Role of Truncated O-GalNAc Glycans in Cancer Progression and Metastasis in Endocrine Cancers
Abstract
Simple Summary
Abstract
1. Introduction
2. Synthesis of Truncated O-Glycans
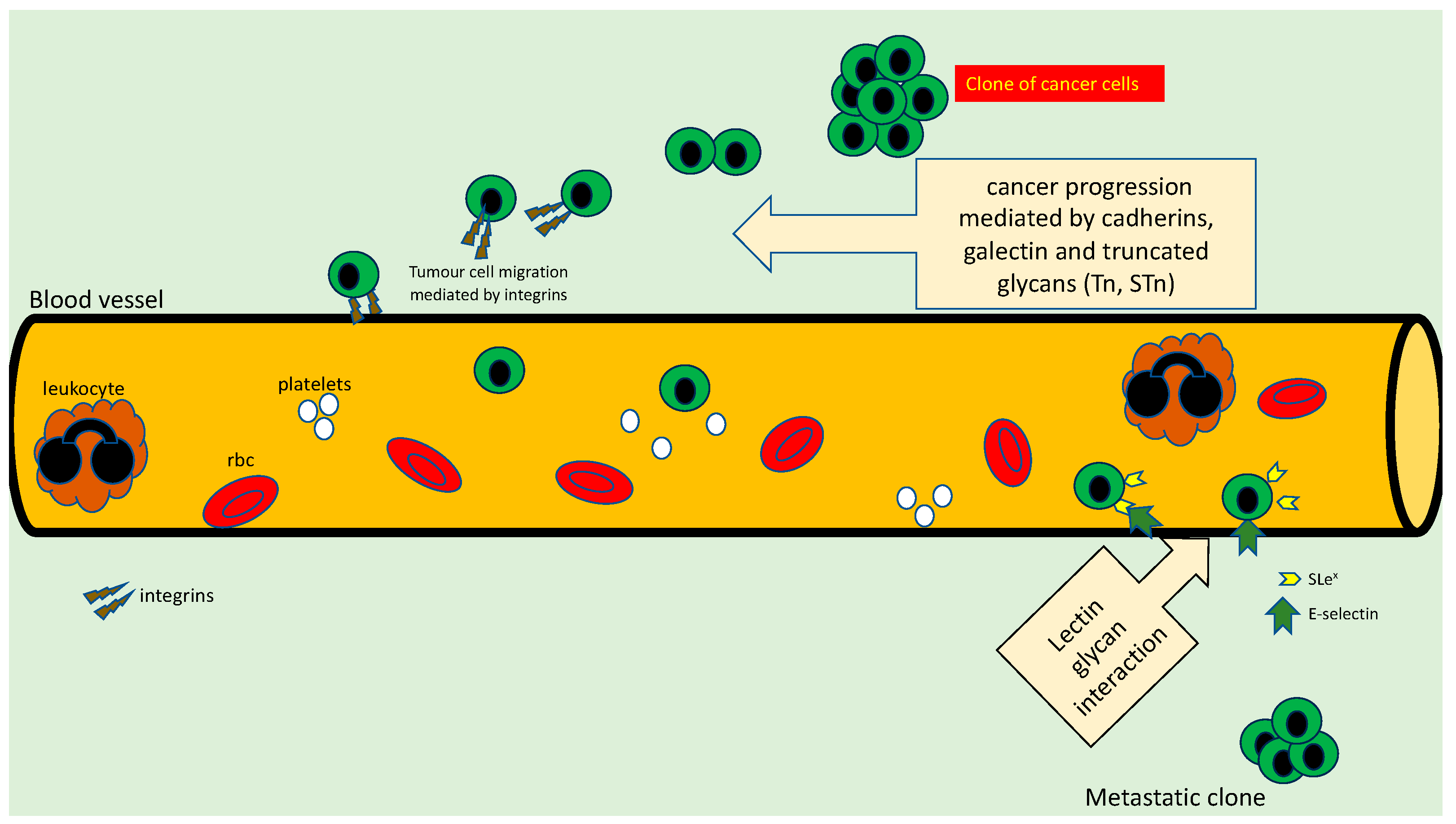
3. Role Played by Glycans in Cancer Cell Adhesion
4. Glycans and Metastasis
5. Role of Truncated Glycans in Immune Modulation in Cancer
6. Aberrant O-GalNAc Glycosylation in Endocrine Cancers
- a.
- Thyroid cancer
- b.
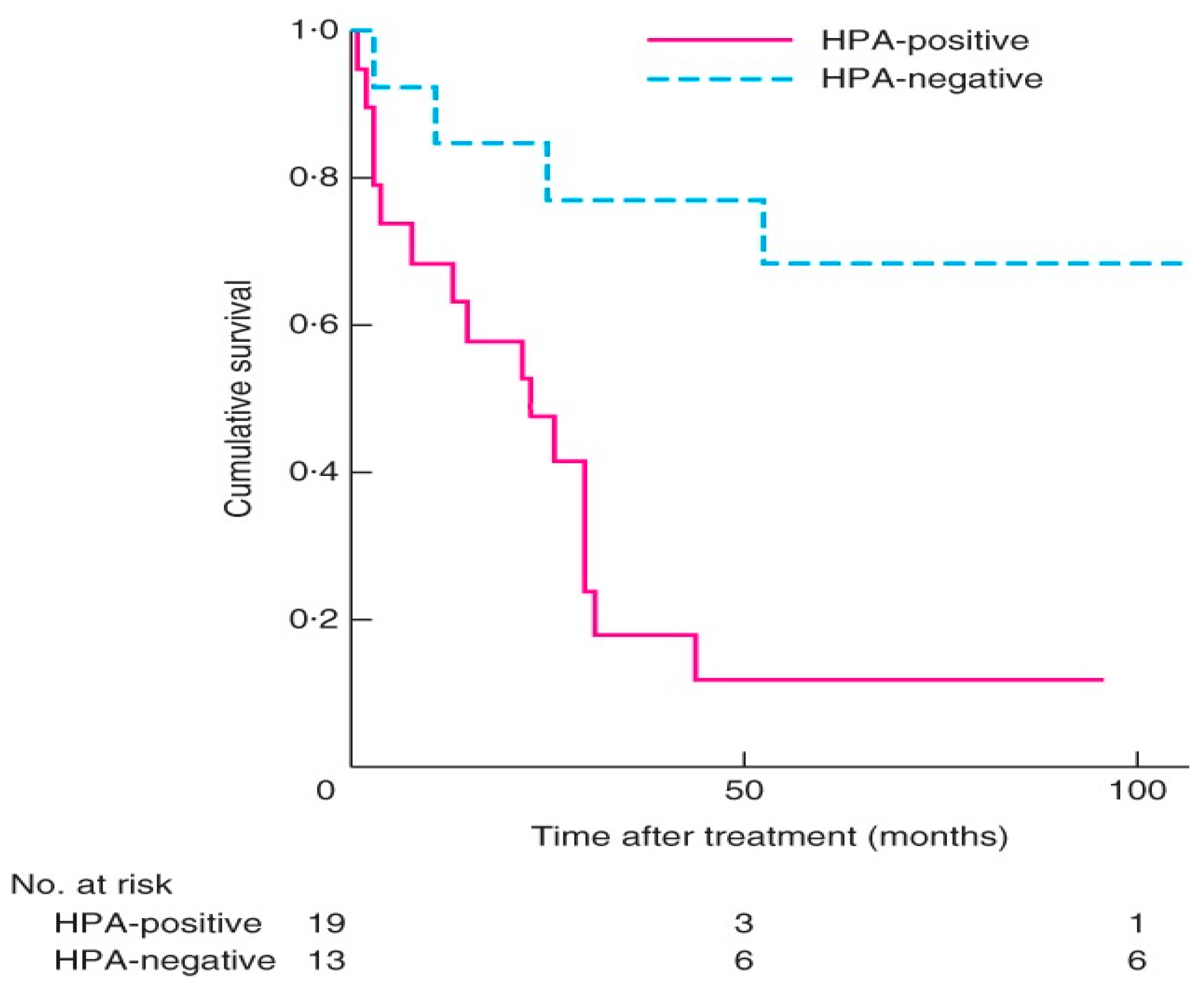
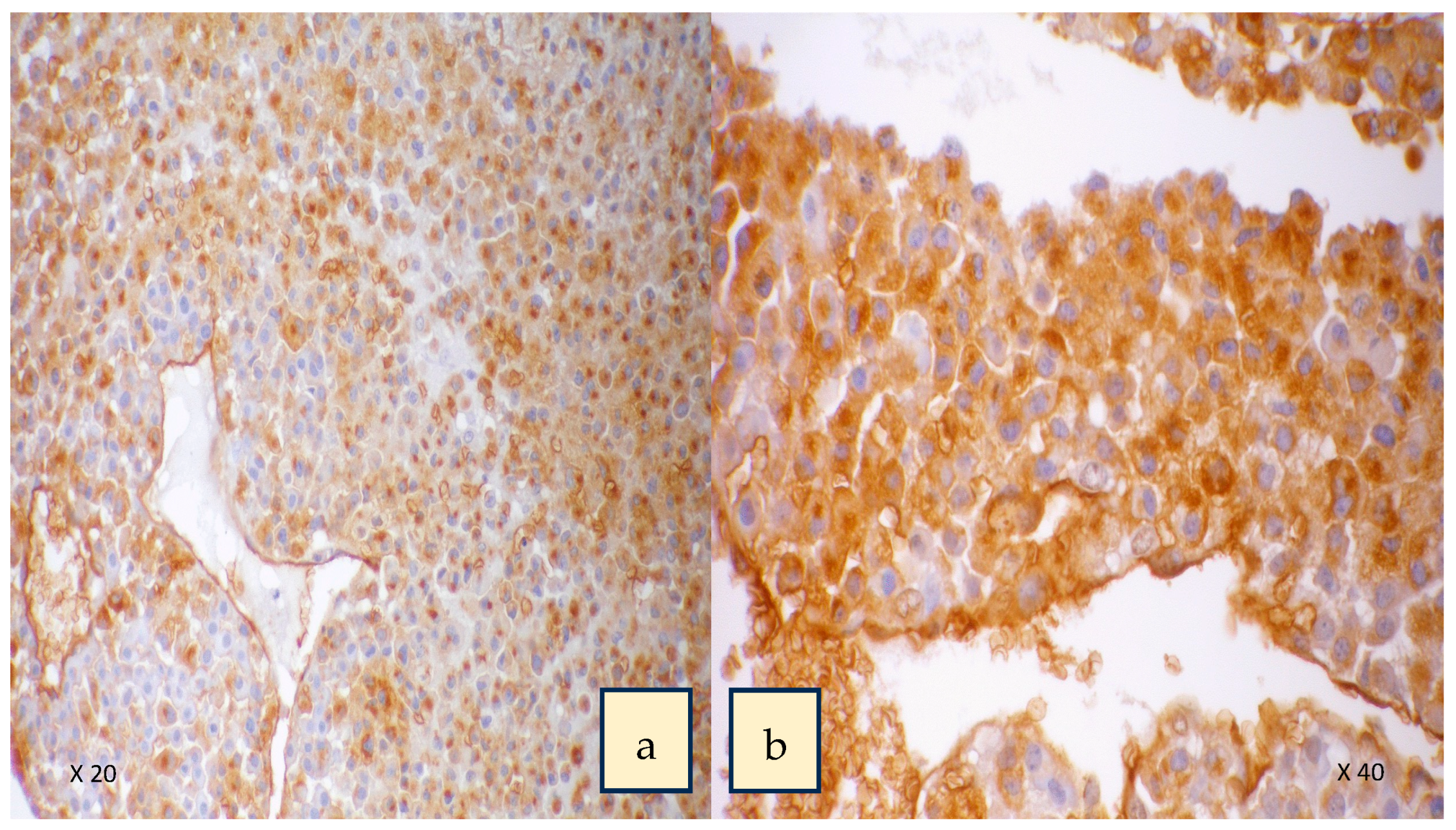
- Adrenal cancer
| Human Cancer Type | % of Truncated Glycans Expressed by Cancers | References |
|---|---|---|
| Breast cancer | Tn (57–92%), STn (33–42%), T (32%) | [87,108,109,110] |
| Gastric cancer | STn (50–78%), Tn (92%), T (20%) | [111,112] |
| Colorectal cancer | Tn (51–98%), STn (75–87%), T (20%) | [87,113,114] |
| Lung cancer | Tn (16%), STn (33%), T (10%) | [87] |
| Pancreatic cancer | Tn (53%), STn (56–97%), T (16–48%) | [87,115] |
| Skin cancer | Tn (33–50%), STn (24%), T (25%) | [87,116] |
| Brain cancer | Tn (51%), STn (80%), T (20%) | [87] |
| Mesenchymal cancers | No expression | [87] |
7. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chatham, J.C.; Zhang, J.; Wende, A.R. Role of O-linked N-acetylglucosamine protein modification in cellular (patho)physiology. Physiol. Rev. 2021, 101, 427–493. [Google Scholar] [CrossRef] [PubMed]
- Hart, G.; Housley, M.; Slawson, C. Cycling of O-linked beta-N-acetylglucosamine on nucleocytoplasmic proteins. Nature 2007, 446, 1017–1022. [Google Scholar] [CrossRef] [PubMed]
- Brockhausen, I.; Wandall, H.; Hagen, K.; Stanley, P. O-GalNAc Glycans. In Essentials of Glycobiology; Jolla, A., Ed.; Cold Spring Harbor Laboratory Press: New York, NY, USA, 2022; pp. 117–128. [Google Scholar]
- Varki, A. Biological roles of glycans. Glycobiology 2016, 27, 3–49. [Google Scholar] [CrossRef]
- Fukuda, M. Possible roles of tumor-associated carbohydrate antigens. Cancer Res. 1996, 56, 2237–2244. [Google Scholar]
- Hakomori, S. Tumor malignancy defined by aberrant glycosylation and sphingo(glyco)lipid metabolism. Cancer Res. 1996, 56, 5309–5318. [Google Scholar]
- Stowell, S.R.; Ju, T.; Cummings, R.D. Protein Glycosylation in Cancer. Annu. Rev. Pathol. Mech. Dis. 2015, 10, 473–510. [Google Scholar] [CrossRef] [PubMed]
- Varki, A.; Cummings, R.; Esko, J.; Stanley, P.; Hart, G.; Aebi, M.; Mohnen, D.; Kinoshita, T.; Packer, N.; Prestegard, J. Essentials of Glycobiology. 2022. Available online: https://www.ncbi.nlm.nih.gov/books/NBK579918/ (accessed on 8 June 2023).
- Marcos, N.T.; Cruz, A.; Silva, F.; Almeida, R.; David, L.; Mandel, U.; Clausen, H.; von Mensdorff-Pouilly, S.; Reis, C.A. Polypeptide GalNAc-transferases, ST6GalNAc-transferase I, and ST3Gal-transferase I Expression in Gastric Carcinoma Cell Lines. J. Histochem. Cytochem. 2003, 51, 761–771. [Google Scholar] [CrossRef]
- Dalziel, M.; Whitehouse, C.; McFarlane, I.; Brockhausen, I.; Gschmeissner, S.; Schwientek, T.; Clausen, H.; Burchell, J.; Taylor-Papadimitriou, J. The Relative Activities of the C2GnT1 and ST3Gal-I Glycosyltransferases Determine O-Glycan Structure and Expression of a Tumor-associated Epitope on MUC1. J. Biol. Chem. 2001, 276, 11007–11015. [Google Scholar] [CrossRef]
- Springer, G.F.; Desai, P.R.; Banatwala, I. Blood group MN antigens and precursors in normal and malignant human breast glandular tissue. Gynecol. Oncol. 1975, 54, 335–339. [Google Scholar]
- Bennett, E.P.; Mandel, U.; Clausen, H.; Gerken, T.; Fritz, T.; Tabak, L.A. Control of mucin-type O-glycosylation: A classification of the polypeptide GalNAc-transferase gene family. Glycobiology 2011, 22, 736–756. [Google Scholar] [CrossRef]
