Natural Products for the Prevention, Treatment and Progression of Breast Cancer
Abstract
Simple Summary
Abstract
1. Introduction
2. Molecular Basis of BC
3. Polyphenols
3.1. Curcumin
3.2. Epigallocatechin-3-Gallate
4. Indole-3-Carbinol
5. Artesunate
6. Ginger and Its Constituents
6.1. 6-Gingerol
6.2. 6-Shogaol (6SG)
7. Flavonoids and Bioflavonoids
7.1. Xanthohumol
7.2. Citrus Bioflavonoids
8. Myrrh
9. Sulforaphane
10. Vitamin D
11. Mushrooms
12. Acetylsalicylic Acid
13. Metformin
14. Clinical Studies
15. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
References
- Rodrigues, E.C.G.; Neris, R.R.; Nascimento, L.C.; de Oliveira-Cardoso, É.A.; Dos Santos, M.A. Body image experience of women with breast cancer: A meta-synthesis. Scand. J. Caring Sci. 2023, 37, 20–36. [Google Scholar] [CrossRef] [PubMed]
- Iacopetta, D.; Ceramella, J.; Baldino, N.; Sinicropi, M.S.; Catalano, A. Targeting breast cancer: An overlook on current strategies. Int. J. Mol. Sci. 2023, 24, 3643. [Google Scholar] [CrossRef] [PubMed]
- Malumbres, M.; Barbacid, M. To cycle or not to cycle: A critical decision in cancer. Nat. Rev. Cancer 2011, 1, 222–231. [Google Scholar] [CrossRef]
- Singh, N.; Baby, D.; Rajguru, J.P.; Patil, P.B.; Thakkannavar, S.S.; Pujari, V.B. Inflammation and cancer. Ann. Afr. Med. 2019, 18, 121. [Google Scholar] [CrossRef] [PubMed]
- Buchta Rosean, C.; Bostic, R.R.; Ferey, J.C.M.; Feng, T.Y.; Azar, F.N.; Tung, K.S.; Dozmorov, M.G.; Smirnova, E.; Bos, P.D.; Rutkowski, M.R. Preexisting commensal dysbiosis is a host-intrinsic regulator of tissue inflammation and tumor cell dissemination in hormone receptor-positive breast cancer. Cancer Res. 2019, 79, 3662–3675. [Google Scholar] [CrossRef]
- Dolcet, X.; Llobet, D.; Pallares, J.; Matias-Guiu, X. NF-kB in development and progression of human cancer. Virchows Arch. 2005, 446, 475–482. [Google Scholar] [CrossRef]
- Gallo, O.; Franchi, A.; Magnelli, L.; Sardi, I.; Vannacci, A.; Boddit, V.; Chiarugi, V.; Masini, E. Cyclooxygenase-2 Pathway Correlates with VEGF Expression in Head and Neck Cancer. Implications for Tumor Angiogenesis and Metastasis. Neoplasia 2001, 3, 53–61. [Google Scholar] [CrossRef]
- Ikeda, H.; Kakeya, H. Targeting hypoxia-inducible factor 1 (HIF-1) signaling with natural products toward cancer chemotherapy. J. Antibiot. 2021, 74, 687–695. [Google Scholar] [CrossRef]
- Nourazarian, A.R.; Kangari, P.; Salmaninejad, A. Roles of oxidative stress in the development and progression of breast cancer. Asian Pac. J. Cancer Prev. 2014, 15, 4745–4751. [Google Scholar] [CrossRef]
- Javadian, M.; Gharibi, T.; Shekari, N.; Abdollahpour-Alitappeh, M.; Mohammadi, A.; Hossieni, A.; Mohammadi, H.; Kazemi, T. The role of microRNAs regulating the expression of matrix metalloproteinases (MMPs) in breast cancer development, progression, and metastasis. J. Cell Physiol. 2019, 234, 5399–5412. [Google Scholar] [CrossRef]
- Biello, F.; Platini, F.; D’Avanzo, F.; Cattrini, C.; Mennitto, A.; Genestroni, S.; Martini, V.; Marzullo, P.; Aimaretti, G.; Gennari, A. Insulin/IGF axis in breast cancer: Clinical evidence and translational insights. Biomolecules 2021, 11, 125. [Google Scholar] [CrossRef] [PubMed]
- Santolla, M.F.; Maggiolini, M. The FGF/FGFR System in Breast Cancer: Oncogenic Features and Therapeutic Perspectives. Cancers 2020, 12, 3029. [Google Scholar] [CrossRef] [PubMed]
- Loh, H.Y.; Norman, B.P.; Lai, K.S.; Rahman, N.M.A.N.A.; Alitheen, N.B.M.; Osman, M.A. The regulatory role of microRNAs in breast cancer. Int. J. Mol. Sci. 2019, 20, 4940. [Google Scholar] [CrossRef]
- Filosa, R.; Peduto, A.; de Caprariis, P.; Saturnino, C.; Festa, M.; Petrella, A.; Pau, A.; Pinna, G.A.; Colla, P.L.; Busonera, B.; et al. Synthesis and antiproliferative properties of N3/8-disubstituted-3,8-diazabicyclo[3.2.1]octane analogues of 3,8-bis[2-(3,4,5-trimethoxyphenyl)pyridin-4-yl]methyl-piperazine. Eur. J. Med. Chem. 2007, 42, 293–306. [Google Scholar] [CrossRef]
- De Cicco, P.; Catani, M.V.; Gasperi, V.; Sibilano, M.; Quaglietta, M.; Savini, I. Nutrition and breast cancer: A literature review on prevention, treatment and recurrence. Nutrients 2019, 11, 1514. [Google Scholar] [CrossRef]
- Li, Y.; Li, S.; Meng, X.; Gan, R.Y.; Zhang, J.J.; Li, H.B. Dietary natural products for prevention and treatment of breast cancer. Nutrients 2017, 9, 728. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs from 1981 to 2014. J. Nat. Prod. 2016, 79, 629–661. [Google Scholar] [CrossRef]
- ESCOP. ESCOP Monographs, 2nd ed.; The European Scientific Cooperative on Phythoterapy: Devon, UK, 2009. [Google Scholar]
- Garcia-Oliveira, P.; Otero, P.; Pereira, A.; Chamorro, F.; Carpena, M.; Echave, J.; Fraga-Corral, M.; Simal-Gandara, J.; Prieto, M. Status and challenges of plant-anticancer compounds in cancer treatment. Pharmaceuticals 2021, 14, 157. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, T.; Dutta, S.; Akter, R.; Rahman, M.; Karthika, C.; Nagaswarupa, H.P.; Murthy, H.C.A.; Fratila, O.; Brata, R.; Bungau, S. Role of phytonutrients in nutrigenetics and nutrigenomic perspective in curing breast cancer. Biomolecules 2021, 11, 1176. [Google Scholar] [CrossRef]
- Haffner, M.C.; Berlato, C.; Doppler, W. Exploiting Our Knowledge of NF-ΚB Signaling for the Treatment of Mammary Cancer. J. Mammary Gland Biol. Neoplasia 2006, 11, 63–73. [Google Scholar] [CrossRef]
- Das, T.; Chen, Z.; Hendriks, R.W.; Kool, M. A20/Tumor Necrosis Factor α-induced protein 3 in immune cells controls development of autoinflammation and autoimmunity: Lessons from mouse models. Front. Immunol. 2018, 9, 104. [Google Scholar] [CrossRef]
- Rohwedder, I.; Wackerbarth, L.M.; Heinig, K.; Ballweg, A.; Altstätter, J.; Ripphahn, M.; Nussbaum, C.; Salvermoser, M.; Bierskhenk, S.; Straub, T.; et al. A20 and the non-canonical NF-κB pathway are key regulators of neutrophil recruitment during fetal ontogeny. JCI Insight, 2023; in press. [Google Scholar] [CrossRef]
- Carvalho, M.I.; Silva-Carvalho, R.; Pires, I.; Prada, J.; Bianchini, R.; Jensen-Jarolim, E.; Queiroga, F.L. A comparative approach of tumor-associated inflammation in mammary cancer between humans and dogs. BioMed Res. Int. 2016, 2016, 4917387. [Google Scholar] [CrossRef] [PubMed]
- Calderwood, S.K.; Gong, J. Molecular chaperones in mammary cancer growth and breast tumor therapy. J. Cell. Biochem. 2012, 113, 1096–1103. [Google Scholar] [CrossRef] [PubMed]
- Jelic, M.D.; Mandic, A.D.; Maricic, S.M.; Srdjenovic, B.U. Oxidative stress and its role in cancer. J. Cancer Res. Ther. 2021, 17, 22–28. [Google Scholar] [CrossRef]
- Maccio, A.; Sanna, E.; Neri, M.; Oppi, S.; Madeddu, C. Cachexia as evidence of the mechanisms of resistance and tolerance during the evolution of cancer disease. Int. J. Mol. Sci. 2021, 22, 2890. [Google Scholar] [CrossRef] [PubMed]
