METTL3-Modulated circUHRF2 Promotes Colorectal Cancer Stemness and Metastasis through Increasing DDX27 mRNA Stability by Recruiting IGF2BP1
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Clinical Specimens
2.2. Cell Culture
2.3. Cell Transfection or Infection
2.4. Subcellular Fractionation
2.5. Sanger Sequencing and RNase R Treatment
2.6. Fluorescence In Situ Hybridization (FISH)
2.7. Tumor Spheroid Formation
2.8. Expression Profiling of CD133 by Flow Cytometry
2.9. Cell Invasion Assay
2.10. Scratch Wound-Healing Assay
2.11. DDX27 mRNA Stability Assay
2.12. Methylated RNA Immunoprecipitation (MeRIP) Assay
2.13. RNA Pull-Down Assay
2.14. RNA-Protein Immunoprecipitation (RIP) Assay
2.15. Animal Experiments
2.16. Hematoxylin and Eosin (H&E) Staining
2.17. Immunohistochemistry (IHC)
2.18. RNA Isolation and Quantitative Real-Time PCR (qRT-PCR)
2.19. Western Blot Analysis
2.20. Statistical Analysis
3. Results
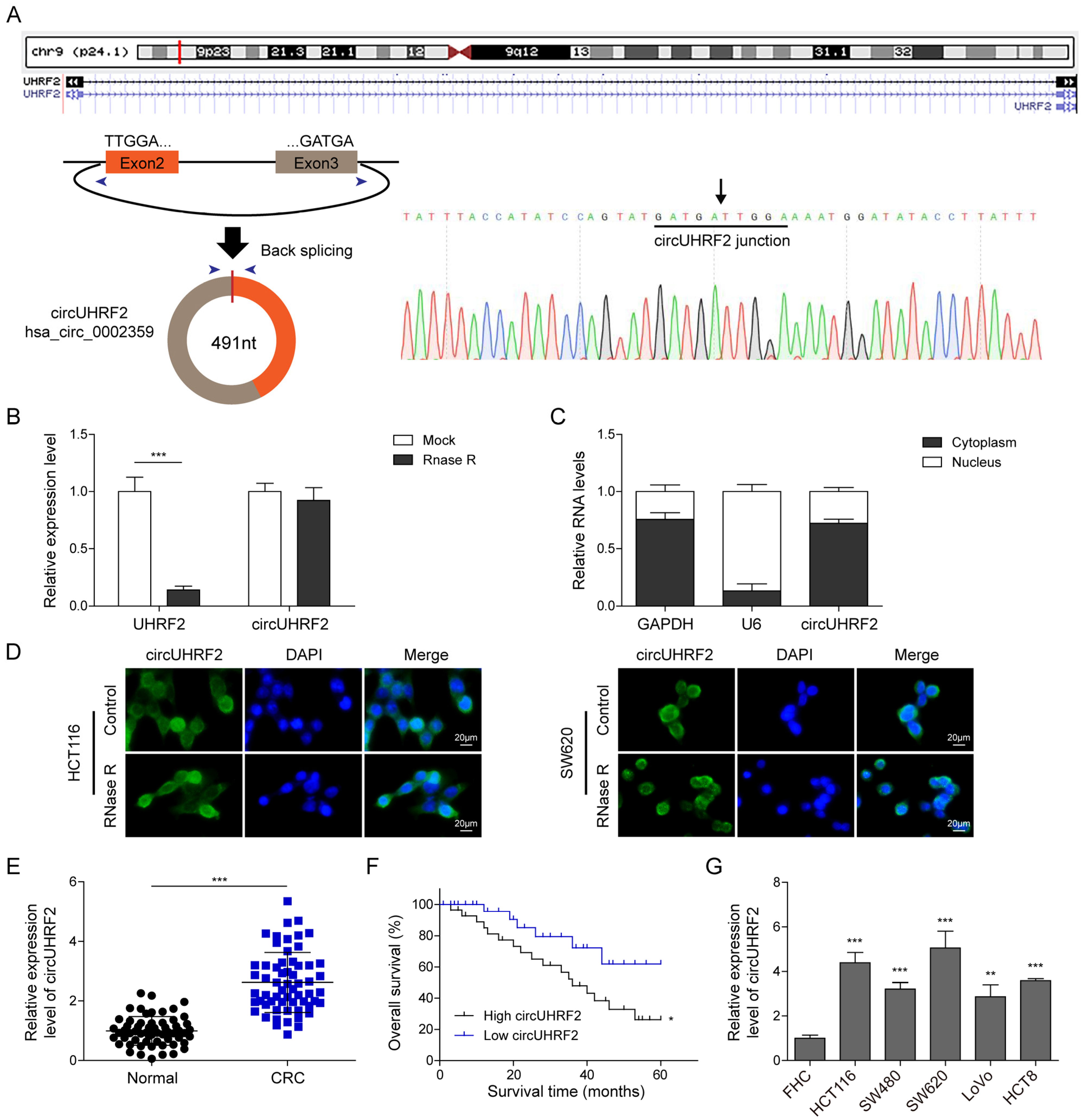
3.1. CircUHRF2 Was Highly Expressed in CRC and Positively Correlated with Poor Prognosis
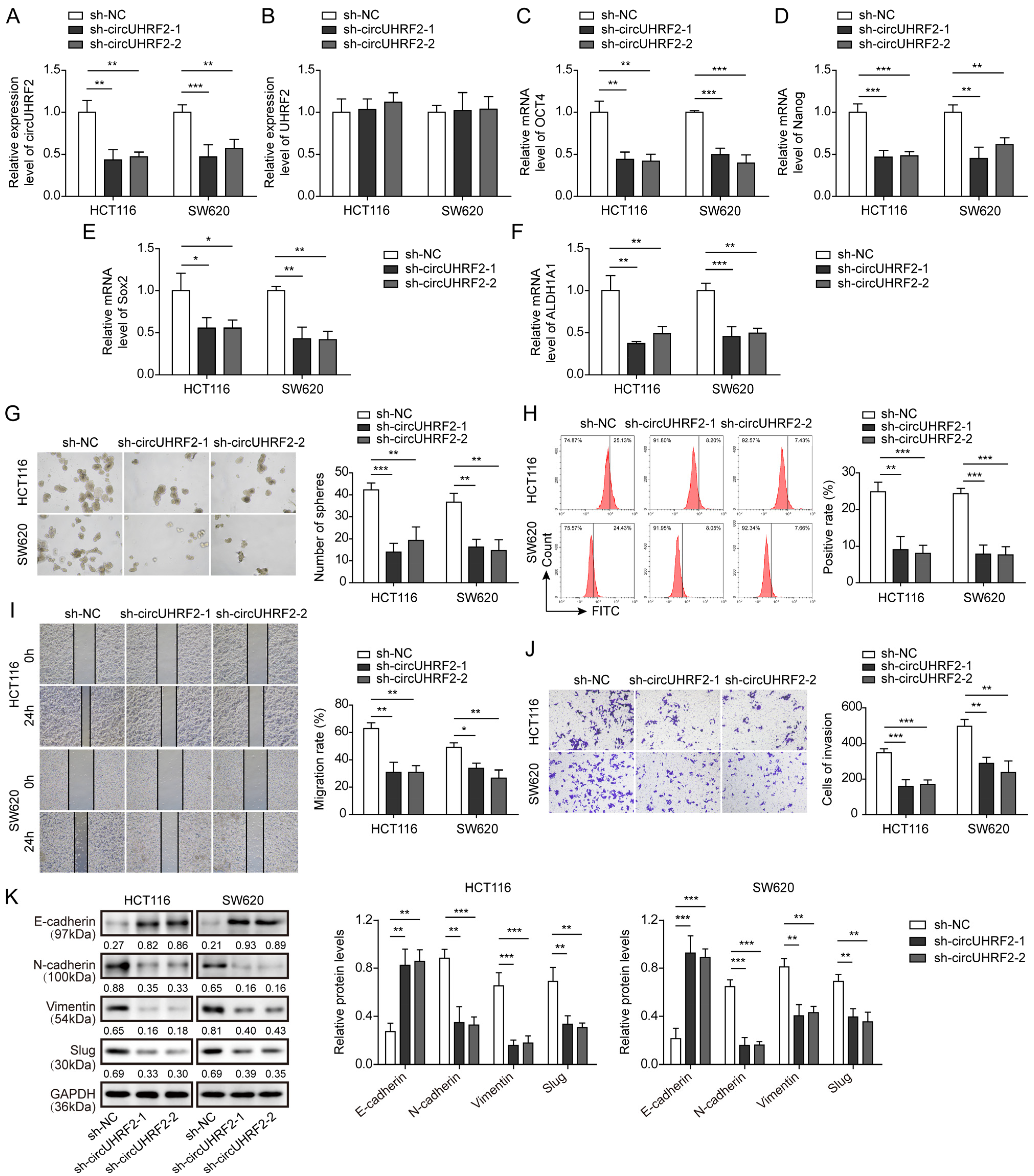
3.2. Knockdown of circUHRF2 Suppressed CRC Stemness, Migration, and EMT
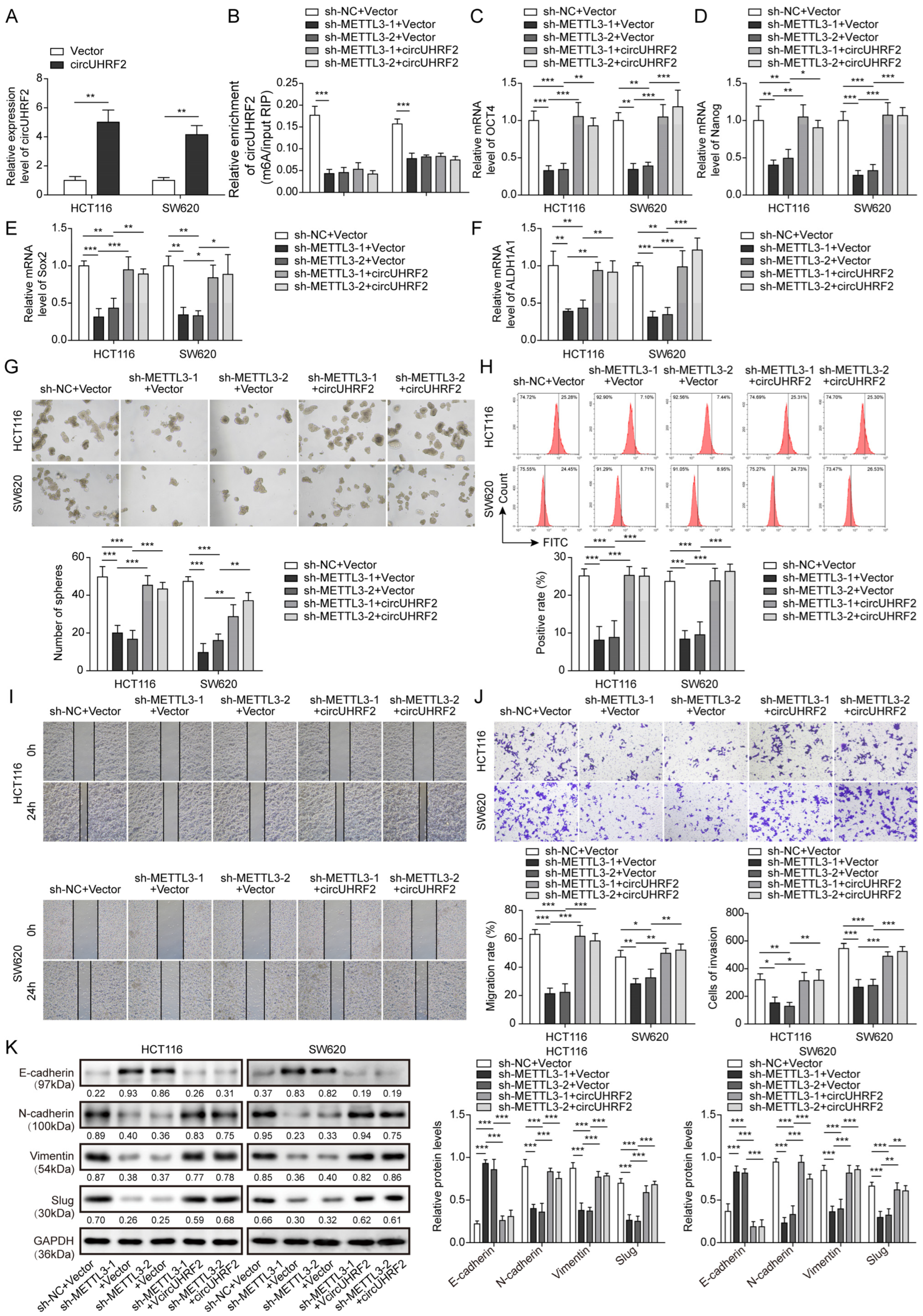
3.3. METTL3 Was Highly Expressed in CRC and Enhanced circUHRF2 Expression through m6A Modification
3.4. Downregulation of METTL3 Suppressed CRC Stemness, Migration, and EMT by Decreasing circUHRF2 Expression
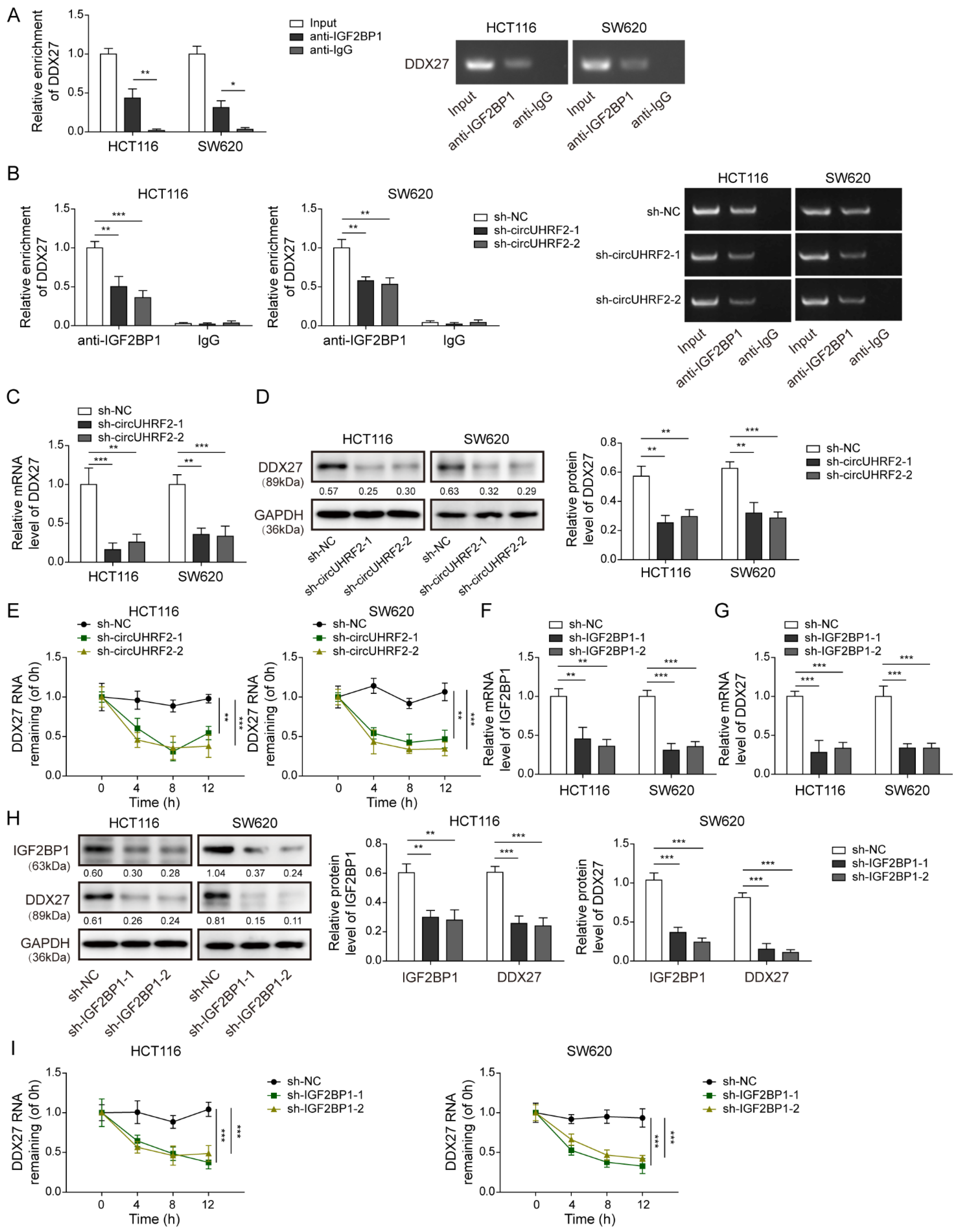
3.5. CircUHRF2 Directly Bound to IGF2BP1
3.6. CircUHRF2 Restrained Loss of DDX27 Protein via Recruitment of IGF2BP1
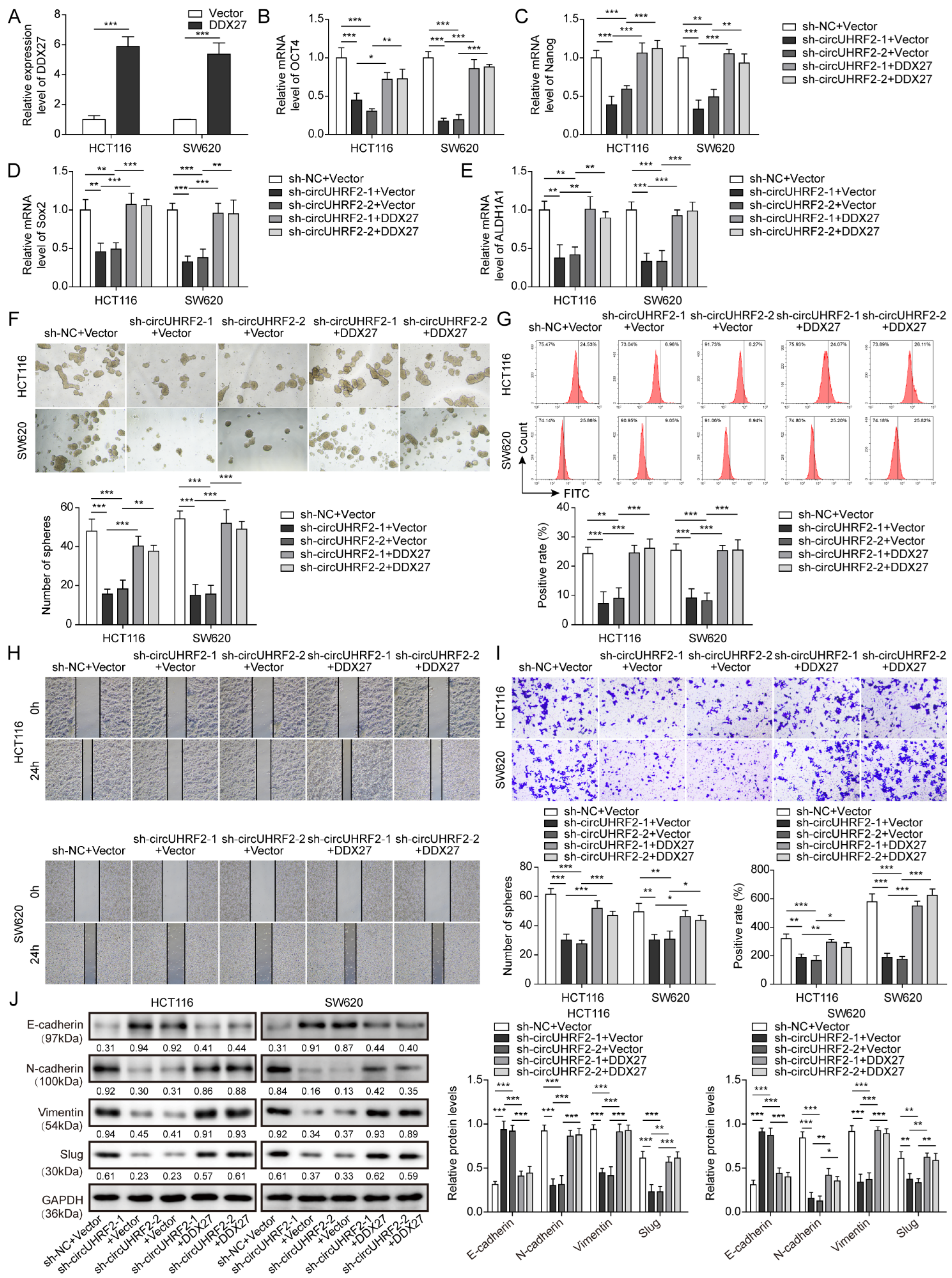
3.7. CircUHRF2 Silencing-Mediated Inhibition in CRC Stemness, Migration, and EMT Was Reversed by DDX27 Overexpression