- Festari, M.F.; Trajtenberg, F.; Berois, N.; Pantano, S.; Revoredo, L.; Kong, Y.; Solari-Saquieres, P.; Narimatsu, Y.; Freire, T.; Bay, S.; et al. Revisiting the human polypeptide GalNAc-T1 and T13 paralogs. Glycobiology 2017, 27, 140–153. [Google Scholar] [CrossRef]
- de Las Rivas, M.; Lira-Navarrete, E.; Gerken, T.; Hurtado-Guerrero, R. Polypeptide GalNAc-Ts: From redundancy to specificity. Curr. Opin. Struct. Biol. 2019, 56, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Ju, T.; Aryal, R.; Kudelka, M.; Wang, Y.; Cummings, R.D. The Cosmc connection to the Tn antigen in cancer. Cancer Biomark. 2014, 14, 63–81. [Google Scholar] [CrossRef]
- Brockhausen, I. Mucin-type O-glycans in human colon and breast cancer: Glycodynamics and functions. EMBO Rep. 2006, 7, 599–604. [Google Scholar] [CrossRef]
- Ju, T.; Cummings, R.D. A unique molecular chaperone Cosmc required for activity of the mammalian core 1 β3-galactosyltransferase. Proc. Natl. Acad. Sci. USA 2002, 99, 16613–16618. [Google Scholar] [CrossRef]
- Radhakrishnan, P.; Dabelsteen, S.; Madsen, F.B.; Francavilla, C.; Kopp, K.L.; Steentoft, C.; Vakhrushev, S.Y.; Olsen, J.V.; Hansen, L.; Bennett, E.P.; et al. Immature truncated O-glycophenotype of cancer directly induces oncogenic features. Proc. Natl. Acad. Sci. USA 2014, 111, E4066–E4075. [Google Scholar] [CrossRef] [PubMed]
- Brockhausen, I. Pathways of O-glycan biosynthesis in cancer cells. Biochim. Biophys. Acta Gen. Subj. 1999, 1473, 67–95. [Google Scholar] [CrossRef]
- Munkley, J. The Role of Sialyl-Tn in Cancer. Int. J. Mol. Sci. 2016, 17, 275. [Google Scholar] [CrossRef]
- Pinho, S.S.; Reis, C.A. Glycosylation in cancer: Mechanisms and clinical implications. Nat. Rev. Cancer 2015, 15, 540–555. [Google Scholar] [CrossRef] [PubMed]
- Munkley, J.; Oltean, S.; Vodák, D.; Wilson, B.T.; Livermore, K.E.; Zhou, Y.; Star, E.; Floros, V.I.; Johannessen, B.; Knight, B.; et al. The androgen receptor controls expression of the cancer-associated sTn antigen and cell adhesion through induction of ST6GalNAc1 in prostate cancer. Oncotarget 2015, 6, 34358–34374. [Google Scholar] [CrossRef]
- Julien, S.; Adriaenssens, E.; Ottenberg, K.; Furlan, A.; Courtand, G.; Vercoutter-Edouart, A.; Hanisch, F.; Delannoy, P.; Le Bourhis, X. ST6GalNAc I expression in MDA-MB-231 breast cancer cells greatly modifies their O-glycosylation pattern and enhances their tumourigenicity. Glycobiology 2006, 16, 54–64. [Google Scholar] [CrossRef]
- Ozaki, H.; Matsuzaki, H.; Ando, H.; Kaji, H.; Nakanishi, H.; Ikehara, Y.; Narimatsu, H. Enhancement of metastatic ability by ectopic expression of ST6GalNAcI on a gastric cancer cell line in a mouse model. Clin. Exp. Metastasis 2012, 29, 229–238. [Google Scholar] [CrossRef]
- Gao, Y.; Aryal, R.; Ju, T.; Cummings, R.; Gahlay, G.; Jarvis, D.; Matta, K.; Vlahakis, J.; Szarek, W.; Brockhausen, I. Acceptor specificities and selective inhibition of recombinant human Gal- and GlcNAc-transferases that synthesize core structures 1, 2, 3 and 4 of O-glycans. Biochim. Biophys. Acta 2013, 1830, 4274–4281. [Google Scholar] [CrossRef]
- Datta, A.K. Comparative sequence analysis in the sialyltransferase protein family: Analysis of motifs. Curr. Drug Targets 2009, 10, 483–498. [Google Scholar] [CrossRef]
- Madunić, K.; Mayboroda, O.; Zhang, T.; Weber, J.; Boons, G.; Morreau, H.; van Vlierberghe, R.; van Wezel, T.; Lageveen-Kammeijer, G.; Wuhrer, M. Specific (sialyl-)Lewis core 2 O-glycans differentiate colorectal cancer from healthy colon epithelium. Theranostics 2022, 12, 4498–4512. [Google Scholar] [CrossRef] [PubMed]
