Chasing Immune Checkpoint Inhibitors in Ovarian Cancer: Novel Combinations and Biomarker Discovery
Abstract
Simple Summary
Abstract
1. Introduction
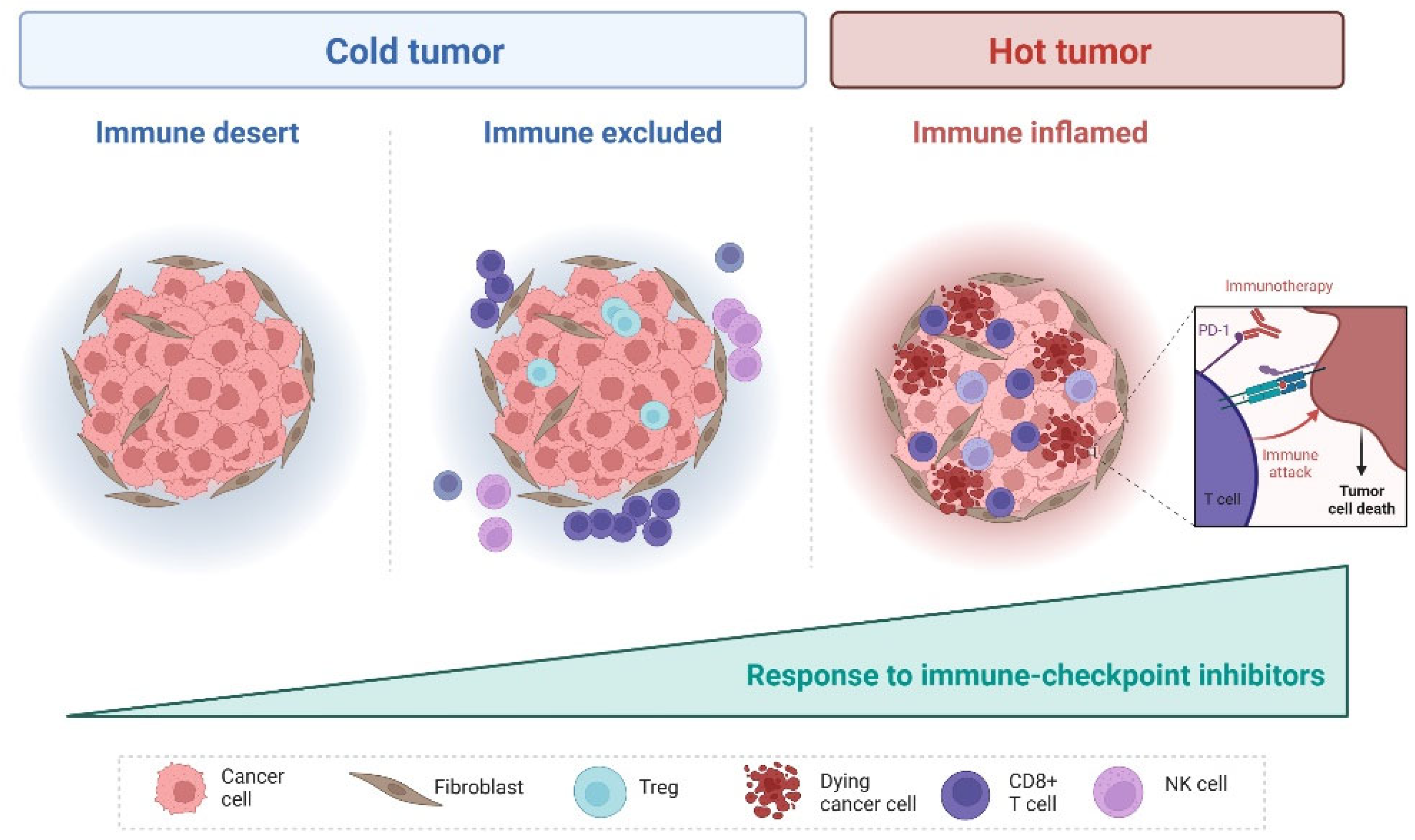
2. Tumor Microenvironment in EOC
3. Immune Checkpoints in EOC
3.1. Single-Agent Strategy
3.2. Combination Strategies
3.2.1. ICIs with Chemotherapy
3.2.2. ICIs with Antiangiogenic Agents
3.2.3. ICIs with PARP Inhibitors
3.2.4. Multimodality Combination
3.2.5. Combinations of ICIs
4. Clear Cell Ovarian Cancer
5. Predictive Biomarkers of ICI Activity
6. Discussion
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cancer Stat Facts: Ovarian Cancer. Available online: https://seer.cancer.gov/statfacts/html/ovary.html (accessed on 10 June 2023).
- Colombo, N.; Sessa, C.; du Bois, A.; Ledermann, J.; McCluggage, W.G.; McNeish, I.; Morice, P.; Pignata, S.; Ray-Coquard, I.; Vergote, I.; et al. ESMO–ESGO consensus conference recommendations on ovarian cancer: Pathology and molecular biology, early and advanced stages, borderline tumours and recurrent disease†. Ann. Oncol. 2019, 30, 672–705. [Google Scholar] [CrossRef]
- Tew, W.P.; Lacchetti, C.; Ellis, A.; Maxian, K.; Banerjee, S.; Bookman, M.; Jones, M.B.; Lee, J.-M.; Lheureux, S.; Liu, J.F.; et al. PARP Inhibitors in the Management of Ovarian Cancer: ASCO Guideline. J. Clin. Oncol. 2020, 38, 3468–3493. [Google Scholar] [CrossRef] [PubMed]
- Tew, W.P.; Lacchetti, C.; Kohn, E.C.; PARP Inhibitors in the Management of Ovarian Cancer Guideline Expert Panel. Poly(ADP-Ribose) Polymerase Inhibitors in the Management of Ovarian Cancer: ASCO Guideline Rapid Recommendation Update. J. Clin. Oncol. 2022, 40, 3878–3881. [Google Scholar] [CrossRef]
- Caruso, G.; Tomao, F.; Parma, G.; Lapresa, M.; Multinu, F.; Palaia, I.; Aletti, G.; Colombo, N. Poly (ADP-ribose) polymerase inhibitors (PARPi) in ovarian cancer: Lessons learned and future directions. Int. J. Gynecol. Cancer Off. J. Int. Gynecol. Cancer Soc. 2023, 33, 431–443. [Google Scholar] [CrossRef]
- Baert, T.; Ferrero, A.; Sehouli, J.; O’Donnell, D.M.; González-Martín, A.; Joly, F.; van der Velden, J.; Blecharz, P.; Tan, D.S.P.; Querleu, D.; et al. The systemic treatment of recurrent ovarian cancer revisited. Ann. Oncol. 2021, 32, 710–725. [Google Scholar] [CrossRef]
- Matulonis, U.A.; Lorusso, D.; Oaknin, A.; Pignata, S.; Dean, A.; Denys, H.; Colombo, N.; Van Gorp, T.; Konner, J.A.; Marin, M.R.; et al. Efficacy and Safety of Mirvetuximab Soravtansine in Patients with Platinum-Resistant Ovarian Cancer with High Folate Receptor Alpha Expression: Results From the SORAYA Study. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2023, 41, 2436–2445. [Google Scholar] [CrossRef]
- Eskander, R.N.; Sill, M.W.; Beffa, L.; Moore, R.G.; Hope, J.M.; Musa, F.B.; Mannel, R.; Shahin, M.S.; Cantuaria, G.H.; Girda, E.; et al. Pembrolizumab plus Chemotherapy in Advanced Endometrial Cancer. N. Engl. J. Med. 2023, 388, 2159–2170. [Google Scholar] [CrossRef]
- Mirza, M.R.; Chase, D.M.; Slomovitz, B.M.; dePont Christensen, R.; Novák, Z.; Black, D.; Gilbert, L.; Sharma, S.; Valabrega, G.; Landrum, L.M.; et al. Dostarlimab for Primary Advanced or Recurrent Endometrial Cancer. N. Engl. J. Med. 2023, 388, 2145–2158. [Google Scholar] [CrossRef]
- Colombo, N.; Dubot, C.; Lorusso, D.; Caceres, M.V.; Hasegawa, K.; Shapira-Frommer, R.; Tewari, K.S.; Salman, P.; Hoyos Usta, E.; Yañez, E.; et al. Pembrolizumab for Persistent, Recurrent, or Metastatic Cervical Cancer. N. Engl. J. Med. 2021, 385, 1856–1867. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, L.; Rodríguez-Abreu, D.; Gadgeel, S.; Esteban, E.; Felip, E.; De Angelis, F.; Domine, M.; Clingan, P.; Hochmair, M.J.; Powell, S.F.; et al. Pembrolizumab plus Chemotherapy in Metastatic Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2018, 378, 2078–2092. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.S.; Mellman, I. Elements of cancer immunity and the cancer-immune set point. Nature 2017, 541, 321–330. [Google Scholar] [CrossRef]
- Chen, D.S.; Mellman, I. Oncology meets immunology: The cancer-immunity cycle. Immunity 2013, 39, 1–10. [Google Scholar] [CrossRef]
- Pitt, J.M.; Vétizou, M.; Daillère, R.; Roberti, M.P.; Yamazaki, T.; Routy, B.; Lepage, P.; Boneca, I.G.; Chamaillard, M.; Kroemer, G.; et al. Resistance Mechanisms to Immune-Checkpoint Blockade in Cancer: Tumor-Intrinsic and -Extrinsic Factors. Immunity 2016, 44, 1255–1269. [Google Scholar] [CrossRef]
- Zhang, L.; Conejo-Garcia, J.R.; Katsaros, D.; Gimotty, P.A.; Massobrio, M.; Regnani, G.; Makrigiannakis, A.; Gray, H.; Schlienger, K.; Liebman, M.N.; et al. Intratumoral T Cells, Recurrence, and Survival in Epithelial Ovarian Cancer. N. Engl. J. Med. 2003, 348, 203–213. [Google Scholar] [CrossRef] [PubMed]
- Hwang, W.-T.; Adams, S.F.; Tahirovic, E.; Hagemann, I.S.; Coukos, G. Prognostic significance of tumor-infiltrating T cells in ovarian cancer: A meta-analysis. Gynecol. Oncol. 2012, 124, 192–198. [Google Scholar] [CrossRef] [PubMed]
- Sato, E.; Olson, S.H.; Ahn, J.; Bundy, B.; Nishikawa, H.; Qian, F.; Jungbluth, A.A.; Frosina, D.; Gnjatic, S.; Ambrosone, C.; et al. Intraepithelial CD8+ tumor-infiltrating lymphocytes and a high CD8+/regulatory T cell ratio are associated with favorable prognosis in ovarian cancer. Proc. Natl. Acad. Sci. USA 2005, 102, 18538–18543. [Google Scholar] [CrossRef]
