Could Oxidative Stress Play a Role in the Development and Clinical Management of Differentiated Thyroid Cancer?
Abstract
Simple Summary
Abstract
1. Introduction
2. The Diagnosis of Thyroid Cancer
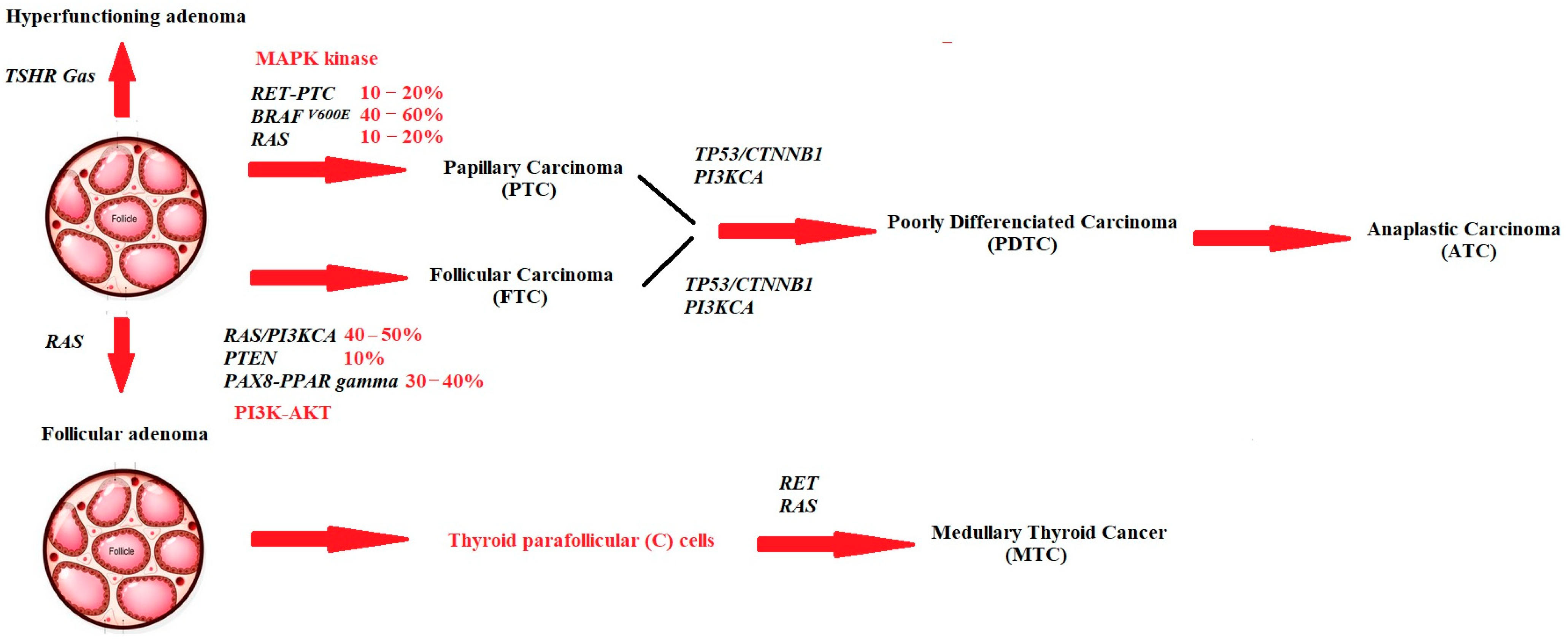
3. The Main Genes Implicated in the Development of Thyroid Cancer
4. Treatment of Differentiated Thyroid Cancer
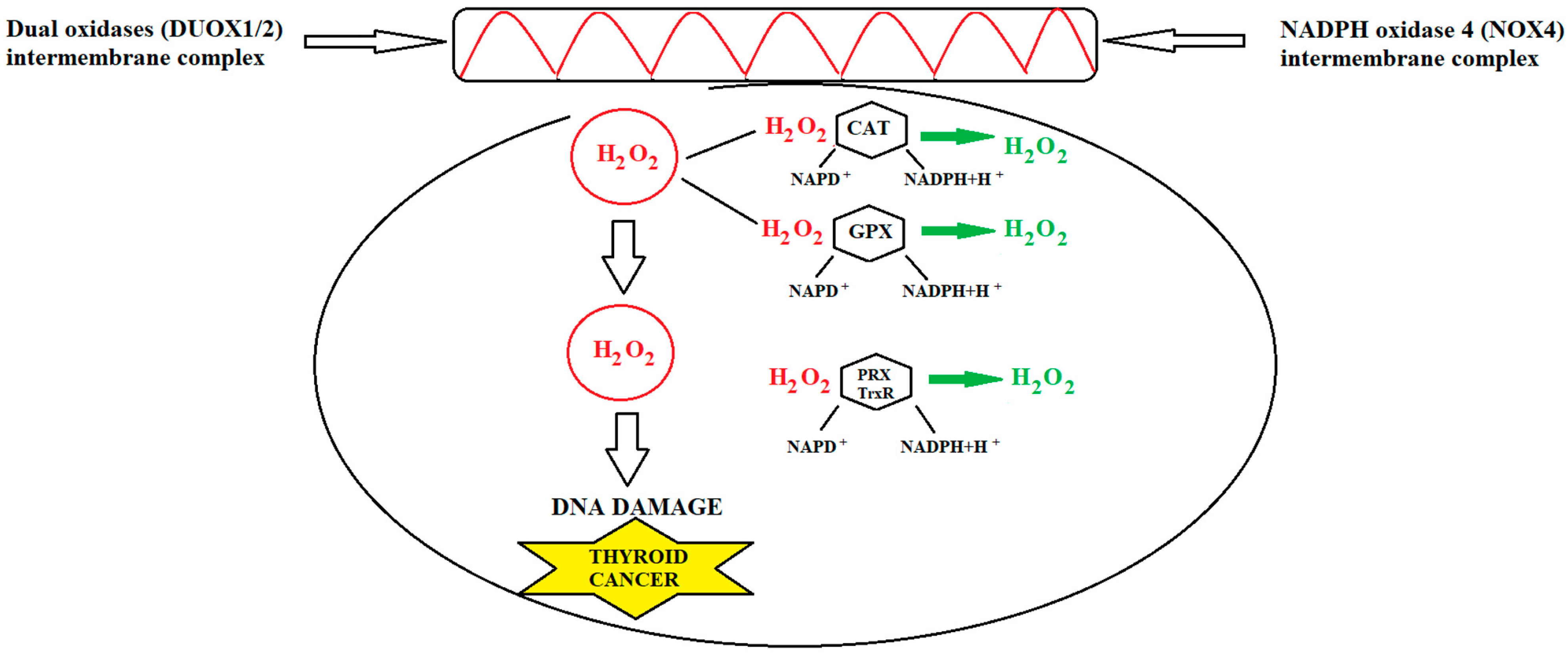
5. Thyroid Cancer and Oxidative Stress
6. RAI Therapy and Oxidative Stress
7. Antioxidants in Thyroid Cancer
8. Future Perspectives
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Seib, C.D.; Sosa, J.A. Evolving Understanding of the Epidemiology of Thyroid Cancer. Endocrinol. Metab. Clin. N. Am. 2019, 48, 23–35. [Google Scholar] [CrossRef]
- Lamartina, L.; Grani, G.; Durante, C.; Filetti, S.; Cooper, D.S. Screening for differentiated thyroid cancer in selected populations. Lancet Diabetes Endocrinol. 2020, 8, 81–88. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Araque, D.V.P.; Bleyer, A.; Brito, J.P. Thyroid cancer in adolescents and young adults. Future Oncol. 2017, 13, 1253–1261. [Google Scholar] [CrossRef] [PubMed]
- Torre, L.A.; Sauer, A.M.; Chen, M.S., Jr.; Kagawa-Singer, M.; Jemal, A.; Siegel, R.L. Cancer statistics for Asian Americans, Native Hawaiians, and Pacific Islanders, 2016: Converging incidence in males and females. CA Cancer J. Clin. 2016, 66, 182–202. [Google Scholar] [CrossRef]
- Liao, D.; Lv, G.; Wang, T.; Min, J.; Wang, Y.; Liu, S. Prognostic value of long non-coding RNA BLACAT1 in patients with papillary thyroid carcinoma. Cancer Cell Int. 2018, 18, 47. [Google Scholar] [CrossRef] [PubMed]
- Haymart, M.R. Progress and Challenges in Thyroid Cancer Management. Endocr. Pract. 2021, 27, 1260–1263. [Google Scholar] [CrossRef]
- Megwalu, U.C.; Moon, P.K. Thyroid Cancer Incidence and Mortality Trends in the United States: 2000–2018. Thyroid 2022, 32, 560–570. [Google Scholar] [CrossRef]
- Baloch, Z.W.; Asa, S.L.; Barletta, J.A.; Ghossein, R.A.; Juhlin, C.C.; Jung, C.K.; LiVolsi, V.A.; Papotti, M.G.; Sobrinho-Simões, M.; Tallini, G.; et al. Overview of the 2022 WHO Classification of Thyroid Neoplasms. Endocr. Pathol. 2022, 33, 27–63. [Google Scholar] [CrossRef]
- Kim, T.H.; Lee, M.; Kwon, A.Y.; Choe, J.H.; Kim, J.H.; Kim, J.S.; Hahn, S.Y.; Shin, J.H.; Chung, M.K.; Son, Y.I.; et al. Molecular genotyping of the non-invasive encapsulated follicular variant of papillary thyroid carcinoma. Histopathology 2018, 72, 648–661. [Google Scholar] [CrossRef]
- Jung, C.K.; Bychkov, A.; Song, D.E.; Kim, J.H.; Zhu, Y.; Liu, Z.; Keelawat, S.; Lai, C.R.; Hirokawa, M.; Kameyama, K.; et al. Molecular correlates and nuclear features of encapsulated follicular-patterned thyroid neoplasms. Endocrinol. Metab. 2021, 36, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Fuchs, T.L.; Ahmadi, S.; Alghamdi, M.; Alzumaili, B.; Bani, M.-A.; Baudin, E.; Chou, A.; De Leo, A.; Fagin, J.A.; et al. International Medullary Thyroid Carcinoma Grading System: A Validated Grading System for Medullary Thyroid Carcinoma. J. Clin. Oncol. 2022, 40, 96–104. [Google Scholar] [CrossRef] [PubMed]
- Harahap, W.A.; Tofrizal, T.; Oktahermoniza, O. Relationship between the Expression of BRAF V600E and Ki-67 with the Recurrence of Well-Differentiated Thyroid Cancer. Asian Pac. J. Cancer Prev. 2022, 23, 3617–3622. [Google Scholar] [CrossRef] [PubMed]
- Pan, D.-H.; Wen, D.-Y.; Luo, Y.-H.; Chen, G.; Yang, H.; Chen, J.-Q.; He, Y. The diagnostic and prognostic values of Ki-67/MIB-1 expression in thyroid cancer: A meta-analysis with 6051 cases. OncoTargets Ther. 2017, 10, 3261–3276. [Google Scholar] [CrossRef] [PubMed]
- Lindfors, H.; Lundgren, C.I.; Zedenius, J.; Juhlin, C.C.; Shabo, I. The Clinical Significance of Lymph Node Ratio and Ki-67 Expression in Papillary Thyroid Cancer. World J. Surg. 2021, 45, 2155–2164. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Guo, T.; Niu, D.; Zhu, Y.; Ren, W.; Yao, Q.; Huang, X.; Feng, Q.; Wang, T.; Ma, X.; et al. Clinical significance and interrelations of PD-L1 expression, Ki-67 index, and molecular alterations in sporadic medullary thyroid carcinoma from a Chinese population. Virchows Arch. 2022, 481, 903–911. [Google Scholar] [CrossRef] [PubMed]