- Hill, C.S.; Baharo-Hassan, D.; Farooqui, M. C2-O-sLeX glycoproteins are E-selectin ligands that regulate invasion of human colon and hepatic carcinoma cells. PLoS ONE 2011, 6, e16281. [Google Scholar]
- Cazet, A.; Julien, S.; Bobowski, M.; Krzewinski-Recchi, M.; Harduin-Lepers, A.; Groux-Degroote, S.; Delannoy, P. Conse-quences of the expression of sialylated antigens in breast cancer. Carbohydr. Res. 2010, 345, 1377–1383. [Google Scholar] [CrossRef] [PubMed]
- Pascoal, C.; Carrascal, M.A.; Barreira, D.F.; Lourenço, R.A.; Granjo, P.; Grosso, A.R.; Borralho, P.; Braga, S.; Videira, P.A. Sialyl LewisX/A and Cytokeratin Crosstalk in Triple Negative Breast Cancer. Cancers 2023, 15, 731. [Google Scholar] [CrossRef]
- Marciel, M.P.; Haldar, B.; Hwang, J.; Bhalerao, N.; Bellis, S.L. Role of tumor cell sialylation in pancreatic cancer progression. In Advances in Cancer Research; Academic Press: Cambridge, MA, USA, 2023; Volume 157, pp. 123–155. [Google Scholar]
- Hasehira, K.; Furuta, T.; Shimomura, O.; Asada, M.; Oda, T.; Tateno, H. Quantitative structural analysis of glycans expressed within tumors derived from pancreatic cancer patient-derived xenograft mouse models. Biochem. Biophys. Res. Commun. 2021, 534, 310–316. [Google Scholar] [CrossRef]
- Tsuboi, S.; Suzuki, Y.; Sutoh, M.; Hatakeyama, S.; Mori, K.; Yamamoto, H.; Koie, T.; Saitoh, H.; Yamaya, K.; Funyu, T.; et al. MUC1 carrying core 2 O-glycans functions as a molecular shield against NK cell attack, promoting bladder tumor metastasis. Int. J. Oncol. 2012, 40, 1831–1838. [Google Scholar] [CrossRef]
- Ju, T.; Wang, Y.; Aryal, R.; Lehoux, S.; Ding, X.; Kudelka, M.; Cutler, C.; Zeng, J.; Wang, J.; Sun, X.; et al. Tn and sialyl-Tn antigens, aberrant O-glycomics as human disease markers. Proteomics. Clin. Appl. 2013, 7, 618–631. [Google Scholar] [CrossRef] [PubMed]
- Freitas, D.; Campos, D.; Gomes, J.; Pinto, F.; Macedo, J.; Matos, R.; Mereiter, S.; Pinto, M.; Polónia, A.; Gartner, F.; et al. O-glycans truncation modulates gastric cancer cell signaling and transcription leading to a more aggressive phenotype. Ebiomedicine 2019, 40, 349–362. [Google Scholar] [CrossRef]
- Parameswaran, R.; Tan, W.B.; Nga, M.E.; Soon, G.S.T.; Ngiam, K.Y.; Brooks, S.A.; Sadler, G.P.; Mihai, R. Binding of aberrant glycoproteins recognizable by Helix pomatia agglutinin in adrenal cancers. BJS Open 2018, 2, 353–359. [Google Scholar] [CrossRef]
- Brooks, S.A. The involvement of Helix pomatia lectin (HPA) binding N-acetylgalactosamine glycans in cancer progression. Histol. Histopathol. 2000, 15, 143–158. [Google Scholar] [PubMed]
- Bevilacqua, M.; Nelson, R. Selectins. J. Clin. Investig. 1993, 91, 379–387. [Google Scholar] [CrossRef] [PubMed]
- Kannagi, R.; Izawa, M.; Koike, T.; Miyazaki, K.; Kimura, N. Carbohydrate-mediated cell adhesion in cancer metastasis and angiogenesis. Cancer Sci. 2004, 95, 377–384. [Google Scholar] [CrossRef] [PubMed]
- Phillips, M.L.; Nudelman, E.; Gaeta, F.C.A.; Perez, M.; Singhal, A.K.; Hakomori, S.-I.; Paulson, J.C. ELAM-1 Mediates Cell Adhesion by Recognition of a Carbohydrate Ligand, Sialyl-Lex. Science 1990, 250, 1130–1132. [Google Scholar] [CrossRef]
- Takada, A.; Ohmori, K.; Takahashi, N.; Tsuyuoka, K.; Yago, A.; Zenita, K.; Hasegawa, A.; Kannagi, R. Adhesion of human cancer cells to vascular endothelium mediated by a carbohydrate antigen, sialyl Lewis A. Biochem. Biophys. Res. Commun. 1991, 179, 713–719. [Google Scholar] [CrossRef]
- Shimura, T.; Takenaka, Y.; Tsutsumi, S.; Hogan, V.; Kikuchi, A.; Raz, A. Galectin-3, a novel binding partner of beta-catenin. Cancer Res. 2004, 64, 6363–6367. [Google Scholar] [CrossRef]
