Prediction of Primary Tumor Sites in Spinal Metastases Using a ResNet-50 Convolutional Neural Network Based on MRI
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
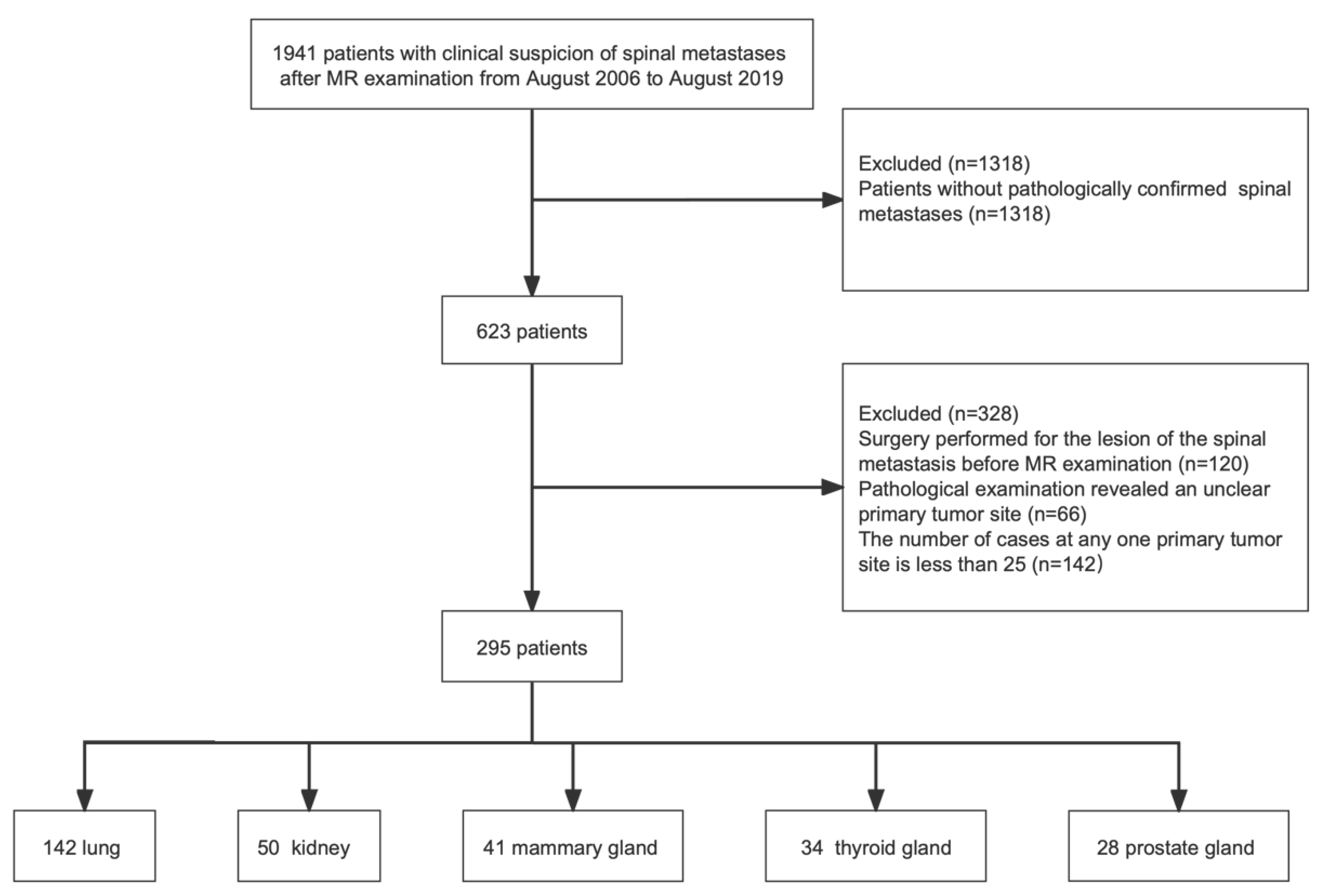
2.1. Study Population
2.2. Imaging Acquisition
2.3. Data Pre-Processing
2.4. Training Set and Testing Set Split
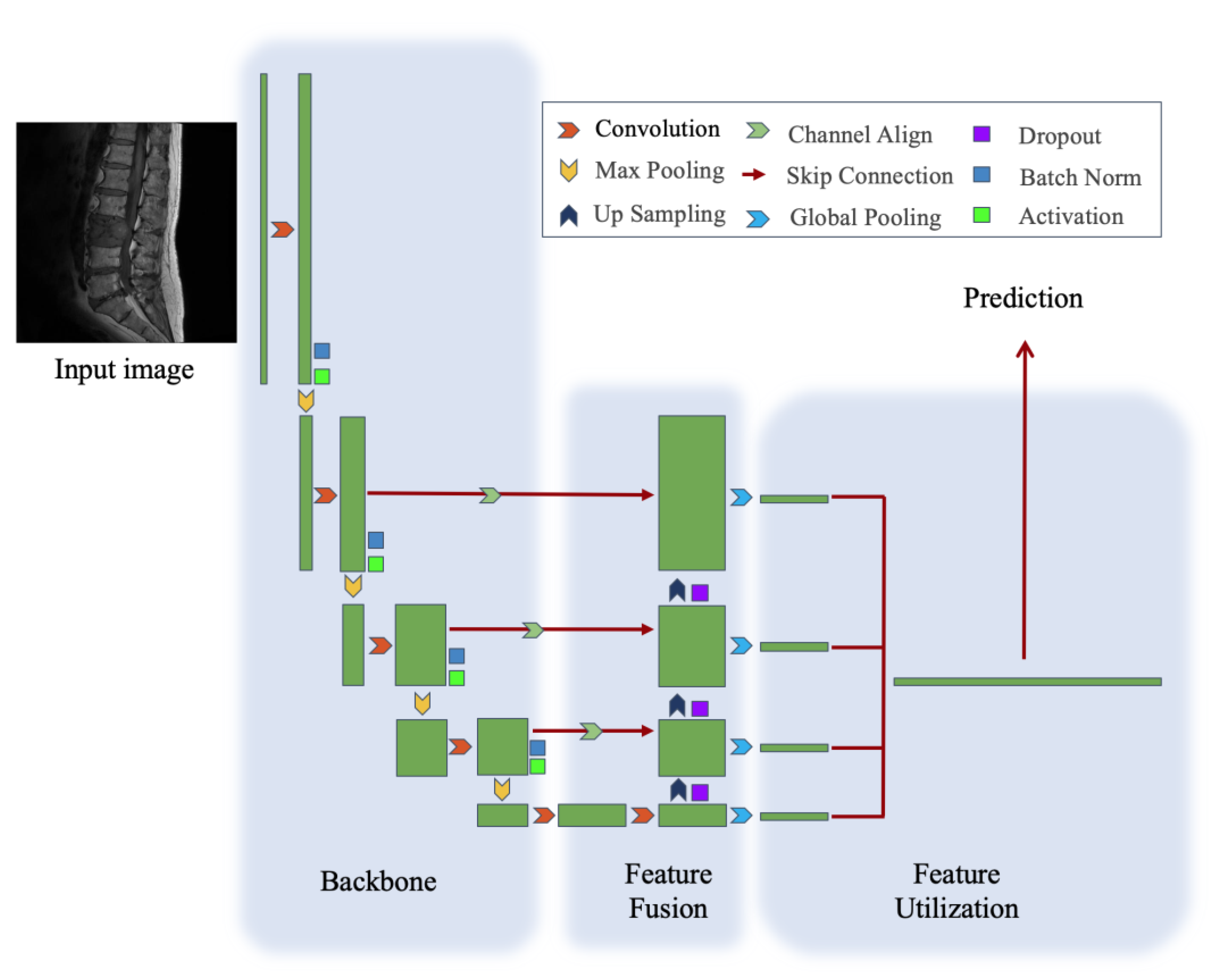
2.5. Model Architecture
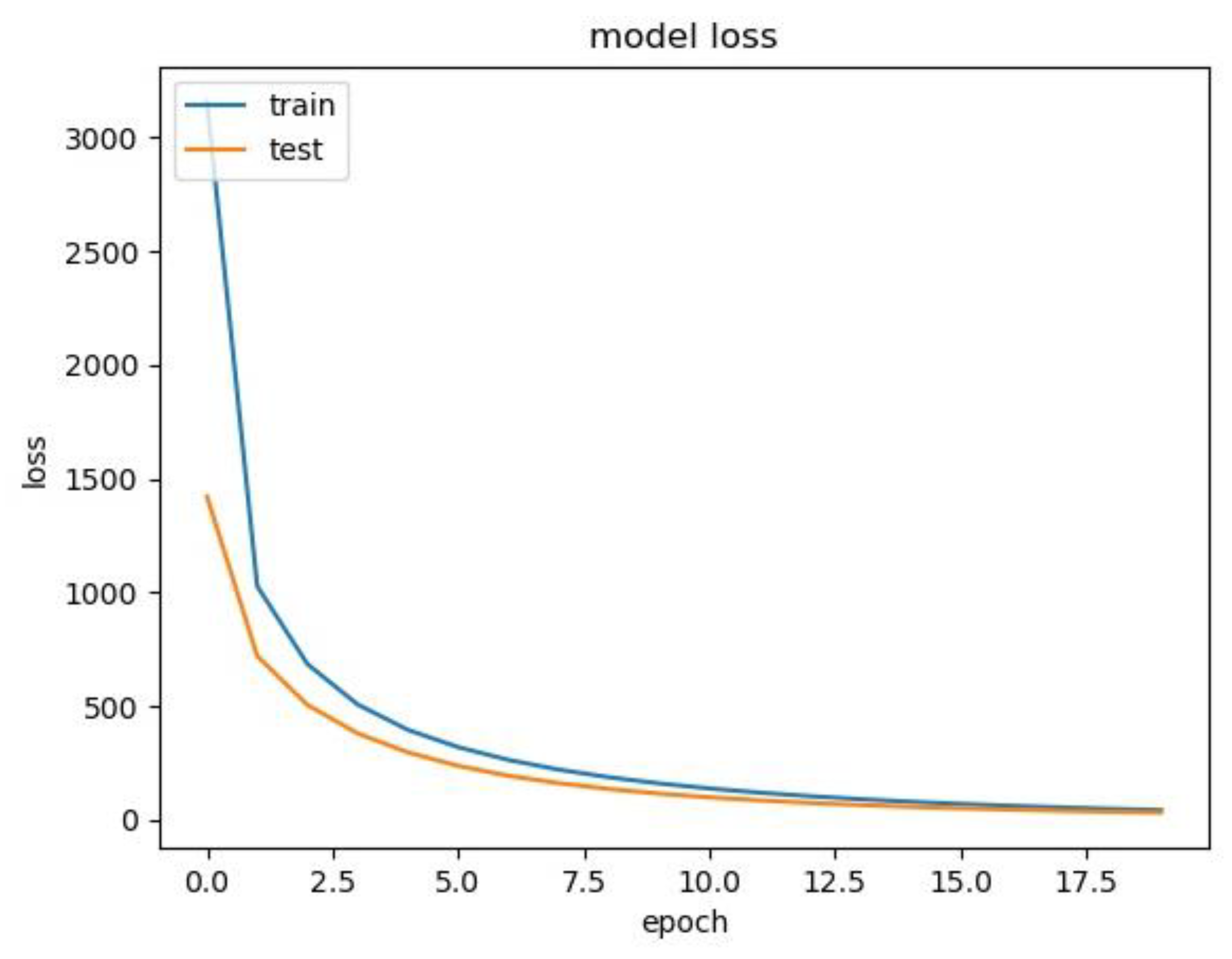
2.6. Training and Evaluation
2.7. Inference and Visualization
2.8. Prior Information from Gender and Age
3. Results
3.1. Patient Characteristics
3.2. Performance of the ResNet-50 Model
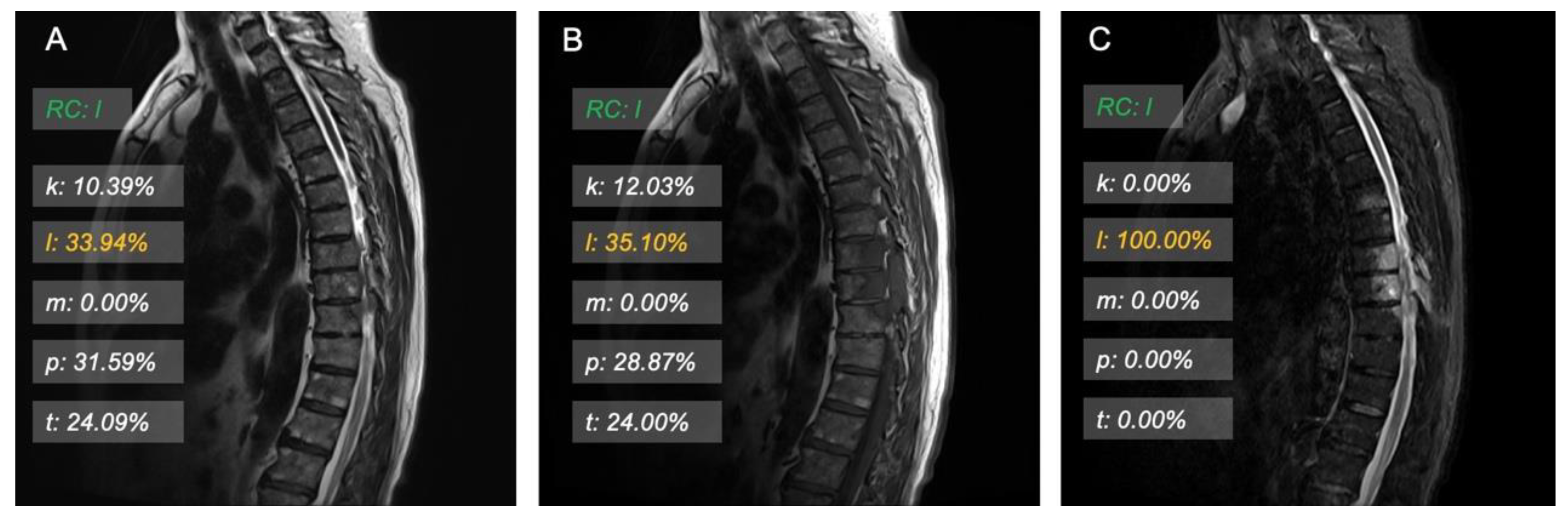