3.8. Knockdown of circUHRF2 or METTL3 Suppressed CRC Growth, Stemness, and Metastasis in Nude Mice through Regulation of DDX27 Protein
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Goding Sauer, A.; Fedewa, S.A.; Butterly, L.F.; Anderson, J.C.; Cercek, A.; Smith, R.A.; Jemal, A. Colorectal cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 145–164. [Google Scholar] [CrossRef] [PubMed]
- Pretzsch, E.; Bosch, F.; Neumann, J.; Ganschow, P.; Bazhin, A.; Guba, M.; Werner, J.; Angele, M. Mechanisms of Metastasis in Colorectal Cancer and Metastatic Organotropism: Hematogenous versus Peritoneal Spread. J. Oncol. 2019, 2019, 7407190. [Google Scholar] [CrossRef]
- Li, S.; Han, Z.; Zhao, N.; Zhu, B.; Zhang, Q.; Yang, X.; Sheng, D.; Hou, J.; Guo, S.; Wei, L.; et al. Inhibition of DNMT suppresses the stemness of colorectal cancer cells through down-regulating Wnt signaling pathway. Cell. Signal. 2018, 47, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yan, Q.; Mo, Y.; Liu, Y.; Zhang, S.; Guo, C.; Wang, F.; Li, G.; Zeng, Z.; Xiong, W. Splicing factor derived circular RNA circCAMSAP1 accelerates nasopharyngeal carcinoma tumorigenesis via a SERPINH1/c-Myc positive feedback loop. Mol. Cancer 2022, 21, 62. [Google Scholar] [CrossRef]
- Yang, K.D.; Wang, Y.; Zhang, F.; Luo, B.H.; Feng, D.Y.; Zeng, Z.J. CircN4BP2L2 promotes colorectal cancer growth and metastasis through regulation of the miR-340-5p/CXCR4 axis. Lab. Investig. 2022, 102, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Dong, X.; Yan, B.; Yu, W.; Shan, L. CircAGFG1 drives metastasis and stemness in colorectal cancer by modulating YY1/CTNNB1. Cell Death Dis. 2020, 11, 542. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Li, X.; Meng, Q.; Sun, H.; Wu, S.; Hu, W.; Liu, G.; Yang, Y.; Chen, R. CircPTK2 (hsa_circ_0005273) as a novel therapeutic target for metastatic colorectal cancer. Mol. Cancer 2020, 19, 13. [Google Scholar] [CrossRef]