- Mishra, P.J.; Banerjee, D. Activation and differentiation of mesenchymal stem cells. Methods Mol. Biol. 2017, 1554, 201–209. [Google Scholar]
- Liu, D.; Chen, Z. The effect of curcumin on breast cancer cells. J. Breast Cancer 2013, 16, 133–137. [Google Scholar] [CrossRef]
- Park, S.-Y.; Choi, J.-H.; Nam, J.-S. Targeting cancer stem cells in triple-negative breast cancer. Cancers 2019, 11, 965. [Google Scholar] [CrossRef]
- Boman, B.M.; Wicha, M.S. Cancer stem cells: A step toward the cure. J. Clin. Oncol. 2008, 26, 2795–2799. [Google Scholar] [CrossRef]
- Lobo, N.A.; Shimono, Y.; Qian, D.; Clarke, M.F. The biology of cancer stem cells. Annu. Rev. Cell Dev. Biol. 2007, 23, 675–699. [Google Scholar] [CrossRef]
- Morrison, S.J.; Spradling, A.C. Stem cells and niches: Mechanisms that promote stem cell maintenance throughout life. Cell 2008, 132, 598–611. [Google Scholar] [CrossRef]
- Han, Y.; Wang, J.; Xu, B. Cytotoxic lymphocyte-related gene signature in triple-negative breast cancer. J. Personal. Med. 2023, 13, 457. [Google Scholar] [CrossRef]
- Taams, L.S.; Palmer, D.B.; Akbar, A.N.; Robinson, D.S.; Brown, Z.; Hawrylowicz, C.M. Regulatory T cells in human disease and their potential for therapeutic manipulation. Immunology 2006, 118, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Hoppe, R.; Fan, P.; Buttner, F.; Winter, S.; Tyagi, A.K.; Cunliffe, H.; Jordan, V.C.; Brauch, H. Profiles of miRNAs matched to biology in aromatase inhibitor resistant breast cancer. Oncotarget 2016, 7, 71235–71254. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.J.; Yang, F.; Ding, J.J.; Yan, D.L.; Wang, D.D.; Yang, S.J.; Ding, L.; Li, J.; Chen, D.; Ma, R.; et al. MiR-31 inhibits migration and invasion by targeting SATB2 in triple negative breast cancer. Gene 2016, 594, 47–58. [Google Scholar] [CrossRef]
- Raza, U.; Saatci, O.; Uhlmann, S.; Ansari, S.A.; Eyupoglu, E.; Yurdusev, E.; Mutlu, M.; Ersan, P.G.; Altundag, M.K.; Zhang, J.D.; et al. The miR-644a/CTBP1/p53 axis suppresses drug resistance by simultaneous inhibition of cell survival and epithelial-mesenchymal transition in breast cancer. Oncotarget 2016, 7, 49859–49877. [Google Scholar] [CrossRef] [PubMed]
- Chu, J.; Zhu, Y.; Liu, Y.; Sun, L.; Lv, X.; Wu, Y.; Liu, Q. E2F7 overexpression leads to tamoxifen resistance in breast cancer cells by competing with E2F1 at miR-15a/16 promoter. Oncotarget 2015, 6, 31944–31957. [Google Scholar] [CrossRef] [PubMed]
- Carbognin, L.; Miglietta, F.; Paris, I.; Dieci, M.V. Prognostic and predictive implications of PTEN in breast cancer: Unfulfilled promises but intriguing perspectives. Cancers 2019, 11, 1401. [Google Scholar] [CrossRef]
- Sobhani, M.; Farzaei, M.H.; Kiani, S.; Khodarahmi, R. Immunomodulatory; anti-inflammatory/antioxidant effects of polyphenols: A comparative review on the parental compounds and their metabolites. Food Rev. Int. 2021, 37, 759–811. [Google Scholar] [CrossRef]
- Do Valle, I.F.; Roweth, H.G.; Malloy, M.W.; Moco, S.; Barron, D.; Battinelli, E.; Loscalzo, J.; Barabasi, A.L. Network medicine framework shows that proximity of polyphenol targets and disease proteins predicts therapeutic effects of polyphenols. Nat. Food 2021, 2, 143. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zheng, J.; Li, Y.; Xu, D.P.; Li, S.; Chen, Y.M.; Li, H.B. Natural polyphenols for prevention and treatment of cancer. Nutrients 2016, 8, 515. [Google Scholar] [CrossRef]
- Ali, M.; Benfante, V.; Stefano, A.; Yezzi, A.; Di Raimondo, D.; Tuttolomondo, A.; Comelli, A. Anti-arthritic and anti-cancer activities of polyphenols: A review of the most recent in vitro assays. Life 2023, 13, 361. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Tan, H.Y.; Wang, N.; Cheung, F.; Hong, M.; Feng, Y. The potential and action mechanism of polyphenols in the treatment of liver diseases. Oxid. Med. Cell. Longev. 2018, 2018, 8394818. [Google Scholar] [CrossRef] [PubMed]
- Yoshioka, Y.; Ohishi, T.; Nakamura, Y.; Fukutomi, R.; Miyoshi, N. Anti-Cancer Effects of Dietary Polyphenols via ROS-mediated pathway with their modulation of microRNAs. Molecules 2022, 27, 3816. [Google Scholar] [CrossRef] [PubMed]
- Anjum, J.; Mitra, S.; Das, R.; Alam, R.; Mojumder, A.; Emran, T.B.; Islam, F.; Rauf, A.; Hossain, M.J.; Aljohani, A.S.M.; et al. A renewed concept on the MAPK signaling pathway in cancers: Polyphenols as a choice of therapeutics. Pharmacol. Res. 2022, 184, 106398. [Google Scholar] [CrossRef]
- Rajendran, P.; Abdelsalam, S.A.; Renu, K.; Veeraraghavan, V.; Ben Ammar, R.; Ahmed, E.A. Polyphenols as potent epigenetics agents for cancer. Int. J. Mol. Sci. 2022, 23, 11712. [Google Scholar] [CrossRef]
- Maleki Dana, P.; Sadoughi, F.; Asemi, Z.; Yousefi, B. The role of polyphenols in overcoming cancer drug resistance: A comprehensive review. Cell. Mol. Biol. Lett. 2022, 27, 1. [Google Scholar] [CrossRef]
- Duda-Chodak, A.; Tarko, T. Possible side effects of polyphenols and their interactions with medicines. Molecules 2023, 28, 2536. [Google Scholar] [CrossRef]
- Jiang, M.; Huang, O.; Zhang, X.; Xie, Z.; Shen, A.; Liu, H.; Geng, M.; Shen, K. Curcumin induces cell death and restores tamoxifen sensitivity in the antiestrogen-resistant breast cancer cell lines MCF-7/LCC2 and MCF-7/LCC9. Molecules 2013, 18, 701–720. [Google Scholar] [CrossRef]
- Kumar, P.; Kadakol, A.; Shasthrula, P.K.; Mundhe, N.A.; Jamdade, V.S.; Barua, C.C.; Gaikwad, A.B. Curcumin as an adjuvant to breast cancer treatment. Anti-Cancer Agents Med. Chem. 2015, 15, 647–656. [Google Scholar] [CrossRef] [PubMed]
- Shehzad, A.; Rehman, G.; Lee, Y.S. Curcumin in inflammatory diseases. Biofactors 2013, 39, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Wright, L.E.; Frye, J.B.; Gorti, B.; Timmermann, B.N.; Funk, J.L. Bioactivity of turmeric-derived curcuminoids and related metabolites in breast cancer. Curr. Pharm. Des. 2013, 19, 6218–6225. [Google Scholar] [CrossRef] [PubMed]
- Nabekura, T.; Kamiyama, S.; Kitagawa, S. Effects of dietary chemopreventive phytochemicals on P-glycoprotein function. Biochem. Biophys. Res. Commun. 2005, 327, 866–870. [Google Scholar] [CrossRef] [PubMed]
- Cojocneanu Petric, R.; Braicu, C.; Raduly, L.; Zanoaga, O.; Dragos, N.; Monroig, P.; Dumitrascu, D.; Berindan-Neagoe, I. Phytochemicals modulate carcinogenic signaling pathways in breast and hormone-related cancers. OncoTargets Ther. 2015, 8, 2053–2066. [Google Scholar] [CrossRef]
- Abadi, A.J.; Mirzaei, S.; Mahabady, M.K.; Hashemi, F.; Zabolian, A.; Hashemi, F.; Raee, P.; Aghamiri, S.; Ashrafizadeh, M.; Aref, A.R.; et al. Curcumin and its derivatives in cancer therapy: Potentiating antitumor activity of cisplatin and reducing side effects. Phytother. Res. 2022, 36, 189–213. [Google Scholar] [CrossRef]
- Mehta, K.; Pantazis, P.; McQueen, T.; Aggarwal, B.B. Antiproliferative effect of curcumin (diferuloylmethane) against human breast tumor cell lines. Anticancer Drugs 1997, 8, 470–481. [Google Scholar] [CrossRef]