- Berx, G.; van Roy, F. Involvement of members of the cadherin superfamily in cancer. Cold Spring Harb. Perspect. Biol. 2009, 1, a003129. [Google Scholar] [CrossRef]
- Guilford, P.; Hopkins, J.; Harraway, J.; McLeod, M.; McLeod, N.; Harawira, P.; Taite, H.; Scoular, R.; Miller, A.; Reeve, A.E. E-cadherin germline mutations in familial gastric cancer. Nature 1998, 392, 402–405. [Google Scholar] [CrossRef]
- Corso, G.; Figueiredo, J.; De Angelis, S.P.; Corso, F.; Girardi, A.; Pereira, J.; Seruca, R.; Bonanni, B.; Carneiro, P.; Pravettoni, G.; et al. E-cadherin deregulation in breast cancer. J. Cell. Mol. Med. 2020, 24, 5930–5936. [Google Scholar] [CrossRef]
- Baranwal, S.; Alahari, S.K. Molecular mechanisms controlling E-cadherin expression in breast cancer. Biochem. Biophys. Res. Commun. 2009, 384, 6–11. [Google Scholar] [CrossRef] [PubMed]
- von Burstin, J.; Eser, S.; Paul, M.; Seidler, B.; Brandl, M.; Messer, M.; von Werder, A.; Schmidt, A.; Mages, J.; Pagel, P.; et al. E-cadherin regulates metastasis of pancreatic cancer in vivo and is suppressed by a SNAIL/HDAC1/HDAC2 repressor complex. Gastroenterology 2009, 137, e1–e5. [Google Scholar] [CrossRef] [PubMed]
- Canel, M.; Serrels, A.; Frame, M.C.; Brunton, V.G. E-cadherin–integrin crosstalk in cancer invasion and metastasis. J. Cell Sci. 2013, 126, 393–401. [Google Scholar] [CrossRef] [PubMed]
- Hynes, R.O. Integrins: Bidirectional, Allosteric Signaling Machines. Cell 2002, 110, 673–687. [Google Scholar] [CrossRef]
- Cavallaro, U.; Christofori, G. Cell adhesion and signalling by cadherins and Ig-CAMs in cancer. Nat. Rev. Cancer 2004, 4, 118–132. [Google Scholar] [CrossRef] [PubMed]
- Aigner, S.; Sthoeger, Z.M.; Fogel, M.; Weber, E.; Zarn, J.; Ruppert, M.; Zeller, Y.; Vestweber, D.; Stahel, R.; Sammar, M.; et al. CD24, a mucin-type glycoprotein, is a ligand for P-selectin on human tumor cells. Blood 1997, 89, 3385–3395. [Google Scholar] [CrossRef]
- Mitsuoka, C.; Sawada-Kasugai, M.; Ando-Furui, K.; Izawa, M.; Nakanishi, H.; Nakamura, S.; Ishida, H.; Kiso, M.; Kannagi, R. Identification of a Major Carbohydrate Capping Group of the L-selectin Ligand on High Endothelial Venules in Human Lymph Nodes as 6-Sulfo Sialyl Lewis X. J. Biol. Chem. 1998, 273, 11225–11233. [Google Scholar] [CrossRef]
- Kannagi, R. Regulatory roles of carbohydrate ligands for selectins in the homing of lymphocytes. Curr. Opin. Struct. Biol. 2002, 12, 599–608. [Google Scholar] [CrossRef]
- Sancho, D.; Sousa, C.R. Sensing of cell death by myeloid C-type lectin receptors. Curr. Opin. Immunol. 2013, 25, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Coombs, P.; Graham, S.A.; Drickamer, K.; Taylor, M.E. Selective Binding of the Scavenger Receptor C-type Lectin to Lewisx Trisaccharide and Related Glycan Ligands. J. Biol. Chem. 2005, 280, 22993–22999. [Google Scholar] [CrossRef]
- Elola, M.; Capurro, M.; Barrio, M.; Coombs, P.; Taylor, M.; Drickamer, K.; Mordoh, J. Lewis x antigen mediates adhesion of human breast carcinoma cells to activated endothelium. Possible involvement of the endothelial scavenger receptor C-type lectin. Breast Cancer Res. Treat. 2007, 101, 161–174. [Google Scholar] [CrossRef]
- Xin, M.; Dong, X.-W.; Guo, X.-L. Role of the interaction between galectin-3 and cell adhesion molecules in cancer metastasis. Biomed. Pharmacother. 2015, 69, 179–185. [Google Scholar] [CrossRef]
- Furtak, V.; Hatcher, F.; Ochieng, J. Galectin-3 mediates the endocytosis of beta-1 integrins by breast carcinoma cells. Biochem. Biophys. Res. Commun. 2001, 289, 845–850. [Google Scholar] [CrossRef] [PubMed]
- Ochieng, J.; Furtak, V.; Lukyanov, P. Extracellular functions of galectin-3. Glycoconj. J. 2002, 19, 527–535. [Google Scholar] [CrossRef]