- Zhou, Y.; Pei, Z.; Maimaiti, A.; Zheng, L.; Zhu, Z.; Tian, M.; Zhou, Z.; Tan, F.; Pei, Q.; Li, Y.; et al. m(6)A methyltransferase KIAA1429 acts as an oncogenic factor in colorectal cancer by regulating SIRT1 in an m(6)A-dependent manner. Cell Death Discov. 2022, 8, 83. [Google Scholar] [CrossRef]
- He, P.C.; He, C. m(6) A RNA methylation: From mechanisms to therapeutic potential. EMBO J. 2021, 40, e105977. [Google Scholar] [CrossRef]
- Hu, Y.; Gao, Q.; Ma, S.; Yu, P.; Ding, S.; Yao, X.; Zhang, Z.; Lu, S.; Lu, M.; Zhang, J.; et al. FMR1 promotes the progression of colorectal cancer cell by stabilizing EGFR mRNA in an m(6)A-dependent manner. Cell Death Dis. 2022, 13, 941. [Google Scholar] [CrossRef]
- Li, F.; Yi, Y.; Miao, Y.; Long, W.; Long, T.; Chen, S.; Cheng, W.; Zou, C.; Zheng, Y.; Wu, X.; et al. N(6)-Methyladenosine Modulates Nonsense-Mediated mRNA Decay in Human Glioblastoma. Cancer Res. 2019, 79, 5785–5798. [Google Scholar] [CrossRef]
- Wang, Q.; Guo, X.; Li, L.; Gao, Z.; Su, X.; Ji, M.; Liu, J. N(6)-methyladenosine METTL3 promotes cervical cancer tumorigenesis and Warburg effect through YTHDF1/HK2 modification. Cell Death Dis. 2020, 11, 911. [Google Scholar] [CrossRef]
- Li, T.; Hu, P.S.; Zuo, Z.; Lin, J.F.; Li, X.; Wu, Q.N.; Chen, Z.H.; Zeng, Z.L.; Wang, F.; Zheng, J.; et al. METTL3 facilitates tumor progression via an m(6)A-IGF2BP2-dependent mechanism in colorectal carcinoma. Mol. Cancer 2019, 18, 112. