Location of Recurrences after Trimodality Treatment for Glioblastoma with Respect to the Delivered Radiation Dose Distribution and Its Influence on Prognosis
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Treatment Sequence, Radiation Therapy Planning and Radiation Therapy
2.2. Margin Definition and Clinical Target Volume Definition
2.3. Identification of Recurrence and Recurrence Pattern
2.4. Statistics, End-Points
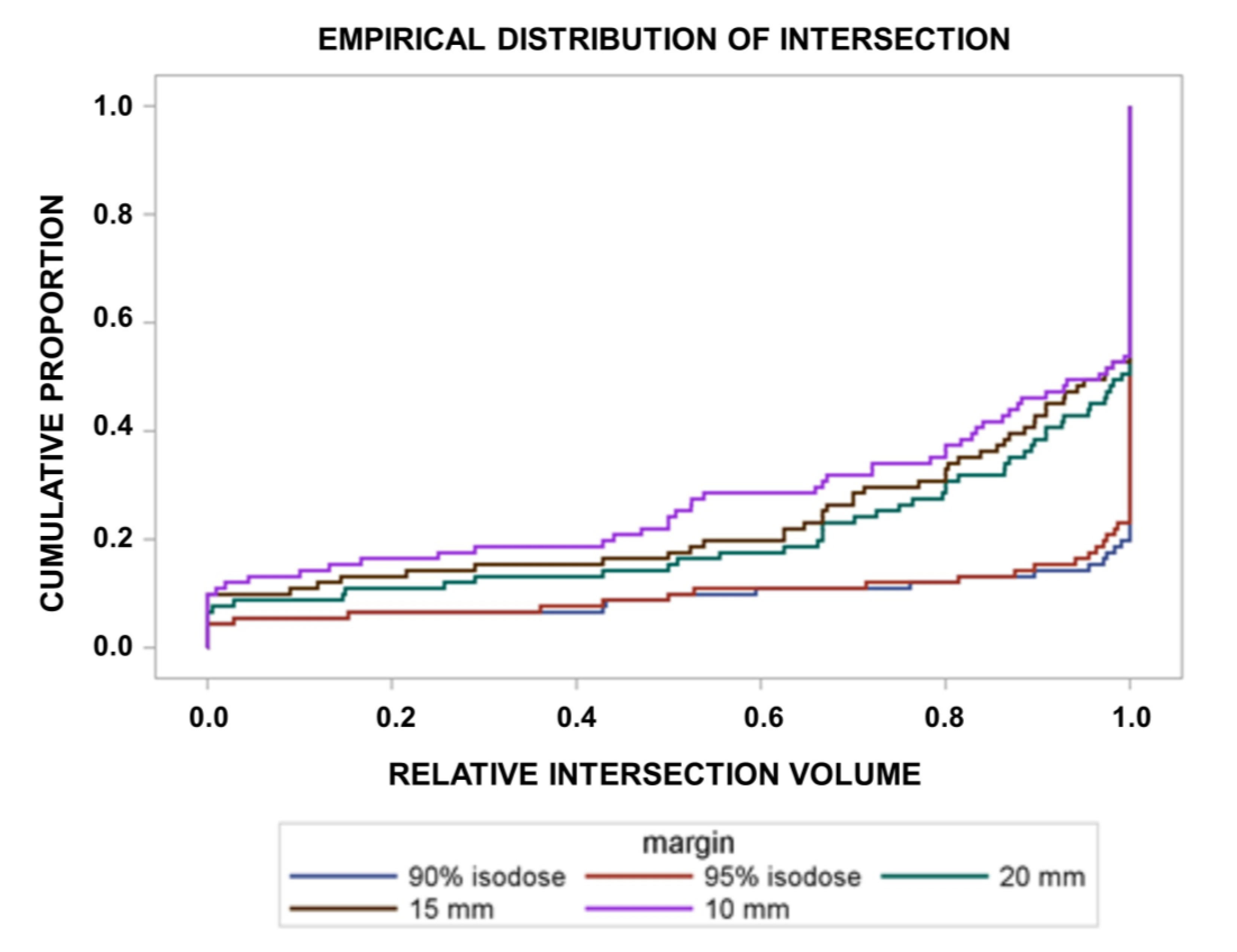
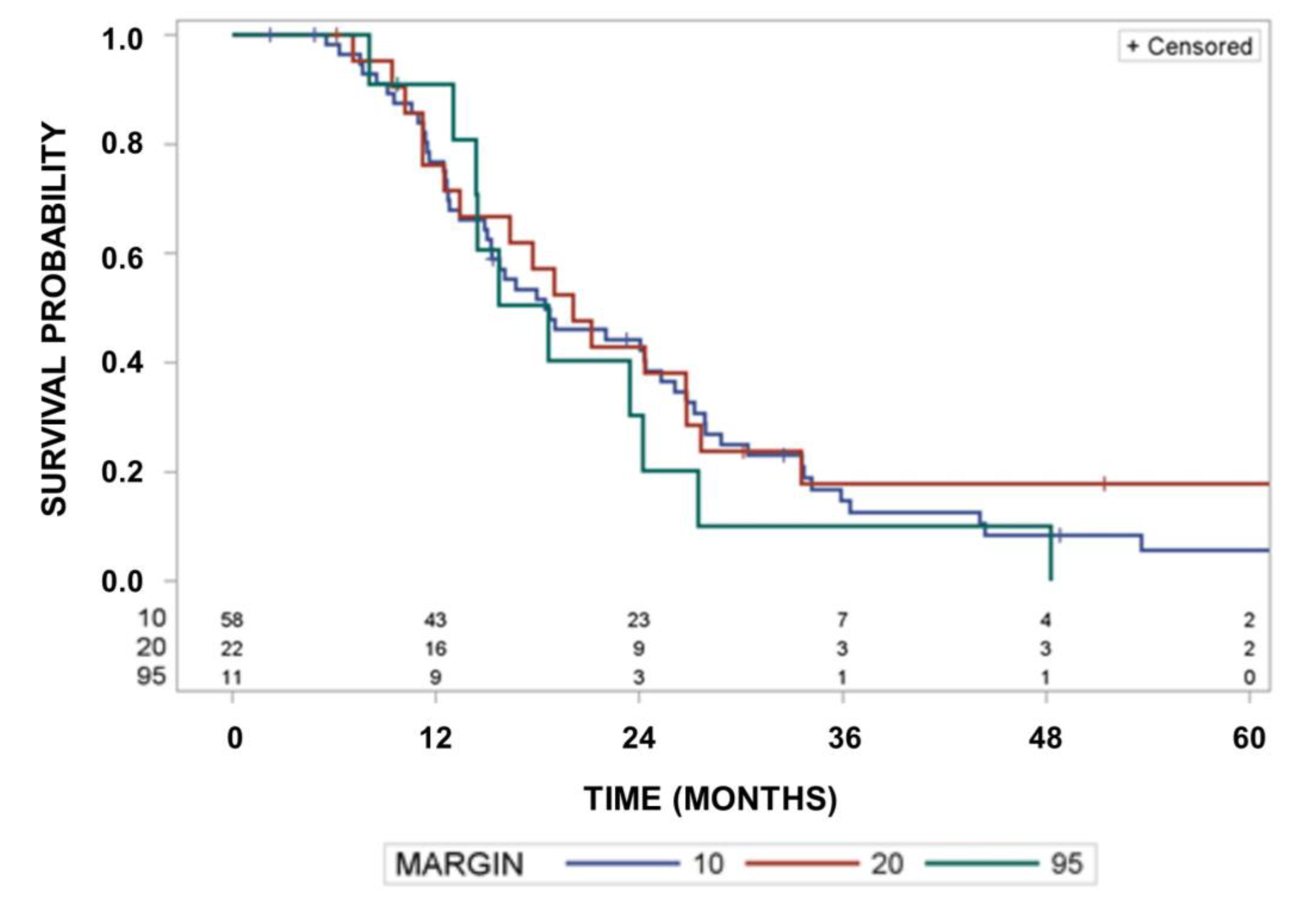
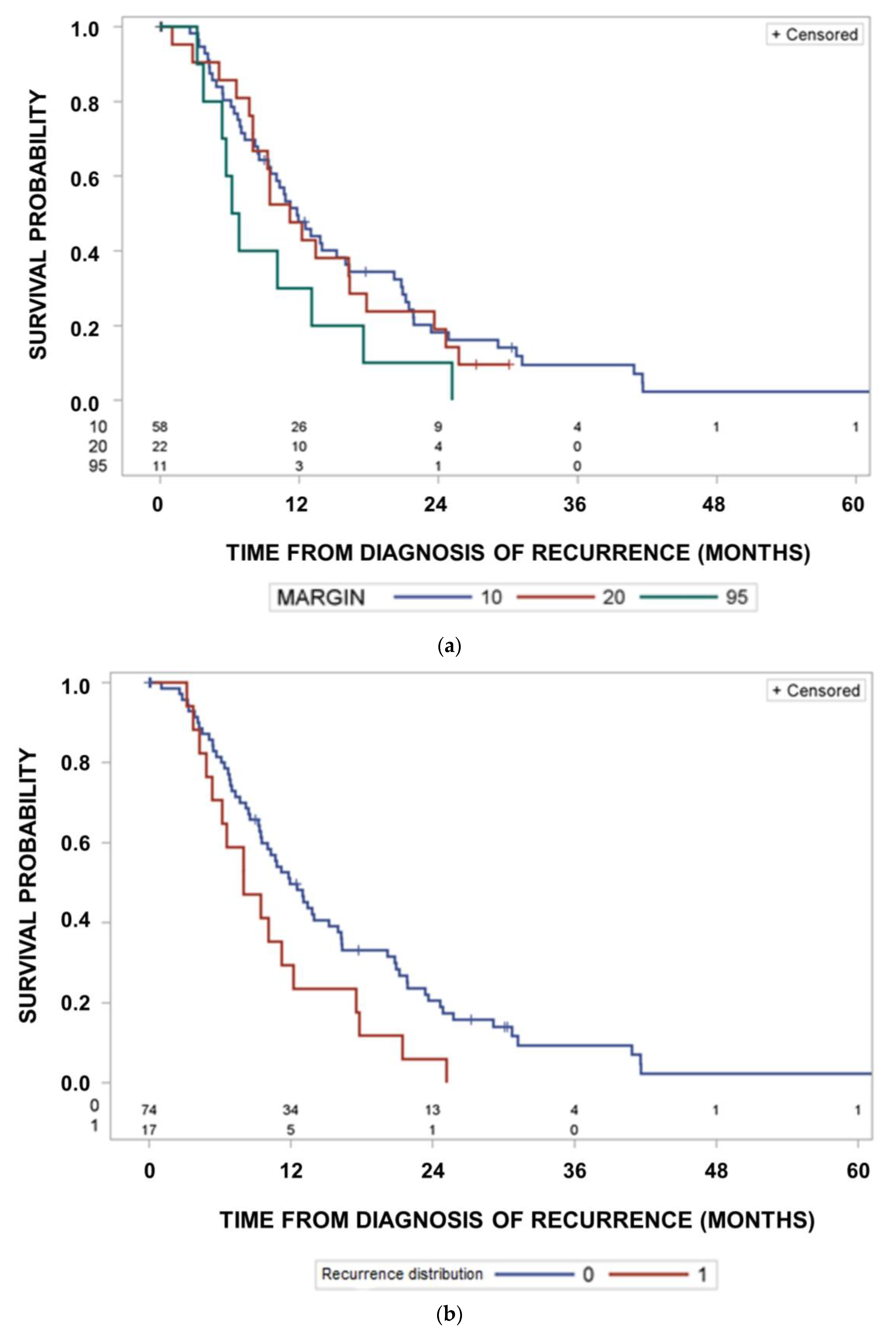
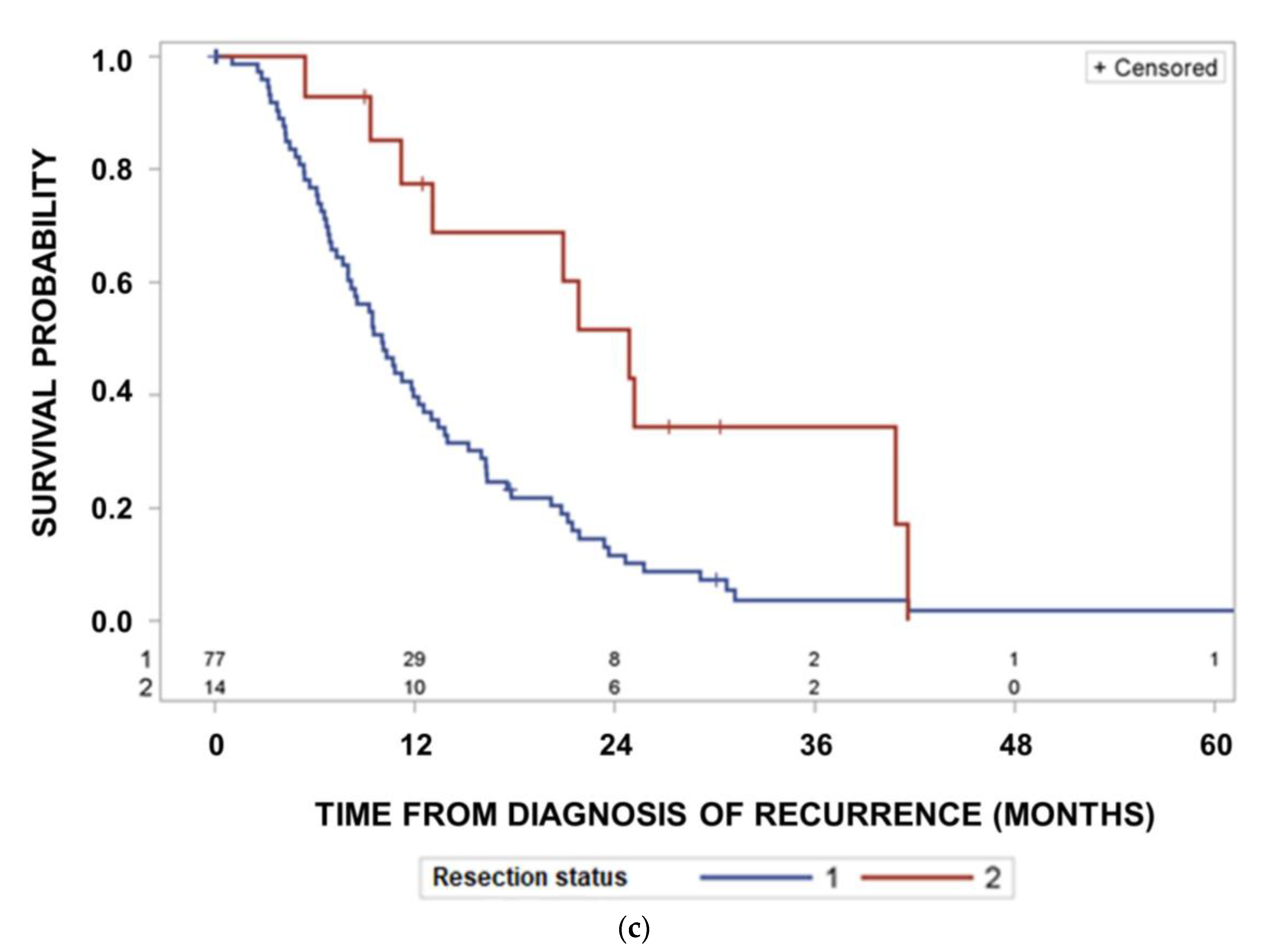
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Niyazi, M.; Brada, M.; Chalmers, A.J.; Combs, S.E.; Erridge, S.C.; Fiorentino, A.; Grosu, A.L.; Lagerwaard, F.J.; Minniti, G.; Mirimanoff, R.-O.; et al. ESTRO-ACROP guideline “target delineation of glioblastomas”. Radiother. Oncol. 2016, 118, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Cabrera, A.R.; Kirkpatrick, J.P.; Fiveash, J.B.; Shih, H.A.; Koay, E.J.; Lutz, S.; Petit, J.; Chao, S.T.; Brown, P.D.; Vogelbaum, M.; et al. Radiation therapy for glioblastoma: Executive summary of an American Society for Radiation Oncology Evidence-Based Clinical Practice Guideline. Pract. Radiat. Oncol. 