- Consortium, O.T.T.A. Dose-Response Association of CD8+ Tumor-Infiltrating Lymphocytes and Survival Time in High-Grade Serous Ovarian Cancer. JAMA Oncol. 2017, 3, e173290. [Google Scholar] [CrossRef]
- Zhang, A.W.; McPherson, A.; Milne, K.; Kroeger, D.R.; Hamilton, P.T.; Miranda, A.; Funnell, T.; Little, N.; de Souza, C.P.E.; Laan, S.; et al. Interfaces of Malignant and Immunologic Clonal Dynamics in Ovarian Cancer. Cell 2018, 173, 1755–1769.e1722. [Google Scholar] [CrossRef]
- Togashi, Y.; Shitara, K.; Nishikawa, H. Regulatory T cells in cancer immunosuppression—Implications for anticancer therapy. Nat. Rev. Clin. Oncol. 2019, 16, 356–371. [Google Scholar] [CrossRef]
- Curiel, T.J.; Coukos, G.; Zou, L.; Alvarez, X.; Cheng, P.; Mottram, P.; Evdemon-Hogan, M.; Conejo-Garcia, J.R.; Zhang, L.; Burow, M.; et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat. Med. 2004, 10, 942–949. [Google Scholar] [CrossRef]
- Toker, A.; Nguyen, L.T.; Stone, S.C.; Yang, S.Y.C.; Katz, S.R.; Shaw, P.A.; Clarke, B.A.; Ghazarian, D.; Al-Habeeb, A.; Easson, A.; et al. Regulatory T Cells in Ovarian Cancer Are Characterized by a Highly Activated Phenotype Distinct from that in Melanoma. Clin. Cancer Res. 2018, 24, 5685–5696. [Google Scholar] [CrossRef] [PubMed]
- Leach, D.; Krummel, M.; Allison, J. Enhancement of Antitumor Immunity by CTLA-4 Blockade. Science 1996, 271, 1734–1736. [Google Scholar] [CrossRef] [PubMed]
- Ishida, Y.; Agata, Y.; Shibahara, K.; Honjo, T. Induced expression of PD-1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. EMBO J. 1992, 11, 3887–3895. [Google Scholar] [CrossRef]
- Larkin, J.; Chiarion-Sileni, V.; Gonzalez, R.; Grob, J.-J.; Rutkowski, P.; Lao, C.D.; Cowey, C.L.; Schadendorf, D.; Wagstaff, J.; Dummer, R.; et al. Five-Year Survival with Combined Nivolumab and Ipilimumab in Advanced Melanoma. N. Engl. J. Med. 2019, 381, 1535–1546. [Google Scholar] [CrossRef] [PubMed]
- Baas, P.; Scherpereel, A.; Nowak, A.K.; Fujimoto, N.; Peters, S.; Tsao, A.S.; Mansfield, A.S.; Popat, S.; Jahan, T.; Antonia, S.; et al. First-line nivolumab plus ipilimumab in unresectable malignant pleural mesothelioma (CheckMate 743): A multicentre, randomised, open-label, phase 3 trial. Lancet 2021, 397, 375–386. [Google Scholar] [CrossRef]
- Socinski, M.A.; Jotte, R.M.; Cappuzzo, F.; Orlandi, F.; Stroyakovskiy, D.; Nogami, N.; Rodríguez-Abreu, D.; Moro-Sibilot, D.; Thomas, C.A.; Barlesi, F.; et al. Atezolizumab for First-Line Treatment of Metastatic Nonsquamous NSCLC. N. Engl. J. Med. 2018, 378, 2288–2301. [Google Scholar] [CrossRef] [PubMed]
- Powles, T.; Park, S.H.; Voog, E.; Caserta, C.; Valderrama, B.P.; Gurney, H.; Kalofonos, H.; Radulović, S.; Demey, W.; Ullén, A.; et al. Avelumab Maintenance Therapy for Advanced or Metastatic Urothelial Carcinoma. N. Engl. J. Med. 2020, 383, 1218–1230. [Google Scholar] [CrossRef]
- Cogdill, A.P.; Andrews, M.C.; Wargo, J.A. Hallmarks of response to immune checkpoint blockade. Br. J. Cancer 2017, 117, 1–7. [Google Scholar] [CrossRef]
- Herbst, R.S.; Soria, J.-C.; Kowanetz, M.; Fine, G.D.; Hamid, O.; Gordon, M.S.; Sosman, J.A.; McDermott, D.F.; Powderly, J.D.; Gettinger, S.N.; et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature 2014, 515, 563–567. [Google Scholar] [CrossRef]
- Disis, M.L.; Taylor, M.H.; Kelly, K.; Beck, J.T.; Gordon, M.; Moore, K.M.; Patel, M.R.; Chaves, J.; Park, H.; Mita, A.C.; et al. Efficacy and Safety of Avelumab for Patients with Recurrent or Refractory Ovarian Cancer: Phase 1b Results From the JAVELIN Solid Tumor Trial. JAMA Oncol. 2019, 5, 393–401. [Google Scholar] [CrossRef] [PubMed]
- Hamanishi, J.; Mandai, M.; Ikeda, T.; Minami, M.; Kawaguchi, A.; Murayama, T.; Kanai, M.; Mori, Y.; Matsumoto, S.; Chikuma, S.; et al. Safety and Antitumor Activity of Anti–PD-1 Antibody, Nivolumab, in Patients with Platinum-Resistant Ovarian Cancer. J. Clin. Oncol. 2015, 33, 4015–4022. [Google Scholar] [CrossRef] [PubMed]
- Varga, A.; Piha-Paul, S.; Ott, P.A.; Mehnert, J.M.; Berton-Rigaud, D.; Morosky, A.; Yang, P.; Ruman, J.; Matei, D. Pembrolizumab in patients with programmed death ligand 1–positive advanced ovarian cancer: Analysis of KEYNOTE-028. Gynecol. Oncol. 2019, 152, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Colombo, I.; Lien, S.; Yang, C.; Clouthier, D.L.; Bonilla, L.; Cyriac, S.; Ethier, J.-L.; Lee, Y.C.; Kanjanapan, Y.; Mandilaras, V.; et al. Immunologic and genomic characterization of high grade serous ovarian cancer (HGSOC) in patients (pts) treated with pembrolizumab (Pembro) in the phase II INSPIRE trial. J. Clin. Oncol. 2017, 35, 5581. [Google Scholar] [CrossRef]
- Matulonis, U.A.; Shapira-Frommer, R.; Santin, A.D.; Lisyanskaya, A.S.; Pignata, S.; Vergote, I.; Raspagliesi, F.; Sonke, G.S.; Birrer, M.; Provencher, D.M.; et al. Antitumor activity and safety of pembrolizumab in patients with advanced recurrent ovarian cancer: Results from the phase II KEYNOTE-100 study. Ann. Oncol. 2019, 30, 1080–1087. [Google Scholar] [CrossRef]
- Matulonis, U.A.; Shapira, R.; Santin, A.; Lisyanskaya, A.S.; Pignata, S.; Vergote, I.; Raspagliesi, F.; Sonke, G.S.; Birrer, M.; Sehouli, J.; et al. Final results from the KEYNOTE-100 trial of pembrolizumab in patients with advanced recurrent ovarian cancer. J. Clin. Oncol. 2020, 38, 6005. [Google Scholar] [CrossRef]