- Benoit, V.; Relic, B.; Leval, X.X.; Chariot, A.; Merville, M.P.; Bours, V. Regulation of HER-2 oncogene expression by cyclooxygenase-2 and prostaglandin E2. Oncogene 2004, 23, 1631–1635. [Google Scholar] [CrossRef]
- Qiao, Q.; Jiang, Y.; Li, G. Curcumin improves the antitumor effect of X-ray irradiation by blocking the NF-kappaB pathway: An in vitro study of lymphoma. Anticancer Drugs 2012, 23, 597–605. [Google Scholar] [CrossRef]
- Jäger, R.; Lowery, R.P.; Calvanese, A.V.; Joy, J.M.; Purpura, M.; Wilson, J.M. Comparative absorption of curcumin formulations. Nutr. J. 2014, 13, 11. [Google Scholar] [CrossRef]
- Chen, A.L.; Hsu, C.H.; Lin, J.K.; Hsu, M.M.; Ho, Y.F.; She, T.S.; Ko, J.Y.; Lin, J.T.; Lin, B.R.; Wu, M.S.; et al. Phase I clinical trial of curcumin, a chemopreventive agent, in patients with high-risk or pre-malignant lesions. Anticancer Res. 2001, 21, e2900. [Google Scholar]
- Kunnumakkara, A.B.; Guha, S.; Krishnan, S.; Diagaradjane, P.; Gelovani, J.; Aggarwal, B.B. Curcumin potentiates antitumor activity of gemcitabine in an orthotopic model of pancreatic cancer through suppression of proliferation, angiogenesis, and inhibition of nuclear factor-κB-regulated gene products. Cancer Res. 2007, 67, 3853–3861. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Gu, Z.L.; Qin, Z.H.; Liang, Z.Q. Effect of curcumin on the adhesion of platelets to brain microvascular endothelial cells in vitro. Acta Pharmacol. Sin. 2008, 29, 800–807. [Google Scholar] [CrossRef]
- Kim, D.C.; Ku, S.K.; Lee, W.; Bae, J.S. Barrier protective activities of curcumin and its derivative. Inflamm. Res. 2012, 61, 437–444. [Google Scholar] [CrossRef]
- Thomas, S.L.; Zhong, D.; Zhou, W.; Malik, S.; Liotta, D.; Snyder, J.P.; Hamel, E.; Giannakakou, P. EF24, a novel curcumin analog, disrupts the microtubule cytoskeleton and inhibits HIF-1. Cell Cycle 2008, 7, 2409–2417. [Google Scholar] [CrossRef]
- Cao, L.; Xiao, X.; Lei, J.; Duan, W.; Ma, Q.; Li, W. Curcumin inhibits hypoxia-induced epithelialmesenchymal transition in pancreatic cancer cells via suppression of the hedgehog signaling pathway. Oncol. Rep. 2016, 35, 3728–3734. [Google Scholar] [CrossRef] [PubMed]
- Angulo, P.; Kaushik, G.; Subramaniam, D.; Dandawate, P.; Neville, K.; Chastain, K.; Anant, S. Natural compounds targeting major cell signaling pathways: A novel paradigm for osteosarcoma therapy. J. Hematol. Oncol. 2017, 10, 10. [Google Scholar] [CrossRef]
- Zhou, M.; Fan, C.; Tian, N. Effects of curcumin on the gene expression profile of L-02 cells. Biomed. Rep. 2015, 3, 519–526. [Google Scholar] [CrossRef]
- Alam, M.; Ali, S.; Ashraf, G.M.; Bilgrami, A.L.; Yadav, D.K.; Hassan, M.I. Epigallocatechin 3-gallate: From green tea to cancer therapeutics. Food Chem. 2022, 379, 132135. [Google Scholar] [CrossRef]
- Wang, L.X.; Shi, Y.L.; Zhang, L.J.; Wang, K.R.; Xiang, L.P.; Cai, Z.Y.; Lu, J.L.; Ye, J.H.; Liang, Y.R.; Zheng, X.Q. Inhibitory effects of (–)-epigallocatechin-3-gallate on esophageal cancer. Molecules 2019, 24, 954. [Google Scholar] [CrossRef]
- Tanabe, H.; Suzuki, T.; Ohishi, T.; Isemura, M.; Nakamura, Y.; Unno, K. Effects of epigallocatechin-3-gallate on matrix metalloproteinases in terms of its anticancer activity. Molecules 2023, 28, 525. [Google Scholar] [CrossRef]
- Li, F.; Qasim, S.; Li, D.; Dou, Q.P. Updated review on green tea polyphenol epigallocatechin-3-gallate as a cancer epigenetic regulator. Semin. Cancer Biol. 2022, 83, 335–352. [Google Scholar] [CrossRef]
- Athanasiou, E.; Verras, G.I.; Papageorgiou, S.; Kelesis, I.; Gatsis, A.; Karaoulani, C.; Stouras, I.; Katanas, P.; Saitani, E.M.; Oikonomou, M.E.; et al. The association between the risk of breast cancer and epigallocatechin-3-gallate intake: A literature review of a potential chemopreventive agent. Curr. Med. Chem. 2022, 29, 6169–6196. [Google Scholar] [CrossRef]
- Zhao, H.; Zhu, W.; Zhao, X.; Li, X.; Zhou, Z.; Zheng, M.; Meng, X.; Kong, L.; Zhang, S.; He, D.; et al. Efficacy of Epigallocatechin-3-gallate in preventing dermatitis in patients with breast cancer receiving postoperative radiotherapy: A double-blind, placebo-controlled, phase 2 randomized clinical trial. JAMA Dermatol. 2022, 158, 779–786. [Google Scholar] [CrossRef] [PubMed]
- Bradlow, H.L.; Zeligs, M.A. Diindolylmethane (DIM) spontaneously forms from indole-3-carbinol (I3C) during cell culture experiments. In Vivo 2010, 24, 387–391. [Google Scholar] [PubMed]
- Marconett, C.N.; Singhal, A.K.; Sundar, S.N.; Firestone, G.L. Indole-3-carbinol disrupts estrogen receptor-alpha dependent expression of insulin-like growth factor-1 receptor and insulin receptor substrate-1 and proliferation of human breast cancer cells. Mol. Cell. Endocrinol. 2012, 363, 74–84. [Google Scholar] [CrossRef]
- Caruso, J.A.; Campana, R.; Wei, C.; Su, C.H.; Hanks, A.M.; Bornmann, W.G.; Keyomarsi, K. Indole-3-carbinol and its N-alkoxy derivatives preferentially target ERα-positive breast cancer cells. Cell Cycle 2014, 13, 2587–2599. [Google Scholar] [CrossRef] [PubMed]
- Chinni, S.R.; Li, Y.; Upadhyay, S.; Koppolu, P.K.; Sarkar, F.H. Indole-3-carbinol (I3C) induced cell growth inhibition, G1 cell cycle arrest and apoptosis in prostate cancer cells. Oncogene 2001, 20, 2927–2937. [Google Scholar] [CrossRef]
- Dandawate, P.R.; Subramaniam, D.; Jensen, R.A.; Anant, S. Targeting cancer stem cells and signaling pathways by phytochemicals: Novel approach for breast cancer therapy. Semin. Cancer Biol. 2016, 40, 192–208. [Google Scholar] [CrossRef]
- Hargraves, K.G.; He, L.; Firestone, G.L. Phytochemical regulation of the tumor suppressive microRNA, miR-34a, by p53-dependent and independent responses in human breast cancer cells. Mol. Carcinog. 2016, 55, 486–498. [Google Scholar] [CrossRef]
- De Santi, M.; Carloni, E.; Galluzzi, L.; Diotallevi, A.; Lucarini, S.; Magnani, M.; Brandi, G. Inhibition of testosterone aromatization by the indole-3-carbinol derivative CTet in CYP19A1-overexpressing MCF-7 breast cancer cells. Anti-Cancer Agents Med. Chem. 2015, 15, 894–902. [Google Scholar] [CrossRef] [PubMed]
- De Santi, M.; Galluzzi, L.; Lucarini, S.; Paoletti, M.F.; Fraternale, A.; Duranti, A.; De Marco, C.; Fanelli, M.; Zaffaroni, N.; Brandi, G.; et al. The indole-3-carbinol cyclic tetrameric derivative CTet inhibits cell proliferation via overexpression of p21/CDKN1A in both estrogen receptor-positive and triple-negative breast cancer cell lines. Breast Cancer Res. 2011, 13, R33. [Google Scholar] [CrossRef]
- Brandi, G.; Fraternale, A.; Lucarini, S.; Paiardini, M.; de Santi, M.; Cervasi, B.; Paoletti, M.F.; Galluzzi, L.; Duranti, A.; Magnani, M. Antitumoral activity of indole-3-carbinol cyclic tri- and tetrameric derivatives mixture in human breast cancer cells: In vitro and in vivo studies. Anti-Cancer Agents Med. Chem. 2013, 13, 654–662. [Google Scholar] [CrossRef] [PubMed]