2016, 6, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, M.R.; Dignan, J.J.; Armstrong, T.S.; Wefel, J.S.; Blumenthal, D.T.; Vogelbaum, M.A.; Colman, H.; Chakravarti, A.; Pugh, S.; Won, W.; et al. A randomized trial of bevacizumab for newly dignosed glioblastoma. N. Engl. J. Med. 2014, 370, 699–708. [Google Scholar] [CrossRef]
- McDonald, M.W.; Shu, H.K.; Curran, W.J., Jr.; Crocker, I.R. Pattern of failure after limited margin radiotherapy and temozolomide for glioblastoma. Int. J. Radiat. Oncol. Biol. Phys. 2011, 79, 130–136. [Google Scholar] [CrossRef] [PubMed]
- Kelly, P.J.; Daumas-Duport, C.; Scheithauer, B.W.; Kall, B.A.; Kispert, D.B. Stereotactic Histologic Correlations of Computed Tomography- and Magnetic Resonance Imaging-Defined Abnormalities in Patients with Glial Neoplasms. Mayo Clin. Proc. 1987, 62, 450–459. [Google Scholar] [CrossRef]
- Levegrün, S.; Garcia, A.S.; Qamhiyeh, S.; Khouya, A.; Huberina, N.; Stuschke, M.; Pöttgen, C. Verbesserte Hippocampus-Schonung durch non-koplanare Techniken im Vergleich zu koplanaren Techniken bei der Stralentherapie von Gliomen. Strahlenther Oncol. 2022, 198 (Suppl. 1), S80. [Google Scholar]