- Saeland, E.; van Vliet, S.J.; Bäckström, M.; van den Berg, V.C.M.; Geijtenbeek, T.B.H.; Meijer, G.A.; van Kooyk, Y. The C-type lectin MGL expressed by dendritic cells detects glycan changes on MUC1 in colon carcinoma. Cancer Immunol. Immunother. 2007, 56, 1225–1236. [Google Scholar] [CrossRef] [PubMed]
- PAllavena; Chieppa, M.; Bianchi, G.; Solinas, G.; Fabbri, M.; Laskarin, G.; Mantovani, A. Engagement of the mannose receptor by tumoral mucins activates an immune suppressive phenotype in human tumor-associated macrophages. Clin. Dev. Immunol. 2010, 2010, 547179. [Google Scholar]
- Dusoswa, S.; Verhoeff, J.; Abels, E.; Méndez-Huergo, S.; Croci, D.; Kuijper, L.; de Miguel, E.; Wouters, V.; Best, M.; Rodriguez, E.; et al. Glioblastomas exploit truncated O-linked glycans for local and distant im-mune modulation via the macrophage galactose-type lectin. Proc. Natl. Acad Sci. USA 2020, 117, 3693–3703. [Google Scholar] [CrossRef]
- Yu, L.-G. The oncofetal Thomsen–Friedenreich carbohydrate antigen in cancer progression. Glycoconj. J. 2007, 24, 411–420. [Google Scholar] [CrossRef]
- Zou, J.; Glinsky, V.V.; Landon, L.A.; Matthews, L.; Deutscher, S.L. Peptides specific to the galectin-3 carbohydrate recognition domain inhibit metastasis-associated cancer cell adhesion. Carcinogenesis 2005, 26, 309–318. [Google Scholar] [CrossRef]
- Nath, S.; Mukherjee, P. MUC1: A multifaceted oncoprotein with a key role in cancer progression. Trends Mol. Med. 2014, 20, 332–342. [Google Scholar] [CrossRef] [PubMed]
- Kufe, D.W. MUC1-C oncoprotein as a target in breast cancer: Activation of signaling pathways and therapeutic approaches. Oncogene 2012, 32, 1073–1081. [Google Scholar] [CrossRef]
- Shimodaira, K.; Nakayama, J.; Nakamura, N.; Hasebe, O.; Katsuyama, T.; Fukuda, M. Carcinoma-associated expression of core 2 beta-1,6-N-acetylglucosaminyltransferase gene in human colorectal cancer: Role of O-glycans in tumor progression. Cancer Res. 1997, 57, 5201–5206. [Google Scholar]
- Nakamura, K.; Niimi, K.; Yamamoto, E.; Ikeda, Y.; Nishino, K.; Suzuki, S.; Kajiyama, H.; Kikkawa, F. Core 2 β1,6-N-acetylglucosaminyltransferases accelerate the escape of choriocarcinoma from natural killer cell immunity. Biochem. Biophys. Rep. 2021, 26, 100951. [Google Scholar] [CrossRef]
- Vavasseur, F.; Dole, K.; Yang, J.; Matta, K.L.; Myerscough, N.; Corfield, A.; Paraskeva, C.; Brockhausen, I. O-glycan biosynthesis in human colorectal adenoma cells during progression to cancer. JBIC J. Biol. Inorg. Chem. 1994, 222, 415–424. [Google Scholar] [CrossRef]
- Tsuboi, S.; Hatakeyama, S.; Ohyama, C.; Fukuda, M. Two opposing roles of O-glycans in tumor metastasis. Trends Mol. Med. 2012, 18, 224–232. [Google Scholar] [CrossRef]
- Bapu, D.; Runions, J.; Kadhim, M.; Brooks, S.A. N-acetylgalactosamine glycans function in cancer cell adhesion to endothelial cells: A role for truncated O-glycans in metastatic mechanisms. Cancer Lett. 2016, 375, 367–374. [Google Scholar] [CrossRef]
- Glinsky, V.; Glinsky, G.V.; Rittenhouse-Olson, K.; Huflejt, M.; Glinskii, O.V.; Deutscher, S.L.; Quinn, T.P. The role of Thomsen-Friedenreich antigen in adhesion of human breast and prostate cancer cells to the endothelium. Cancer Res. 2001, 61, 4851–4857. [Google Scholar] [PubMed]
- Gill, D.; Tham, K.; Chia, J.; Wang, S.; Steentoft, C.; Clausen, H.; Bard-Chapeau, E.; Bard, F. Initiation of GalNAc-type O-glycosylation in the endoplasmic reticulum promotes cancer cell invasiveness. Proc. Natl. Acad. Sci. USA 2013, 110, E3152–E3161. [Google Scholar] [CrossRef]