- Li, W.-S.; Wang, C.-H.; Ko, S.; Chang, T.T.; Jen, Y.C.; Yao, C.-F.; More, S.V.; Jao, S.-C. Synthesis and evaluation of the cytotoxicities of tetraindoles: Observation that the 5-hydroxy tetraindole (SK228) induces G2 arrest and apoptosis in human breast cancer cells. J. Med. Chem. 2012, 55, 1583–1592. [Google Scholar] [CrossRef] [PubMed]
- Doisneau-Sixou, S.F.; Sergio, C.M.; Carroll, J.S.; Hui, R.; Musgrove, E.A.; Sutherland, R.L. Estrogen and antiestrogen regulation of cell cycle progression in breast cancer cells. Endocr.-Relat. Cancer 2003, 10, 179–186. [Google Scholar] [CrossRef]
- Singh, A.A.; Jo, S.H.; Kiddane, A.T.; Niyonizigiye, I.; Kim, G.D. Indole-3-carbinol induces apoptosis in AGS cancer cells via mitochondrial pathway. Chem. Biol. Drug Des. 2023; in press. [Google Scholar] [CrossRef]
- Lin, H.; Gao, X.; Chen, G.; Sun, J.; Chu, J.; Jing, K.; Li, P.; Zeng, R.; Wei, B. Indole-3-carbinol as inhibitors of glucocorticoid-induced apoptosis in osteoblastic cells through blocking ROS-mediated Nrf2 pathway. Biochem. Biophys. Res. Commun. 2015, 460, 422–427. [Google Scholar] [CrossRef]
- Ruwizhi, N.; Maseko, R.B.; Aderibigbe, B.A. Recent advances in the therapeutic efficacy of artesunate. Pharmaceutics 2022, 14, 504. [Google Scholar] [CrossRef]
- Pirali, M.; Taheri, M.; Zarei, S.; Majidi, M.; Ghafouri, H. Artesunate, as a HSP70 ATPase activity inhibitor, induces apoptosis in breast cancer cells. Int. J. Biol. Macromol. 2020, 164, 3369–3375. [Google Scholar] [CrossRef]
- Zeng, H.B.; Dong, L.Q.; Xu, C.; Zhao, X.H.; Wu, L.G. Artesunate promotes osteoblast differentiation through MiR-34a/DKK1 axis. Acta Histochem. 2020, 122, 151601. [Google Scholar] [CrossRef]
- Zhang, L.; Liao, Y.; Tang, L. MicroRNA-34 family: A potential tumor suppressor and therapeutic candidate in cancer. J. Exp. Clin. Cancer Res. 2019, 38, 53. [Google Scholar] [CrossRef]
- Shishodia, S.; Sethi, G.; Aggarwal, B.B. Curcumin: Getting back to the roots. Ann. N. Y. Acad. Sci. 2005, 1056, 206–217. [Google Scholar] [CrossRef] [PubMed]
- Promdam, N.; Panichayupakaranant, P. [6]-Gingerol: A narrative review of its beneficial effect on human health. Food Chem. Adv. 2022, 1, 100043. [Google Scholar] [CrossRef]
- Teng, H.; Seuseu, K.T.; Lee, W.-Y.; Chen, L. Comparing the effects of microwave radiation on 6-gingerol and 6-shogaol from ginger rhizomes (Zingiber officinale Rosc). PLoS ONE 2019, 14, e0214893. [Google Scholar] [CrossRef]
- Zhan, K.Y.; Xu, K.; Yin, H.Z. Preparative separation and purification of gingerols from ginger (Zingiber officinale Roscoe) by high-speed counter-current chromatography. Food Chem. 2011, 126, 1959–1963. [Google Scholar] [CrossRef] [PubMed]
- Garza-Cadena, C.; Ortega-Rivera, D.M.; Machorro-García, G.; Gonzalez-Zermeño, E.M.; Homma-Dueñas, D.; Plata-Gryl, M.; Castro-Muñoz, R. A comprehensive review on Ginger (Zingiber officinale) as a potential source of nutraceuticals for food formulations: Towards the polishing of gingerol and other present biomolecules. Food Chem. 2023, 413, 135629. [Google Scholar] [CrossRef] [PubMed]
- Dang, J.; Lv, Y.; Li, C.Z.; Fang, Y.; Li, G.; Wang, Q.L. Integrated chromatographic approach for the discovery of gingerol antioxidants from Dracocephalum heterophyllum and their potential targets. Anal. Methods 2022, 14, 4133–4145. [Google Scholar] [CrossRef]
- De Lima, R.M.T.; dos Reis, A.C.; de Menezes, A.A.P.M.; Santos, J.V.; Filho, J.W.G.D.O.; Ferreira, J.R.D.O.; de Alencar, M.V.O.B.; da Mata, A.M.O.F.; Khan, I.N.; Islam, A.; et al. Protective and therapeutic potential of ginger (Zingiber officinale) extract and [6]-gingerol in cancer: A comprehensive review. Phyther. Res. 2018, 32, 1885–1907. [Google Scholar] [CrossRef]
- Ibrahim, A.S.; Sobh, M.A.; Eid, H.M.; Salem, A.; Elbelasi, H.H.; El-Naggar, M.H.; AbdelBar, F.M.; Sheashaa, H.; Sobh, M.A.; Badria, F.A. Gingerol-derivatives: Emerging new therapy against human drug-resistant MCF-7. Tumor Biol. 2014, 35, 9941–9948. [Google Scholar] [CrossRef]
- Fan, J.; Yang, X.; Bi, Z. 6-Gingerol inhibits osteosarcoma cell proliferation through apoptosis and AMPK activation. Tumor Biol. 2015, 36, 1135–1141. [Google Scholar] [CrossRef]
- Angelini, A.; Conti, P.; Ciofani, G.; Cuccurullo, F.; Di Ilio, C. Modulation of multidrug resistance P-glycoprotein activity by antiemetic compounds in human doxorubicin-resistant sarcoma cells (MES-SA/Dx-5): Implications on cancer therapy. J. Boil. Regul. Homeost. Agents 2014, 27, 1029–1037. [Google Scholar]
- Lee, D.H.; Kim, D.W.; Jung, C.H.; Lee, Y.J.; Park, D. Gingerol sensitizes TRAIL-induced apoptotic cell death of glioblastoma cells. Toxicol. Appl. Pharmacol. 2014, 279, 253–265. [Google Scholar] [CrossRef] [PubMed]
- Poltronieri, J.; Becceneri, A.B.; Fuzer, A.M.; Cesar, J.C.; Martin, A.C.B.M.; Vieira, P.C.; Pouliot, N.; Cominetti, M.R. [6]-gingerol as a cancer chemopreventive agent: A review of its activity on different steps of the metastatic process. Mini-Rev. Med. Chem. 2014, 14, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.S.; Seo, E.Y.; Kang, N.E.; Kim, W.K. [6]-Gingerol Inhibits Metastasis of MDA-MB-231 Human Breast Cancer Cells. J. Nutr. Biochem. 2008, 19, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Vichakshana, G.A.D.; Young, D.J.; Choo, W.S. Extraction, purification, food applications, and recent advances for enhancing the bioavailability of 6-gingerol from ginger–A review. Qual. Assur. Saf. Crops Foods 2022, 14, 67–83. [Google Scholar] [CrossRef]
- Poli, V.; Aparna, Y.; Motireddy, S.R. 6-Gingerol, new insights into its anti-diabetic potential with special reference to AMPK pathway: A review. J. Food Nutr. Res. 2022, 10, 681–695. [Google Scholar] [CrossRef]
- Wu, C.H.; Hong, B.H.; Ho, C.T.; Yen, G.C. Targeting cancer stem cells in breast cancer: Potential anticancer properties of 6-shogaol and pterostilbene. J. Agric. Food Chem. 2015, 63, 2432–2441. [Google Scholar] [CrossRef]
- Kim, S.M.; Kim, C.; Bae, H.; Lee, J.H.; Baek, S.H.; Nam, D.; Chung, W.S.; Shim, B.S.; Lee, S.G.; Kim, S.H.; et al. 6-Shogaol exerts anti-proliferative and pro-apoptotic effects through the modulation of STAT3 and MAPKs signaling pathways. Mol. Carcinog. 2015, 54, 1132–1146. [Google Scholar] [CrossRef]
- Hafuth, S.; Randhawa, S. Investigating the anti-cancer properties of 6-shogaol in Zingiber officinale. Crit. Rev. Oncogen. 2022, 7, 15–22. [Google Scholar] [CrossRef]
- Bawadood, A.S.; Al-Abbasi, F.A.; Anwar, F.; El-Halawany, A.M.; Al-Abd, A.M. 6-Shogaol suppresses the growth of breast cancer cells by inducing apoptosis and suppressing autophagy via targeting notch signaling pathway. Biomed. Pharmacother. 2020, 128, 110302. [Google Scholar] [CrossRef]
- Carbone, K.; Gervasi, F. An updated review of the genus Humulus: A valuable source of bioactive compounds for health and disease prevention. Plants 2022, 11, 3434. [Google Scholar] [CrossRef] [PubMed]