- Gondi, V.; Hermann, B.P.; Mehta, M.P.; Tomé, W.A. Hippocampal Dosimetry Predicts Neurocognitive Function Impairment after Fractionated Stereotactic Radiotherapy for Benign or Low-Grade Adult Brain Tumors. Int. J. Radiat. Oncol. 2013, 85, 348–354. [Google Scholar] [CrossRef]
- Stupp, R.; Taillibert, S.; Kanner, A.; Read, W.; Steinberg, D.M.; Lhermitte, B.; Toms, S.; Idbaih, A.; Ahluwalia, M.S.; Fink, K.; et al. Effect of tumor-treating fields plus maintenance Temozolomide vs maintenance Temozolomide Alone on survival in patients with glioblastoma: A randomized clinical trial. JAMA 2017, 318, 2306–2316. [Google Scholar] [CrossRef]
- Gebhardt, B.J.; Dobelbower, M.C.; Ennis, W.H.; Bag, A.K.; Markert, J.M.; Fiveash, J.B. Patterns of failure for glioblastoma multiforme following limited-margin radiation and concurrent temozolomide. Radiat. Oncol. 2014, 9, 140. [Google Scholar] [CrossRef]
- Paulsson, A.K.; McMullen, K.P.; Peiffer, A.M.; Hinson, W.H.; Kearns, W.T.; Johnson, A.J.; Lesser, G.J.; Ellis, T.L.; Tatter, S.B.; Debinski, W.; et al. Limited margins using modern radiation techniques does not increase marginal failure rate of glioblastoma. Am. J. Clin. Oncol. 2014, 37, 177–181. [Google Scholar] [CrossRef]