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Yuan, W.; Zhou, Q.; Shao, B.; Guo, Y.; Wang, W.; Yang, S.; Zhao, L.; Dang, Q.; Yang, X.; et al. N6-methyladenosine-induced circ1662 promotes metastasis of colorectal cancer by accelerating YAP1 nuclear localization. Theranostics 2021, 11, 4298–4315. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.X.; Chen, X.; Xia, L.P.; Zhang, J.X.; Pan, Z.Z.; Ma, X.D.; Han, K.; Chen, J.W.; Judde, J.G.; Deas, O.; et al. N(6)-methyladenosine modification of circNSUN2 facilitates cytoplasmic export and stabilizes HMGA2 to promote colorectal liver metastasis. Nat. Commun. 2019, 10, 4695. [Google Scholar] [CrossRef]
- Chen, H.M.; Lin, C.C.; Chen, W.S.; Jiang, J.K.; Yang, S.H.; Chang, S.C.; Ho, C.L.; Yang, C.C.; Huang, S.C.; Chao, Y.; et al. Insulin-Like Growth Factor 2 mRNA-Binding Protein 1 (IGF2BP1) Is a Prognostic Biomarker and Associated with Chemotherapy Responsiveness in Colorectal Cancer. Int. J. Mol. Sci. 2021, 22, 6940. [Google Scholar] [CrossRef]
- Zhang, X.L.; Li, K.J.; Feng, J.X.; Liu, G.J.; Feng, Y.L. Blocking the IGF2BP1-promoted glucose metabolism of colon cancer cells via direct de-stabilizing mRNA of the LDHA enhances anticancer effects. Mol. Ther. Nucleic Acids 2021, 23, 835–846. [Google Scholar] [CrossRef]
- Xie, F.; Huang, C.; Liu, F.; Zhang, H.; Xiao, X.; Sun, J.; Zhang, X.; Jiang, G. CircPTPRA blocks the recognition of RNA N(6)-methyladenosine through interacting with IGF2BP1 to suppress bladder cancer progression. Mol. Cancer 2021, 20, 68. [Google Scholar] [CrossRef]
- Gavert, N.; Sheffer, M.; Raveh, S.; Spaderna, S.; Shtutman, M.; Brabletz, T.; Barany, F.; Paty, P.; Notterman, D.; Domany, E.; et al. Expression of L1-CAM and ADAM10 in human colon cancer cells induces metastasis. Cancer Res. 2007, 67, 7703–7712. [Google Scholar] [CrossRef] [PubMed]
