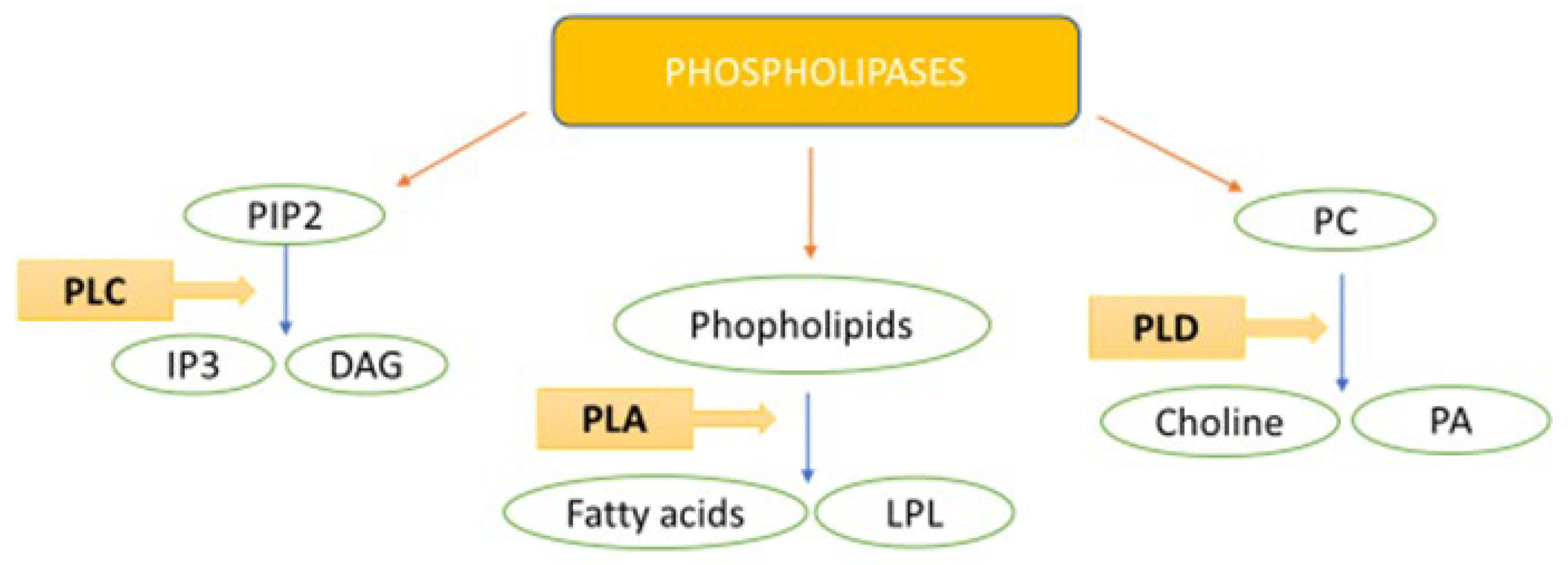
Phospholipase Family Enzymes in Lung Cancer: Looking for Novel Therapeutic Approaches
Abstract
Simple Summary
Abstract
1. Introduction: Lung Cancer Features
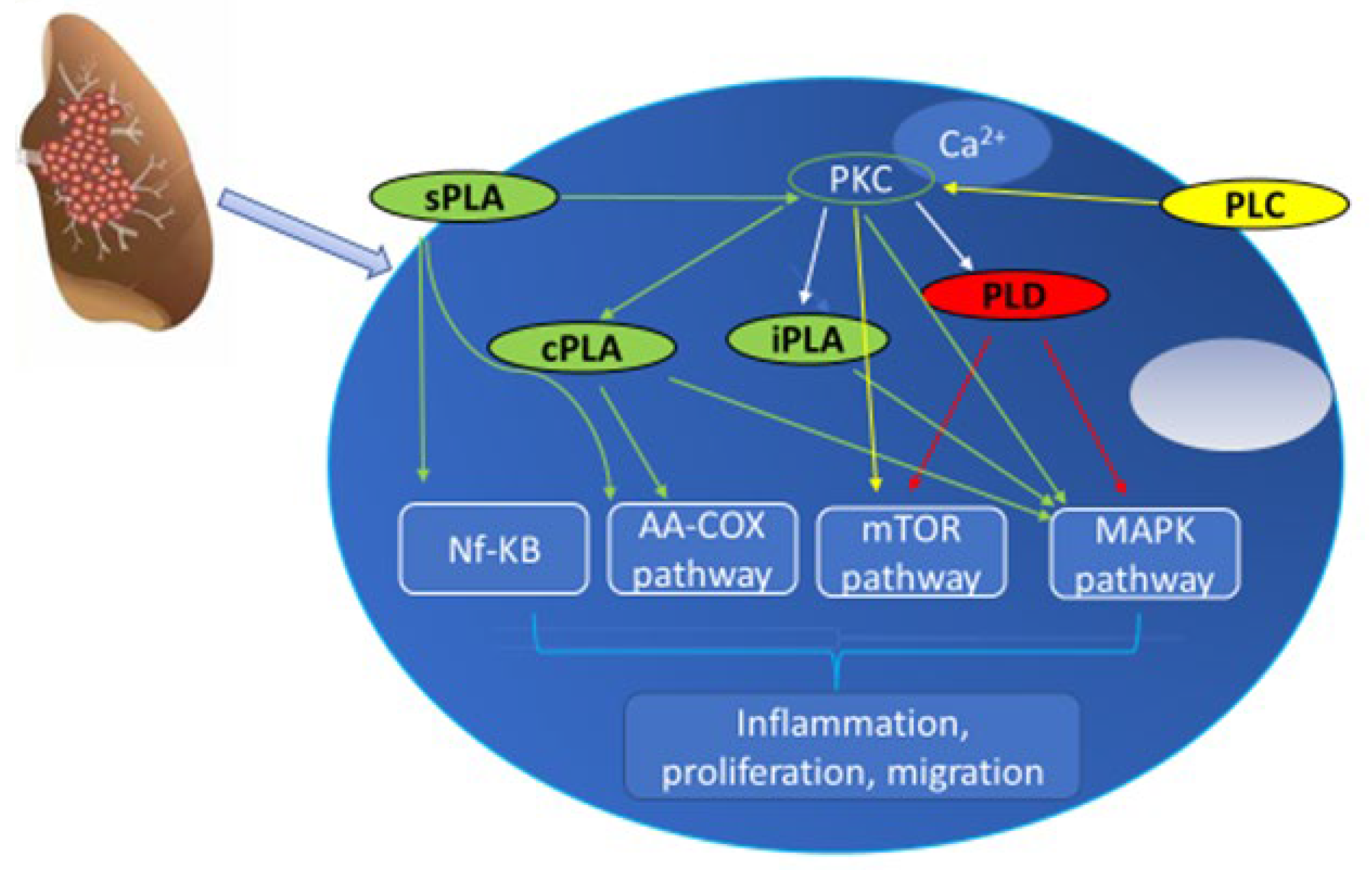
2. PLC and Lung Cancer
2.1. PLCβ
2.2. PLCε
2.3. PLC δ
2.4. PLC γ
2.5. PLC Concluding Remarks
3. PLD and Lung Cancer
PLD Concluding Remarks
4. PLA and Lung Cancer
4.1. sPLA2
4.2. cPLA2
4.3. iPLA2
4.4. PLA Concluding Remarks
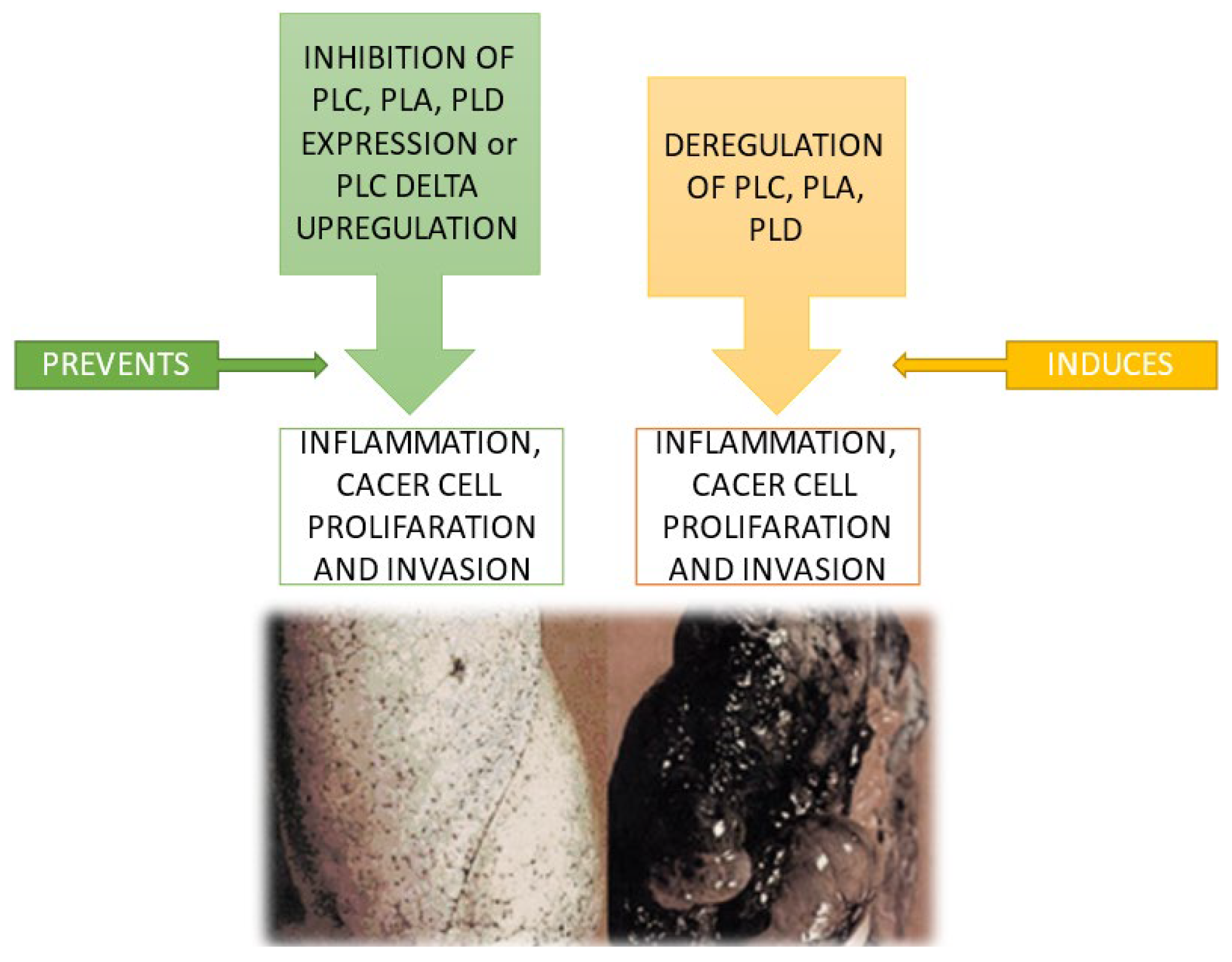
5. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shah, S.C.; Kayamba, V.; Peek, R.M.; Heimburger, D. Cancer Control in Low- and Middle-Income Countries: Is It Time to Consider Screening? J. Glob. Oncol. 2019, 5, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Haier, J.; Schaefers, J. Economic Perspective of Cancer Care and Its Consequences for Vulnerable Groups. Cancers 2022, 14, 3158. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Thandra, K.C.; Barsouk, A.; Saginala, K.; Aluru, J.S.; Barsouk, A. Epidemiology of Lung Cancer. Contemp. Oncol. 2021, 25, 45–52. [Google Scholar] [CrossRef]
- Samet, J.M.; Avila-Tang, E.; Boffetta, P.; Hannan, L.M.; Olivo-Marston, S.; Thun, M.J.; Rudin, C.M. Lung Cancer in Never Smokers: Clinical Epidemiology and Environmental Risk Factors. Clin. Cancer Res. 2009, 15, 5626–5645. [Google Scholar] [CrossRef]
- Lemjabbar-Alaoui, H.; Hassan, O.U.; Yang, Y.-W.; Buchanan, P. Lung Cancer: Biology and Treatment Options. Biochim. Biophys. Acta 2015, 1856, 189–210. [Google Scholar] [CrossRef]
- Chen, B.T.; Chen, Z.; Ye, N.; Mambetsariev, I.; Fricke, J.; Daniel, E.; Wang, G.; Wong, C.W.; Rockne, R.C.; Colen, R.R.; et al. Differentiating Peripherally-Located Small Cell Lung Cancer from Non-Small Cell Lung Cancer Using a CT Radiomic Approach. Front. Oncol. 2020, 10, 593. [Google Scholar] [CrossRef]
- Adib, E.; Nassar, A.H.; Abou Alaiwi, S.; Groha, S.; Akl, E.W.; Sholl, L.M.; Michael, K.S.; Awad, M.M.; Jӓnne, P.A.; Gusev, A.; et al. Variation in Targetable Genomic Alterations in Non-Small Cell Lung Cancer by Genetic Ancestry, Sex, Smoking History, and Histology. Genome Med. 2022, 14, 39. [Google Scholar] [CrossRef]
- Chevallier, M.; Borgeaud, M.; Addeo, A.; Friedlaender, A. Oncogenic Driver Mutations in Non-Small Cell Lung Cancer: Past, Present and Future. World J. Clin. Oncol. 2021, 12, 217–237. [Google Scholar] [CrossRef]
- Dong, J.; Li, B.; Lin, D.; Zhou, Q.; Huang, D. Advances in Targeted Therapy and Immunotherapy for Non-small Cell Lung Cancer Based on Accurate Molecular Typing. Front. Pharmacol. 2019, 10, 230. [Google Scholar] [CrossRef]
- Janssens, R.; Arnou, R.; Schoefs, E.; Petrocchi, S.; Cincidda, C.; Ongaro, G.; Oliveri, S.; Smith, M.Y.; Louis, E.; Vandevelde, M.; et al. Key Determinants of Health-Related Quality of Life Among Advanced Lung Cancer Patients: A Qualitative Study in Belgium and Italy. Front. Pharmacol. 2021, 12, 710518. [Google Scholar] [CrossRef] [PubMed]
- Steeghs, E.M.P.; Groen, H.J.M.; Schuuring, E.; Aarts, M.J.; Damhuis, R.A.M.; Voorham, Q.J.M.; PATH consortium; Ligtenberg, M.J.L.; Grünberg, K. Mutation-Tailored Treatment Selection in Non-Small Cell Lung Cancer Patients in Daily Clinical Practice. Lung Cancer 2022, 167, 87–97. [Google Scholar] [CrossRef] [PubMed]
- Borrelli, G.M.; Trono, D. Recombinant Lipases and Phospholipases and Their Use as Biocatalysts for Industrial Applications. Int. J. Mol. Sci. 2015, 16, 20774–20840. [Google Scholar] [CrossRef]
- Aloulou, A.; Rahier, R.; Arhab, Y.; Noiriel, A.; Abousalham, A. Phospholipases: An Overview. Methods Mol. Biol. 2018, 1835, 69–105. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Dai, L.; Liu, D.; Du, W. Progress & Prospect of Enzyme-Mediated Structured Phospholipids Preparation. Catalysts 2022, 12, 795. [Google Scholar] [CrossRef]
- Bruntz, R.C.; Lindsley, C.W.; Brown, H.A. Phospholipase D Signaling Pathways and Phosphatidic Acid as Therapeutic Targets in Cancer. Pharmacol. Rev. 2014, 66, 1033–1079. [Google Scholar] [CrossRef] [PubMed]
- De Freitas Saito, R.; de Andrade, L.N.S.; Bustos, S.O.; Chammas, R. Phosphatidylcholine-Derived Lipid Mediators: The Crosstalk Between Cancer Cells and Immune Cells. Front. Immunol. 2022, 13, 768606. [Google Scholar] [CrossRef]
- Vecchi, L.; Araújo, T.G.; Azevedo, F.V.P.d.V.; Mota, S.T.S.; Ávila, V.d.M.R.; Ribeiro, M.A.; Goulart, L.R. Phospholipase A2 Drives Tumorigenesis and Cancer Aggressiveness through Its Interaction with Annexin A1. Cells 2021, 10, 1472. [Google Scholar] [CrossRef]