- Guram, K.; Smith, M.; Ginader, T.; Bodeker, K.; Pelland, D.; Pennington, E.; Buatti, J.M. Using Smaller-Than-Standard Radiation Treatment Margins Does Not Change Survival Outcomes in Patients with High-Grade Gliomas. Pract. Radiat. Oncol. 2018, 9, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Hassanzadeh, C.; Rudra, S.; Ma, S.; Brenneman, R.; Huang, Y.; Henke, L.; Abraham, C.; Campian, J.; Tsien, C.; Huang, J. Evaluation of interim MRI changes during limited-field radiation therapy for glioblastoma and implications for treatment planning. Radiother. Oncol. 2021, 158, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Kumar, R.; Sharma, S.C.; Mukherjee, A.; Khandelwal, N.; Tripathi, M.; Miriyala, R.; Oinam, A.S.; Madan, R.; Yadav, B.S.; et al. Impact of volume of irradiation on survival and quality of life in glioblastoma: A prospective, phase 2, randomized comparison of RTOG and MDACC protocols. Neuro-Oncol. Pract. 2020, 7, 89–93. [Google Scholar] [CrossRef] [PubMed]
- Ellingson, B.M.; Wen, P.Y.; Cloughesy, T.F. Modified Criteria for Radiographic Response Assessment in Glioblastoma Clinical Trials. Neurotherapeutics 2017, 14, 307–320. [Google Scholar] [CrossRef] [PubMed]
- EORTC 26981/22981. Available online: https://clinicaltrials.gov/ct2/show/NCT00006353 (accessed on 22 September 2022).