- Cheriyamundath, S.; Kumar, A.; Gavert, N.; Brabletz, T.; Ben-Ze’ev, A. The Collagen-Modifying Enzyme PLOD2 Is Induced and Required during L1-Mediated Colon Cancer Progression. Int. J. Mol. Sci. 2021, 22, 3552. [Google Scholar] [CrossRef]
- Li, H.; Cao, B.; Zhao, R.; Li, T.; Xu, X.; Cui, H.; Deng, H.; Gao, J.; Wei, B. circDNMT1 Promotes Malignant Progression of Gastric Cancer Through Targeting miR-576-3p/Hypoxia Inducible Factor-1 Alpha Axis. Front. Oncol. 2022, 12, 817192. [Google Scholar] [CrossRef] [PubMed]
- Malvezzi, M.; Carioli, G.; Bertuccio, P.; Boffetta, P.; Levi, F.; La Vecchia, C.; Negri, E. European cancer mortality predictions for the year 2018 with focus on colorectal cancer. Ann. Oncol. 2018, 29, 1016–1022. [Google Scholar] [CrossRef] [PubMed]
- Kristensen, L.S.; Jakobsen, T.; Hager, H.; Kjems, J. The emerging roles of circRNAs in cancer and oncology. Nat. Rev. Clin. Oncol. 2022, 19, 188–206. [Google Scholar] [CrossRef]
- Zhou, J.; Wang, L.; Sun, Q.; Chen, R.; Zhang, C.; Yang, P.; Tan, Y.; Peng, C.; Wang, T.; Jin, C.; et al. Hsa_circ_0001666 suppresses the progression of colorectal cancer through the miR-576-5p/PCDH10 axis. Clin. Transl. Med. 2021, 11, e565. [Google Scholar] [CrossRef]
- Zhang, X.; Yao, J.; Shi, H.; Gao, B.; Zhou, H.; Zhang, Y.; Zhao, D.; Gao, S.; Wang, C.; Zhang, L. Hsa_circ_0026628 promotes the development of colorectal cancer by targeting SP1 to activate the Wnt/beta-catenin pathway. Cell Death Dis. 2021, 12, 802. [Google Scholar] [CrossRef]
- Lu, S.; Yan, D.; Wu, Z.; Jiang, T.; Chen, J.; Yuan, L.; Lin, J.; Peng, Z.; Tang, H. Ubiquitin-like with PHD and ring finger domains 2 is a predictor of survival and a potential therapeutic target in colon cancer. Oncol. Rep. 2014, 31, 1802–1810. [Google Scholar] [CrossRef]