- Omatsu, J.H.K.; Katsumata, N.; Nishio, S.; Sawada, K.; Takeuchi, S.; Aoki, D.; Fujiwara, K.; Sugiyama, T.; Konishi, I. Nivolumab versus gemcitabine or pegylated liposomal doxorubicin for patients with platinum-resistant (advanced or recurrent) ovarian cancer: Open-label, randomized trial in Japan (NINJA trial). Ann. Oncol. 2020, 31 (Suppl. S4), S551–S589. [Google Scholar] [CrossRef]
- Lee, E.K.; Xiong, N.; Cheng, S.C.; Barry, W.T.; Penson, R.T.; Konstantinopoulos, P.A.; Hoffman, M.A.; Horowitz, N.; Dizon, D.S.; Stover, E.H.; et al. Combined pembrolizumab and pegylated liposomal doxorubicin in platinum resistant ovarian cancer: A phase 2 clinical trial. Gynecol. Oncol. 2020, 159, 72–78. [Google Scholar] [CrossRef]
- O’Cearbhaill, R.E.; Wolfer, A.; Disilvestro, P.; O’Malley, D.M.; Sabbatini, P.; Shohara, L.; Schwarzenberger, P.O.; Ricciardi, T.; Macri, M.; Ryan, A.; et al. 945P—A phase I/II study of chemo-immunotherapy with durvalumab (durva) and pegylated liposomal doxorubicin (PLD) in platinum-resistant recurrent ovarian cancer (PROC). Ann. Oncol. 2018, 29, viii337. [Google Scholar] [CrossRef]
- Pujade-Lauraine, E.; Fujiwara, K.; Ledermann, J.A.; Oza, A.M.; Kristeleit, R.; Ray-Coquard, I.-L.; Richardson, G.E.; Sessa, C.; Yonemori, K.; Banerjee, S.; et al. Avelumab alone or in combination with chemotherapy versus chemotherapy alone in platinum-resistant or platinum-refractory ovarian cancer (JAVELIN Ovarian 200): An open-label, three-arm, randomised, phase 3 study. Lancet Oncol. 2021, 22, 1034–1046. [Google Scholar] [CrossRef]
- Monk, B.J.; Colombo, N.; Oza, A.M.; Fujiwara, K.; Birrer, M.J.; Randall, L.; Poddubskaya, E.V.; Scambia, G.; Shparyk, Y.V.; Lim, M.C.; et al. Chemotherapy with or without avelumab followed by avelumab maintenance versus chemotherapy alone in patients with previously untreated epithelial ovarian cancer (JAVELIN Ovarian 100): An open-label, randomised, phase 3 trial. Lancet Oncol. 2021, 22, 1275–1289. [Google Scholar] [CrossRef] [PubMed]
- Ray-Coquard, I.L.; Savoye, A.M.; Mouret-Reynier, M.-A.; Chabaud, S.; Derbel, O.; Kalbacher, E.; Leheurteur, M.; Martinez, A.; Cornila, C.; Martinez, M.; et al. Efficacy and safety results from neopembrov study, a randomized phase II trial of neoadjuvant chemotherapy (CT) with or without pembrolizumab (P) followed by interval debulking surgery and standard systemic therapy ± P for advanced high-grade serous carcinoma (HGSC): A GINECO study. J. Clin. Oncol. 2021, 39 (Suppl. S15), 5500. [Google Scholar]
- Moroney, J.W.; Powderly, J.; Lieu, C.H.; Bendell, J.C.; Eckhardt, S.G.; Chang, C.-W.; Molinero, L.; Spahn, J.; Williams, P.; Lin, Y.G.; et al. Safety and Clinical Activity of Atezolizumab Plus Bevacizumab in Patients with Ovarian Cancer: A Phase Ib Study. Clin. Cancer Res. 2020, 26, 5631–5637. [Google Scholar] [CrossRef] [PubMed]
- Moore, K.N.; Bookman, M.; Sehouli, J.; Miller, A.; Anderson, C.; Scambia, G.; Myers, T.; Taskiran, C.; Robison, K.; Mäenpää, J.; et al. Atezolizumab, Bevacizumab, and Chemotherapy for Newly Diagnosed Stage III or IV Ovarian Cancer: Placebo-Controlled Randomized Phase III Trial (IMagyn050/GOG 3015/ENGOT-OV39). J. Clin. Oncol. 2021, 39, 1842–1855. [Google Scholar] [CrossRef]
- Zsiros, E.; Lynam, S.; Attwood, K.M.; Wang, C.; Chilakapati, S.; Gomez, E.C.; Liu, S.; Akers, S.; Lele, S.; Frederick, P.J.; et al. Efficacy and Safety of Pembrolizumab in Combination with Bevacizumab and Oral Metronomic Cyclophosphamide in the Treatment of Recurrent Ovarian Cancer: A Phase 2 Nonrandomized Clinical Trial. JAMA Oncol. 2021, 7, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.F.; Herold, C.; Gray, K.P.; Penson, R.T.; Horowitz, N.; Konstantinopoulos, P.A.; Castro, C.M.; Hill, S.J.; Curtis, J.; Luo, W.; et al. Assessment of Combined Nivolumab and Bevacizumab in Relapsed Ovarian Cancer: A Phase 2 Clinical Trial. JAMA Oncol. 2019, 5, 1731–1738. [Google Scholar] [CrossRef]
- Lwin, Z.; Gomez-Roca, C.; Saada-Bouzid, E.; Yanez, E.; Muñoz, F.L.; Im, S.-A.; Castanon, E.; Senellart, H.; Graham, D.; Voss, M.; et al. LEAP-005: Phase II study of lenvatinib (len) plus pembrolizumab (pembro) in patients (pts) with previously treated advanced solid tumours. Ann. Oncol. 2020, 31 (Suppl. S4), S1142–S1215. [Google Scholar] [CrossRef]
- Kurtz, J.E.; Pujade-Lauraine, E.; Oaknin, A.; Belin, L.; Tsibulak, I.; Cibula, D.; Vergote, I.B.; Rosengarten, O.S.; Rodrigues, M.J.; de Gregorio, N.; et al. Phase III ATALANTE/ov29 trial: Atezolizumab (Atz) versus placebo with platinum-based chemotherapy (Cx) plus bevacizumab (bev) in patients (pts) with platinum-sensitive relapse (PSR) of epithelial ovarian cancer (OC). Ann. Oncol. 2022, 33 (Suppl. S7), S808–S869. [Google Scholar] [CrossRef]
- Konstantinopoulos, P.A.; Waggoner, S.; Vidal, G.A.; Mita, M.; Moroney, J.W.; Holloway, R.; Van Le, L.; Sachdev, J.C.; Chapman-Davis, E.; Colon-Otero, G.; et al. Single-Arm Phases 1 and 2 Trial of Niraparib in Combination with Pembrolizumab in Patients with Recurrent Platinum-Resistant Ovarian Carcinoma. JAMA Oncol. 2019, 5, 1141–1149. [Google Scholar] [CrossRef]
- Drew, Y.; Kaufman, B.; Banerjee, S.; Lortholary, A.; Hong, S.H.; Park, Y.H.; Zimmermann, S.; Roxburgh, P.; Ferguson, M.; Alvarez, R.H.; et al. 1190PD—Phase II study of olaparib + durvalumab (MEDIOLA): Updated results in germline BRCA-mutated platinum-sensitive relapsed (PSR) ovarian cancer (OC). Ann. Oncol. 2019, 30, v485–v486. [Google Scholar] [CrossRef]