- De Andrade Silva, G.V.; Demaman Arend, G.; Ferreira Zielinski, A.A.; Di Luccio, M.; Ambrosi, A. Xanthohumol Properties and Strategies for Extraction from Hops and Brewery Residues: A Review. Food Chem. 2023, 404, 134629. [Google Scholar] [CrossRef] [PubMed]
- Harish, V.; Haque, E.; Śmiech, M.; Taniguchi, H.; Jamieson, S.; Tewari, D.; Bishayee, A. Xanthohumol for human malignancies: Chemistry, pharmacokinetics and molecular targets. Int. J. Mol. Sci. 2021, 22, 4478. [Google Scholar] [CrossRef]
- Miranda, C.L.; Stevens, J.F.; Helmrich, A.; Henderson, M.C.; Rodriguez, R.J.; Yang, Y.H.; Deinzer, M.L.; Barnes, D.W.; Buhler, D.R. Antiproliferative and cytotoxic effects of prenylated flavonoids from hops (Humulus lupulus) in human cancer cell lines. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 1999, 37, 271–285. [Google Scholar] [CrossRef] [PubMed]
- Yoo, Y.B.; Park, K.S.; Kim, J.B.; Kang, H.J.; Yang, J.H.; Lee, E.K.; Kim, H.Y. Xanthohumol inhibits cellular proliferation in a breast cancer cell line (MDA-MB231) through an intrinsic mitochondrial-dependent pathway. Indian J. Cancer 2014, 51, 518–523. [Google Scholar]
- Kim, S.Y.; Lee, I.-S.; Moon, A. 2-Hydroxychalcone and xanthohumol inhibit invasion of triple negative breast cancer cells. Chem. Biol. Interact. 2013, 203, 565–572. [Google Scholar] [CrossRef]
- Tan, K.W.; Cooney, J.; Jensen, D.; Li, Y.; Paxton, J.W.; Birch, N.P.; Scheepens, A. Hop-derived prenylflavonoids are substrates and inhibitors of the efflux transporter breast cancer resistance protein (BCRP/ABCG2). Mol. Nutr. Food Res. 2014, 14, 2099–2110. [Google Scholar] [CrossRef]
- Kang, Y.; Park, M.A.; Heo, S.W.; Park, S.Y.; Kang, K.W.; Park, P.H.; Kim, J.A. The radio-sensitizing effect of xanthohumol is mediated by STAT3 and EGFR suppression in doxorubicin-resistant MCF-7 human breast cancer cells. Biochim. Biophys. Acta 2013, 1830, 2638–2648. [Google Scholar] [CrossRef]
- Hemachandra, L.; Madhubhani, P.; Chandrasena, R.; Esala, P.; Chen, S.-N.; Main, M.; Lankin, D.C.; Scism, R.A.; Dietz, B.M.; Pauli, G.F. Hops (Humulus lupulus) inhibits oxidative estrogen metabolism and estrogen-induced malignant transformation in human mammary epithelial cells (MCF-10A). Cancer Prev. Res. 2012, 5, 73–81. [Google Scholar] [CrossRef]
- Monteiro, R.; Faria, A.; Azevedo, I.; Calhau, C. Modulation of breast cancer cell survival by aromatase inhibiting hop (Humulus lupulus L.) flavonoids. J. Steroid Biochem. Mol. Biol. 2007, 105, 124–130. [Google Scholar] [CrossRef]
- Yoshimaru, T.; Komatsu, M.; Tashiro, E.; Imoto, M.; Osada, H.; Miyoshi, Y.; Honda, J.; Sasa, M.; Katagiri, T. Xanthohumol suppresses oestrogen-signalling in breast cancer through the inhibition of BIG3-PHB2 interactions. Sci. Rep. 2014, 4, 7355. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Dhawan, S.; Girase, P.S.; Awolade, P.; Shinde, S.R.; Karpoormath, R.; Singh, P. Recent Advances in Chalcone-Based Anticancer Heterocycles: A Structural and Molecular Target Perspective. Curr. Med. Chem. 2021, 28, 6805–6845. [Google Scholar] [CrossRef] [PubMed]
- Viola, K.; Kopf, S.; Rarova, L.; Jarukamjorn, K.; Kretschy, N.; Teichmann, M.; Vonach, C.; Atanasov, A.G.; Giessrigl, B.; Huttary, N.; et al. Xanthohumol attenuates tumour cell-mediated breaching of the lymphendothelial barrier and prevents intravasation and metastasis. Arch. Toxicol. 2013, 87, 1301–1312. [Google Scholar] [CrossRef] [PubMed]
- Neumann, H.F.; Frank, J.; Venturelli, S.; Egert, S. Bioavailability and cardiometabolic effects of xanthohumol: Evidence from animal and human studies. Mol. Nutr. Food Res. 2022, 66, e2100831. [Google Scholar] [CrossRef]
- Langley, B.O.; Ryan, J.J.; Hanes, D.; Phipps, J.; Stack, E.; Metz, T.O.; Stevens, J.F.; Bradley, R. Xanthohumol microbiome and signature in healthy adults (the XMaS Trial): Safety and tolerability results of a phase i triple-masked, placebo-controlled clinical trial. Mol. Nutr. Food Res. 2021, 65, 2001170. [Google Scholar] [CrossRef]
- Singhal, J.; Chikara, S.; Horne, D.; Salgia, R.; Awasthi, S.Z.; Singhal, S.S. 2′-Hydroxyflavanone inhibits in vitro and in vivo growth of breast cancer cells by targeting RLIP76. Mol. Carcinog. 2018, 57, 1751–1762. [Google Scholar] [CrossRef]
- El-Kersh, D.M.; Ezzat, S.M.; Salama, M.M.; Mahrous, E.A.; Attia, Y.M.; Ahmed, M.S.; Elmazar, M.M. Anti-estrogenic and anti-aromatase activities of citrus peels major compounds in breast cancer. Sci. Rep. 2021, 11, 7121. [Google Scholar] [CrossRef]
- Iriti, M.; Kubina, R.; Cochis, A.; Sorrentino, R.; Varoni, E.M.; Kabała-Dzik, A.; Azzimonti, B.; Dziedzic, A.; Rimondini, L.; Wojtyczka, R.D. Rutin, a quercetin glycoside, restores chemosensitivity in human breast cancer cells. Phytother. Res. 2017, 31, 1529–1538. [Google Scholar] [CrossRef]
- Pandey, P.; Khan, F. A mechanistic review of the anticancer potential of hesperidin, a natural flavonoid from citrus fruits. Nutr. Res. 2021, 92, 21–31. [Google Scholar] [CrossRef]
- Garg, A.; Garg, S.; Zaneveld, L.J.D.; Singla, A.K. Chemistry and pharmacology of the Citrus bioflavonoid hesperidin. Phytother. Res. 2001, 15, 655–669. [Google Scholar] [CrossRef]
- Alam, F.; Mohammadin, K.; Shafique, Z.; Amjad, S.T.; Asad, M. Citrus flavonoids as potential therapeutic agents: A review. Phytother. Res. 2022, 36, 1417–1441. [Google Scholar] [CrossRef]
- Ahmed, O.M.; AbouZid, S.F.; Ahmed, N.A.; Zaky, M.Y.; Liu, H. An up-to-date review on citrus flavonoids: Chemistry and benefits in health and diseases. Curr. Pharm. Des. 2021, 27, 513–530. [Google Scholar] [CrossRef] [PubMed]
- Guthrie, N.; Carroll, K.K. Inhibition of Mammary Cancer by Citrus Flavonoids. Flavonoids Living Syst. 1998, 439, 227–236. [Google Scholar]
- Nguyen, M.; Osipo, C. Targeting breast cancer stem cells using naturally occurring phytoestrogens. Int. J. Mol. Sci. 2022, 23, 6813. [Google Scholar] [CrossRef] [PubMed]
- Tipton, D.A.; Lyle, B.; Babich, H.; Dabbous, M.K. In Vitro cytotoxic and anti-inflammatory effects of myrrh oil on human gingival fibroblasts and epithelial cells. Toxicol. In Vitro 2003, 17, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Hassan, A.B.; Shahid, S.M.A.; Alkhamaiseh, S.I.; Ahmed, M.Q.; Albalwi, F.O.; Al-Gholaigah, M.H.; Alqahtani, M.M.; Alshammari, M.G.; Kausar, M.A. Antibacterial activity of Commiphora molmol in wound infections. Biochem. Cell. Arch. 2017, 17, 639–644. [Google Scholar]
- Alqahtani, A.S.; Nasr, F.A.; Noman, O.M.; Farooq, M.; Alhawassi, T.; Qamar, W.; El-Gamal, A. Cytotoxic evaluation and anti-angiogenic effects of two furano-sesquiterpenoids from Commiphora myrrh resin. Molecules 2020, 25, 1318. [Google Scholar] [CrossRef]
- US Food and Drug Administration. CFR—Code of Federal Regulations Title 21; FDA: Silver Spring, MD, USA, 2015. Available online: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/cfrsearch.cfm (accessed on 19 May 2023).