- Brown, H.A.; Thomas, P.G.; Lindsley, C.W. Targeting Phospholipase D in Cancer, Infection and Neurodegenerative Disorders. Nat. Rev. Drug Discov. 2017, 16, 351–367. [Google Scholar] [CrossRef]
- Lyu, M.S.; Park, D.J.; Rhee, S.G.; Kozak, C.A. Genetic Mapping of the Human and Mouse Phospholipase C Genes. Mamm. Genome 1996, 7, 501–504. [Google Scholar] [CrossRef] [PubMed]
- Rhee, S.G.; Choi, K.D. Multiple Forms of Phospholipase C Isozymes and Their Activation Mechanisms. Adv. Second Messenger Phosphoprot. Res. 1992, 26, 35–61. [Google Scholar]
- Rhee, S.G.; Bae, Y.S. Regulation of Phosphoinositide-Specific Phospholipase C Isozymes. J. Biol. Chem. 1997, 272, 15045–15048. [Google Scholar] [CrossRef]
- Hannan, A.J.; Kind, P.C.; Blakemore, C. Phospholipase C-Β1 Expression Correlates with Neuronal Differentiation and Synaptic Plasticity in Rat Somatosensory Cortex. Neuropharmacology 1998, 37, 593–605. [Google Scholar] [CrossRef]
- Lee, S.B.; Rao, A.K.; Lee, K.H.; Yang, X.; Bae, Y.S.; Rhee, S.G. Decreased Expression of Phospholipase C-Beta 2 Isozyme in Human Platelets with Impaired Function. Blood 1996, 88, 1684–1691. [Google Scholar] [CrossRef]
- Jiang, H.; Lyubarsky, A.; Dodd, R.; Vardi, N.; Pugh, E.; Baylor, D.; Simon, M.I.; Wu, D. Phospholipase C Β4 Is Involved in Modulating the Visual Response in Mice. Proc. Natl. Acad. Sci. USA 1996, 93, 14598–14601. [Google Scholar] [CrossRef]
- Strassheim, D.; Shafer, S.H.; Phelps, S.H.; Williams, C.L. Small Cell Lung Carcinoma Exhibits Greater Phospholipase C-Beta1 Expression and Edelfosine Resistance Compared with Non-Small Cell Lung Carcinoma. Cancer Res. 2000, 60, 2730–2736. [Google Scholar] [PubMed]
- Zhang, T.; Song, X.; Liao, X.; Wang, X.; Zhu, G.; Yang, C.; Xie, X. Distinct Prognostic Values of Phospholipase C Beta Family Members for Non-Small Cell Lung Carcinoma. BioMed Res. Int. 2019, 2019, 4256524. [Google Scholar] [CrossRef]
- Mandal, S.; Bandyopadhyay, S.; Tyagi, K.; Roy, A. Recent Advances in Understanding the Molecular Role of Phosphoinositide-Specific Phospholipase C Gamma 1 as an Emerging Onco-Driver and Novel Therapeutic Target in Human Carcinogenesis. Biochim. Biophys. Acta Rev. Cancer 2021, 1876, 188619. [Google Scholar] [CrossRef] [PubMed]
- Rebecchi, M.J.; Pentyala, S.N. Structure, Function, and Control of Phosphoinositide-Specific Phospholipase C. Physiol. Rev. 2000, 80, 1291–1335. [Google Scholar] [CrossRef] [PubMed]
- Fiume, R.; Faenza, I.; Sheth, B.; Poli, A.; Vidalle, M.C.; Mazzetti, C.; Abdul, S.H.; Campagnoli, F.; Fabbrini, M.; Kimber, S.T.; et al. Nuclear Phosphoinositides: Their Regulation and Roles in Nuclear Functions. Int. J. Mol. Sci. 2019, 20, 2991. [Google Scholar] [CrossRef] [PubMed]
- Rhee, S.G. Regulation of Phosphoinositide-Specific Phospholipase C. Annu. Rev. Biochem. 2001, 70, 281–312. [Google Scholar] [CrossRef]
- Katan, M.; Cockcroft, S. Phospholipase C Families: Common Themes and Versatility in Physiology and Pathology. Prog. Lipid Res. 2020, 80, 101065. [Google Scholar] [CrossRef]
- Faenza, I.; Bavelloni, A.; Fiume, R.; Santi, P.; Martelli, A.M.; Maria Billi, A.; Rita Lo Vasco, V.; Manzoli, L.; Cocco, L. Expression of Phospholipase C Beta Family Isoenzymes in C2C12 Myoblasts during Terminal Differentiation. J. Cell. Physiol. 2004, 200, 291–296. [Google Scholar] [CrossRef]
- Jackson, L.; Qifti, A.; Pearce, K.M.; Scarlata, S. Regulation of Bifunctional Proteins in Cells: Lessons from the Phospholipase Cβ/G Protein Pathway. Protein Sci. 2020, 29, 1258–1268. [Google Scholar] [CrossRef]
- Muralidharan, K.; Van Camp, M.M.; Lyon, A.M. Structure and Regulation of Phospholipase Cβ and ε at the Membrane. Chem. Phys. Lipids 2021, 235, 105050. [Google Scholar] [CrossRef]
- Böhm, D.; Schwegler, H.; Kotthaus, L.; Nayernia, K.; Rickmann, M.; Köhler, M.; Rosenbusch, J.; Engel, W.; Flügge, G.; Burfeind, P. Disruption of PLC-Β1-Mediated Signal Transduction in Mutant Mice Causes Age-Dependent Hippocampal Mossy Fiber Sprouting and Neurodegeneration. Mol. Cell. Neurosci. 2002, 21, 584–601. [Google Scholar] [CrossRef]
- Arthur, J.F.; Matkovich, S.J.; Mitchell, C.J.; Biden, T.J.; Woodcock, E.A. Evidence for Selective Coupling of Alpha 1-Adrenergic Receptors to Phospholipase C-Beta 1 in Rat Neonatal Cardiomyocytes. J. Biol. Chem. 2001, 276, 37341–37346. [Google Scholar] [CrossRef]