- Tsuboi, S.; Sutoh, M.; Hatakeyama, S.; Hiraoka, N.; Habuchi, T.; Horikawa, Y.; Hashimoto, Y.; Yoneyama, T.; Mori, K.; Koie, T.; et al. A novel strategy for evasion of NK cell immunity by tumours expressing core2 O-glycans. EMBO J. 2011, 30, 3173–3185. [Google Scholar] [CrossRef]
- Lanier, L.L. Up on the tightrope: Natural killer cell activation and inhibition. Nat. Immunol. 2008, 9, 495–502. [Google Scholar] [CrossRef]
- Ogata, S.; Maimonis, P.J.; Itzkowitz, S.H. Mucins bearing the cancer-associated sialosyl-Tn antigen mediate inhibition of natural killer cell cytotoxicity. Cancer Res. 1992, 52, 4741–4746. [Google Scholar]
- Smith, B.A.H.; Bertozzi, C.R. The clinical impact of glycobiology: Targeting selectins, Siglecs and mammalian glycans. Nat. Rev. Drug Discov. 2021, 20, 217–243. [Google Scholar] [CrossRef] [PubMed]
- Beatson, R.; Maurstad, G.; Picco, G.; Arulappu, A.; Coleman, J.; Wandell, H.H.; Clausen, H.; Mandel, U.; Taylor-Papadimitriou, J.; Sletmoen, M.; et al. The Breast Cancer-Associated Glycoforms of MUC1, MUC1-Tn and sialyl-Tn, Are Expressed in COSMC Wild-Type Cells and Bind the C-Type Lectin MGL. PLoS ONE 2015, 10, e0125994. [Google Scholar] [CrossRef]
- Lenos, K.; Goos, J.A.C.M.; Vuist, I.M.; Den Uil, S.H.; Diemen, P.M.D.-V.; Belt, E.J.T.; Stockmann, H.B.A.C.; Bril, H.; De Wit, M.; Carvalho, B.; et al. MGL ligand expression is correlated to BRAF mutation and associated with poor survival of stage III colon cancer patients. Oncotarget 2015, 6, 26278–26290. [Google Scholar] [CrossRef]
- Varki, A.; Angata, T. Siglecs—The major subfamily of I-type lectins. Glycobiology 2005, 16, 1R–27R. [Google Scholar] [CrossRef]
- Ohta, M.; Ishida, A.; Toda, M.; Akita, K.; Inoue, M.; Yamashita, K.; Watanabe, M.; Murata, T.; Usui, T.; Nakada, H. Immuno-modulation of monocyte-derived dendritic cells through ligation of tumor-produced mucins to Siglec-9. Biochem. Biophys. Res. Commun. 2010, 402, 663–669. [Google Scholar] [CrossRef] [PubMed]
- Rajesh, C.; Radhakrishnan, P. The (Sialyl) Tn antigen: Contributions to immunosuppression in gastrointestinal cancers. Front. Oncol. 2022, 12, 1093496. [Google Scholar] [CrossRef]
- Macauley, M.S.; Crocker, P.R.; Paulson, J.C. Siglec-mediated regulation of immune cell function in disease. Nat. Rev. Immunol. 2014, 14, 653–666. [Google Scholar] [CrossRef] [PubMed]
- Hudak, J.; Canham, S.; Bertozzi, C. Glycocalyx engineering reveals a Siglec-based mechanism for NK cell immunoevasion. Nat. Chem. Biol. 2014, 10, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Nicoll, G.; Avril, T.; Lock, K.; Furukawa, K.; Bovin, N.; Crocker, P.R. Ganglioside GD3 expression on target cells can modulate NK cell cytotoxicity via siglec-7-dependent and -independent mechanisms. Eur. J. Immunol. 2003, 33, 1642–1648. [Google Scholar] [CrossRef] [PubMed]
- Jandus, C.; Boligan, K.; Chijioke, O.; Liu, H.; Dahlhaus, M.; Démoulins, T.; Schneider, C.; Wehrli, M.; Hunger, R.; Baerlocher, G.; et al. Interactions between Siglec-7/9 receptors and ligands influence NK cell-dependent tumor immunosurveillance. J. Clin. Investig. 2014, 124, 1810–1820. [Google Scholar] [CrossRef] [PubMed]
- Rømer, T.B.; Aasted, M.K.M.; Dabelsteen, S.; Groen, A.; Schnabel, J.; Tan, E.; Pedersen, J.W.; Haue, A.D.; Wandall, H.H. Mapping of truncated O-glycans in cancers of epithelial and non-epithelial origin. Br. J. Cancer 2021, 125, 1239–1250. [Google Scholar] [CrossRef]
- Lugo, R.; Ávila-Nava, A.; García-Pérez, R.; Herrera-Escalante, S.; De la Cruz-Acosta, J.; Gutiérrez-Solis, A. Systematic review and meta-analysis of the clinical survival significance of Sialyl-Tn expression in histological tissues from cancer patients. Transl. Cancer Res. 2020, 9, 547–555. [Google Scholar] [CrossRef]