- Ito, Y.; Hirokawa, M.; Miyauchi, A.; Higashiyama, T.; Kihara, M.; Miya, A. Prognostic Significance of the Proportion of Tall Cell Components in Papillary Thyroid Carcinoma. World J. Surg. 2017, 41, 742–747. [Google Scholar] [CrossRef]
- Chen, J.-H.; Faquin, W.C.; Lloyd, R.V.; Nosé, V. Clinicopathological and molecular characterization of nine cases of columnar cell variant of papillary thyroid carcinoma. Mod. Pathol. 2011, 24, 739–749. [Google Scholar] [CrossRef]
- Haroon Al Rasheed, M.R.H.; Xu, B. Molecular Alterations in Thyroid Carcinoma. Surg. Pathol. Clin. 2019, 12, 921–930. [Google Scholar] [CrossRef]
- Howitt, B.E.; Jia, Y.; Sholl, L.M.; Barletta, J.A. Molecular alterations in partially-encapsulated or well-circumscribed follicular variant of papillary thyroid carcinoma. Thyroid 2013, 23, 1256–1262. [Google Scholar] [CrossRef]
- Scheffel, R.S.; Dora, J.M.; Maia, A.L. BRAF mutations in thyroid cancer. Curr. Opin. Oncol. 2022, 34, 9–18. [Google Scholar] [CrossRef]
- Rajagopalan, H.; Bardelli, A.; Lengauer, C.; Kinzler, K.W.; Vogelstein, B.; Velculescu, V.E. RAF/RAS oncogenes and mismatch-repair status. Nature 2002, 418, 934. [Google Scholar] [CrossRef] [PubMed]
- Al Hamad, M.A.; Albisher, H.M.; Al Saeed, W.R.; Almumtin, A.T.; Allabbad, F.M.; Shawarby, M.A. BRAF gene mutations in synchronous papillary thyroid carcinoma and Langerhans cell histiocytosis co-existing in the thyroid gland: A case report and literature review. BMC Cancer 2019, 19, 170. [Google Scholar] [CrossRef] [PubMed]
- Shiu, J.; Lander, A.D. When oncogenes do not cause cancer. Elife 2021, 10, e74912. [Google Scholar] [CrossRef] [PubMed]
- Fakhruddin, N.; Jabbour, M.; Novy, M.; Tamim, H.; Bahmad, H.; Farhat, F.; Zaatari, G.; Aridi, T.; Kriegshauser, G.; Oberkanins, C.; et al. BRAF and NRAS Mutations in Papillary Thyroid Carcinoma and Concordance in BRAF Mutations Between Primary and Corresponding Lymph Node Metastases. Sci. Rep. 2017, 7, 4666. [Google Scholar] [CrossRef]
- Xing, M.; Westra, W.H.; Tufano, R.P.; Cohen, Y.; Rosenbaum, E.; Rhoden, K.J.; Carson, K.A.; Vasko, V.; Larin, A.; Tallini, G.; et al. BRAF Mutation Predicts a Poorer Clinical Prognosis for Papillary Thyroid Cancer. J. Clin. Endocrinol. Metab. 2005, 90, 6373–6379. [Google Scholar] [CrossRef]
- Rusinek, D.; Swierniak, M.; Chmielik, E.; Kowal, M.; Kowalska, M.; Cyplinska, R.; Czarniecka, A.; Piglowski, W.; Korfanty, J.; Chekan, M.; et al. BRAFV600E-Associated Gene Expression Profile: Early Changes in the Transcriptome, Based on a Transgenic Mouse Model of Papillary Thyroid Carcinoma. PLoS ONE 2015, 10, e0143688. [Google Scholar] [CrossRef]
- Zhu, X.; Peng, X.; Zhu, L.; Xie, L.; Cheng, F.; Zhou, B. Evaluation of the diagnostic performance of contrast-enhanced ultrasound combined with BRAF V600E gene detection in nodules of unclear significance by thyroid fine-needle aspiration. Gland. Surg. 2021, 10, 328–335. [Google Scholar] [CrossRef]
- Scheffel, R.S.; de Cristo, A.P.; Romitti, M.; Vargas, C.V.F.; Ceolin, L.; Zanella, A.B.; Dora, J.M.; Maia, A.L. The BRAFV600E mutation analysis and risk stratification in papillary thyroid carcinoma. Arq. Bras. Endocrinol. Metabol. 2021, 64, 751–757. [Google Scholar] [CrossRef]
- Soares, P.; Póvoa, A.A.; Melo, M.; Vinagre, J.; Máximo, V.; Eloy, C.; Cameselle-Teijeiro, J.M.; Sobrinho-Simões, M. Molecular Pathology of Non-familial Follicular Epithelial–Derived Thyroid Cancer in Adults: From RAS/BRAF-like Tumor Designations to Molecular Risk Stratification. Endocr. Pathol. 2021, 32, 44–62. [Google Scholar] [CrossRef]
- Lanfredini, S.; Thapa, A.; O’Neill, E. RAS in pancreatic cancer. Biochem. Soc. Trans. 2019, 47, 961–972. [Google Scholar] [CrossRef]