- Ramazzotti, G.; Bavelloni, A.; Blalock, W.; Piazzi, M.; Cocco, L.; Faenza, I. BMP-2 Induced Expression of PLCβ1 That Is a Positive Regulator of Osteoblast Differentiation. J. Cell Physiol. 2016, 231, 623–629. [Google Scholar] [CrossRef] [PubMed]
- Lyon, A.M.; Tesmer, J.J.G. Structural Insights into Phospholipase C-β Function. Mol. Pharmacol. 2013, 84, 488–500. [Google Scholar] [CrossRef] [PubMed]
- Bertagnolo, V.; Grassilli, S.; Volinia, S.; Al-Qassab, Y.; Brugnoli, F.; Vezzali, F.; Lambertini, E.; Palomba, M.; Piubello, Q.; Orvieto, E.; et al. Ectopic Expression of PLC-Β2 in Non-Invasive Breast Tumor Cells Plays a Protective Role against Malignant Progression and Is Correlated with the Deregulation of MiR-146a. Mol. Carcinog. 2019, 58, 708–721. [Google Scholar] [CrossRef] [PubMed]
- Adamski, F.M.; Timms, K.M.; Shieh, B.H. A Unique Isoform of Phospholipase Cbeta4 Highly Expressed in the Cerebellum and Eye. Biochim. Biophys. Acta 1999, 1444, 55–60. [Google Scholar] [CrossRef]
- Martelli, A.M.; Gilmour, R.S.; Bertagnolo, V.; Neri, L.M.; Manzoli, L.; Cocco, L. Nuclear localization and signalling activity of PLCβ in Swiss 3T3 cells. Nature 1992, 358, 242–245. [Google Scholar] [CrossRef] [PubMed]
- Faenza, I.; Billi, A.M.; Follo, M.Y.; Fiume, R.; Martelli, A.M.; Cocco, L.; Manzoli, L. Nuclear Phospholipase C Signaling through Type 1 IGF Receptor and Its Involvement in Cell Growth and Differentiation. Anticancer. Res. 2005, 25, 2039–2041. [Google Scholar] [PubMed]
- Faenza, I.; Fiume, R.; Piazzi, M.; Colantoni, A.; Cocco, L. Nuclear Inositide Specific Phospholipase C Signalling—Interactions and Activity. FEBS J. 2013, 280, 6311–6321. [Google Scholar] [CrossRef]
- Faenza, I.; Matteucci, A.; Manzoli, L.; Billi, A.M.; Aluigi, M.; Peruzzi, D.; Vitale, M.; Castorina, S.; Suh, P.-G.; Cocco, L. A Role for Nuclear Phospholipase Cbeta 1 in Cell Cycle Control. J. Biol. Chem. 2000, 275, 30520–30524. [Google Scholar] [CrossRef] [PubMed]
- Faenza, I.; Ramazzotti, G.; Bavelloni, A.; Fiume, R.; Gaboardi, G.C.; Follo, M.Y.; Gilmour, R.S.; Martelli, A.M.; Ravid, K.; Cocco, L. Inositide-Dependent Phospholipase C Signaling Mimics Insulin in Skeletal Muscle Differentiation by Affecting Specific Regions of the Cyclin D3 Promoter. Endocrinology 2007, 148, 1108–1117. [Google Scholar] [CrossRef] [PubMed]
- Faenza, I.; Matteucci, A.; Bavelloni, A.; Marmiroli, S.; Martelli, A.M.; Gilmour, R.S.; Suh, P.-G.; Manzoli, L.; Cocco, L. Nuclear PLCβ1 Acts as a Negative Regulator of P45/NF-E2 Expression Levels in Friend Erythroleukemia Cells. Biochim. Biophys. Acta 2002, 1589, 305–310. [Google Scholar] [CrossRef]
- Park, J.B.; Lee, C.S.; Jang, J.-H.; Ghim, J.; Kim, Y.-J.; You, S.; Hwang, D.; Suh, P.-G.; Ryu, S.H. Phospholipase Signalling Networks in Cancer. Nat. Rev. Cancer 2012, 12, 782–792. [Google Scholar] [CrossRef]
- Lee, Y.J.; Shin, K.J.; Jang, H.-J.; Noh, D.-Y.; Ryu, S.H.; Suh, P.-G. Phospholipase Signaling in Breast Cancer. Adv. Exp. Med. Biol. 2021, 1187, 23–52. [Google Scholar] [CrossRef]
- Chao, C.-C.; Lee, W.-F.; Wang, S.-W.; Chen, P.-C.; Yamamoto, A.; Chang, T.-M.; Weng, S.-L.; Liu, J.-F. CXC Chemokine Ligand-13 Promotes Metastasis via CXCR5-Dependent Signaling Pathway in Non-Small Cell Lung Cancer. J. Cell. Mol. Med. 2021, 25, 9128–9140. [Google Scholar] [CrossRef]
- Yue, Q.-Y.; Zhao, W.; Tan, Y.; Deng, X.-L.; Zhang, Y.-H. PLCE1 Inhibits Apoptosis of Non-Small Cell Lung Cancer via Promoting PTEN Methylation. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 6211–6216. [Google Scholar] [CrossRef] [PubMed]
- Martins, M.; McCarthy, A.; Baxendale, R.; Guichard, S.; Magno, L.; Kessaris, N.; El-Bahrawy, M.; Yu, P.; Katan, M. Tumor Suppressor Role of Phospholipase Cε in Ras-Triggered Cancers. Proc. Natl. Acad. Sci. USA 2014, 111, 4239–4244. [Google Scholar] [CrossRef] [PubMed]
- Luo, X. Phospholipase C ε-1 Inhibits P53 Expression in Lung Cancer. Cell Biochem. Funct. 2014, 32, 294–298. [Google Scholar] [CrossRef] [PubMed]
- Tyutyunnykova, A.; Telegeev, G.; Dubrovska, A. The Controversial Role of Phospholipase C Epsilon (PLCε) in Cancer Development and Progression. J. Cancer 2017, 8, 716–729. [Google Scholar] [CrossRef]
- Yang, Y.-C.; Chiou, P.-C.; Chen, P.-C.; Liu, P.-Y.; Huang, W.-C.; Chao, C.-C.; Tang, C.-H. Melatonin Reduces Lung Cancer Stemness through Inhibiting of PLC, ERK, P38, β-Catenin, and Twist Pathways. Environ. Toxicol. 2019, 34, 203–209. [Google Scholar] [CrossRef]
- Yagisawa, H.; Okada, M.; Naito, Y.; Sasaki, K.; Yamaga, M.; Fujii, M. Coordinated Intracellular Translocation of Phosphoinositide-Specific Phospholipase C-δ with the Cell Cycle. Biochim. Biophys. Acta 2006, 1761, 522–534. [Google Scholar] [CrossRef]