- Stupp, R.; Mason, W.P.; van den Bent, M.J.; Weller, M.; Fisher, B.; Taphoorn, M.J.B.; Belanger, K.; Brandes, A.A.; Marosi, C.; Bogdahn, U.; et al. Radiotherapy plus Concomitant and Adjuvant Temozolomide for Glioblastoma. N. Engl. J. Med. 2005, 352, 987–996. [Google Scholar] [CrossRef]
- Ataman, F.; Poortmans, P.; Stupp, R.; Fisher, B.; Mirimanoff, R.-O. Quality assurance of the EORTC 26981/22981; NCIC CE3 intergroup trial on radiotherapy with or without temozolomide for newly-diagnosed glioblastoma multiforme: The individual case review. Eur. J. Cancer 2004, 40, 1724–1730. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.W.; Fraass, B.A.; Marsh, L.H.; Herbort, K.; Gebarski, S.S.; Martel, M.K.; Radany, E.H.; Lichter, A.S.; Sandler, H.M. Patterns of failure following high-dose 3-D conformal radiotherapy for high-grade astrocytomas: A quantitative dosimetric study. Int. J. Radiat. Oncol. Biol. Phys. 1999, 43, 79–88. [Google Scholar] [CrossRef]
- Niyazi, M.; Jansen, N.L.; Rottler, M.; Ganswindt, U.; Belka, C. Recurrence pattern analysis after re-irradiation with bevacizumab in recurrent malignant glioma patients. Radiat. Oncol. 2014, 9, 299. [Google Scholar] [CrossRef]
- Brandes, A.A.; Tosoni, A.; Franceschi, E.; Sotti, G.; Frezza, G.; Amistà, P.; Morandi, L.; Spagnolli, F.; Ermani, M. Recurrence Pattern After Temozolomide Concomitant with and Adjuvant to Radiotherapy in Newly Diagnosed Patients With Glioblastoma: Correlation with MGMT Promoter Methylation Status. J. Clin. Oncol. 2009, 27, 1275–1279. [Google Scholar] [CrossRef]
- Pepe, M.S.; Mori, M. Kaplan-Meier, marginal or conditional probability curves in summarizing competing risks failure time data? Stat. Med. 1993, 12, 737–751. [Google Scholar] [CrossRef]
- Wen, P.Y.; Chang, S.M.; Bent, M.J.V.D.; Vogelbaum, M.A.; Macdonald, D.R.; Lee, E.Q. Response Assessment in Neuro-Oncology Clinical Trials. J. Clin. Oncol. 2017, 35, 2439–2449. [Google Scholar] [CrossRef] [PubMed]
- Buglione, M.; Pedretti, S.; Poliani, P.L.; Liserre, R.; Gipponi, S.; Spena, G.; Borghetti, P.; Pegurri, L.; Saiani, F.; Spiazzi, L.; et al. Pattern of relapse of glioblastoma multiforme treated with radical radio-chemotherapy: Could a margin reduction be proposed? J. Neurooncol. 2016, 128, 303–312. [Google Scholar] [CrossRef] [PubMed]
- Faustino, A.C.; Viani, G.A.; Hamamura, A.C. Patterns of recurrence and outcomes of glioblastoma multiforme treated with chemoradiation and adjuvant temozolomide. Clinics 2020, 75, e1553. [Google Scholar] [CrossRef] [PubMed]
- Gunjur, A.; Bressel, M.; Ryan, G. The addition of temozolomide does not change the pattern of progression of glioblastoma multiforme post-radiotherapy. J. Med. Imaging Radiat. Oncol. 2012, 56, 567–573. [Google Scholar] [CrossRef] [PubMed]
- Milano, M.T.; Okunieff, P.; Donatello, R.S.; Mohile, N.A.; Sul, J.; Walter, K.A.; Korones, D.N. Patterns and Timing of Recurrence After Temozolomide-Based Chemoradiation for Glioblastoma. Int. J. Radiat. Oncol. 2010, 78, 1147–1155. [Google Scholar] [CrossRef]
- Minniti, G.; Amelio, D.; Amichetti, M.; Salvati, M.; Muni, R.; Bozzao, A.; Lanzetta, G.; Scarpino, S.; Arcella, A.; Enrici, R.M. Patterns of failure and comparison of different target volume delineations in patients with glioblastoma treated with conformal radiotherapy plus concomitant and adjuvant temozolomide. Radiother. Oncol. 2010, 97, 377–381. [Google Scholar] [CrossRef]