- Zheng, W.; Dong, X.; Zhao, Y.; Wang, S.; Jiang, H.; Zhang, M.; Zheng, X.; Gu, M. Multiple Functions and Mechanisms Underlying the Role of METTL3 in Human Cancers. Front. Oncol. 2019, 9, 1403. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Gao, S.; Liu, W.; Wong, C.C.; Wu, J.; Liu, D.; Gou, H.; Kang, W.; Zhai, J.; Li, C.; et al. RNA N(6)-Methyladenosine Methyltransferase METTL3 Facilitates Colorectal Cancer by Activating the m(6)A-GLUT1-mTORC1 Axis and Is a Therapeutic Target. Gastroenterology 2021, 160, 1284–1300.e16. [Google Scholar] [CrossRef]
- Tian, J.; Ying, P.; Ke, J.; Zhu, Y.; Yang, Y.; Gong, Y.; Zou, D.; Peng, X.; Yang, N.; Wang, X.; et al. ANKLE1 N(6) -Methyladenosine-related variant is associated with colorectal cancer risk by maintaining the genomic stability. Int. J. Cancer 2020, 146, 3281–3293. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Wu, J.; Liu, F.; He, J.; Wu, F.; Chen, J.; Jiang, Z. IGF2BP1 Promotes the Liver Cancer Stem Cell Phenotype by Regulating MGAT5 mRNA Stability by m6A RNA Methylation. Stem Cells Dev. 2021, 30, 1115–1125. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wan, Y.; Zhang, Z.; Jiang, Y.; Gu, Z.; Ma, X.; Nie, S.; Yang, J.; Lang, J.; Cheng, W.; et al. IGF2BP1 overexpression stabilizes PEG10 mRNA in an m6A-dependent manner and promotes endometrial cancer progression. Theranostics 2021, 11, 1100–1114. [Google Scholar] [CrossRef]
- Zhu, S.; Wang, J.Z.; Chen, D.; He, Y.T.; Meng, N.; Chen, M.; Lu, R.X.; Chen, X.H.; Zhang, X.L.; Yan, G.R. An oncopeptide regulates m(6)A recognition by the m(6)A reader IGF2BP1 and tumorigenesis. Nat. Commun. 2020, 11, 1685. [Google Scholar] [CrossRef]