- Karmakar, S.; Kaushik, G.; Nimmakayala, R.; Rachagani, S.; Ponnusamy, M.P.; Batra, S.K. MicroRNA regulation of K-Ras in pancreatic cancer and opportunities for therapeutic intervention. Semin. Cancer Biol. 2019, 54, 63–71. [Google Scholar] [CrossRef]
- Moscatello, C.; Di Marcantonio, M.C.; Savino, L.; D’amico, E.; Spacco, G.; Simeone, P.; Lanuti, P.; Muraro, R.; Mincione, G.; Cotellese, R.; et al. Emerging Role of Oxidative Stress on EGFR and OGG1-BER Cross-Regulation: Implications in Thyroid Physiopathology. Cells 2022, 11, 822. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, M. RET receptor signaling: Function in development, metabolic disease, and cancer. Proc. Jpn. Acad. Ser. B 2022, 98, 112–125. [Google Scholar] [CrossRef] [PubMed]
- Carrillo, C.F.B.; Rojas, J.A.P.; Barrionuevo, C.; Zambrano, S.C.; Cárdenas, A.P.; Santibañez, I.Q.; Mujica, M.D.C.C. Cáncer Medular de Tiroides familiar: Reporte de un caso y revisión de la literatura. Rev. Fac. Cienc. Med. Cordoba 2018, 75, 303–309. [Google Scholar] [CrossRef]
- Fallahi, P.; Ferrari, S.M.; Galdiero, M.R.; Varricchi, G.; Elia, G.; Ragusa, F.; Paparo, S.R.; Benvenga, S.; Antonelli, A. Molecular targets of tyrosine kinase inhibitors in thyroid cancer. Semin. Cancer Biol. 2022, 79, 180–196. [Google Scholar] [CrossRef]
- Li, A.Y.; McCusker, M.G.; Russo, A.; Scilla, K.A.; Gittens, A.; Arensmeyer, K.; Mehra, R.; Adamo, V.; Rolfo, C. RET fusions in solid tumors. Cancer Treat. Rev. 2019, 81, 101911. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, M.; Kawai, K.; Asai, N. Roles of the RET Proto-oncogene in Cancer and Development. JMA J. 2020, 3, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.S.; Qadri, Q.; Makhdoomi, M.J.; Wani, M.A.; Malik, A.A.; Niyaz, M.; Masoodi, S.R.; Andrabi, K.I.; Ahmad, R.; Mudassar, S. RET/PTC Gene Rearrangements in Thyroid Carcinogenesis: Assessment and Clinico-Pathological Correlations. Pathol. Oncol. Res. 2020, 26, 507–513. [Google Scholar] [CrossRef]
- Jaber, T.; Dadu, R.; Hu, M.I. Medullary thyroid carcinoma. Curr. Opin. Endocrinol. Diabetes 2021, 28, 540–546. [Google Scholar] [CrossRef]
- Hernandez-Prera, J.C. Molecular Pathology of Thyroid Tumors. Surg. Pathol. Clin. 2021, 14, 493–506. [Google Scholar] [CrossRef] [PubMed]
- Zhulai, G.; Oleinik, E.; Shibaev, M.; Ignatev, K. Adenosine-Metabolizing Enzymes, Adenosine Kinase and Adenosine Deaminase, in Cancer. Biomolecules 2022, 12, 418. [Google Scholar] [CrossRef] [PubMed]
- Carney, J.A.; Lyssikatos, C.; Seethala, R.R.; Lakatos, P.; Perez-Atayde, A.; Lahner, H.; Stratakis, C.A. The Spectrum of Thyroid Gland Pathology in Carney Complex: The Importance of Follicular Carcinoma. Am. J. Surg. Pathol. 2018, 42, 587–594. [Google Scholar] [CrossRef]
- Curylova, L.; Ramos, H.; Saraiva, L.; Skoda, J. Noncanonical roles of p53 in cancer stemness and their implications in sarcomas. Cancer Lett. 2022, 525, 131–145. [Google Scholar] [CrossRef]
- Loureiro, J.; Abrantes, M.; Oliveira, P.; Saraiva, L. P53 in skin cancer: From a master player to a privileged target for prevention and therapy. Biochim. Biophys. Acta (BBA)—Rev. Cancer 2020, 1874, 188438. [Google Scholar] [CrossRef]
- Oduah, E.I.; Grossman, S.R. Harnessing the vulnerabilities of p53 mutants in lung cancer—Focusing on the proteasome: A new trick for an old foe? Cancer Biol. Ther. 2020, 21, 293–302. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Aljahdali, I.A.M.; Zhang, R.; Nastiuk, K.L.; Krolewski, J.J.; Ling, X. Kidney cancer biomarkers and targets for therapeutics: Survivin (BIRC5), XIAP, MCL-1, HIF1α, HIF2α, NRF2, MDM2, MDM4, p53, KRAS and AKT in renal cell carcinoma. J. Exp. Clin. Cancer Res. 2021, 40, 254. [Google Scholar] [CrossRef]
- Malaguarnera, R.; Vella, V.; Vigneri, R.; Frasca, F. p53 family proteins in thyroid cancer. Endocr.-Relat. Cancer 2007, 14, 43–60. [Google Scholar] [CrossRef]
- Cancer Genome Atlas Research Network. Integrated genomic characterization of papillary thyroid carcinoma. Cell 2014, 159, 676–690. [Google Scholar] [CrossRef]