- Wallner, K.E.; Galicich, J.H.; Krol, G.; Arbit, E.; Malkin, M.G. Patterns of failure following treatment for glioblastoma multiforme and anaplastic astrocytoma. Int. J. Radiat. Oncol. 1989, 16, 1405–1409. [Google Scholar] [CrossRef]
- Jiang, H.; Yu, K.; Li, M.; Cui, Y.; Ren, X.; Yang, C.; Zhao, X.; Lin, S. Classification of Progression Patterns in Glioblastoma: Analysis of Predictive Factors and Clinical Implications. Front. Oncol. 2020, 10, 590648. [Google Scholar] [CrossRef] [PubMed]
- Lasocki, A.; Gaillard, F. Non-Contrast-Enhancing Tumor: A New Frontier in Glioblastoma Research. Am. J. Neuroradiol. 2019, 40, 758–765. [Google Scholar] [CrossRef]
- Eidel, O.; Burth, S.; Neumann, J.-O.; Kieslich, P.J.; Sahm, F.; Jungk, C.; Kickingereder, P.; Bickelhaupt, S.; Mundiyanapurath, S.; Bäumer, P.; et al. Tumor Infiltration in Enhancing and Non-Enhancing Parts of Glioblastoma: A Correlation with Histopathology. PLoS ONE 2017, 12, e0169292. [Google Scholar] [CrossRef]
- Lohmann, P.; Stavrinou, P.; Lipke, K.; Bauer, E.K.; Ceccon, G.; Werner, J.-M.; Neumaier, B.; Fink, G.R.; Shah, N.J.; Langen, K.-J.; et al. FET PET reveals considerable spatial differences in tumour burden compared to conventional MRI in newly diagnosed glioblastoma. Eur. J. Nucl. Med. 2018, 46, 591–602. [Google Scholar] [CrossRef]
- Kiesel, B.; Mischkuling, M.; Wohrer, A.; Martinez-Moreno, M.; Millesi, M.; Mallouhi, A.; Czech, T.; Preusser, M.; Hainfellner, J.A.; Wolfsberger, S.; et al. Systematic histopathologic analysis of different 5-aminolevolinic acid-induced fluorescence levels in newly dignosed glioblastomas. J. Neurosurg. 2018, 129, 341–353. [Google Scholar] [CrossRef] [PubMed]
- Abou-El-Ardat, K.; Seifert, M.; Becker, K.; Eisenreich, S.; Lehmann, M.; Hackmann, K.; Rump, A.; Meijer, G.; Carvalho, B.; Temme, A.; et al. Comprehensive molecular characteriza-tion of multifocal glioblastoma proves its monoclonal origin and reveals novel in-sights into clonal evolution and heterogeneity of glioblastomas. Neuro Oncol. 2017, 19, 546–557. [Google Scholar] [CrossRef]
- Shimizu, K.; Tamura, K.; Hara, S.; Inaji, M.; Tanaka, Y.; Kobayashi, D.; Sugawara, T.; Wakimoto, H.; Nariai, T.; Ishii, K.; et al. Correlation of Intraoperative 5-ALA-Induced Fluorescence Intensity and Preoperative 11C-Methionine PET Uptake in Glioma Surgery. Cancers 2022, 14, 1449. [Google Scholar] [CrossRef] [PubMed]
- Mills, S.J.; Du Plessis, D.; Pal, P.; Thompson, G.; Buonacorrsi, G.; Soh, C.; Parker, G.J.M.; Jackson, A. Mitotic Activity in Glioblastoma Correlates with Estimated Extravascular Extracellular Space Derived from Dynamic Contrast-Enhanced MR Imaging. Am. J. Neuroradiol. 2015, 37, 811–817. [Google Scholar] [CrossRef] [PubMed]
- Liesche-Starnecker, F.; Prokop, G.; Yakushev, I.; Preibisch, C.; Delbridge, C.; Meyer, H.S.; Aftahy, K.; Barz, M.; Meyer, B.; Zimmer, C.; et al. Visualizing cellularity and angiogenesis in newly-diagnosed glioblastoma with diffusion and perfusion MRI and FET-PET imaging. EJNMMI Res. 2021, 11, 72. [Google Scholar] [CrossRef]