- Huang, Q.; Guo, H.; Wang, S.; Ma, Y.; Chen, H.; Li, H.; Li, J.; Li, X.; Yang, F.; Qiu, M.; et al. A novel circular RNA, circXPO1, promotes lung adenocarcinoma progression by interacting with IGF2BP1. Cell Death Dis. 2020, 11, 1031. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Ma, J.; Zheng, A.; Song, X.; Chen, S.; Jin, F. DEAD-box helicase 27 enhances stem cell-like properties with poor prognosis in breast cancer. J. Transl. Med. 2021, 19, 334. [Google Scholar] [CrossRef]
- Tang, J.; Chen, H.; Wong, C.C.; Liu, D.; Li, T.; Wang, X.; Ji, J.; Sung, J.J.; Fang, J.Y.; Yu, J. DEAD-box helicase 27 promotes colorectal cancer growth and metastasis and predicts poor survival in CRC patients. Oncogene 2018, 37, 3006–3021. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Li, D.; Bai, Y.; Song, S.; Yan, P.; Wu, R.; Zhang, Y.; Hu, G.; Lin, C.; Li, X.; et al. DEAD-box helicase 27 plays a tumor-promoter role by regulating the stem cell-like activity of human colorectal cancer cells. Onco Targets Ther. 2019, 12, 233–241. [Google Scholar] [CrossRef]



| Genes | Primer Sequences (5′-3′) |
|---|---|
| circUHRF2 | F: 5′-TTCAGACTGTGTTGCTGCTGAT-3′ |
| R: 5′-CAGGAAGATGATCAGGGTCTGG-3′ | |
| OCT4 | F: 5′-CCCGAAAGAGAAAGCGAACC-3′ |
| R: 5′-GCAGCCTCAAAATCCTCTCG-3′ | |
| Nanog | F: 5′-GTCCCAAAGGCAAACAACCC-3′ |
| R: 5′-ATCCCTGCGTCACACCATTG-3′ | |
| Sox2 | F: 5′-GCCCTGCAGTACAACTCCAT-3′ |
| R: 5′-GACTTGACCACCGAACCCAT-3′ | |
| ALDH1A1 | F: 5′-GATCCCCGTGGCGTACTATG-3′ |
| R: 5′-TGGATCTTGTCAGCCCAACC-3′ | |
| METTL3 | F: 5′-GAGTGCATGAAAGCCAGTGA-3′ |
| R: 5′-CTGGAATCACCTCCGACACT-3′ | |
| DDX27 | F: 5′-CCGCAGTGCTGATTTCAACC-3′ |
| R: 5′-GCTCCAGGCTGAGGAAATGG-3′ | |
| GAPDH | F: 5′-CCAGGTGGTCTCCTCTGA-3′ |
| R: 5′-GCTGTAGCCAAATCGTTGT-3′ |
| Clinical Parameters | Cases (n) | circUHRF2 Expression | p-Value (* p < 0.05) | |