- Batiha, G.E.; Wasef, L.; Teibo, J.O.; Shaheen, H.M.; Zakariya, A.M.; Akinfe, O.A.; Teibo, T.K.A.; Al-Kuraishy, H.M.; Al-Garbee, A.I.; Alexiou, A.; et al. Commiphora myrrh: A phytochemical and pharmacological update. Naunyn. Schmiedebergs Arch. Pharmacol. 2023, 396, 405–420. [Google Scholar] [CrossRef]
- Rasheed, N.; Ghafoor, S. Commiphora wightii and molmol have therapeutic effects in oral cancers and COVID-19 disease by modulating anti-apoptotic proteins and inflammatory pathways. Biomedica 2022, 38, 193–197. [Google Scholar] [CrossRef]
- Al-Harbi, M.M.; Qureshi, S.; Raza, M.; Ahmed, M.M.; Giangreco, A.B.; Shah, A.H. Anticarcinogenic effect of Commiphora molmol on solid tumors induced by Ehrlich carcinoma cells in mice. Chemotherapy 1994, 40, 337–347. [Google Scholar] [CrossRef]
- Suliman, R.S.; Alghamdi, S.S.; Ali, R.; Aljatli, D.; Aljammaz, N.A.; Huwaizi, S.; Rahman, I. The Role of Myrrh Metabolites in Cancer, Inflammation, and Wound Healing: Prospects for a Multi-Targeted Drug Therapy. Pharmaceuticals 2022, 15, 944. [Google Scholar] [CrossRef]
- Kuck, K.; Unterholzner, A.; Lipowicz, B.; Schwindl, S.; Jürgenliemk, G.; Schmidt, T.J.; Heilmann, J. Terpenoids from myrrh and their cytotoxic activity against HeLa cells. Molecules 2023, 28, 1637. [Google Scholar] [CrossRef]
- Wu, Y.; Zhou, T.; Qian, D.; Liu, X.; Xu, Y.; Hong, W.; Meng, X.; Tang, H. Z-Guggulsterone induces cell cycle arrest and apoptosis by targeting the p53/CCNB1/PLK1 pathway in triple-negative breast cancer. ACS Omega 2023, 8, 2780–2792. [Google Scholar] [CrossRef]
- Shehata, T.M.; Elsewedy, H.S. Paclitaxel and myrrh oil combination therapy for enhancement of cytotoxicity against breast cancer; QbD approach. Processes 2022, 10, 907. [Google Scholar] [CrossRef]
- Messina, G.; Scarpa, S.; Rovelli, F.; Colciago, M.; Merli, N.; Lissoni, P. A randomized study of complementary supportive medicine with Aloe arborescens vs. Aloe plus myrrh in metastatic solid tumor patients who did not respond to the standard anticancer therapies. Med. Clin. Sci. 2022, 4, 1–3. [Google Scholar] [CrossRef]
- Kaiser, A.E.; Baniasadi, M.; Giansiracusa, D.; Giansiracusa, M.; Garcia, M.; Fryda, Z.; Wong, T.L.; Bishayee, A. Sulforaphane: A broccoli bioactive phytocompound with cancer preventive potential. Cancers 2021, 13, 4796. [Google Scholar] [CrossRef]
- Khan, S.; Awan, K.A.; Iqbal, M.J. Sulforaphane as a potential remedy against cancer: Comprehensive mechanistic review. J. Food Biochem. 2022, 46, e13886. [Google Scholar]
- Saavedra-Leos, M.Z.; Jordan-Alejandre, E.; Puente-Rivera, J.; Silva-Cázares, M.B. Molecular pathways related to sulforaphane as adjuvant treatment: A nanomedicine perspective in breast cancer. Medicina 2022, 58, 1377. [Google Scholar] [CrossRef] [PubMed]
- Ho, E.; Clarke, J.D.; Dashwood, R.H. Dietary sulforaphane, a histone deacetylase inhibitor for cancer prevention. J. Nutr. 2009, 139, 2393–2396. [Google Scholar] [CrossRef] [PubMed]
- Appari, M.; Babu, K.R.; Kaczorowski, A.; Gross, W.; Herr, I. Sulforaphane, quercetin and catechins complement each other in elimination of advanced pancreatic cancer by mir-let-7 induction and k-ras inhibition. Int. J. Oncol. 2014, 45, 1391–1400. [Google Scholar] [CrossRef] [PubMed]
- Nallasamy, P.; Si, H.; Babu, P.V.A.; Pan, D.; Fu, Y.; Brooke, E.A.; Shah, H.; Zhen, W.; Zhu, H.; Liu, D. Sulforaphane reduces vascular inflammation in mice and prevents TNF-α-induced monocyte adhesion to primary endothelial cells through interfering with the NF-κB pathway. J. Nutr. Biochem. 2014, 25, 824–833. [Google Scholar] [CrossRef]
- Kaczyńska, A.; Świerczyńska, J.; Herman-Antosiewicz, A. Sensitization of HER2 positive breast cancer cells to lapatinib using plants-derived isothiocyanates. Nutr. Cancer 2015, 67, 976–986. [Google Scholar] [CrossRef] [PubMed]
- Ou, J.; Smith, R.C.; Tobe, R.H.; Lin, J.; Arriaza, J.; Fahey, J.W.; Liu, R.; Zeng, Y.; Liu, Y.; Huang, L.; et al. Efficacy of sulforaphane in treatment of children with autism spectrum disorder: A randomized double-blind placebo-controlled multi-center trial. J. Autism Develop. Disord. 2022; in press. [Google Scholar] [CrossRef] [PubMed]
- Bose, C.; Awasthi, S.; Sharma, R.; Benes, H.; Hauer-Jensen, M.; Boerma, M.; Singh, S.P. Sulforaphane potentiates anticancer effects of doxorubicin and attenuates its cardiotoxicity in a breast cancer model. PLoS ONE 2018, 13, e0193918. [Google Scholar] [CrossRef] [PubMed]
- Mordecai, J.; Ullah, S.; Ahmad, I. Sulforaphane and its protective role in prostate cancer: A mechanistic approach. Int. J. Mol. Sci. 2023, 24, 6979. [Google Scholar] [CrossRef] [PubMed]
- Bozic, D.; Baralić, K.; Živančević, K.; Miljaković, E.A.; Ćurčić, M.; Antonijević, B.; Djordjević, A.B.; Bulat, Z.; Zhang, Y.; Yang, L.; et al. Predicting sulforaphane-induced adverse effects in colon cancer patients via in silico investigation. Biomed. Pharmacother. 2022, 146, 112598. [Google Scholar] [CrossRef] [PubMed]
- Yao, S.; Kwan, M.L.; Ergas, I.J.; Roh, J.M.; Cheng, T.D.; Hong, C.C.; McCann, S.E.; Tang, L.; Davis, W.; Liu, S.; et al. Association of serum level of vitamin D at diagnosis with breast cancer survival: A case-cohort analysis in the pathways study. JAMA Oncol. 2017, 3, 351–357. [Google Scholar] [CrossRef] [PubMed]
- Chopra, H.; Mishra, A.K.; Baig, A.A.; Mohanta, T.K. Narrative review: Bioactive potential of various mushrooms as the treasure of versatile therapeutic natural product. J. Fungi 2021, 7, 728. [Google Scholar] [CrossRef]
- Lu, C.C.; Hsu, Y.J.; Chang, C.J.; Lin, C.S.; Martel, J.; Ojcius, D.M.; Ko, Y.F.; Lai, H.C.; Young, J.D. Immunomodulatory properties of medicinal mushrooms: Differential effects of water and ethanol extracts on NK cell-mediated cytotoxicity. Innate Immun. 2016, 22, 522–533. [Google Scholar] [CrossRef]