- Drew, Y.; Penson, R.T.; O’Malley, D.M.; Kim, J.; Zimmermann, S.; Roxburgh, P.; Sohn, J.; Stemmer, S.M.; Bastia, S.; Ferguson, M.; et al. Phase II study of olaparib (O) plus durvalumab (D) and bevacizumab (B) (MEDIOLA): Initial results in patients (pts) with non-germline BRCA-mutated (non-gBRCAm) platinum sensitive relapsed (PSR) ovarian cancer (OC). Ann. Oncol. 2020, 31 (Suppl. S4), S551–S589. [Google Scholar] [CrossRef]
- Liu, J. An open-label phase II study of dostarlimab (TSR-042), bevacizumab (bev), and niraparib combination in patients (pts) with platinum-resistant ovarian cancer (PROC): Cohort A of the OPAL trial. In Proceedings of the Women’s Cancer, Society of Gynecology Oncology (SGO) 2021 Conference, Virtually, 19–21 March 2021. Abstract 10415. [Google Scholar]
- Zamarin, D.; Burger, R.A.; Sill, M.W.; Powell, D.J., Jr.; Lankes, H.A.; Feldman, M.D.; Zivanovic, O.; Gunderson, C.; Ko, E.; Mathews, C.; et al. Randomized Phase II Trial of Nivolumab Versus Nivolumab and Ipilimumab for Recurrent or Persistent Ovarian Cancer: An NRG Oncology Study. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2020, 38, 1814–1823. [Google Scholar] [CrossRef]
- Lee, J.-Y. KGOG 3046/TRU-D: A phase II study of durvalumab and tremelimumab with front-line neoadjuvant chemotherapy in patients with advanced-stage epithelial ovarian cancer. In Proceedings of the Women’s Cancer, Society of Gynecology Oncology 2021, Virtually, 19–21 March 2021. Abstract 10565. [Google Scholar]
- Hinchcliff, E. Randomized phase II trial of durvalumab (anti-PDL1) and tremelimumab (anti-CTLA4) administered in combination versus sequentially for the treatment of recurrent platinum-resistant non-clear cell ovarian cancer (NCT03026062). In Proceedings of the Women’s Cancer, Society of Gynecology Oncology 2021 Conference, Virtually, 19–21 March 2021. Abstract 10601. [Google Scholar]
- Abiko, K.; Mandai, M.; Hamanishi, J.; Yoshioka, Y.; Matsumura, N.; Baba, T.; Yamaguchi, K.; Murakami, R.; Yamamoto, A.; Kharma, B.; et al. PD-L1 on tumor cells is induced in ascites and promotes peritoneal dissemination of ovarian cancer through CTL dysfunction. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2013, 19, 1363–1374. [Google Scholar] [CrossRef] [PubMed]
- Apetoh, L.; Ladoire, S.; Coukos, G.; Ghiringhelli, F. Combining immunotherapy and anticancer agents: The right path to achieve cancer cure? Ann. Oncol. 2015, 26, 1813–1823. [Google Scholar] [CrossRef] [PubMed]
- Lesterhuis, W.J.; Punt, C.J.A.; Hato, S.V.; Eleveld-Trancikova, D.; Jansen, B.J.H.; Nierkens, S.; Schreibelt, G.; de Boer, A.; Van Herpen, C.M.L.; Kaanders, J.H.; et al. Platinum-based drugs disrupt STAT6-mediated suppression of immune responses against cancer in humans and mice. J. Clin. Investig. 2011, 121, 3100–3108. [Google Scholar] [CrossRef]
- Vincent, J.; Mignot, G.; Chalmin, F.; Ladoire, S.; Bruchard, M.; Chevriaux, A.; Martin, F.; Apetoh, L.; Rébé, C.; Ghiringhelli, F. 5-Fluorouracil selectively kills tumor-associated myeloid-derived suppressor cells resulting in enhanced T cell–dependent antitumor immunity. Cancer Res. 2010, 70, 3052–3061. [Google Scholar] [CrossRef]
- Ding, Z.-C.; Blazar, B.R.; Mellor, A.L.; Munn, D.H.; Zhou, G. Chemotherapy rescues tumor-driven aberrant CD4+ T-cell differentiation and restores an activated polyfunctional helper phenotype. Blood 2010, 115, 2397–2406. [Google Scholar] [CrossRef]
- Zhang, L.; Dermawan, K.; Jin, M.; Liu, R.; Zheng, H.; Xu, L.; Zhang, Y.; Cai, Y.; Chu, Y.; Xiong, S. Differential impairment of regulatory T cells rather than effector T cells by paclitaxel-based chemotherapy. Clin. Immunol. 2008, 129, 219–229. [Google Scholar] [CrossRef]
- Rios-Doria, J.; Durham, N.; Wetzel, L.; Rothstein, R.; Chesebrough, J.; Holoweckyj, N.; Zhao, W.; Leow, C.C.; Hollingsworth, R. Doxil synergizes with cancer immunotherapies to enhance antitumor responses in syngeneic mouse models. Neoplasia 2015, 17, 661–670. [Google Scholar] [CrossRef]
- O’Cearbhaill, R.E.; Homicsko, K.; Wolfer, A.; DiSilvestro, P.A.; O’Malley, D.M.; Sabbatini, P.; Orcurto, A.; Barras, D.; Shohara, L.; Ricciardi, T.; et al. A Phase I/II Study of Chemo-Immunotherapy with Durvalumab (durva) and Pegylated Liposomal Doxorubicin (PLD) in PlatinumResistant Recurrent Ovarian Cancer (PROC): Genomic Sequencing and Updated Efficacy Results. In Proceedings of the Society of Gynecology Oncology Meeting 2020, Toronto, ON, Canada, 28–31 March 2020. [Google Scholar]
- Casey, S.C.; Tong, L.; Li, Y.; Do, R.; Walz, S.; Fitzgerald, K.N.; Gouw, A.M.; Baylot, V.; Gütgemann, I.; Eilers, M.; et al. MYC regulates the antitumor immune response through CD47 and PD-L1. Science 2016, 352, 227–231. [Google Scholar] [CrossRef]
- Jain, R.K. Antiangiogenesis strategies revisited: From starving tumors to alleviating hypoxia. Cancer Cell 2014, 26, 605–622. [Google Scholar] [CrossRef] [PubMed]
- Motzer, R.J.; Penkov, K.; Haanen, J.; Rini, B.; Albiges, L.; Campbell, M.T.; Venugopal, B.; Kollmannsberger, C.; Negrier, S.; Uemura, M.; et al. Avelumab plus Axitinib versus Sunitinib for Advanced Renal-Cell Carcinoma. N. Engl. J. Med. 2019, 380, 1103–1115. [Google Scholar] [CrossRef]
- Finn, R.S.; Qin, S.; Ikeda, M.; Galle, P.R.; Ducreux, M.; Kim, T.-Y.; Lim, H.Y.; Kudo, M.; Breder, V.V.; Merle, P.; et al. IMbrave150: Updated overall survival (OS) data from a global, randomized, open-label phase III study of atezolizumab (atezo) + bevacizumab (bev) versus sorafenib (sor) in patients (pts) with unresectable hepatocellular carcinoma (HCC). J. Clin. Oncol. 2021, 39, 267. [Google Scholar] [CrossRef]