|---|---|---|---|---|
| High (n) | Low (n) | |||
| Age | 0.796 | |||
| <60 years | 29 | 15 | 14 | |
| ≥60 years | 31 | 15 | 16 | |
| Gender | 0.559 | |||
| Male | 44 | 23 | 21 | |
| Female | 16 | 7 | 9 | |
| Tumor size (cm) | 0.018 * | |||
| <5 | 25 | 8 | 17 | |
| ≥5 | 35 | 22 | 13 | |
| TNM stage | 0.035 * | |||
| I/II | 24 | 8 | 16 | |
| III/IV | 36 | 22 | 14 | |
| Local invasion | 0.414 | |||
| T1/T2 | 20 | 9 | 11 | |
| T3/T4 | 40 | 21 | 19 | |
| Differentiation | 0.069 | |||
| Poor | 27 | 17 | 10 | |
| Moderate/High | 33 | 13 | 20 | |
| Lymph node metastasis | 0.19 | |||
| Yes | 35 | 20 | 15 | |
| No | 25 | 10 | 15 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, M.; Tian, B.; Hu, G.; Guo, Y. METTL3-Modulated circUHRF2 Promotes Colorectal Cancer Stemness and Metastasis through Increasing DDX27 mRNA Stability by Recruiting IGF2BP1. Cancers 2023, 15, 3148. https://doi.org/10.3390/cancers15123148
Chen M, Tian B, Hu G, Guo Y. METTL3-Modulated circUHRF2 Promotes Colorectal Cancer Stemness and Metastasis through Increasing DDX27 mRNA Stability by Recruiting IGF2BP1. Cancers. 2023; 15(12):3148. https://doi.org/10.3390/cancers15123148
Chicago/Turabian StyleChen, Miao, Buning Tian, Gui Hu, and Yihang Guo. 2023. "METTL3-Modulated circUHRF2 Promotes Colorectal Cancer Stemness and Metastasis through Increasing DDX27 mRNA Stability by Recruiting IGF2BP1" Cancers 15, no. 12: 3148. https://doi.org/10.3390/cancers15123148
APA StyleChen, M., Tian, B., Hu, G., & Guo, Y. (2023). METTL3-Modulated circUHRF2 Promotes Colorectal Cancer Stemness and Metastasis through Increasing DDX27 mRNA Stability by Recruiting IGF2BP1. Cancers, 15(12), 3148. https://doi.org/10.3390/cancers15123148