- Dralle, H.; Machens, A.; Basa, J.; Fatourechi, V.; Franceschi, S.; Hay, I.D.; Nikiforov, Y.E.; Pacini, F.; Pasieka, J.L.; Sherman, S.I. Follicular cell-derived thyroid cancer. Nat. Rev. Dis. Prim. 2015, 1, 15077. [Google Scholar] [CrossRef]
- Hu, J.; Cao, J.; Topatana, W.; Juengpanich, S.; Li, S.; Bin Zhang, B.; Shen, J.; Cai, L.; Cai, X.; Chen, M. Targeting mutant p53 for cancer therapy: Direct and indirect strategies. J. Hematol. Oncol. 2021, 14, 157. [Google Scholar] [CrossRef] [PubMed]
- Recondo, G.; Che, J.; Jänne, P.A.; Awad, M.M. Targeting MET Dysregulation in Cancer. Cancer Discov. 2020, 10, 922–934. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Su, X.; Li, Z.; Deng, L.; Liu, X.; Feng, X.; Peng, J. HGF/c-MET pathway in cancer: From molecular characterization to clinical evidence. Oncogene 2021, 40, 4625–4651. [Google Scholar] [CrossRef] [PubMed]
- Comoglio, P.M.; Trusolino, L.; Boccaccio, C. Known and novel roles of the MET oncogene in cancer: A coherent approach to targeted therapy. Nat. Rev. Cancer 2018, 18, 341–358. [Google Scholar] [CrossRef]
- Uchikawa, E.; Chen, Z.; Xiao, G.-Y.; Zhang, X.; Bai, X.-C. Structural basis of the activation of c-MET receptor. Nat. Commun. 2021, 12, 4074. [Google Scholar] [CrossRef]
- Stanley, J.; Neelamohan, R.; Suthagar, E.; Vengatesh, G.; Jayakumar, J.; Chandrasekaran, M.; Banu, S.; Aruldhas, M. Lipid peroxidation and antioxidants status in human malignant and non-malignant thyroid tumours. Hum. Exp. Toxicol. 2016, 35, 585–597. [Google Scholar] [CrossRef]
- Pacini, F.; Fuhrer, D.; Elisei, R.; Handkiewicz-Junak, D.; Leboulleux, S.; Luster, M.; Schlumberger, M.; Smit, J.W. 2022 ETA Consensus Statement: What are the indications for post-surgical radioiodine therapy in differentiated thyroid cancer? Eur. Thyroid. J. 2022, 11, e210046. [Google Scholar] [CrossRef]
- Haugen, B.R.; Alexander, E.K.; Bible, K.C.; Doherty, G.M.; Mandel, S.J.; Nikiforov, Y.E.; Pacini, F.; Randolph, G.W.; Sawka, A.M.; Schlumberger, M.; et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 2016, 26, 1–133. [Google Scholar] [CrossRef]
- Thiagarajan, S.; Yousuf, A.; Shetty, R.; Dhar, H.; Mathur, Y.; Nair, D.; Basu, S.; Patil, A.; Kane, S.; Ghosh-Laskar, S.; et al. Poorly differentiated thyroid carcinoma (PDTC) characteristics and the efficacy of radioactive iodine (RAI) therapy as an adjuvant treatment in a tertiary cancer care center. Eur. Arch. Oto-Rhino-Laryngol. 2020, 277, 1807–1814. [Google Scholar] [CrossRef]
- TuttleMD, R.M.; Ahuja, S.; Avram, A.M.; Bernet, V.J.; Bourguet, P.; Daniels, G.H.; Dillehay, G.; Draganescu, C.; Flux, G.; Fuehrer, D.; et al. Controversies, Consensus, and Collaboration in the Use of 131I Therapy in Differentiated Thyroid Cancer: A Joint Statement from the American Thyroid Association, the European Association of Nuclear Medicine, the Society of Nuclear Medicine and Molecular Imaging, and the European Thyroid Association. Thyroid 2019, 29, 461–470. [Google Scholar] [CrossRef]
- Sun, Y.-Q.; Sun, D.; Zhang, X.; Zhang, Y.-Q.; Lin, Y.-S. Radioiodine adjuvant therapy in differentiated thyroid cancer: An update and reconsideration. Front. Endocrinol. 2022, 13, 994288. [Google Scholar] [CrossRef] [PubMed]
- Paluskievicz, C.M.; Chang, D.R.; Blackburn, K.W.; Turner, D.J.; Munir, K.M.; Mullins, C.D.; Olson, J.A.; Hu, Y. Low-Risk Papillary Thyroid Cancer: Treatment De-Escalation and Cost Implications. J. Surg. Res. 2022, 275, 273–280. [Google Scholar] [CrossRef] [PubMed]
- Ain, K.B. Radioiodine-remnant ablation in low-risk differentiated thyroid cancer: Pros. Endocrine 2015, 50, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Verburg, F.A.; Flux, G.; Giovanella, L.; van Nostrand, D.; Muylle, K.; Luster, M. Differentiated thyroid cancer patients potentially benefitting from postoperative I-131 therapy: A review of the literature of the past decade. Eur. J. Nucl. Med. 2020, 47, 78–83. [Google Scholar] [CrossRef]
- Liu, J.; Liu, Y.; Lin, Y.; Liang, J. Radioactive Iodine-Refractory Differentiated Thyroid Cancer and Redifferentiation Therapy. Endocrinol. Metab. 2019, 34, 215–225. [Google Scholar] [CrossRef] [PubMed]