- Makker, V.; Taylor, M.H.; Aghajanian, C.; Oaknin, A.; Mier, J.; Cohn, A.L.; Romeo, M.; Bratos, R.; Brose, M.S.; DiSimone, C.; et al. Lenvatinib Plus Pembrolizumab in Patients with Advanced Endometrial Cancer. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2020, 38, 2981–2992. [Google Scholar] [CrossRef]
- Carmeliet, P.; Jain, R.K. Molecular mechanisms and clinical applications of angiogenesis. Nature 2011, 473, 298–307. [Google Scholar] [CrossRef]
- Facciabene, A.; Peng, X.; Hagemann, I.S.; Balint, K.; Barchetti, A.; Wang, L.P.; Gimotty, P.A.; Gilks, C.B.; Lal, P.; Zhang, L.; et al. Tumour hypoxia promotes tolerance and angiogenesis via CCL28 and T(reg) cells. Nature 2011, 475, 226–230. [Google Scholar] [CrossRef]
- Lee, W.S.; Yang, H.; Chon, H.J.; Kim, C. Combination of anti-angiogenic therapy and immune checkpoint blockade normalizes vascular-immune crosstalk to potentiate cancer immunity. Exp. Mol. Med. 2020, 52, 1475–1485. [Google Scholar] [CrossRef] [PubMed]
- Facciabene, A.; Motz, G.T.; Coukos, G. T-regulatory cells: Key players in tumor immune escape and angiogenesis. Cancer Res. 2012, 72, 2162–2171. [Google Scholar] [CrossRef] [PubMed]
- Kammertoens, T.; Friese, C.; Arina, A.; Idel, C.; Briesemeister, D.; Rothe, M.; Ivanov, A.; Szymborska, A.; Patone, G.; Kunz, S.; et al. Tumour ischaemia by interferon-γ resembles physiological blood vessel regression. Nature 2017, 545, 98–102. [Google Scholar] [CrossRef]
- Oza, A.M.; Cook, A.D.; Pfisterer, J.; Embleton, A.; Ledermann, J.A.; Pujade-Lauraine, E.; Kristensen, G.; Carey, M.S.; Beale, P.; Cervantes, A.; et al. Standard chemotherapy with or without bevacizumab for women with newly diagnosed ovarian cancer (ICON7): Overall survival results of a phase 3 randomised trial. Lancet Oncol. 2015, 16, 928–936. [Google Scholar] [CrossRef]
- Tewari, K.S.; Burger, R.A.; Enserro, D.; Norquist, B.M.; Swisher, E.M.; Brady, M.F.; Bookman, M.A.; Fleming, G.F.; Huang, H.; Homesley, H.D.; et al. Final Overall Survival of a Randomized Trial of Bevacizumab for Primary Treatment of Ovarian Cancer. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2019, 37, 2317–2328. [Google Scholar] [CrossRef] [PubMed]
- Aghajanian, C.; Blank, S.V.; Goff, B.A.; Judson, P.L.; Teneriello, M.G.; Husain, A.; Sovak, M.A.; Yi, J.; Nycum, L.R. OCEANS: A randomized, double-blind, placebo-controlled phase III trial of chemotherapy with or without bevacizumab in patients with platinum-sensitive recurrent epithelial ovarian, primary peritoneal, or fallopian tube cancer. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2012, 30, 2039–2045. [Google Scholar] [CrossRef]
- Poveda, A.M.; Selle, F.; Hilpert, F.; Reuss, A.; Savarese, A.; Vergote, I.; Witteveen, P.; Bamias, A.; Scotto, N.; Mitchell, L.; et al. Bevacizumab Combined with Weekly Paclitaxel, Pegylated Liposomal Doxorubicin, or Topotecan in Platinum-Resistant Recurrent Ovarian Cancer: Analysis by Chemotherapy Cohort of the Randomized Phase III AURELIA Trial. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2015, 33, 3836–3838. [Google Scholar] [CrossRef]
- Iglehart, J.D.; Silver, D.P. Synthetic Lethality—A New Direction in Cancer-Drug Development. N. Engl. J. Med. 2009, 361, 189–191. [Google Scholar] [CrossRef]
- Sen, T.; Rodriguez, B.L.; Chen, L.; Corte, C.M.D.; Morikawa, N.; Fujimoto, J.; Cristea, S.; Nguyen, T.; Diao, L.; Li, L.; et al. Targeting DNA Damage Response Promotes Antitumor Immunity through STING-Mediated T-cell Activation in Small Cell Lung Cancer. Cancer Discov. 2019, 9, 646–661. [Google Scholar] [CrossRef]
- Pantelidou, C.; Sonzogni, O.; De Oliveria Taveira, M.; Mehta, A.K.; Kothari, A.; Wang, D.; Visal, T.; Li, M.K.; Pinto, J.; Castrillon, J.A.; et al. PARP Inhibitor Efficacy Depends on CD8(+) T-cell Recruitment via Intratumoral STING Pathway Activation in BRCA-Deficient Models of Triple-Negative Breast Cancer. Cancer Discov. 2019, 9, 722–737. [Google Scholar] [CrossRef]
- Jiao, S.; Xia, W.; Yamaguchi, H.; Wei, Y.; Chen, M.K.; Hsu, J.M.; Hsu, J.L.; Yu, W.H.; Du, Y.; Lee, H.H.; et al. PARP Inhibitor Upregulates PD-L1 Expression and Enhances Cancer-Associated Immunosuppression. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2017, 23, 3711–3720. [Google Scholar] [CrossRef] [PubMed]
- Gelmon, K.A.; Tischkowitz, M.; Mackay, H.; Swenerton, K.; Robidoux, A.; Tonkin, K.; Hirte, H.; Huntsman, D.; Clemons, M.; Gilks, B.; et al. Olaparib in patients with recurrent high-grade serous or poorly differentiated ovarian carcinoma or triple-negative breast cancer: A phase 2, multicentre, open-label, non-randomised study. Lancet Oncol. 2011, 12, 852–861. [Google Scholar] [CrossRef]
- Moore, K.N.; Secord, A.A.; Geller, M.A.; Miller, D.S.; Cloven, N.; Fleming, G.F.; Wahner Hendrickson, A.E.; Azodi, M.; DiSilvestro, P.; Oza, A.M.; et al. Niraparib monotherapy for late-line treatment of ovarian cancer (QUADRA): A multicentre, open-label, single-arm, phase 2 trial. Lancet Oncol. 2019, 20, 636–648. [Google Scholar] [CrossRef]
- Caruso, G.; Gigli, F.; Parma, G.; Lapresa, M.; Derio, S.; Palaia, I.; Colombo, N. Myeloid neoplasms post PARP inhibitors for ovarian cancer. Int. J. Gynecol. Cancer Off. J. Int. Gynecol. Cancer Soc. 2023, 33, 598–606. [Google Scholar] [CrossRef] [PubMed]
- AstraZeneca. Available online: https://www.astrazeneca.com/media-centre/press-releases/2023/lynparza-imfinzi-met-endpoint-in-ovarian-cancer (accessed on 5 April 2023).