- Maurer, G.D.; Tichy, J.; Harter, P.N.; Nöth, U.; Weise, L.; Quick-Weller, J.; Deichmann, R.; Steinbach, J.P.; Bähr, O.; Hattingen, E. Matching Quantitative MRI Parameters with Histological Features of Treatment-Naïve IDH Wild-Type Glioma. Cancers 2021, 13, 4060. [Google Scholar] [CrossRef]
- Jackson, C.; Choi, J.; Khalafallah, A.; Price, C.; Bettegowda, C.; Lim, M.; Gallia, G.; Weingart, J.; Brem, H.; Mukherjee, D. A systematic review and meta-analysis of supratotal versus gross total resection for glioblastoma. J. Neuro-Oncol. 2020, 148, 419–431. [Google Scholar] [CrossRef]
- Mampre, D.; Ehresman, J.; Pinilla-Monsalve, G.D.; Osorio, M.A.G.; Olivi, A.; Quinones-Hinojosa, A.; Chaichana, K.L. Extending the resection beyond the contrast-enhancement for glioblastoma: Feasibility, efficacy, and outcomes. Br. J. Neurosurg. 2018, 32, 528–535. [Google Scholar] [CrossRef]
- Kim, M.M.; Sun, Y.; Aryal, M.P.; Parmar, H.A.; Piert, M.; Rosen, B.; Mayo, C.S.; Balter, J.M.; Schipper, M.; Gabel, N.; et al. A phase II study of dose-intensified chemoradiation using biologically-based target volume definition in patients with newly diagnosed gliobalstoma. Int. J. Radiat. Oncol. Biol. Phys. 2021, 110, 792–803. [Google Scholar] [CrossRef]
- Glas, M.; Ballo, M.T.; Bomzon, Z.; Urman, N.; Levi, S.; Lavy-Shahaf, G.; Jeyapalan, S.; Sio, T.T.; DeRose, P.M.; Misch, M.; et al. The Impact of Tumor Treating Fields on Glioblastoma Progression Patterns. Int. J. Radiat. Oncol. 2021, 112, 1269–1278. [Google Scholar] [CrossRef] [PubMed]
- Khan, L.; Soliman, H.; Sahgal, A.; Perry, J.; Xu, W.; Tsao, M.N. External beam dose escalation for high grade Glioma. Cochrane Database Syst. Rev. 2020, 5, CD011475. [Google Scholar] [PubMed]
- Souhami, L.; Seiferheld, W.; Brachman, D.; Podgorsak, E.B.; Werner-Wasik, M.; Lustig, R.; Schultz, C.J.; Sause, W.; Okunieff, P.; Buckner, J.; et al. Randomized comparison of stereotactic radiosurgery followed by conventional radiotherapy with carmustine to conventional with carmustine for patients with gliobastoma multiforme: Report of Radiation Therapy Oncology Group 93-05 protocol. Int. J. Radiat. Oncol. Biol. Phys. 2004, 60, 853–860. [Google Scholar] [CrossRef] [PubMed]


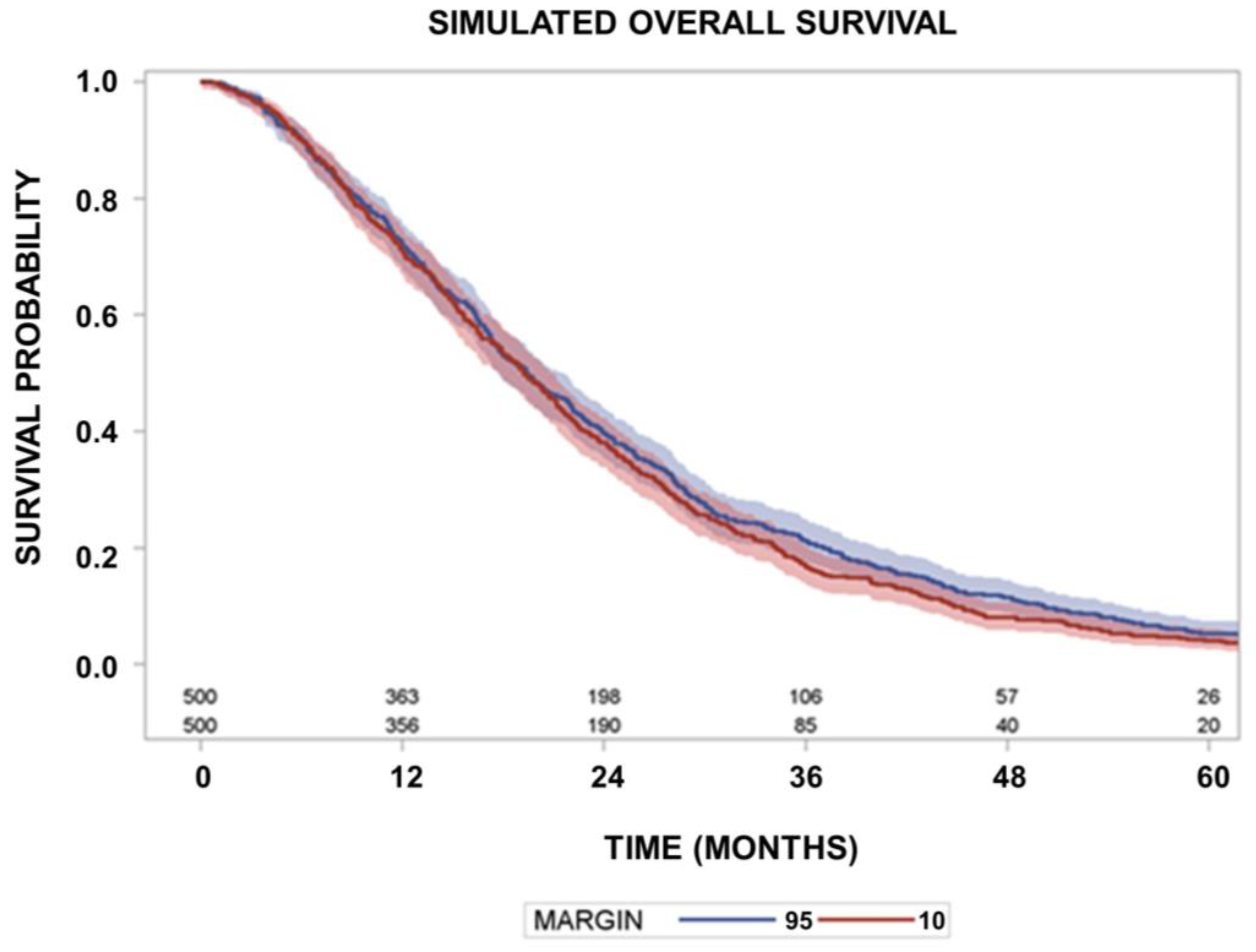
| Characteristic | Patient Numbers | |
|---|---|---|
| Age | 59 y (37 y–80 y) | |
| Sex | Male vs. female | 50/41 |
| Laterality of the tumor | Left vs. right, vs. bilateral | 38/52/1 |
| Location of the primary | frontal/temporal/parietal/parietooccipital/occipital/parietotemporal/other | 30/22/13/7/7/6/6 |
| Type of resection | gross total vs. partial resection | 47/44 |
| MGMT promoter methylation status | Unmethylated/methylated/not determined | 57/30/4 |
| Analysis of IDH-mutation status | Performed/not performed | 71/20 |
| Time from surgery to start of radiotherapy | 28 d (7 d–97 d) | |
| Volume of the postoperative GTV | 26.5 cm3 (0.5 cm3–195.4 cm3) | |
| Conventional fractionation vs. Hypofractionation of radiotherapy | 85/6 | |
| Total dose | Conventional Hypofractionated | 60 Gy (56 Gy–60 Gy) 40 Gy (40 Gy–40 Gy) |
| Concurrent single drug Temozolomide | yes/no | 77/14 |
| Concurrent Temozolomide and Lomustin | yes/no | 5/86 |
| TT-Fields consolidation | yes/no | 13/78 |
| Recurrence pattern | Uni- vs. multifocal recurrence | 74/17 |