- Orzechowski, A.; Cywińska, A.; Rostagno, A.A.; Rizzi, F.M. Oxidative Stress, Chronic Inflammation, and Amyloidoses. Oxidative Med. Cell. Longev. 2019, 2019, 6024975. [Google Scholar] [CrossRef] [PubMed]
- Morshed, S.A.; Davies, T.F. Understanding Thyroid Cell Stress. J. Clin. Endocrinol. Metab. 2020, 105, e66–e69. [Google Scholar] [CrossRef]
- Dizdaroglu, M.; Jaruga, P. Mechanisms of free radical-induced damage to DNA. Free. Radic. Res. 2012, 46, 382–419. [Google Scholar] [CrossRef]
- Singh, V.; Ubaid, S. Role of Silent Information Regulator 1 (SIRT1) in Regulating Oxidative Stress and Inflammation. Inflammation 2020, 43, 1589–1598, Erratum in Inflammation 2021, 44, 2142. [Google Scholar] [CrossRef]
- Nogueira, J.E. Recent Advances in Molecular Hydrogen Research Reducing Exercise-Induced Oxidative Stress and Inflammation. Curr. Pharm. Des. 2021, 27, 731–736. [Google Scholar] [CrossRef]
- Pisoschi, A.M.; Pop, A. The role of antioxidants in the chemistry of oxidative stress: A review. Eur. J. Med. Chem. 2015, 97, 55–74. [Google Scholar] [CrossRef] [PubMed]
- Tafani, M.; Sansone, L.; Limana, F.; Arcangeli, T.; De Santis, E.; Polese, M.; Fini, M.; Russo, M.A. The Interplay of Reactive Oxygen Species, Hypoxia, Inflammation, and Sirtuins in Cancer Initiation and Progression. Oxidative Med. Cell. Longev. 2016, 2016, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Maier, J.; Van Steeg, H.; Van Oostrom, C.; Karger, S.; Paschke, R.; Krohn, K. Deoxyribonucleic Acid Damage and Spontaneous Mutagenesis in the Thyroid Gland of Rats and Mice. Endocrinology 2006, 147, 3391–3397. [Google Scholar] [CrossRef] [PubMed]
- Kundaktepe, B.P.; Sozer, V.; Durmus, S.; Kocael, P.C.; Kundaktepe, F.O.; Papila, C.; Gelisgen, R.; Uzun, H. The evaluation of oxidative stress parameters in breast and colon cancer. Medicine 2021, 100, e25104. [Google Scholar] [CrossRef]
- Ren, Y.; Liang, H.; Wang, X.; Cao, Z.; Ma, Y.; Liu, X. Alterations in mitochondrial function and energy metabolism-related properties in thyroid cancer stem cells. Acta Biochim. Pol. 2021, 69, 11–17. [Google Scholar] [CrossRef]
- Colin, I.M.; Denef, J.-F.; Lengelé, B.; Many, M.-C.; Gérard, A.-C. Recent Insights into the Cell Biology of Thyroid Angiofollicular Units. Endocr. Rev. 2013, 34, 209–238. [Google Scholar] [CrossRef]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative Stress: Harms and Benefits for Human Health. Oxid. Med. Cell. Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef]
- Mehran, L.; Amouzegar, A.; Rahimabad, P.K.; Tohidi, M.; Tahmasebinejad, Z.; Azizi, F. Thyroid Function and Metabolic Syndrome: A Population-Based Thyroid Study. Horm. Metab. Res. 2017, 49, 192–200. [Google Scholar] [CrossRef]
- Luo, Y.; Ma, J.; Lu, W. The Significance of Mitochondrial Dysfunction in Cancer. Int. J. Mol. Sci. 2020, 21, 5598. [Google Scholar] [CrossRef]
- Wang, N.; Feng, J.-F.; Zeng, P.; Yang, Y.-H.; Luo, J.; Yang, Y.-W. Total oxidant/antioxidant status in sera of patients with thyroid cancers. Endocr.-Relat. Cancer 2011, 18, 773–782. [Google Scholar] [CrossRef]
- Rašić, I.; Rašić, A.; Akšamija, G.; Radović, S. The Relationship Between Serum Level of Malondialdehyde and Progression of Colorectal Cancer. Acta Clin. Croat. 2018, 57, 411–416. [Google Scholar] [CrossRef] [PubMed]
- Dogan, R.; Dogan, E.E.; Guler, E.M.; Senturk, E.; Yenigun, A.; Celik, I.; Aksoy, F.; Ozturan, O. Oxidative stress values of tumor core, edge, and healthy thyroid tissue in thyroid masses. Eur. Arch. Oto-Rhino-Laryngol. 2021, 278, 2953–2960. [Google Scholar] [CrossRef]
- Mancini, A.; Di Segni, C.; Raimondo, S.; Olivieri, G.; Silvestrini, A.; Meucci, E.; Currò, D. Thyroid Hormones, Oxidative Stress, and Inflammation. Mediat. Inflamm. 2016, 2016, 6757154. [Google Scholar] [CrossRef] [PubMed]