- Overman, M.J.; Lonardi, S.; Wong, K.Y.M.; Lenz, H.J.; Gelsomino, F.; Aglietta, M.; Morse, M.A.; Van Cutsem, E.; McDermott, R.; Hill, A.; et al. Durable Clinical Benefit with Nivolumab Plus Ipilimumab in DNA Mismatch Repair-Deficient/Microsatellite Instability-High Metastatic Colorectal Cancer. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2018, 36, 773–779. [Google Scholar] [CrossRef] [PubMed]
- Motzer, R.J.; Tannir, N.M.; McDermott, D.F.; Arén Frontera, O.; Melichar, B.; Choueiri, T.K.; Plimack, E.R.; Barthélémy, P.; Porta, C.; George, S.; et al. Nivolumab plus Ipilimumab versus Sunitinib in Advanced Renal-Cell Carcinoma. N. Engl. J. Med. 2018, 378, 1277–1290. [Google Scholar] [CrossRef] [PubMed]
- Sung, P.-L.; Chang, Y.-H.; Chao, K.-C.; Chuang, C.-M. Global distribution pattern of histological subtypes of epithelial ovarian cancer: A database analysis and systematic review. Gynecol. Oncol. 2014, 133, 147–154. [Google Scholar] [CrossRef]
- Sugiyama, T.; Kamura, T.; Kigawa, J.; Terakawa, N.; Kikuchi, Y.; Kita, T.; Suzuki, M.; Sato, I.; Taguchi, K. Clinical characteristics of clear cell carcinoma of the ovary: A distinct histologic type with poor prognosis and resistance to platinum-based chemotherapy. Cancer 2000, 88, 2584–2589. [Google Scholar] [CrossRef]
- Okamoto, A.; Glasspool, R.M.; Mabuchi, S.; Matsumura, N.; Nomura, H.; Itamochi, H.; Takano, M.; Takano, T.; Susumu, N.; Aoki, D.; et al. Gynecologic Cancer InterGroup (GCIG) Consensus Review for Clear Cell Carcinoma of the Ovary. Int. J. Gynecol. Cancer 2014, 24, S20–S25. [Google Scholar] [CrossRef] [PubMed]
- Maru, Y.; Tanaka, N.; Ohira, M.; Itami, M.; Hippo, Y.; Nagase, H. Identification of novel mutations in Japanese ovarian clear cell carcinoma patients using optimized targeted NGS for clinical diagnosis. Gynecol. Oncol. 2017, 144, 377–383. [Google Scholar] [CrossRef]
- Shibuya, Y.; Tokunaga, H.; Saito, S.; Shimokawa, K.; Katsuoka, F.; Bin, L.; Kojima, K.; Nagasaki, M.; Yamamoto, M.; Yaegashi, N. Identification of somatic genetic alterations in ovarian clear cell carcinoma with next generation sequencing. Genes Chromosom. Cancer 2018, 57, 51–60. [Google Scholar] [CrossRef]
- Sung, C.O.; Choi, C.H.; Ko, Y.H.; Ju, H.; Choi, Y.L.; Kim, N.; Kang, S.Y.; Ha, S.Y.; Choi, K.; Bae, D.S.; et al. Integrative analysis of copy number alteration and gene expression profiling in ovarian clear cell adenocarcinoma. Cancer Genet. 2013, 206, 145–153. [Google Scholar] [CrossRef]
- Marabelle, A.; Le, D.T.; Ascierto, P.A.; Giacomo, A.M.D.; Jesus-Acosta, A.D.; Delord, J.-P.; Geva, R.; Gottfried, M.; Penel, N.; Hansen, A.R.; et al. Efficacy of Pembrolizumab in Patients with Noncolorectal High Microsatellite Instability/Mismatch Repair–Deficient Cancer: Results from the Phase II KEYNOTE-158 Study. J. Clin. Oncol. 2020, 38, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Cai, K.Q.; Albarracin, C.; Rosen, D.; Zhong, R.; Zheng, W.; Luthra, R.; Broaddus, R.; Liu, J. Microsatellite instability and alteration of the expression of hMLH1 and hMSH2 in ovarian clear cell carcinoma. Hum. Pathol. 2004, 35, 552–559. [Google Scholar] [CrossRef]
- Howitt, B.E.; Strickland, K.C.; Sholl, L.M.; Rodig, S.; Ritterhouse, L.L.; Chowdhury, D.; D’Andrea, A.D.; Matulonis, U.A.; Konstantinopoulos, P.A. Clear cell ovarian cancers with microsatellite instability: A unique subset of ovarian cancers with increased tumor-infiltrating lymphocytes and PD-1/PD-L1 expression. Oncoimmunology 2017, 6, e1277308. [Google Scholar] [CrossRef] [PubMed]
- Kristeleit, R.; Clamp, A.R.; Gourley, C.; Roux, R.; Hall, M.; Devlin, M.J.; Nirsimloo, R.; Kounnis, V.; Hughes, L.; Counsell, N.; et al. 521MO Efficacy of pembrolizumab monotherapy (PM) for advanced clear cell gynaecological cancer (CCGC): Phase II PEACOCC trial. Ann. Oncol. 2022, 33, S783. [Google Scholar] [CrossRef]
- Bellone, S.; Buza, N.; Choi, J.; Zammataro, L.; Gay, L.; Elvin, J.; Rimm, D.L.; Liu, Y.; Ratner, E.S.; Schwartz, P.E.; et al. Exceptional Response to Pembrolizumab in a Metastatic, Chemotherapy/Radiation-Resistant Ovarian Cancer Patient Harboring a PD-L1-Genetic Rearrangement. Clin. Cancer Res. 2018, 24, 3282–3291. [Google Scholar] [CrossRef]
- Liu, X.; Xia, B.; Zhang, W.; Sun, L.; Feng, C.; Huang, Y.; Gao, Y.; Jiang, J.; Li, G.; Gao, Q. 522MO Preliminary results of sintilimab (Sin)+bevacizumab (Bev) in recurrent/persistent ovarian clear cell carcinoma (INOVA): A multicenter, single-arm, phase II trial. Ann. Oncol. 2022, 33, S783. [Google Scholar] [CrossRef]
- Ngoi, N.Y.; Heong, V.; Ow, S.; Chay, W.Y.; Kim, H.S.; Choi, C.H.; Goss, G.; Goh, J.C.; Tai, B.C.; Lim, D.G.; et al. A multicenter phase II randomized trial of durvalumab (MEDI-4736) versus physician’s choice chemotherapy in recurrent ovarian clear cell adenocarcinoma (MOCCA). Int. J. Gynecol. Cancer Off. J. Int. Gynecol. Cancer Soc. 2020, 30, 1239–1242. [Google Scholar] [CrossRef] [PubMed]
- Davis, A.A.; Patel, V.G. The role of PD-L1 expression as a predictive biomarker: An analysis of all US Food and Drug Administration (FDA) approvals of immune checkpoint inhibitors. J. Immunother. Cancer 2019, 7, 278. [Google Scholar] [CrossRef] [PubMed]
- Pujade-Lauraine, E.; Fujiwara, K.; Ledermann, J.A.; Oza, A.M.; Kristeleit, R.S.; Ray-Coquard, I.L.; Richardson, G.E.; Sessa, C.; Yonemori, K.; Banerjee, S.; et al. Avelumab alone or in combination with pegylated liposomal doxorubicin versus pegylated liposomal doxorubicin alone in platinum-resistant or refractory epithelial ovarian cancer: Primary and biomarker analysis of the phase III JAVELIN Ovarian 200 trial. Gynecol. Oncol. 2019, 154, 21–22. [Google Scholar] [CrossRef]
- Le, D.T.; Uram, J.N.; Wang, H.; Bartlett, B.R.; Kemberling, H.; Eyring, A.D.; Skora, A.D.; Luber, B.S.; Azad, N.S.; Laheru, D.; et al. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. N. Engl. J. Med. 2015, 372, 2509–2520. [Google Scholar] [CrossRef]
- Cancer Genome Atlas Research Network. Integrated genomic analyses of ovarian carcinoma. Nature 2011, 474, 609–615. [Google Scholar] [CrossRef]
- Cristescu, R.; Mogg, R.; Ayers, M.; Albright, A.; Murphy, E.; Yearley, J.; Sher, X.; Liu, X.Q.; Lu, H.; Nebozhyn, M.; et al. Pan-tumor genomic biomarkers for PD-1 checkpoint blockade-based immunotherapy. Science 2018, 362, eaar3593. [Google Scholar] [CrossRef]
- McGrail, D.J.; Pilié, P.G.; Rashid, N.U.; Voorwerk, L.; Slagter, M.; Kok, M.; Jonasch, E.; Khasraw, M.; Heimberger, A.B.; Lim, B.; et al. High tumor mutation burden fails to predict immune checkpoint blockade response across all cancer types. Ann. Oncol. 2021, 32, 661–672. [Google Scholar] [CrossRef]