- Priyadarshini, D.K.S.; Kumar, M.T. Immunomodulatory and anti-cancer properties of pharmacologically relevant mushroom glycans. Recent Pat. Biotechnol. 2016, 10, 72–78. [Google Scholar] [CrossRef]
- Jeff, I.B.; Fan, E.; Tian, M.; Song, C.; Yan, J.; Zhou, Y. In vivo anticancer and immunomodulating activities of mannogalactoglucan-type polysaccharides from Lentinus edodes (Berkeley) Singer. Cent. Eur. J. Immunol. 2016, 1, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Sarup Singh, R.; Preet Kaur, H.; Rakesh Kanwar, J. Mushroom lectins as promising anticancer substances. Curr. Protein Pept. Sci. 2016, 17, 797–807. [Google Scholar] [CrossRef] [PubMed]
- Frouws, M.A.; Bastiaannet, E.; Langley, R.E.; Chia, W.K.; van Herk-Sukel, M.P.; Lemmens, V.E.; Putter, H.; Hartgrink, H.H.; Bonsing, B.A.; Van de Velde, C.J.; et al. Effect of low-dose aspirin use on survival of patients with gastrointestinal malignancies; an observational study. Br. J. Cancer 2017, 116, 405–413. [Google Scholar] [CrossRef]
- Todoric, J.; Antonucci, L.; Karin, M. Targeting inflammation in cancer prevention and therapy. Cancer Prev. Res. 2016, 9, 895–905. [Google Scholar] [CrossRef]
- Ventura, L.; Miccinesi, G.; Barchielli, A.; Manneschi, G.; Puliti, D.; Mantellini, P.; Orso, F.; Zappa, M. Does low-dose aspirin use for cardiovascular disease prevention reduce colorectal cancer deaths? A comparison of two cohorts in the Florence district, Italy. Eur. J. Cancer Prev. 2018, 27, 2034–2039. [Google Scholar] [CrossRef]
- Boutaud, O.; Sosa, I.R.; Amin, T.; Oram, D.; Adler, D.; Hwang, H.S.; Crews, B.C.; Milne, G.; Harris, B.K.; Hoeksema, M.; et al. Inhibition of the biosynthesis of prostaglandin E2 by low-dose aspirin: Implications for adenocarcinoma metastasis. Cancer Prev. Res. 2016, 9, 855–865. [Google Scholar] [CrossRef] [PubMed]
- Milani, D.; Khorramymehr, S.; Vasaghi-Gharamaleki, B. The effect of acetylsalicylic acid (Asa) on the mechanical properties of breast cancer epithelial cells. Recent Pat. Anti-Cancer Drug Discov. 2022, 17, 410–415. [Google Scholar] [CrossRef] [PubMed]
- Abo-Elmatty, D.M.; Ahmed, E.A.; Tawfik, M.K.; Helmy, S.A. Metformin enhancing the antitumor efficacy of carboplatin against Ehrlich solid carcinoma grown in diabetic mice: Effect on IGF-1 and tumoral expression of IGF-1 receptors. Int. Immunopharmacol. 2017, 44, 72–86. [Google Scholar] [CrossRef]
- Leonel, C.; Borin, T.F.; de Carvalho Ferreira, L.; Moschetta, M.G.; Bajgelman, M.C.; Viloria-Petit, A.M.; de Campos Zuccari, D.A.P. Inhibition of Epithelial-Mesenchymal Transition and Metastasis by Combined TGFbeta Knockdown and Metformin Treatment in a Canine Mammary Cancer Xenograft Model. J. Mammary Gland Biol. Neoplasia 2017, 22, 27–41. [Google Scholar] [CrossRef]
- Leonel, C.; Ferreira, L.C.; Borin, T.F.; Moschetta, M.G.; Freitas, G.S.; Haddad, M.R.; de Camargos Pinto Robles, J.A.; Aparecida Pires de Campos Zuccari, D. Inhibition of epithelial-mesenchymal transition in response to treatment with metformin and Y27632 in breast cancer cell lines. Anticancer Agents Med. Chem. 2017, 17, 1113–1125. [Google Scholar] [CrossRef]
- Rico, M.; Baglioni, M.; Bondarenko, M.; Cesatti Laluce, N.; Rozados, V.; André, N.; Carré, M.; Graciela Scharovsky, O.; Menacho Márquez, M. Metformin and propranolol combination prevents cancer progression and metastasis in different breast cancer models. Oncotarget 2016, 8, 2874. [Google Scholar] [CrossRef] [PubMed]
- Xie, W.; Wang, L.; Sheng, H.; Qiu, J.; Zhang, D.; Zhang, L.; Yang, F.; Tang, D.; Zhang, K. Metformin induces growth inhibition and cell cycle arrest by upregulating microRNA34a in renal cancer cells. Med. Sci. Monit. 2017, 23, 29–37. [Google Scholar] [CrossRef]
- Micallef, D.; Micallef, S.; Schembri-Wismayer, P.; Calleja-Agius, J. Novel applications of COX-2 inhibitors, metformin, and statins for the primary chemoprevention of breast cancer. J. Turk. Ger. Gynecol. Assoc. 2016, 17, 214–223. [Google Scholar] [CrossRef] [PubMed]
- Grossmann, M.E.; Yang, D.Q.; Guo, Z.; Potter, D.A.; Cleary, M.P. Metformin treatment for the prevention and/or treatment of breast/mammary tumorigenesis. Curr. Pharmacol. Rep. 2015, 1, 312–323. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Mao, W.; Zhai, Y.; Tong, C.; Liu, M.; Ma, L.; Yu, X.; Li, S. Anti-tumor activity of metformin: From metabolic and epigenetic perspectives. Oncotarget 2017, 8, 5619–5628. [Google Scholar] [CrossRef]
- Sacco, F.; Calderone, A.; Castagnoli, L.; Cesareni, G. The cell-autonomous mechanisms underlying the activity of metformin as an anticancer drug. Br. J. Cancer 2016, 115, 1451–1456. [Google Scholar] [CrossRef]
- Chai, X.; Chu, H.; Yang, X.; Meng, Y.; Shi, P.; Gou, S. Metformin Increases Sensitivity of Pancreatic Cancer Cells to Gemcitabine by Reducing CD133+ Cell Populations and Suppressing ERK/P70S6K Signaling. Sci. Rep. 2015, 5, 14404. [Google Scholar] [CrossRef]
- Soo, J.S.; Ng, C.H.; Tan, S.H.; Malik, R.A.; Teh, Y.C.; Tan, B.S.; Ho, G.F.; See, M.H.; Taib, N.A.; Yip, C.H.; et al. Metformin synergizes 5-fluorouracil, epirubicin, and cyclophosphamide (FEC) combination therapy through impairing intracellular ATP production and DNA repair in breast cancer stem cells. Apoptosis 2015, 20, 1373–1387. [Google Scholar] [CrossRef]
- Zhang, Y.; Storr, S.J.; Johnson, K.; Green, A.R.; Rakha, E.A.; Ellis, I.O.; Morgan, D.A.L.; Martin, S.G. Involvement of metformin and AMPK in the radioresponse and prognosis of luminal versus basal-like breast cancer treated with radiotherapy. Oncotarget 2014, 5, 12936–12949. [Google Scholar] [CrossRef]
- Kangwan, N.; Park, J.M.; Kim, E.H.; Hahm, K.B. Chemoquiescence for ideal cancer treatment and prevention: Where are we now? J. Cancer Prev. 2014, 19, 89–96. [Google Scholar] [CrossRef]
| Structure | Name |
|---|---|
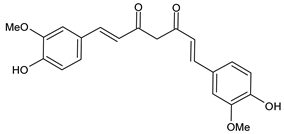 | Curcumin |
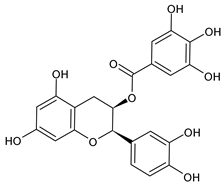 | Epigallocatechin gallate (EGCG) |