- Khosrowabadi, E.; Wenta, T.; Keskitalo, S.; Manninen, A.; Kellokumpu, S. Altered glycosylation of several metasta-sis-associated glycoproteins with terminal GalNAc defines the highly invasive cancer cell phenotype. Oncotarget 2022, 13, 73–89. [Google Scholar] [CrossRef]
- Brooks, S.; Leathem, A. Expression of alpha-GalNAc glycoproteins by breast cancers. Br. J. Cancer 1995, 71, 1033–1038. [Google Scholar] [CrossRef]
- Ito, N.; Imai, S.; Haga, S.; Nagaike, C.; Morimura, Y.; Hatake, K. Localization of binding sites of Ulex europaeus I, Helix pomatia and Griffonia simplicifolia I-B4 lectins and analysis of their backbone structures by several glycosidases and poly-N-acetyllactosamine-specific lectins in human breast carcinomas. Histochem. Cell Biol. 1996, 106, 331–339. [Google Scholar]
- Springer, G. Tn epitope (N-acetyl-D-galactosamine alpha-O-serine/threonine) density in primary breast carcinoma: A functional predictor of aggressiveness. Mol. Immunol. 1989, 26, 1–5. [Google Scholar] [CrossRef]
- AWu, M.; Song, S.; Sugii, S.; Herp, A. Differential binding properties of Gal/GalNAc specific lectins available for characterization of glycoreceptors. Indian J. Biochem. Biophys. 1997, 34, 61–71. [Google Scholar]
- Parameswaran, R.; Sadler, G.; Brooks, S. Helix pomatia Agglutinin Binding Glycoproteins in Thyroid Tumors. World J. Surg. 2011, 35, 2219–2227. [Google Scholar] [CrossRef]
- Lee, K.Y.; Sreenivasan, D.K.; Feng, P.; Yang, P.; Parameswaran, R. MON-LB85 Aberrant Glycan Expression Changes with the Phenotype of Follicular Thyroid Tumours. J. Endocr. Soc. 2020, 4, 2093. [Google Scholar] [CrossRef]
- Kitahara, C.M.; Sosa, J.A. The changing incidence of thyroid cancer. Nat. Rev. Endocrinol. 2016, 12, 646–653. [Google Scholar] [CrossRef]
- Shulin, J.H.; Aizhen, J.; Kuo, S.M.; Tan, W.B.; Ngiam, K.Y.; Parameswaran, R. Rising incidence of thyroid cancer in Singapore not solely due to micropapillary subtype. Ann. R Coll. Surg. Engl. 2018, 100, 295–300. [Google Scholar] [CrossRef]
- Chmielik, E.; Rusinek, D.; Oczko-Wojciechowska, M.; Jarzab, M.; Krajewska, J.; Czarniecka, A.; Jarzab, B. Heterogeneity of Thyroid Cancer. Pathobiology 2018, 85, 117–129. [Google Scholar] [CrossRef] [PubMed]
- Parameswaran, R.; Brooks, S.; Sadler, G.P. Molecular pathogenesis of follicular cell derived thyroid cancers. Int. J. Surg. 2010, 8, 186–193. [Google Scholar] [CrossRef] [PubMed]
- Brooks, S.A.; Carter, T.M. N-acetylgalactosamine, N-acetylglucosamine and sialic acid expression in primary breast cancers. Acta Histochem. 2001, 103, 37–51. [Google Scholar] [CrossRef] [PubMed]
- Else, T.; Kim, A.; Sabolch, A.; Raymond, V.; Kandathil, A.; Caoili, E.; Jolly, S.; Miller, B.; Giordano, T.; Hammer, G. Adrenocortical carcinoma. Endocr. Rev. 2014, 35, 282–326. [Google Scholar] [CrossRef]
- Crona, J.; Beuschlein, F. Adrenocortical carcinoma—Towards genomics guided clinical care. Nat. Rev. Endocrinol. 2019, 15, 548–560. [Google Scholar] [CrossRef]
- Fassnacht, M.; Allolio, B. Clinical management of adrenocortical carcinoma. Best Pract. Res. Clin. Endocrinol. Metab. 2009, 23, 273–289. [Google Scholar] [CrossRef]
- Luton, J.-P.; Cerdas, S.; Billaud, L.; Thomas, G.; Guilhaume, B.; Bertagna, X.; Laudat, M.-H.; Louvel, A.; Chapuis, Y.; Blondeau, P.; et al. Clinical Features of Adrenocortical Carcinoma, Prognostic Factors, and the Effect of Mitotane Therapy. N. Engl. J. Med. 1990, 322, 1195–1201. [Google Scholar] [CrossRef]
- Nakamura, Y.; Yamazaki, Y.; Felizola, S.; Ise, K.; Morimoto, R.; Satoh, F.; Arai, Y.; Sasano, H. Adrenocortical carcinoma: Review of the pathologic features, production of adrenal steroids, and molecular pathogenesis. Endocrinol. Metab. Clin. N. Am. 2015, 44, 399–410. [Google Scholar] [CrossRef]