- Marabelle, A.; Fakih, M.; Lopez, J.; Shah, M.; Shapira-Frommer, R.; Nakagawa, K.; Chung, H.C.; Kindler, H.L.; Lopez-Martin, J.A.; Miller, W.H., Jr.; et al. Association of tumour mutational burden with outcomes in patients with advanced solid tumours treated with pembrolizumab: Prospective biomarker analysis of the multicohort, open-label, phase 2 KEYNOTE-158 study. Lancet. Oncol. 2020, 21, 1353–1365. [Google Scholar] [CrossRef]
- Konstantinopoulos, P.A.; Ceccaldi, R.; Shapiro, G.I.; D’Andrea, A.D. Homologous Recombination Deficiency: Exploiting the Fundamental Vulnerability of Ovarian Cancer. Cancer Discov. 2015, 5, 1137–1154. [Google Scholar] [CrossRef] [PubMed]
- Clouthier, D.L.; Lien, S.C.; Yang, S.Y.C.; Nguyen, L.T.; Manem, V.S.K.; Gray, D.; Ryczko, M.; Razak, A.R.A.; Lewin, J.; Lheureux, S.; et al. An interim report on the investigator-initiated phase 2 study of pembrolizumab immunological response evaluation (INSPIRE). J. Immunother. Cancer 2019, 7, 72. [Google Scholar] [CrossRef]
- Darvin, P.; Toor, S.M.; Sasidharan Nair, V.; Elkord, E. Immune checkpoint inhibitors: Recent progress and potential biomarkers. Exp. Mol. Med. 2018, 50, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Ritchie, G.; Gasper, H.; Man, J.; Lord, S.; Marschner, I.; Friedlander, M.; Lee, C.K. Defining the Most Appropriate Primary End Point in Phase 2 Trials of Immune Checkpoint Inhibitors for Advanced Solid Cancers: A Systematic Review and Meta-analysis. JAMA Oncol. 2018, 4, 522–528. [Google Scholar] [CrossRef]
- Anagnostou, V.; Yarchoan, M.; Hansen, A.R.; Wang, H.; Verde, F.; Sharon, E.; Collyar, D.; Chow, L.Q.M.; Forde, P.M. Immuno-oncology Trial Endpoints: Capturing Clinically Meaningful Activity. Clin. Cancer Res. 2017, 23, 4959–4969. [Google Scholar] [CrossRef]
- Sagawa, T.; Sato, Y.; Hamaguchi, K.; Hirakawa, M.; Nagashima, H.; Waga, E.; Fujikawa, K.; Takahashi, Y. Improved efficacy to cytotoxic agents chemotherapy after immune checkpoint inhibitors exposure in metastatic gastric cancer. J. Clin. Oncol. 2020, 38, 297. [Google Scholar] [CrossRef]
- Dwary, A.D.; Master, S.; Patel, A.; Cole, C.; Mansour, R.; Mills, G.; Koshy, N.; Peddi, P.; Burton, G.; Hammoud, D.; et al. Excellent response to chemotherapy post immunotherapy. Oncotarget 2017, 8, 91795–91802. [Google Scholar] [CrossRef]
- Martin-Romano, P.; Ammari, S.; El-Dakdoukti, Y.; Baldini, C.; Varga, A.; Vuagnat, P.; Angevin, E.; Bahleda, R.; Gazzah, A.; Champiat, S.; et al. Chemotherapy beyond immune checkpoint inhibitors in patients with metastatic colorectal cancer. Eur. J. Cancer 2020, 137, 117–126. [Google Scholar] [CrossRef]
- Szabados, B.; van Dijk, N.; Tang, Y.Z.; van der Heijden, M.S.; Wimalasingham, A.; de Liano, A.G.; Chowdhury, S.; Hughes, S.; Rudman, S.; Linch, M.; et al. Response Rate to Chemotherapy after Immune Checkpoint Inhibition in Metastatic Urothelial Cancer. Eur. Urol. 2018, 73, 149–152. [Google Scholar] [CrossRef] [PubMed]
- Saleh, K.; Daste, A.; Martin, N.; Pons-Tostivint, E.; Auperin, A.; Herrera-Gomez, R.G.; Baste-Rotllan, N.; Bidault, F.; Guigay, J.; Le Tourneau, C.; et al. Response to salvage chemotherapy after progression on immune checkpoint inhibitors in patients with recurrent and/or metastatic squamous cell carcinoma of the head and neck. Eur. J. Cancer 2019, 121, 123–129. [Google Scholar] [CrossRef]
- Bonilla, L.; Oza, A.M.; Lheureux, S.; Saibil, S.; Chen, S.; Lee, Y.C.; Gray, D.; Colombo, I.; Madariaga, A.; Dhani, N.C.; et al. Clinical outcome of sequential chemotherapy after immune checkpoint inhibitors in advanced ovarian cancer. J. Clin. Oncol. 2019, 37, 5580. [Google Scholar] [CrossRef]
- Veneziani, A.; Lheureux, S.; Alqaisi, H.; Bhat, G.; Colombo, I.; Gonzalez, E.; Newton, S.; Msan, A.; Quintos, J.; Ramsahai, J.; et al. Pembrolizumab, maveropepimut-S, and low-dose cyclophosphamide in advanced epithelial ovarian cancer: Results from phase 1 and expansion cohort of PESCO trial. J. Clin. Oncol. 2022, 40, 5505. [Google Scholar] [CrossRef]
- Van Nieuwenhuysen, E.; O’Malley, D.; O’Cearbhaill, R.E.; Moore, K.N.; Hamilton, E.P.; Yeku, O.; Bouberhan, S.; Hou, J.Y.; Yoo, S.Y.; Brouwer-Visser, J.; et al. 523MO Ubamatamab (REGN4018, MUC16xCD3 bispecific antibody) monotherapy in patients with recurrent ovarian cancer (OC): Phase I dose-escalation analysis. Ann. Oncol. 2022, 33, S784. [Google Scholar] [CrossRef]
- Winer, I.; Vaishampayan, U.; Gilbert, L.; Rosen, S.; Gandhi, S.; Wang, Y.; Du, Y.; Sun, L.; Dalal, R.; Desai, M.; et al. Clinical outcomes of ovarian cancer patients treated with the novel engineered cytokine nemvaleukin alfa in combination with the PD-1 inhibitor pembrolizumab: Recent data from ARTISTRY-1 (077). Gynecol. Oncol. 2022, 166, S49. [Google Scholar] [CrossRef]

| First Author/ Study Name | Agents | Phase | Setting | Histology | Biomarkers for Pts Inclusion | n | ORR | mPFS (Months) | Exploratory Biomarkers |
|---|---|---|---|---|---|---|---|---|---|
| Single agent | |||||||||
| Disis et al. [32] JAVELIN Solid Tumor | Avelumab | 1b | PSOC/PROC median previous lines: 3 | all | no | 125 | All: 9.6% PSOC: 3.6% PROC:5.3% | 2.6 (95% CI, 1.4–2.8) |
PD-L1 neg: ORR 7.9% PD-L1 > 5%: 12.5%
PD-L1 neg: ORR 12.2%
|
| Hamanishi et al. [33] | Nivolumab | 2 | PROC | all | no | 20 | 15% | 3.5 (95% CI, 1.7–3.9) | PD-L1 in tumor cells (archival tissue): no correlation |
| Omatsu et al. [38] NINJA | Nivolumab vs. gemcitabine or PLD | 3 | PROC | all | no | 316 | 8 vs. 13% | 2.0 vs. 3.8, HR 1.5, (95% CI: 1.2–1.9) | PD-L1: no correlation BRCA status: no correlation |
| Varga et al. [34] KEYNOTE-028 | Pembrolizumab | 1b | PSOC/PROC | all | PD-L1 ≥ 1% in tumor and immune cells | 26 | 11.5% | 1.9 (95% CI, 1.8–3.5) | NA |
| Colombo et al. [35] (INSPIRE-ovarian cohort) | Pembrolizumab | 2 | PSOC/PROC | HGSOC | no | 21 | 0% | 1.9 | PD-L1: no correlation Other: under investigation |
| Matulonis et al. [37] KEYNOTE-100 | Pembrolizumab | 2 | PSOC/PROC 2 cohorts: (A) 1–3 prior lines (TFI 3–12 months) (B) 4–6 prior lines (TFI > 3 months) | all | no | Cohort A 285 cohort B 91 | A + B: 8.5% A: 8.1% B: 9.9% | 2.1 in both cohorts (95% CI, cohort A 2.1–2.2 and cohort B 2.1–2.6)) | PD-L1 as CPS score (archival tissue) in both cohorts CPS < 1: ORR 5% CPS ≥ 1: ORR 8% CPS ≥ 10: ORR 13.8% |
| Combinations | |||||||||
| Chemotherapy | |||||||||