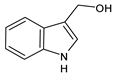 | Indole-3-carbinol (I3C) |
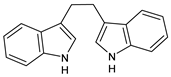 | Diindolylmethane |
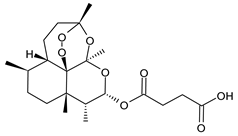 | Artesunate |
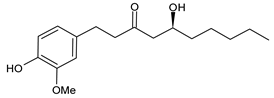 | 6-Gingerol |
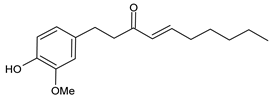 | 6-Shogaol (6SG) |
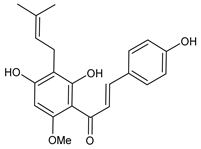 | Xanthohumol |
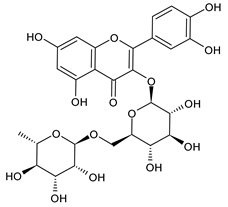 | Rutin |
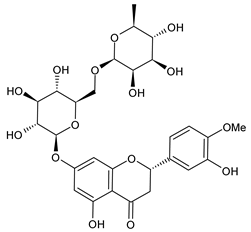 | Hesperidin |
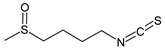 | Sulforaphane |
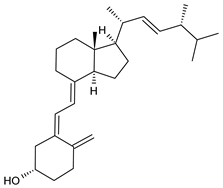 | Vitamin D2 |
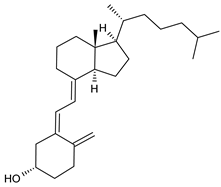 | Vitamin D3 |
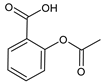 | Acetylsalicylic acid (ASA) |
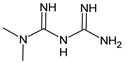 | Metformin |
| Natural Product | Name of the Clinical Trial | NCT Number | Doses | Phase | Trial Status |
|---|---|---|---|---|---|
| Curcumin | “Curcumin” in Combination With Chemotherapy in Advanced Breast Cancer | NCT03072992 | 300 mg i.v. | Phase 2 | Completed |
| Curcumin | Phase II Study of Curcumin vs. Placebo for Chemotherapy-Treated Breast Cancer Patients Undergoing Radiotherapy | NCT01740323 | 500 mg BID MERIVA corresponding to ~90 mg of curcumin | Phase 2 | Completed |
| Epigallocatechin-3-gallate (EGCG) | Study of Topically Applied Green Tea Extract for Radio Dermatitis and Radiation Mucositis | NCT01481818 | Application of 0.01~0.05 mL/cm2 3 times a day to the area under treatment during radiotherapy | Phase 2 | Unknown |
| Indole-3-Carbinol | Indole-3-Carbinol in Preventing Breast Cancer in Nonsmoking Women Who Are at High Risk For Breast Cancer (clinical trial: NCT00033345) | NCT00033345 | 400–800 mg pill taken daily | Phase 1 | Completed |
| Artesunate | Study of Artesunate in Metastatic Breast Cancer | NCT00764036 | Add-on therapy with daily single oral doses: 100, 150 or 200 mg | Phase 1 | Completed |
| Ginger (6-gingerol and 6-shogaol) | Ginger in Treating Nausea in Patients Receiving Chemotherapy for Cancer | NCT00040742 | 0.5–1.5 g oral high-dose ginger twice daily | Phase 3 | Completed |
| Xanthohumol | Xanthohumol and Prevention of DNA Damage | NCT02432651 | 6–24 mg xanthohumol per day | Phase 1 | Completed |
| Bioflavonoids | Defined Green Tea Catechin Extract in Treating Women With Hormone Receptor Negative Stage I-III Breast Cancer | NCT00516243 | Green tea catechin extract PO BID (amount not given) | Phase 1 | Completed |
| Bioflavonoids | Green Tea and Reduction of Breast Cancer Risk | NCT00917735 | Two green tea extract capsules, each containing 80.7% total catechins (51.7% EGCG) twice daily | Phase 2 | Completed |
| Bioflavonoids | Disposition of Dietary Polyphenols and Methylxanthines in Mammary Tissues From Breast Cancer Patients (POLYSEN) | NCT03482401 | 3 capsules/day (474 mg phenolics/day) | NA | Completed |
| Sulforaphane | Study to Evaluate the Effect of Sulforaphane in Broccoli Sprout Extract on Breast Tissue | NCT00982319 | 100 µmol of sulforaphane (dissolvable) in Broccoli sprout extract | Phase 2 | Completed |
| SFX-01 (Sulforaphane + alpha-cyclodextrin) | SFX-01 in the Treatment and Evaluation of Metastatic Breast Cancer (STEM) | NCT02970682 | SFX-01, provided as 300 mg capsules, one to be taken twice daily | Phase 2 | Completed |
| Vitamin D | Vitamin D can Increase Pathological Response of the Breast Cancer Patients Treated with Neoadjuvant Therapy | NCT03986268 | 50,000 IU weekly | NA | Unknown |
| Mushrooms | White Button Mushroom Extract in Preventing the Recurrence of Breast Cancer in Postmenopausal Breast Cancer Survivors | NCT00709020 | Escalating doses: 5 g/day, then 8 g/day, then 10 g/day, then 13 g/day | Phase 1 | Completed |
| Acetylsalicylic acid | Aspirin in Preventing Recurrence of Cancer in Patients with HER2 Negative Stage II-III Breast Cancer After Chemotherapy, Surgery, and/or Radiation Therapy | NCT02927249 | 300 mg daily | Phase 3 | Terminated |
| Acetylsalicylic acid | Low Dose Chemotherapy With Aspirin in Patients With Breast Cancer After Neoadjuvant Chemotherapy | NCT01612247 | 325 mg PO daily | NA | Unknown |
| Metformin Hydrocloride | Metformin Hydrochloride vs. Placebo in Overweight or Obese Patients at Elevated Risk for Breast Cancer | NCT01793948 | 850 mg PO BID | Early phase 1 | Completed |
| Metformin | Metformin Hydrochloride in Preventing Breast Cancer in Patients With Atypical Hyperplasia or in Situ Breast Cancer | NCT01905046 | 850 mg PO BID | Phase 3 | Active, not recruiting |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Svolacchia, F.; Brongo, S.; Catalano, A.; Ceccarini, A.; Svolacchia, L.; Santarsiere, A.; Scieuzo, C.; Salvia, R.; Finelli, F.; Milella, L.; et al. Natural Products for the Prevention, Treatment and Progression of Breast Cancer. Cancers 2023, 15, 2981. https://doi.org/10.3390/cancers15112981
Svolacchia F, Brongo S, Catalano A, Ceccarini A, Svolacchia L, Santarsiere A, Scieuzo C, Salvia R, Finelli F, Milella L, et al. Natural Products for the Prevention, Treatment and Progression of Breast Cancer. Cancers. 2023; 15(11):2981. https://doi.org/10.3390/cancers15112981
Chicago/Turabian StyleSvolacchia, Fabiano, Sergio Brongo, Alessia Catalano, Agostino Ceccarini, Lorenzo Svolacchia, Alessandro Santarsiere, Carmen Scieuzo, Rosanna Salvia, Francesca Finelli, Luigi Milella, and et al. 2023. "Natural Products for the Prevention, Treatment and Progression of Breast Cancer" Cancers 15, no. 11: 2981. https://doi.org/10.3390/cancers15112981
APA StyleSvolacchia, F., Brongo, S., Catalano, A., Ceccarini, A., Svolacchia, L., Santarsiere, A., Scieuzo, C., Salvia, R., Finelli, F., Milella, L., Saturnino, C., Sinicropi, M. S., Fabrizio, T., & Giuzio, F. (2023). Natural Products for the Prevention, Treatment and Progression of Breast Cancer. Cancers, 15(11), 2981. https://doi.org/10.3390/cancers15112981