- Akbari, B.; Baghaei-Yazdi, N.; Bahmaie, M.; Abhari, F.M. The role of plant-derived natural antioxidants in reduction of oxidative stress. Biofactors 2022, 48, 611–633. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.-M.; Qi, T.-G. The role of TXNL1 in disease: Treatment strategies for cancer and diseases with oxidative stress. Mol. Biol. Rep. 2021, 48, 2929–2934. [Google Scholar] [CrossRef] [PubMed]
- Szanto, I.; Pusztaszeri, M.; Mavromati, M. H2O2 Metabolism in Normal Thyroid Cells and in Thyroid Tumorigenesis: Focus on NADPH Oxidases. Antioxidants 2019, 8, 126. [Google Scholar] [CrossRef]
- Karger, S.; Krause, K.; Engelhardt, C.; Weidinger, C.; Gimm, O.; Dralle, H.; Sheu-Grabellus, S.-Y.; Schmid, K.W.; Fuhrer, D. Distinct pattern of oxidative DNA damage and DNA repair in follicular thyroid tumours. J. Mol. Endocrinol. 2012, 48, 193–202. [Google Scholar] [CrossRef]
- Muzza, M.; Pogliaghi, G.; Colombo, C.; Carbone, E.; Cirello, V.; Palazzo, S.; Frattini, F.; Gentilini, D.; Gazzano, G.; Persani, L.; et al. Oxidative Stress Correlates with More Aggressive Features in Thyroid Cancer. Cancers 2022, 14, 5857. [Google Scholar] [CrossRef]
- Martinez-Cadenas, C.; Bosch, N.; Peñas, L.; Flores-Couce, E.; Ochoa, E.; Munárriz, J.; Aracil, J.P.; Tajahuerce, M.; Royo, R.; Lozoya, R.; et al. Malignant melanoma arising from a perianal fistula and harbouring a BRAFgene mutation: A case report. BMC Cancer 2011, 11, 343. [Google Scholar] [CrossRef]
- Chung, J.H. BRAF and TERT promoter mutations: Clinical application in thyroid cancer. Endocr. J. 2020, 67, 577–584. [Google Scholar] [CrossRef]
- El Hassani, R.A.; Buffet, C.; Leboulleux, S.; Dupuy, C. Oxidative stress in thyroid carcinomas: Biological and clinical significance. Endocr.-Relat. Cancer 2019, 26, R131–R143. [Google Scholar] [CrossRef]
- Azouzi, N.; Cailloux, J.; Cazarin, J.M.; Knauf, J.A.; Cracchiolo, J.; Al Ghuzlan, A.; Hartl, D.; Polak, M.; Carré, A.; El Mzibri, M.; et al. NADPH Oxidase NOX4 Is a Critical Mediator of BRAFV600E-Induced Downregulation of the Sodium/Iodide Sym-porter in Papillary Thyroid Carcinomas. Antioxid Redox Signal 2017, 26, 864–877. [Google Scholar] [CrossRef] [PubMed]
- Dalton, T.P.; Chen, Y.; Schneider, S.N.; Nebert, D.W.; Shertzer, H.G. Genetically altered mice to evaluate glutathione homeostasis in health and disease. Free. Radic. Biol. Med. 2004, 37, 1511–1526. [Google Scholar] [CrossRef] [PubMed]
- Metere, A.; Frezzotti, F.; Graves, C.E.; Vergine, M.; De Luca, A.; Pietraforte, D.; Giacomelli, L. A possible role for selenoprotein glutathione peroxidase (GPx1) and thioredoxin reductases (TrxR1) in thyroid cancer: Our experience in thyroid surgery. Cancer Cell Int. 2018, 18, 7. [Google Scholar] [CrossRef] [PubMed]
- Akinci, M.; Kosova, F.; Çetin, B.; Sepici, A.; Altan, N.; Aslan, S.; Cetin, A.; Çetin, B. Oxidant/antioxidant balance in patients with thyroid cancer. Acta Cir. Bras. 2008, 23, 551–554. [Google Scholar] [CrossRef] [PubMed]
- Sekhar, K.R.; Cyr, S.; Baregamian, N. Ferroptosis Inducers in Thyroid Cancer. World J. Surg. 2022, 47, 371–381. [Google Scholar] [CrossRef]
- Sekhar, K.R.; Hanna, D.N.; Cyr, S.; Baechle, J.J.; Kuravi, S.; Balusu, R.; Rathmell, K.; Baregamian, N. Glutathione peroxidase 4 inhibition induces ferroptosis and mTOR pathway suppression in thyroid cancer. Sci. Rep. 2022, 12, 19396. [Google Scholar] [CrossRef]
- Ock, C.-Y. 8-Hydroxydeoxyguanosine: Not mere biomarker for oxidative stress, but remedy for oxidative stress-implicated gastrointestinal diseases. World J. Gastroenterol. 2012, 18, 302–308. [Google Scholar] [CrossRef]
- Tabur, S.; Aksoy, Ş.N.; Korkmaz, H.; Ozkaya, M.; Aksoy, N.; Akarsu, E. Investigation of the role of 8-OHdG and oxidative stress in papillary thyroid carcinoma. Tumor Biol. 2015, 36, 2667–2674. [Google Scholar] [CrossRef]
- Koduru, B.; Tejaswini Thakur, A.; Kamath, S.U.; Shenoy, K.R.; Kamath, U.; Reshma, K. Indicators of oxidative stress in thyroid cancer. Indian J. Biochem. Biophys. 2010, 47, 121–123. [Google Scholar]
- Liu, P.; Wang, Y.; Yang, G.; Zhang, Q.; Meng, L.; Xin, Y.; Jiang, X. The role of short-chain fatty acids in intestinal barrier function, inflammation, oxidative stress, and colonic carcinogenesis. Pharmacol. Res. 2021, 165, 105420. [Google Scholar] [CrossRef]