- Mu, H.; Wang, N.; Zhao, L.; Li, S.; Li, Q.; Chen, L.; Luo, X.; Qiu, Z.; Li, L.; Ren, G.; et al. Methylation of PLCD1 and Adenovirus-Mediated PLCD1 Overexpression Elicits a Gene Therapy Effect on Human Breast Cancer. Exp. Cell Res. 2015, 332, 179–189. [Google Scholar] [CrossRef]
- He, X.; Meng, F.; Yu, Z.-J.; Zhu, X.-J.; Qin, L.-Y.; Wu, X.-R.; Liu, Z.; Li, Y.; Zheng, Y.-F. PLCD1 Suppressed Cellular Proliferation, Invasion, and Migration via Inhibition of Wnt/β-Catenin Signaling Pathway in Esophageal Squamous Cell Carcinoma. Dig. Dis. Sci. 2021, 66, 442–451. [Google Scholar] [CrossRef]
- Ji, J.; Fu, J. MiR-17-3p Facilitates Aggressive Cell Phenotypes in Colon Cancer by Targeting PLCD1 Through Affecting KIF14. Appl. Biochem. Biotechnol. 2023, 195, 1723–1735. [Google Scholar] [CrossRef]
- Jian, Y.; Qiao, Q.; Tang, J.; Qin, X. Origin Recognition Complex 1 Regulates Phospholipase Cδ1 to Inhibit Cell Proliferation, Migration and Epithelial-Mesenchymal Transition in Lung Adenocarcinoma. Oncol. Lett. 2022, 24, 252. [Google Scholar] [CrossRef]
- Sala, G.; Dituri, F.; Raimondi, C.; Previdi, S.; Maffucci, T.; Mazzoletti, M.; Rossi, C.; Iezzi, M.; Lattanzio, R.; Piantelli, M.; et al. Phospholipase Cgamma1 Is Required for Metastasis Development and Progression. Cancer Res. 2008, 68, 10187–10196. [Google Scholar] [CrossRef]
- Lu, X.; Fu, H.; Chen, R.; Wang, Y.; Zhan, Y.; Song, G.; Hu, T.; Xia, C.; Tian, X.; Zhang, B. Phosphoinositide Specific Phospholipase Cγ1 Inhibition-Driven Autophagy Caused Cell Death in Human Lung Adenocarcinoma A549 Cells in Vivo and in Vitro. Int. J. Biol. Sci. 2020, 16, 1427–1440. [Google Scholar] [CrossRef]
- Timsah, Z.; Berrout, J.; Suraokar, M.; Behrens, C.; Song, J.; Lee, J.J.; Ivan, C.; Gagea, M.; Shires, M.; Hu, X.; et al. Expression Pattern of FGFR2, Grb2 and Plcγ1 Acts as a Novel Prognostic Marker of Recurrence Recurrence-Free Survival in Lung Adenocarcinoma. Am. J. Cancer Res. 2015, 5, 3135–3148. [Google Scholar]
- Sharma, P.; Allison, J.P. The Future of Immune Checkpoint Therapy. Science 2015, 348, 56–61. [Google Scholar] [CrossRef]
- Ghosh, S.; Nataraj, N.B.; Noronha, A.; Patkar, S.; Sekar, A.; Mukherjee, S.; Winograd-Katz, S.; Kramarski, L.; Verma, A.; Lindzen, M.; et al. PD-L1 Recruits Phospholipase C and Enhances Tumorigenicity of Lung Tumors Harboring Mutant Forms of EGFR. Cell Rep. 2021, 35, 109181. [Google Scholar] [CrossRef]
- Kim, K.-B.; Kim, Y.; Rivard, C.J.; Kim, D.-W.; Park, K.-S. FGFR1 Is Critical for RBL2 Loss-Driven Tumor Development and Requires PLCG1 Activation for Continued Growth of Small Cell Lung Cancer. Cancer Res. 2020, 80, 5051–5062. [Google Scholar] [CrossRef]
- Pasquale, E.B. Eph Receptors and Ephrins in Cancer: Bidirectional Signalling and Beyond. Nat. Rev. Cancer 2010, 10, 165–180. [Google Scholar] [CrossRef] [PubMed]
- Song, W.; Kim, L.C.; Han, W.; Hou, Y.; Edwards, D.N.; Wang, S.; Blackwell, T.S.; Cheng, F.; Brantley-Sieders, D.M.; Chen, J. Phosphorylation of PLCγ1 by EphA2 Receptor Tyrosine Kinase Promotes Tumor Growth in Lung Cancer. Mol. Cancer Res. 2020, 18, 1735–1743. [Google Scholar] [CrossRef]
- Saliakoura, M.; Rossi Sebastiano, M.; Pozzato, C.; Heidel, F.H.; Schnöder, T.M.; Savic Prince, S.; Bubendorf, L.; Pinton, P.; Schmid, R.A.; Baumgartner, J.; et al. PLCγ1 Suppression Promotes the Adaptation of KRAS-Mutant Lung Adenocarcinomas to Hypoxia. Nat. Cell Biol. 2020, 22, 1382–1395. [Google Scholar] [CrossRef] [PubMed]
- Bill, C.A.; Vines, C.M. Phospholipase C. Adv. Exp. Med. Biol. 2020, 1131, 215–242. [Google Scholar] [PubMed]
- Rosse, C.; Linch, M.; Kermorgant, S.; Cameron, A.J.M.; Boeckeler, K.; Parkeret, P.J. PKC and the control of localized signal dynamics. Nat. Rev. Mol. Cell Biol. 2010, 11, 103–112. [Google Scholar] [CrossRef]
- Gomez-Cambronero, J. Phosphatidic Acid, Phospholipase D and Tumorigenesis. Adv. Biol. Regul. 2014, 54, 197–206. [Google Scholar] [CrossRef]
- Saito, M.; Iwadate, M.; Higashimoto, M.; Ono, K.; Takebayashi, Y.; Takenoshita, S. Expression of Phospholipase D2 in Human Colorectal Carcinoma. Oncol. Rep. 2007, 18, 1329–1334. [Google Scholar] [CrossRef]
- Hui, L.; Zheng, Y.; Yan, Y.; Bargonetti, J.; Foster, D.A. Mutant P53 in MDA-MB-231 Breast Cancer Cells Is Stabilized by Elevated Phospholipase D Activity and Contributes to Survival Signals Generated by Phospholipase D. Oncogene 2006, 25, 7305–7310. [Google Scholar] [CrossRef]