- Peiris, D.; Ossondo, M.; Fry, S.; Loizidou, M.; Smith-Ravin, J.; Dwek, M.V. Identification of O-Linked Glycoproteins Binding to the Lectin Helix pomatia Agglutinin as Markers of Metastatic Colorectal Cancer. PLoS ONE 2015, 10, e0138345. [Google Scholar] [CrossRef] [PubMed]
- Rambaruth, N.; Greenwell, P.; Dwek, M. The lectin Helix pomatia agglutinin recognizes O-GlcNAc containing glycoproteins in human breast cancer. Glycobiology 2012, 22, 839–848. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.R.; Sauter, E.R.; Quinn, T.P.; Deutscher, S.L. Thomsen-Friedenreich and Tn Antigens in Nipple Fluid: Carbohydrate Biomarkers for Breast Cancer Detection. Clin. Cancer Res. 2005, 11, 6868–6871. [Google Scholar] [CrossRef]
- Leivonen, M.; Nordling, S.; Lundin, J.; von Boguslawski, K.; Haglund, C. STn and Prognosis in Breast Cancer. Oncology 2001, 61, 299–305. [Google Scholar] [CrossRef]
- Xu, F.; Zhao, H.; Li, J.; Jiang, H. Mucin-type sialyl-Tn antigen is associated with PD-L1 expression and predicts poor clinical prognosis in breast cancer. Gland. Surg. 2021, 10, 2159–2169. [Google Scholar] [CrossRef] [PubMed]
- Victorzon, M.; Nordling, S.; Nilsson, O.; Roberts, P.J.; Haglund, C. Sialyl Tn antigen is an independent predictor of outcome in patients with gastric cancer. Int. J. Cancer 1996, 65, 295–300. [Google Scholar] [CrossRef]
- Werther, J.L.; Tatematsu, M.; Klein, R.; Kurihara, M.; Kumagat, K.; Llorens, P.; Neto, J.G.; Bodian, C.; Pertsemeidis, D.; Yamachika, T.; et al. Sialosyl-Tn, antigen as a marker of gastric cancer progression: An international study. Int. J. Cancer 1996, 69, 193–199. [Google Scholar] [CrossRef]
- Dombek, G.; Ore, A.; Cheng, J.; Matsumoto, Y.; Glickman, J.; Fleishman, A.; Heimburg-Molinaro, J.; Poylin, V.; Fabrizio, A.; Cataldo, T.; et al. Immunohistochemical analysis of Tn antigen expression in colorectal adenocar-cinoma and precursor lesions. BMC Cancer 2022, 22, 1281. [Google Scholar] [CrossRef]
- Xu, F.; Fan, C.; Fan, S.; Liu, F.; Wen, T.; An, G.; Feng, G. Expression profile of mucin-associated sialyl-Tn antigen in Chinese patients with different colorectal lesions (adenomas, carcinomas). Int. J. Clin. Exp. Pathol. 2015, 8, 11549–11554. [Google Scholar] [PubMed]
- Itzkowitz, S.; Kjeldsen, T.; Friera, A.; Hakomori, S.-I.; Yang, U.-S.; Kim, Y.S. Expression of Tn, sialosyl Tn, and T antigens in human pancreas. Gastroenterology 1991, 100, 1691–1700. [Google Scholar] [CrossRef]
- Kanitakis, J.; Al-Rifai, I.; Euvrard, S.; Faure, M.; Claudy, A. Differential expression of the cancer-associated antigens T (Thomsen-Friedenreich) and Tn in primary and recurring squamous cell carcinomas of the skin. Anticancer Res. 1999, 19, 619–620. [Google Scholar] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pinto, D.; Parameswaran, R. Role of Truncated O-GalNAc Glycans in Cancer Progression and Metastasis in Endocrine Cancers. Cancers 2023, 15, 3266. https://doi.org/10.3390/cancers15133266
Pinto D, Parameswaran R. Role of Truncated O-GalNAc Glycans in Cancer Progression and Metastasis in Endocrine Cancers. Cancers. 2023; 15(13):3266. https://doi.org/10.3390/cancers15133266
Chicago/Turabian StylePinto, Diluka, and Rajeev Parameswaran. 2023. "Role of Truncated O-GalNAc Glycans in Cancer Progression and Metastasis in Endocrine Cancers" Cancers 15, no. 13: 3266. https://doi.org/10.3390/cancers15133266
APA StylePinto, D., & Parameswaran, R. (2023). Role of Truncated O-GalNAc Glycans in Cancer Progression and Metastasis in Endocrine Cancers. Cancers, 15(13), 3266. https://doi.org/10.3390/cancers15133266