- Erdamar, H.; Çimen, B.; Gülcemal, H.; Saraymen, R.; Yerer, B.; Demirci, H. Increased lipid peroxidation and impaired enzymatic antioxidant defense mechanism in thyroid tissue with multinodular goiter and papillary carcinoma. Clin. Biochem. 2010, 43, 650–654. [Google Scholar] [CrossRef]
- Donmez-Altuntas, H.; Bayram, F.; Bitgen, N.; Ata, S.; Hamurcu, Z.; Baskol, G. Increased Chromosomal and Oxidative DNA Damage in Patients with Multinodular Goiter and Their Association with Cancer. Int. J. Endocrinol. 2017, 2017, 2907281. [Google Scholar] [CrossRef] [PubMed]
- James, D.L.; Ryan, É.J.; Davey, M.G.; Quinn, A.J.; Heath, D.P.; Garry, S.J.; Boland, M.R.; Young, O.; Lowery, A.J.; Kerin, M.J. Radioiodine Remnant Ablation for Differentiated Thyroid Cancer: A Systematic Review and Meta-analysis. JAMA Otolaryngol. Head Neck Surg. 2021, 147, 544. [Google Scholar] [CrossRef] [PubMed]
- Abshire, D.; Lang, M.K. The Evolution of Radiation Therapy in Treating Cancer. Semin. Oncol. Nurs. 2018, 34, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Brierley, J.; Sherman, E. The Role of External Beam Radiation and Targeted Therapy in Thyroid Cancer. Semin. Radiat. Oncol. 2012, 22, 254–262. [Google Scholar] [CrossRef]
- Bugris, V.; Harmat, V.; Ferenc, G.; Brockhauser, S.; Carmichael, I.; Garman, E.F. Radiation-damage investigation of a DNA 16-mer. J. Synchrotron Radiat. 2019, 26 Pt 4, 998–1009. [Google Scholar] [CrossRef]
- Signore, A.; Campagna, G.; Marinaccio, J.; de Vitis, M.; Lauri, C.; Berardinelli, F.; Tofani, A.; Chianelli, M.; Borro, M.; Gentile, G.; et al. Analysis of Short-Term and Stable DNA Damage in Patients with Differentiated Thyroid Cancer Treated with 131I in Hypothyroidism or with Recombinant Human Thyroid-Stimulating Hormone for Remnant Ablation. J. Nucl. Med. 2022, 63, 1515–1522. [Google Scholar] [CrossRef]
- Baronia, R.; Singh, M.; Gupta, R.B.; Karuppiah, S.; Kumar, R.; Belz, J.; Shanker, R.; Sridhar, S.; Singh, S.P. Synthesis and characterization of multifunctional gold nanoclusters for application in radiation therapy. Int. J. Nanomed. 2018, 13, 113–115. [Google Scholar] [CrossRef]
- Walters, K. Modelling the probability distribution of the number of DNA double-strand breaks due to sporadic alkylation of nucleotide bases. J. Theor. Biol. 2007, 245, 161–168. [Google Scholar] [CrossRef]
- Singh, A.; Ham, J.; Po, J.; Niles, N.; Roberts, T.; Lee, C. The Genomic Landscape of Thyroid Cancer Tumourigenesis and Implications for Immunotherapy. Cells 2021, 10, 1082. [Google Scholar] [CrossRef]
- Vrndic, O.B.; Radivojevic, S.D.; Jovanovic, M.D.; Djukic, S.M.; Teodorovic, L.C.M.; Simonovic, S.T.Z. Oxidative stress in pa-tients with differentiated thyroid cancer: Early effects of radioiodine therapy. Indian J. Biochem. Biophys. 2014, 51, 223–229. [Google Scholar]
- Buczyńska, A.; Sidorkiewicz, I.; Rogucki, M.; Siewko, K.; Adamska, A.; Kościuszko, M.; Maliszewska, K.; Kozłowska, G.; Szumowski, P.; Myśliwiec, J.; et al. Oxidative stress and radioiodine treatment of differentiated thyroid cancer. Sci. Rep. 2021, 11, 17126. [Google Scholar] [CrossRef]
- Leoni, S.G.; Kimura, E.T.; Santisteban, P.; De la Vieja, A. Regulation of Thyroid Oxidative State by Thioredoxin Reductase Has a Crucial Role in Thyroid Responses to Iodide Excess. Mol. Endocrinol. 2011, 25, 1924–1935. [Google Scholar] [CrossRef] [PubMed]
- Snezhkina, A.V.; Kudryavtseva, A.V.; Kardymon, O.L.; Savvateeva, M.V.; Melnikova, N.V.; Krasnov, G.S.; Dmitriev, A.A. ROS Generation and Antioxidant Defense Systems in Normal and Malignant Cells. Oxidative Med. Cell. Longev. 2019, 2019, 6175804. [Google Scholar] [CrossRef] [PubMed]
- Cazarin, J.; Dupuy, C.; de Carvalho, D.P. Redox Homeostasis in Thyroid Cancer: Implications in Na+/I− Symporter (NIS) Regulation. Int. J. Mol. Sci. 2022, 23, 6129. [Google Scholar] [CrossRef] [PubMed]