- Lingrand, M.; Lalonde, S.; Jutras-Carignan, A.; Bergeron, K.-F.; Rassart, E.; Mounier, C. SCD1 Activity Promotes Cell Migration via a PLD-MTOR Pathway in the MDA-MB-231 Triple-Negative Breast Cancer Cell Line. Breast Cancer 2020, 27, 594–606. [Google Scholar] [CrossRef]
- Borel, M.; Cuvillier, O.; Magne, D.; Mebarek, S.; Brizuela, L. Increased Phospholipase D Activity Contributes to Tumorigenesis in Prostate Cancer Cell Models. Mol. Cell. Biochem. 2020, 473, 263–279. [Google Scholar] [CrossRef] [PubMed]
- Toschi, A.; Edelstein, J.; Rockwell, P.; Ohh, M.; Foster, D.A. HIF Alpha Expression in VHL-Deficient Renal Cancer Cells Is Dependent on Phospholipase D. Oncogene 2008, 27, 2746–2753. [Google Scholar] [CrossRef] [PubMed]
- Bowling, F.Z.; Frohman, M.A.; Airola, M.V. Structure and Regulation of Human Phospholipase D. Adv. Biol. Regul. 2021, 79, 100783. [Google Scholar] [CrossRef] [PubMed]
- Kang, D.W.; Choi, K.-Y.; Min, D.S. Functional Regulation of Phospholipase D Expression in Cancer and Inflammation. J. Biol. Chem. 2014, 289, 22575–22582. [Google Scholar] [CrossRef] [PubMed]
- Meacci, E.; Nuti, F.; Catarzi, S.; Vasta, V.; Donati, C.; Bourgoin, S.; Bruni, P.; Moss, J.; Vaughan, M. Activation of Phospholipase D by Bradykinin and Sphingosine 1-Phosphate in A549 Human Lung Adenocarcinoma Cells via Different GTP-Binding Proteins and Protein Kinase C Delta Signaling Pathways. Biochemistry 2003, 42, 284–292. [Google Scholar] [CrossRef]
- Cho, J.H.; Han, J.-S. Phospholipase D and Its Essential Role in Cancer. Mol. Cells 2017, 40, 805–813. [Google Scholar] [CrossRef]
- Chang, Y.-C.; Chang, P.M.-H.; Li, C.-H.; Chan, M.-H.; Lee, Y.-J.; Chen, M.-H.; Hsiao, M. Aldolase A and Phospholipase D1 Synergistically Resist Alkylating Agents and Radiation in Lung Cancer. Front. Oncol. 2022, 11, 811635. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.-H.; Kim, D.; Jin, E.-J. Down-Regulation of Phospholipase D Stimulates Death of Lung Cancer Cells Involving Up-Regulation of the Long NcRNA ANRIL. Anticancer Res. 2015, 35, 2795–2803. [Google Scholar] [PubMed]
- El Osta, M.; Liu, M.; Adada, M.; Senkal, C.E.; Idkowiak-Baldys, J.; Obeid, L.M.; Clarke, C.J.; Hannun, Y.A. Sustained PKCβII Activity Confers Oncogenic Properties in a Phospholipase D- and MTOR-Dependent Manner. FASEB J. 2014, 28, 495–505. [Google Scholar] [CrossRef] [PubMed]
- Wangpaichitr, M.; Savaraj, N.; Maher, J.; Kurtoglu, M.; Lampidis, T.J. Intrinsically Lower AKT, Mammalian Target of Rapamycin, and Hypoxia-Inducible Factor Activity Correlates with Increased Sensitivity to 2-Deoxy-D-Glucose under Hypoxia in Lung Cancer Cell Lines. Mol. Cancer Ther. 2008, 7, 1506–1513. [Google Scholar] [CrossRef] [PubMed]
- Orditura, M.; De Vita, F.; Catalano, G.; Infusino, S.; Lieto, E.; Martinelli, E.; Morgillo, F.; Castellano, P.; Pignatelli, C.; Galizia, G. Elevated Serum Levels of Interleukin-8 in Advanced Non-Small Cell Lung Cancer Patients: Relationship with Prognosis. J. Interferon Cytokine Res. 2002, 22, 1129–1135. [Google Scholar] [CrossRef] [PubMed]
- Hsu, Y.-L.; Hung, J.-Y.; Ko, Y.-C.; Hung, C.-H.; Huang, M.-S.; Kuo, P.-L. Phospholipase D Signaling Pathway Is Involved in Lung Cancer-Derived IL-8 Increased Osteoclastogenesis. Carcinogenesis 2010, 31, 587–596. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Hongu, T.; Sato, T.; Zhang, Y.; Ali, W.; Cavallo, J.-A.; van der Velden, A.; Tian, H.; Di Paolo, G.; Nieswandt, B.; et al. Key Roles for the Lipid Signaling Enzyme Phospholipase D1 in the Tumor Microenvironment during Tumor Angiogenesis and Metastasis. Sci. Signal. 2012, 5, ra79. [Google Scholar] [CrossRef]
- Pazhouhandeh, M.; Samiee, F.; Boniadi, T.; Khedmat, A.F.; Vahedi, E.; Mirdamadi, M.; Sigari, N.; Siadat, S.D.; Vaziri, F.; Fateh, A.; et al. Comparative Network Analysis of Patients with Non-Small Cell Lung Cancer and Smokers for Representing Potential Therapeutic Targets. Sci. Rep. 2017, 7, 13812. [Google Scholar] [CrossRef]
- Leblanc, R.; Peyruchaud, O. New Insights into the Autotaxin/LPA Axis in Cancer Development and Metastasis. Exp. Cell Res. 2015, 333, 183–189. [Google Scholar] [CrossRef]
- Ahn, M.-J.; Park, S.-Y.; Kim, W.K.; Cho, J.H.; Chang, B.J.; Kim, D.J.; Ahn, J.S.; Park, K.; Han, J.-S. A Single Nucleotide Polymorphism in the Phospholipase D1 Gene Is Associated with Risk of Non-Small Cell Lung Cancer. Int. J. Biomed. Sci. 2012, 8, 121–128. [Google Scholar]