| Re-resection as salvage therapy | No vs. complete vs. partial | 58/14/19 |
| Volume of the recurrence at diagnosis of recurrence | 4.0 cm3 (0.1 cm3–115.8 cm3) | |
| Relative Risk of Out-Field Recurrences | P (Exact Fisher Test) | ||
|---|---|---|---|
| Age | ≤60 vs. >60 | 1.14 (0.43–3.03) | 1.0000 |
| Sex | Male vs. female | 3.01 (0.90–10.06) | 0.0790 |
| Laterality of the tumor | left vs. right | 1.37 (0.52–3.58) | 0.5658 |
| Location of the primary (over all locations) | 0.2013 | ||
| Resection status | Gross total vs. partial | 1.69 (0.61–4.64) | 0.3884 |
| IDH-mutation status | Negative vs. not determined | 1.69 (0.41–6.94) | 0.7267 |
| Temozolomide daily | Not concurrent vs. concurrent | 1.50 (0.49–4.70) | 0.4461 |
| Lomustin and Temozolomide | Not concurrent vs. concurrent | 0.76 (0.12–4.70) | 0.5751 |
| Radiation dose fractionation | Conventional vs. hypofractionated | 0.92 (0.14–5.88) | 1.0000 |
| TT-Fields | No-consolidation vs. consolidation | 2.17 (0.31 –15.19) | 0.6829 |
| MGMT methylation status | Methylated vs. unmethylated | 1.06 (0.39–2.87) | 1.0000 |
| Recurrence pattern | Multi- vs. unifocal recurrence | 4.35 (95%CI: 1.76–10.77) | 0.0037 |
| Re-resection as salvage therapy | No vs. incomplete | 1.09 (0.26–4.48) 0.98 (0.30–3.26) | 1.0000 |
| Log (volume) of the recurrence | 1.20 (0.85–1.73) 1 | 0.3210 3 | |
| Log (volume) of the postoperative GTV (resection cavity + CM enhancement | 0.86 (0.56–1.37) 1 | 0.5075 3 | |
| Time from start of radiotherapy to recurrence | 1.00 (0.998–1.002) 2 | 0.6904 3 | |
| Waiting time between surgery and start of radiotherapy | 1.00 (0.96–1.04) 2 | 0.8156 3 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guberina, N.; Padeberg, F.; Pöttgen, C.; Guberina, M.; Lazaridis, L.; Jabbarli, R.; Deuschl, C.; Herrmann, K.; Blau, T.; Wrede, K.H.; et al. Location of Recurrences after Trimodality Treatment for Glioblastoma with Respect to the Delivered Radiation Dose Distribution and Its Influence on Prognosis. Cancers 2023, 15, 2982. https://doi.org/10.3390/cancers15112982
Guberina N, Padeberg F, Pöttgen C, Guberina M, Lazaridis L, Jabbarli R, Deuschl C, Herrmann K, Blau T, Wrede KH, et al. Location of Recurrences after Trimodality Treatment for Glioblastoma with Respect to the Delivered Radiation Dose Distribution and Its Influence on Prognosis. Cancers. 2023; 15(11):2982. https://doi.org/10.3390/cancers15112982
Chicago/Turabian StyleGuberina, Nika, Florian Padeberg, Christoph Pöttgen, Maja Guberina, Lazaros Lazaridis, Ramazan Jabbarli, Cornelius Deuschl, Ken Herrmann, Tobias Blau, Karsten H. Wrede, and et al. 2023. "Location of Recurrences after Trimodality Treatment for Glioblastoma with Respect to the Delivered Radiation Dose Distribution and Its Influence on Prognosis" Cancers 15, no. 11: 2982. https://doi.org/10.3390/cancers15112982
APA StyleGuberina, N., Padeberg, F., Pöttgen, C., Guberina, M., Lazaridis, L., Jabbarli, R., Deuschl, C., Herrmann, K., Blau, T., Wrede, K. H., Keyvani, K., Scheffler, B., Hense, J., Layer, J. P., Glas, M., Sure, U., & Stuschke, M. (2023). Location of Recurrences after Trimodality Treatment for Glioblastoma with Respect to the Delivered Radiation Dose Distribution and Its Influence on Prognosis. Cancers, 15(11), 2982. https://doi.org/10.3390/cancers15112982