- Dinkova-Kostova, A.T.; Talalay, P. Direct and indirect antioxidant properties of inducers of cytoprotective proteins. Mol. Nutr. Food Res. 2008, 52 (Suppl. S1), S128–S138. [Google Scholar] [CrossRef]
- Hagymási, K.; Egresi, A.; Lengyel, G.; Information, R. Antioxidánsok—Antioxidánssokk: Tények és kérdések, 2015 [Antioxidants—antioxidative stress: Facts and questions, 2015]. Orvosi Hetil. 2015, 156, 1884–1887. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, J.; Zhou, H.; Du, Z. Anticancer effects of natural phytochemicals in anaplastic thyroid cancer (Review). Oncol. Rep. 2022, 48, 156. [Google Scholar] [CrossRef]
- Kang, H.J.; Youn, Y.-K.; Hong, M.-K.; Kim, L.S. Antiproliferation and Redifferentiation in Thyroid Cancer Cell Lines by Polyphenol Phytochemicals. J. Korean Med. Sci. 2011, 26, 893–899. [Google Scholar] [CrossRef]
- Zhang, L.; Xu, S.; Cheng, X.; Wu, J.; Wu, L.; Wang, Y.; Wang, X.; Bao, J.; Yu, H. Curcumin induces autophagic cell death in human thyroid cancer cells. Toxicol. Vitr. 2021, 78, 887–2333. [Google Scholar] [CrossRef]
- Birden, N.; Gunel, N.S.; Ozates, N.P.; Bagca, B.G.; Gunduz, C.; Takanlou, L.S.; Takanlou, M.S.; Avci, C.B. The effects of Epigallocatechin-3-gallate and Dabrafenib combination on apoptosis and the genes involved in epigenetic events in anaplastic thyroid cancer cells. Med. Oncol. 2022, 39, 98. [Google Scholar] [CrossRef]
- Murakami, Y.; Nakabeppu, Y.; Sonoda, K.-H. Oxidative Stress and Microglial Response in Retinitis Pigmentosa. Int. J. Mol. Sci. 2020, 21, 7170. [Google Scholar] [CrossRef]
- Bronowicka-Szydełko, A.; Kotyra, Ł.; Lewandowski, Ł.; Gamian, A.; Kustrzeba-Wójcicka, I. Role of Advanced Glycation End-Products and Other Ligands for AGE Receptors in Thyroid Cancer Progression. J. Clin. Med. 2021, 10, 4084. [Google Scholar] [CrossRef]
- Cheng, S.-Y.; Wu, A.T.H.; Batiha, G.E.-S.; Ho, C.-L.; Lee, J.-C.; Lukman, H.Y.; Alorabi, M.; AlRasheedi, A.N.; Chen, J.-H. Identification of DPP4/CTNNB1/MET as a Theranostic Signature of Thyroid Cancer and Evaluation of the Therapeutic Potential of Sitagliptin. Biology 2022, 11, 324. [Google Scholar] [CrossRef]
- Liberti, M.V.; Locasale, J.W. The Warburg Effect: How Does it Benefit Cancer Cells? Trends Biochem. Sci. 2016, 41, 211–218, Erratum in Trends Biochem. Sci. 2016, 41, 287. [Google Scholar] [CrossRef] [PubMed]
- Wright, E.M. SGLT2 and cancer. Pflügers Arch.—Eur. J. Physiol. 2020, 472, 1407–1414, Erratum in Pflügers Arch.—Eur. J. Physiol. 2021, 473, 1807. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yang, L.; Mao, L.; Zhang, L.; Zhu, Y.; Xu, Y.; Cheng, Y.; Sun, R.; Zhang, Y.; Ke, J.; et al. SGLT2 inhibition restrains thyroid cancer growth via G1/S phase transition arrest and apoptosis mediated by DNA damage response signaling pathways. Cancer Cell Int. 2022, 22, 74. [Google Scholar] [CrossRef] [PubMed]

| ATA high-risk (>20%) category |
|
| ATA intermediate risk (5–20%) category |
|
| ATA low risk (<5%) category |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kościuszko, M.; Buczyńska, A.; Krętowski, A.J.; Popławska-Kita, A. Could Oxidative Stress Play a Role in the Development and Clinical Management of Differentiated Thyroid Cancer? Cancers 2023, 15, 3182. https://doi.org/10.3390/cancers15123182
Kościuszko M, Buczyńska A, Krętowski AJ, Popławska-Kita A. Could Oxidative Stress Play a Role in the Development and Clinical Management of Differentiated Thyroid Cancer? Cancers. 2023; 15(12):3182. https://doi.org/10.3390/cancers15123182
Chicago/Turabian StyleKościuszko, Maria, Angelika Buczyńska, Adam Jacek Krętowski, and Anna Popławska-Kita. 2023. "Could Oxidative Stress Play a Role in the Development and Clinical Management of Differentiated Thyroid Cancer?" Cancers 15, no. 12: 3182. https://doi.org/10.3390/cancers15123182
APA StyleKościuszko, M., Buczyńska, A., Krętowski, A. J., & Popławska-Kita, A. (2023). Could Oxidative Stress Play a Role in the Development and Clinical Management of Differentiated Thyroid Cancer? Cancers, 15(12), 3182. https://doi.org/10.3390/cancers15123182