- Liao, J.; Yang, z.; Carter-Cooper, B.; Chang, E.T.; Choi, E.Y.; Kallakury, B.; Liu, X.; Lapidus, R.G.; Cullen, K.J.; Dan, H. Suppression of migration, invasion, and metastasis of cisplatin-resistant head and neck squamous cell carcinoma through IKKβ inhibition. Clin. Exp. Metastasis 2020, 37, 283–292. [Google Scholar] [CrossRef]
- Shi, M.; Zheng, Y.; Garcia, a.; Xu, L.; Foster, D.A. Phospholipase D provides a survival signal in human cancer cells with activated H-Ras or K-Ras. Cancer Lett. 2007, 258, 268–275. [Google Scholar] [CrossRef]
- Heinze, M.; Roos, W. Assay of Phospholipase a Activity. In Plant Lipid Signaling Protocols; Munnik, T., Heilmann, I., Eds.; Methods in Molecular Biology; Humana Press: Totowa, NJ, USA, 2013; Volume 1009, pp. 241–249. [Google Scholar] [CrossRef]
- Scott, K.F.; Sajinovic, M.; Hein, J.; Nixdorf, S.; Galettis, P.; Liauw, W.; de Souza, P.; Dong, Q.; Graham, G.G.; Russell, P.J. Emerging Roles for Phospholipase A2 Enzymes in Cancer. Biochimie 2010, 92, 601–610. [Google Scholar] [CrossRef] [PubMed]
- De Vasconcelos Azevedo, F.V.P.; Zóia, M.A.P.; Lopes, D.S.; Gimenes, S.N.; Vecchi, L.; Alves, P.T.; Rodrigues, R.S.; Silva, A.C.A.; Yoneyama, K.A.G.; Goulart, L.R.; et al. Antitumor and Antimetastatic Effects of PLA2-BthTX-II from Bothrops Jararacussu Venom on Human Breast Cancer Cells. Int. J. Biol. Macromol. 2019, 135, 261–273. [Google Scholar] [CrossRef] [PubMed]
- Yaginuma, S.; Kawana, H.; Aoki, J. Current Knowledge on Mammalian Phospholipase A1, Brief History, Structures, Biochemical and Pathophysiological Roles. Molecules 2022, 27, 2487. [Google Scholar] [CrossRef]
- El Alaoui, M.; Soulère, L.; Noiriel, A.; Popowycz, F.; Khatib, A.; Queneau, Y.; Abousalham, A. A Continuous Spectrophotometric Assay That Distinguishes between Phospholipase A1 and A2 Activities. J. Lipid Res. 2016, 57, 1589–1597. [Google Scholar] [CrossRef]
- Law, S.-H.; Chan, M.-L.; Marathe, G.K.; Parveen, F.; Chen, C.-H.; Ke, L.-Y. An Updated Review of Lysophosphatidylcholine Metabolism in Human Diseases. Int. J. Mol. Sci. 2019, 20, 1149. [Google Scholar] [CrossRef] [PubMed]
- Filkin, S.Y.; Lipkin, A.V.; Fedorov, A.N. Phospholipase Superfamily: Structure, Functions, and Biotechnological Applications. Biochemistry 2020, 85 (Suppl. S1), S177–S195. [Google Scholar] [CrossRef] [PubMed]
- Murakami, M.; Nakatani, Y.; Atsumi, G.-I.; Inoue, K.; Kudo, I. Regulatory Functions of Phospholipase A2. Crit. Rev. Immunol. 2017, 37, 127–195. [Google Scholar] [CrossRef]
- Casale, J.; Kacimi, S.E.O.; Varacallo, M. Biochemistry, Phospholipase A2. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Murakami, M.; Sato, H.; Taketomi, Y. Updating Phospholipase A2 Biology. Biomolecules 2020, 10, 1457. [Google Scholar] [CrossRef] [PubMed]
- Slatter, D.A.; Aldrovandi, M.; O’Connor, A.; Allen, S.M.; Brasher, C.J.; Murphy, R.C.; Mecklemann, S.; Ravi, S.; Darley-Usmar, V.; O’Donnell, V.B. Mapping the Human Platelet Lipidome Reveals Cytosolic Phospholipase A2 as a Regulator of Mitochondrial Bioenergetics during Activation. Cell Metab. 2016, 23, 930–944. [Google Scholar] [CrossRef] [PubMed]
- Psarra, A.; Kokotou, M.G.; Galiatsatou, G.; Mouchlis, V.D.; Dennis, E.A.; Kokotos, G. Highly Potent 2-Oxoester Inhibitors of Cytosolic Phospholipase A2 (GIVA CPLA2). ACS Omega 2018, 3, 8843–8853. [Google Scholar] [CrossRef] [PubMed]
- Corn, K.C.; Windham, M.A.; Rafat, M. Lipids in the Tumor Microenvironment: From Cancer Progression to Treatment. Prog. Lipid Res. 2020, 80, 101055. [Google Scholar] [CrossRef] [PubMed]
- Balijepalli, P.; Sitton, C.C.; Meier, K.E. Lysophosphatidic Acid Signaling in Cancer Cells: What Makes LPA So Special? Cells 2021, 10, 2059. [Google Scholar] [CrossRef] [PubMed]
- Benesch, M.G.K.; Ko, Y.M.; McMullen, T.P.W.; Brindley, D.N. Autotaxin in the Crosshairs: Taking Aim at Cancer and Other Inflammatory Conditions. FEBS Lett. 2014, 588, 2712–2727. [Google Scholar] [CrossRef] [PubMed]
- Peng, Z.; Chang, Y.; Fan, J.; Ji, W.; Su, C. Phospholipase A2 Superfamily in Cancer. Cancer Lett. 2021, 497, 165–177. [Google Scholar] [CrossRef] [PubMed]
- Mouchlis, V.D.; Dennis, E.A. Phospholipase A2 Catalysis and Lipid Mediator Lipidomics. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2019, 1864, 766–771. [Google Scholar] [CrossRef]
- Dennis, E.A.; Cao, J.; Hsu, Y.-H.; Magrioti, V.; Kokotos, G. Phospholipase A2 Enzymes: Physical Structure, Biological Function, Disease Implication, Chemical Inhibition, and Therapeutic Intervention. Chem. Rev. 2011, 111, 6130–6185. [Google Scholar] [CrossRef]