| Lee et al. [39] | Pembrolizumab + PLD | 2 | PROC | all | no | 26 | 26.1% | 5.6 (95% CI 1.7–10.1) | PD-L1 archival tissue, MPS: no correlation T-cell inflamed GEP score: no correlation |
| O’Cearbhaill et al. [40] | Durvalumab + PLD | 1/2 | PROC | all | no | 40 | 22.5% | 5.5 (95% CI 0.3 to 28.8+) | MYC amplification (resistance) |
| Pujade-Lauraine et al. [41] JAVELIN200 | Avelumab + PLD vs. PLD vs. avelumab | 3 | PROC | all | no | 566 | Ave + PLD: 13% PLD: 4% Ave: 4% | Ave + PLD 3.7 (95% CI 3·3–5·1) PLD 3.5 (2·1–4·0), HR 0.78 Ave 1.9 (1.8–1.9), HR 1.68 | PD-L1 pos (≥1% tumor cells or ≥5% immune cells) and CD8 pos (≥1% immune cells), archival tissue: trend toward better PFS. |
| Monk et al. [42] JAVELIN100 | Platinum-based chemotherapy + avelumab, followed by avelumab maintenance vs. platinum-based chemotherapy, followed by avelumab maintenance vs. platinum-based chemotherapy | 3 | First-line | all | no | 998 | Ave combination 36% vs. Ave maintenance 30% vs. 30% | Ave combination 18.1 (95% CI 14·8-NE), HR 1.14 Ave maintenance 16.8 (13·5-NE), HR 1.43 PLD NE | NA |
| Ray-Coquard et al. [43] NeoPembOv | Carboplatin + paclitaxel +/− pembrolizumab | 2 | Neoadjuvant | HGSOC | no | 91 | 73.3% vs. 62.1% Rate or complete resection: 73.8% vs. 70% | 19.3 (95%CI 15–24.5) vs. 20.8 (17–23.4) | NA |
| Antiangiogenic (+/− chemotherapy) | |||||||||
| Moroney et al. [44] | Atezolizumab + bevacizumab | 1b | PROC | all | no | 20 | 15% | 4.9 (range 1.2–20.2) | PD-L1: no correlation |
| Moore et al. [45] IMagyn 50 | Carboplatin + paclitaxel + bevacizumab + atezolizumab/placebo | 3 | First-line | all | PD-L1 on immune cells (1% vs. ≥1%), stratification factor | 1301 | 93 vs. 89% | 19.5 vs. 18.4, HR 0.92 (95% CI, 0.79–1.07) | PD-L1 ≥ 1%: PFS 20.8 vs. 18.5 months (95% CI, 0.65 to 0.99) PD-L1 > 5%: PFS NR vs. 20.2 months |
| Zsiros et al. [46] | Pembrolizumab + bevacizumab + cyclophosphamide | 2 | PSOC/PRSOC | all | no | 40 | 47.5% | 10 (95% CI 1.3–5.7) | NA |
| Liu et al. [47] | Nivolumab + bevacizumab | 2 | PSOC/PROC | all | no | 38 | 28.9% | 9.4 (95% CI, 6.3–14.7) |
PD-L1 ≥ 1%: ORR 14.3% |
| Lwin et al. [48] LEAP005 (ovarian cohort) | Lenvatinib + pembrolizumab | 2 | PSOC/PROC (4L) | all | no | 31 | 32.3% | 4.4 (95% CI 4.0–8.5) | NA |
| Kurtz et al. [49] ATALANTE | Carboplatin-based chemotherapy+ bevacizumab+ atezolizumab/placebo | 3 | PSOC | all (non-mucinous) | no | 614 | NA | 13.5 vs. 11.3 HR 0.83 (95% CI 0.69–0.99) | PD-L1 ≥ 1%: PFS 15.2 vs. 13.1 months, HR: 0.86 (0.63–1.13) |
| PARP Inhibitors | |||||||||
| Konstantinopoulos et al. [50] TOPACIO | Niraparib + pembrolizumab | 1/2 | PROC or platinum ineligible | all | no | 62 | 18% | 3.4 (95% CI, 2.1–5.1) |
PD-L1 ≥ 1%: ORR 14.3%
|
| Drew et al. [51] MEDIOLA (doublet) | Olaparib + durvalumab | 2 | PSOC | gBRCA mutant | no | 32 | 71.9% | 11.1 (95% CI 8.2–15.9) | |
| gBRCA wild type | no | 32 | 34.4% | 5.5 (95% CI 3.6–7.5) | Genomic instability status (GIS) GIS-pos: ORR 50% GIS-neg: ORR 16.7% | ||||
| PARP inhibitors + antiangiogenic | |||||||||
| Drew et al. [52] MEDIOLA (triplet) | Olaparib + durvalumab + bevacizumab | 2 | PSOC | gBRCA wild type ≤2 prior lines of chemo | no | 31 | 87.1% | 14.7 (95% CI 10–18.1) | Genomic instability status (GIS) GIS-pos: ORR 100% GIS-neg: ORR 75% |
| Liu et al. [53] OPAL (cohort A) | Dostarlimab + bevacizumab + niraparib | 2 | PROC | High grade or carcinosarcoma ≤2 prior lines | no | 41 | 17.9% | 7.6 (95% CI 4.2–10.6) | PD-L1 as CPS score: CPS pos (≥1%): ORR 15% CPS neg (<1%): ORR 22% tBRCA status: BRCA mutant: ORR 25% BRCA wild type: ORR 16% |
| Double ICIs | |||||||||
| Zamarin et al. [54] NRG-GY-003 | Ipilimumab + nivolumab vs. nivolumab | 2 | PFI < 12 months ≤3 prior lines | all | no | 100 | 31.4 vs. 12.2 % | 3.9 vs. 2, HR 0.53 (95% CI, 0.34 to 0.82) |
PD-L1 neg: ORR 26.9 vs. 20%
PD-L1 neg: ORR 27.6 vs. 21.4% |
| Lee et al. [55] KGOG 3046/TRU-D | Carboplatin and paclitaxel + durvalumab + tremelimumab | 2 | Neoadjuvant | all | no | 23 | 100% No residual disease after surgery: 74% | NA | NA |
| Hinchcliff et al. [56] | Durvalumab + tremelimumab concomitant vs. sequential | 2 | PROC | HGSOC | no | 61 | 8.7 vs. 0% | 1.87 (95% Ci 1.77–2.17) vs. 1.84 (95% CI 1.77–2.43) | NA |
| Study | Agents | Setting | Histology | n | Biomarkers for Inclusion/Stratification | NCT Number |
|---|---|---|---|---|---|---|
| KEYLYNK-001/ ENGOT Ov43/ GOG3036 | CP +/− bevacizumab + pembrolizumab/placebo + olaparib/placebo | First-line | BRCA wild type | 1284 | PD-L1 (CPS > 10): stratification | NCT03740165 |
| FIRST/ ENGOT Ov44 | CP +/− bevacizumab + niraparib + dostarlimab/placebo | First-line | mucinous and low-grade excluded | 1228 | PD-L1: stratification factor | NCT03602859 |
| ATHENA/ ENGOT Ov45 | Rucaparib/placebo + nivolumab/placebo | Maintenance after first-line | Mucinous excluded | 1000 | HRR status by mutation analysis | NCT03522246 |
| DUO-O/ ENGOT Ov46 | CP + bevacizumab + durvalumab/placebo + olaparib/placebo | First-line | High grade | 1104 | BRCA status * | NCT03737643 |
| ANITA/ ENGOT Ov41/ GEICO 69-O | Platinum-based chemotherapy + atezolizumab/placebo + niraparib | Recurrent PSOC | High-grade serous or endometrioid ≤2 prior lines | 414 | BRCA status: stratification | NCT03598270 |
| NItCHE-MITO33 | Niraparib + dostarlimab vs. chemotherapy of physician choice (+/− bevacizumab) | Recurrent non platinum eligible ≤2 prior lines | all | 427 | PD-L1 and HRD status: stratification | NCT04679064 |
| AGO-OVAR 2.29/ ENGOT Ov34 | PLD or paclitaxel + bevacizumab + atezolizumab | Recurrent PROC ≤3 prior lines | all | 664 | PD-L1: stratification | NCT03353831 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Colombo, I.; Karakasis, K.; Suku, S.; Oza, A.M. Chasing Immune Checkpoint Inhibitors in Ovarian Cancer: Novel Combinations and Biomarker Discovery. Cancers 2023, 15, 3220. https://doi.org/10.3390/cancers15123220
Colombo I, Karakasis K, Suku S, Oza AM. Chasing Immune Checkpoint Inhibitors in Ovarian Cancer: Novel Combinations and Biomarker Discovery. Cancers. 2023; 15(12):3220. https://doi.org/10.3390/cancers15123220
Chicago/Turabian StyleColombo, Ilaria, Katherine Karakasis, Sneha Suku, and Amit M. Oza. 2023. "Chasing Immune Checkpoint Inhibitors in Ovarian Cancer: Novel Combinations and Biomarker Discovery" Cancers 15, no. 12: 3220. https://doi.org/10.3390/cancers15123220
APA StyleColombo, I., Karakasis, K., Suku, S., & Oza, A. M. (2023). Chasing Immune Checkpoint Inhibitors in Ovarian Cancer: Novel Combinations and Biomarker Discovery. Cancers, 15(12), 3220. https://doi.org/10.3390/cancers15123220





