhotic or A | 17/28 vs. 54/121 | 2.67 (1.53–4.63) | 0.0005 | 0.0005 | |
| ALBI score | ALBI 2 vs. ALBI 1 | 11/19 vs. 59/128 | 3.95 (2.03–7.7) | <0.0001 | 0.0002 | |
| ALBI 3 vs. ALBI 1 | 1/2 vs. 59/128 | 2.9 (0.4–21.26) | 0.2943 | |||
| Suboptimal location | Yes vs. No | 34/70 vs. 37/79 | 0.99 (0.62–1.58) | 0.9617 | 0.9617 | |
| Nodule diameter | >20–≤30 mm vs. 10–≤20 mm | 32/61 vs. 39/88 | 1.27 (0.79–2.04) | 0.3164 | 0.3164 | |
| Change of treatment strategy | Age | ≥65 vs. <65 | 34/86 vs. 23/63 | 0.82 (0.48–1.40) | 0.4641 | 0.4641 |
| Age (cont.) | Increase of 1 year | 57/149 | 0.99 (0.97–1.02) | 0.5468 | 0.5468 | |
| AFP | Increase of 100 units | 57/149 | 1.41 (0.77–2.58) | 0.2666 | 0.2666 | |
| BCLC | A vs. 0 | 22/61 vs. 35/88 | 0.97 (0.56–1.66) | 0.8982 | 0.8982 | |
| Child-Pugh | B or C vs. non-cirrhotic or A | 7/28 vs. 50/121 | 1.01 (0.45–2.24) | 0.9831 | 0.9831 | |
| ALBI score | ALBI 2 vs. ALBI 1 | 4/19 vs. 53/128 | 1 (0.36–2.81) | 0.9938 | >0.9999 | |
| ALBI 3 vs. ALBI 1 | 0/2 vs. 53/128 | NE | - | |||
| Suboptimal location | Yes vs. No | 28/70 vs. 29/79 | 1.15 (0.68–1.93) | 0.6057 | 0.6057 | |
| Nodule diameter | >20–<30 mm vs. 10–≤20 mm | 22/61 vs. 35/88 | 0.97 (0.56–1.66) | 0.8982 | 0.8982 | |
| Reason [n, (%)] | <65 Years (n = 10) | ≥65 Years (n = 29) | p-Value (Dichotomized Parameter) | p-Value |
|---|---|---|---|---|
| Symptomatic progression | 4 (44.4) | 5 (55.6) | 0.1970 | 0.0608 |
| Liver dysfunction | 1 (6.7) | 14 (93.3) | 0.0574 | |
| Comorbidities | 5 (33.3) | 10 (66.7) | 0.4633 |
| Event | Parameter | Contrasts | HR (95%CI) | p-Value (Contrast) | p-Value (Parameter) |
|---|---|---|---|---|---|
| Death | Change of treatment strategy (td) | BSC for comorbidities vs. for HCC treatment | 2 (1.04–3.82) | 0.0369 | <0.0001 |
| BSC for symptomatic progression vs. for liver dysfunction | 3.23 (1.36–7.65) | 0.0079 | |||
| BSC for symptomatic progression vs. for comorbidities | 3.65 (1.53–8.26) | 0.0035 | |||
| BSC for liver dysfunction vs. for comorbidities | 1.13 (0.53–2.42) | 0.7470 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muñoz-Martínez, S.; Sapena, V.; García-Criado, Á.; Darnell, A.; Forner, A.; Belmonte, E.; Sanduzzi-Zamparelli, M.; Rimola, J.; Soler, A.; Llarch, N.; et al. Risk of Treatment Failure and Death after Ablation in Hepatocellular Carcinoma Patients—A Multiparametric Prediction. Cancers 2023, 15, 3269. https://doi.org/10.3390/cancers15133269
Muñoz-Martínez S, Sapena V, García-Criado Á, Darnell A, Forner A, Belmonte E, Sanduzzi-Zamparelli M, Rimola J, Soler A, Llarch N, et al. Risk of Treatment Failure and Death after Ablation in Hepatocellular Carcinoma Patients—A Multiparametric Prediction. Cancers. 2023; 15(13):3269. https://doi.org/10.3390/cancers15133269
Chicago/Turabian StyleMuñoz-Martínez, Sergio, Victor Sapena, Ángeles García-Criado, Anna Darnell, Alejandro Forner, Ernest Belmonte, Marco Sanduzzi-Zamparelli, Jordi Rimola, Alexandre Soler, Neus Llarch, and et al. 2023. "Risk of Treatment Failure and Death after Ablation in Hepatocellular Carcinoma Patients—A Multiparametric Prediction" Cancers 15, no. 13: 3269. https://doi.org/10.3390/cancers15133269
APA StyleMuñoz-Martínez, S., Sapena, V., García-Criado, Á., Darnell, A., Forner, A., Belmonte, E., Sanduzzi-Zamparelli, M., Rimola, J., Soler, A., Llarch, N., Iserte, G., Mauro, E., Ayuso, C., Rios, J., Bruix, J., Vilana, R., & Reig, M. (2023). Risk of Treatment Failure and Death after Ablation in Hepatocellular Carcinoma Patients—A Multiparametric Prediction. Cancers, 15(13), 3269. https://doi.org/10.3390/cancers15133269