- Qu, J.; Zhao, X.; Wang, J.; Liu, C.; Sun, Y.; Cai, H.; Liu, J. Plasma Phospholipase A2 Activity May Serve as a Novel Diagnostic Biomarker for the Diagnosis of Breast Cancer. Oncol. Lett. 2018, 15, 5236–5242. [Google Scholar] [CrossRef]
- Yu, J.A.; Li, H.; Meng, X.; Fullerton, D.A.; Nemenoff, R.A.; Mitchell, J.D.; Weyant, M.J. Group IIa Secretory Phospholipase Expression Correlates with Group IIa Secretory Phospholipase Inhibition-Mediated Cell Death in K-Ras Mutant Lung Cancer Cells. J. Thorac. Cardiovasc. Surg. 2012, 144, 1479–1485. [Google Scholar] [CrossRef]
- Cai, Q.; Zhao, Z.; Antalis, C.; Yan, L.; Del Priore, G.; Hamed, A.H.; Stehman, F.B.; Schilder, J.M.; Xu, Y. Elevated and Secreted Phospholipase A₂ Activities as New Potential Therapeutic Targets in Human Epithelial Ovarian Cancer. FASEB J. 2012, 26, 3306–3320. [Google Scholar] [CrossRef]
- Khan, S.A.; Ilies, M.A. The Phospholipase A2 Superfamily: Structure, Isozymes, Catalysis, Physiologic and Pathologic Roles. Int. J. Mol. Sci. 2023, 24, 1353. [Google Scholar] [CrossRef]
- Halpern, A.L.; Kohtz, P.D.; White, A.M.; Houk, A.K.; Rehring, J.F.; Hanson, L.; McCarter, M.D.; Joshi, M.; Meng, X.; Fullerton, D.A.; et al. Secretory Phospholipase A2 IIa Mediates Expression of Growth Factor Receptors in Esophageal Adenocarcinoma. Dig. Dis. Sci. 2021, 66, 784–795. [Google Scholar] [CrossRef]
- Jespersen, S.S.; Stovgaard, E.S.; Nielsen, D.; Christensen, T.D.; Buhl, A.S.K.; Christensen, I.J.; Balslev, E. Expression of Secretory Phospholipase A2 Group IIa in Breast Cancer and Correlation to Prognosis in a Cohort of Advanced Breast Cancer Patients. Appl. Immunohistochem. Mol. Morphol. 2021, 29, e5–e9. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Dong, Z. Overexpression of Secretory Phospholipase A2-IIa Supports Cancer Stem Cell Phenotype via HER/ERBB-Elicited Signaling in Lung and Prostate Cancer Cells. Int. J. Oncol. 2017, 50, 2113–2122. [Google Scholar] [CrossRef]
- Santarpia, L.; Lippman, S.M.; El-Naggar, A.K. Targeting the MAPK-RAS-RAF Signaling Pathway in Cancer Therapy. Expert Opin. Ther. Targets 2012, 16, 103–119. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.-Y.; Li, R.-Z.; Xu, C.; Fan, X.-X.; Li, J.-X.; Meng, W.-Y.; Wang, X.-R.; Liang, T.-L.; Guan, X.-X.; Pan, H.-D.; et al. Emodin Induces Apoptosis and Suppresses Non-Small-Cell Lung Cancer Growth via Downregulation of SPLA2-IIa. Phytomedicine 2022, 95, 153786. [Google Scholar] [CrossRef] [PubMed]
- Dong, Z.; Meller, J.; Succop, P.; Wang, J.; Wikenheiser-Brokamp, K.; Starnes, S.; Lu, S. Secretory Phospholipase A2-IIa Upregulates HER/HER2-Elicited Signaling in Lung Cancer Cells. Int. J. Oncol. 2014, 45, 978–984. [Google Scholar] [CrossRef] [PubMed]
- Menschikowski, M.; Hagelgans, A.; Schuler, U.; Froeschke, S.; Rosner, A.; Siegert, G. Plasma Levels of Phospholipase A2-IIA in Patients with Different Types of Malignancies: Prognosis and Association with Inflammatory and Coagulation Biomarkers. Pathol. Oncol. Res. 2013, 19, 839–846. [Google Scholar] [CrossRef]
- Yu, J.A.; Kalatardi, S.; Dohse, J.; Sadaria, M.R.; Meng, X.; Fullerton, D.A.; Weyant, M.J. Group IIa SPLA2 Inhibition Attenuates NF-ΚB Activity and Promotes Apoptosis of Lung Cancer Cells. Anticancer Res. 2012, 32, 3601–3607. [Google Scholar] [PubMed]
- Sakai, M.; Kakutani, S.; Horikawa, C.; Tokuda, H.; Kawashima, H.; Shibata, H.; Okubo, H.; Sasaki, S. Arachidonic Acid and Cancer Risk: A Systematic Review of Observational Studies. BMC Cancer 2012, 12, 606. [Google Scholar] [CrossRef]
- Lee, S.; Kim, D.; Kang, J.; Kim, E.; Kim, W.; Youn, H.; Youn, B. Surfactant Protein B Suppresses Lung Cancer Progression by Inhibiting Secretory Phospholipase A2 Activity and Arachidonic Acid Production. Cell Physiol. Biochem. 2017, 42, 1684–1700. [Google Scholar] [CrossRef]
- Zhang, S.; Da, L.; Yang, X.; Feng, D.; Yin, R.; Li, M.; Zhang, Z.; Jiang, F.; Xu, L. Celecoxib Potentially Inhibits Metastasis of Lung Cancer Promoted by Surgery in Mice, via Suppression of the PGE2-Modulated β-Catenin Pathway. Toxicol. Lett. 2014, 225, 201–207. [Google Scholar] [CrossRef]
- Wang, M.; Hao, F.-Y.; Wang, J.-G.; Xiao, W. Group IIa Secretory Phospholipase A2 (SPLA2IIa) and Progression in Patients with Lung Cancer. Eur. Rev. Med. Pharmacol. Sci. 2014, 18, 2648–2654. [Google Scholar] [PubMed]
- Leslie, C.C. Cytosolic Phospholipase A2: Physiological Function and Role in Disease. J. Lipid Res. 2015, 56, 1386–1402. [Google Scholar] [CrossRef] [PubMed]
- Menschikowski, M.; Hagelgans, A.; Nacke, B.; Jandeck, C.; Mareninova, O.A.; Asatryan, L.; Siegert, G. Epigenetic Control of Group V Phospholipase A2 Expression in Human Malignant Cells. Tumour. Biol. 2016, 37, 8097–8105. [Google Scholar] [CrossRef]
- Xin, C.; Chu, L.; Zhang, L.; Geng, D.; Wang, Y.; Sun, D.; Sui, P.; Zhao, X.; Gong, Z.; Sui, M.; et al. Expression of Cytosolic Phospholipase A2 (CPLA2)-Arachidonic Acid (AA)-Cyclooxygenase-2 (COX-2) Pathway Factors in Lung Cancer Patients and Its Implication in Lung Cancer Early Detection and Prognosis. Med. Sci. Monit. 2019, 25, 5543–5551. [Google Scholar] [CrossRef]
- Crusz, S.M.; Balkwill, F.R. Inflammation and Cancer: Advances and New Agents. Nat. Rev. Clin. Oncol. 2015, 12, 584–596. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, X.; Zhang, L.; Geng, D.; Wang, Y.; Sun, D.; Sui, P.; Zhao, X.; Xin, C.; Jiang, J.; et al. Inhibition of PLA2G4A Reduces the Expression of Lung Cancer-Related Cytokines. DNA Cell Biol. 2018, 37, 1076–1081. [Google Scholar] [CrossRef]
- Blaine, S.A.; Wick, M.; Dessev, C.; Nemenoff, R.A. Induction of CPLA2 in Lung Epithelial Cells and Non-Small Cell Lung Cancer Is Mediated by Sp1 and c-Jun. J. Biol. Chem. 2001, 276, 42737–42743. [Google Scholar] [CrossRef]
- Hall, Z.; Ament, Z.; Wilson, C.H.; Burkhart, D.L.; Ashmore, T.; Koulman, A.; Littlewood, T.; Evan, G.I.; Griffin, J.L. Myc Expression Drives Aberrant Lipid Metabolism in Lung Cancer. Cancer Res. 2016, 76, 4608–4618. [Google Scholar] [CrossRef] [PubMed]
- Ramanadham, S.; Ali, T.; Ashley, J.W.; Bone, R.N.; Hancock, W.D.; Lei, X. Calcium-Independent Phospholipases A2 and Their Roles in Biological Processes and Diseases. J. Lipid Res. 2015, 56, 1643–1668. [Google Scholar] [CrossRef] [PubMed]
- Hooks, S.B.; Cummings, B.S. Role of Ca2+-Independent Phospholipase A2 in Cell Growth and Signaling. Biochem. Pharmacol. 2008, 76, 1059–1067. [Google Scholar] [CrossRef] [PubMed]
- Yun, H.-M.; Park, K.-R.; Lee, H.P.; Lee, D.H.; Jo, M.; Shin, D.H.; Yoon, D.-Y.; Han, S.B.; Hong, J.T. PRDX6 Promotes Lung Tumor Progression via Its GPx and IPLA2 Activities. Free Radic. Biol. Med. 2014, 69, 367–376. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Gong, L.; Liu, X.; Zhu, T.; Zhou, W.; Kong, L.; Luo, J. Identification of Peroxiredoxin 6 as a Direct Target of Withangulatin A by Quantitative Chemical Proteomics in Non-Small Cell Lung Cancer. Redox Biol. 2021, 46, 102130. [Google Scholar] [CrossRef] [PubMed]
- Kumari, A.; Chowhan, R.K.; Kakchingtabam, P.; Shahnaj, S.; Rahaman, H.; Ansari, M.S.; Singh, L.R. Peroxiredoxin-6: A Guardian of Lung Pathophysiologies. Curr. Protein Pept. Sci. 2021, 22, 666–674. [Google Scholar] [CrossRef]
- Vázquez-Medina, J.P.; Tao, J.-Q.; Patel, P.; Bannitz-Fernandes, R.; Dodia, C.; Sorokina, E.M.; Feinstein, S.I.; Chatterjee, S.; Fisher, A.B. Genetic Inactivation of the Phospholipase A2 Activity of Peroxiredoxin 6 in Mice Protects against LPS-Induced Acute Lung Injury. Am. J. Physiol. Lung Cell Mol. Physiol. 2019, 316, L656–L668. [Google Scholar] [CrossRef]
- Yu, J.A.; Mauchley, D.; Li, H.; Meng, X.; Nemenoff, R.A.; Fullerton, D.A.; Weyant, M.J. Knockdown of secretory phospholipase A2 IIa reduces lung cancer growth in vitro and in vivo. J. Thorac. Cardiovasc. Surg. 2012, 144, 1185–1191. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salucci, S.; Aramini, B.; Bartoletti-Stella, A.; Versari, I.; Martinelli, G.; Blalock, W.; Stella, F.; Faenza, I. Phospholipase Family Enzymes in Lung Cancer: Looking for Novel Therapeutic Approaches. Cancers 2023, 15, 3245. https://doi.org/10.3390/cancers15123245
Salucci S, Aramini B, Bartoletti-Stella A, Versari I, Martinelli G, Blalock W, Stella F, Faenza I. Phospholipase Family Enzymes in Lung Cancer: Looking for Novel Therapeutic Approaches. Cancers. 2023; 15(12):3245. https://doi.org/10.3390/cancers15123245
Chicago/Turabian StyleSalucci, Sara, Beatrice Aramini, Anna Bartoletti-Stella, Ilaria Versari, Giovanni Martinelli, William Blalock, Franco Stella, and Irene Faenza. 2023. "Phospholipase Family Enzymes in Lung Cancer: Looking for Novel Therapeutic Approaches" Cancers 15, no. 12: 3245. https://doi.org/10.3390/cancers15123245
APA StyleSalucci, S., Aramini, B., Bartoletti-Stella, A., Versari, I., Martinelli, G., Blalock, W., Stella, F., & Faenza, I. (2023). Phospholipase Family Enzymes in Lung Cancer: Looking for Novel Therapeutic Approaches. Cancers, 15(12), 3245. https://doi.org/10.3390/cancers15123245