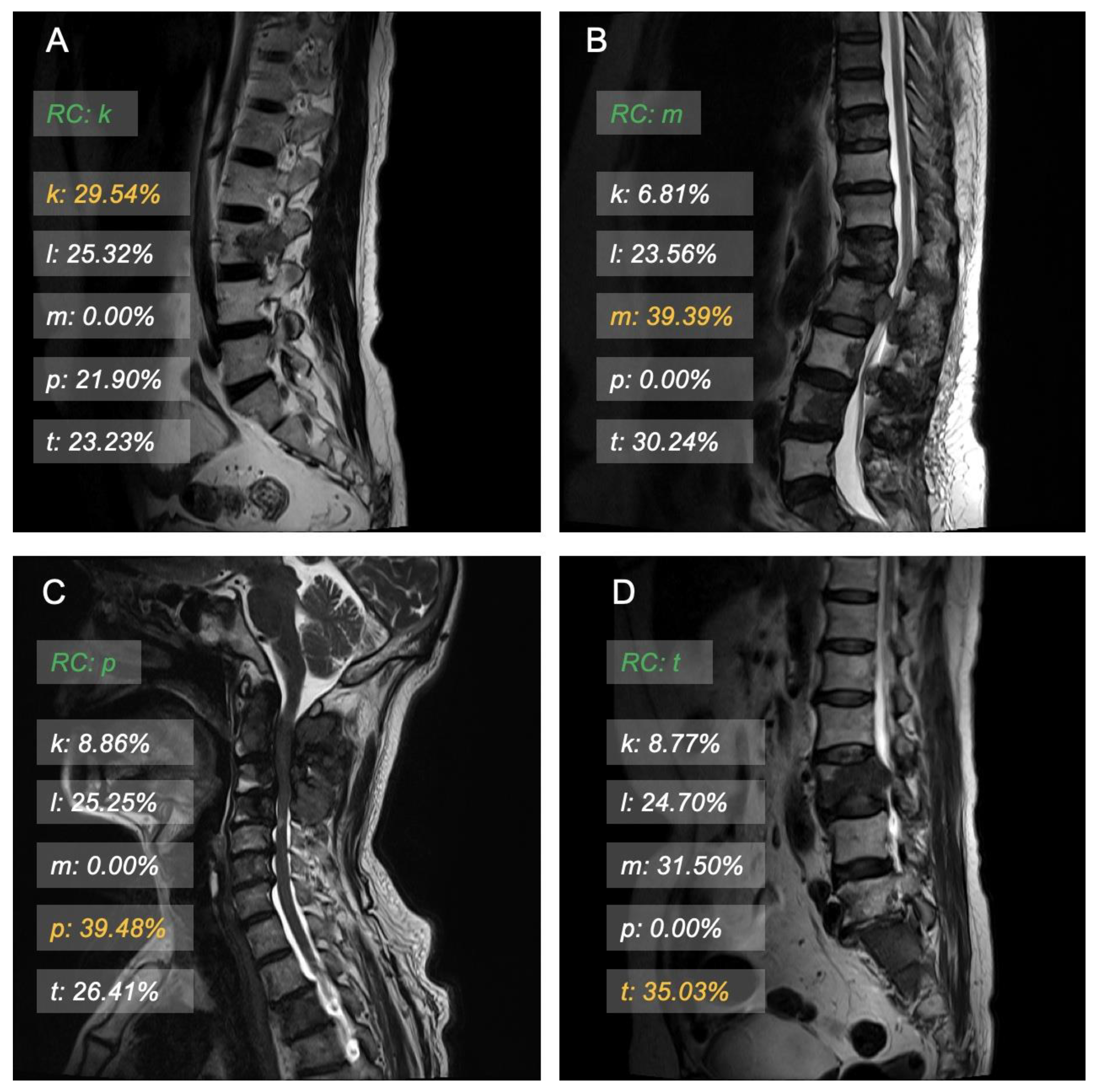
3.3. Model Interpretation and Examples
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Onken, J.S.; Fekonja, L.S.; Wehowsky, R.; Hubertus, V.; Vajkoczy, P. Metastatic Dissemination Patterns of Different Primary Tumors to the Spine and Other Bones. Clin. Exp. Metastasis 2019, 36, 493–498. [Google Scholar] [CrossRef] [PubMed]
- Wewel, J.T.; O’Toole, J.E. Epidemiology of Spinal Cord and Column Tumors. Neuro-Oncol. Pract. 2020, 7, i5–i9. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global Cancer Statistics 2018: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA A Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Wright, E.; Ricciardi, F.; Arts, M.; Buchowski, J.M.; Chung, C.K.; Coppes, M.; Crockard, A.; Depreitere, B.; Fehlings, M.; Kawahara, N.; et al. Metastatic Spine Tumor Epidemiology: Comparison of Trends in Surgery Across Two Decades and Three Continents. World Neurosurg. 2018, 114, e809–e817. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.-R.; Qiao, R.-Q.; Yang, X.-G.; Hu, Y.-C. A Multicenter, Descriptive Epidemiologic Survey of the Clinical Features of Spinal Metastatic Disease in China. Neurol. Res. 2020, 42, 749–759. [Google Scholar] [CrossRef]
- Wang, F.; Zhang, H.; Yang, L.; Yang, X.; Zhang, H.; Li, J.; Qiao, R.; Hu, Y. Epidemiological Characteristics of 1196 Patients with Spinal Metastases: A Retrospective Study. Orthop. Surg. 2019, 11, 1048–1053. [Google Scholar] [CrossRef]
- Choi, S.H.; Koo, J.W.; Choe, D.; Kang, C.-N. The Incidence and Management Trends of Metastatic Spinal Tumors in South Korea: A Nationwide Population-Based Study. Spine 2020, 45, E856–E863. [Google Scholar] [CrossRef]
- Ahmed, A.K.; Goodwin, C.R.; Heravi, A.; Kim, R.; Abu-Bonsrah, N.; Sankey, E.; Kerekes, D.; De la Garza Ramos, R.; Schwab, J.; Sciubba, D.M. Predicting Survival for Metastatic Spine Disease: A Comparison of Nine Scoring Systems. Spine J. 2018, 18, 1804–1814. [Google Scholar] [CrossRef]
- Dardic, M.; Wibmer, C.; Berghold, A.; Stadlmueller, L.; Froehlich, E.V.; Leithner, A. Evaluation of Prognostic Scoring Systems for Spinal Metastases in 196 Patients Treated during 2005–2010. Eur. Spine J. 2015, 24, 2133–2141. [Google Scholar] [CrossRef]
- Yao, A.; Sarkiss, C.A.; Ladner, T.R.; Jenkins, A.L. Contemporary Spinal Oncology Treatment Paradigms and Outcomes for Metastatic Tumors to the Spine: A Systematic Review of Breast, Prostate, Renal, and Lung Metastases. J. Clin. Neurosci. 2017, 41, 11–23. [Google Scholar] [CrossRef]
- Lu, M.Y.; Chen, T.Y.; Williamson, D.F.K.; Zhao, M.; Shady, M.; Lipkova, J.; Mahmood, F. AI-Based Pathology Predicts Origins for Cancers of Unknown Primary. Nature 2021, 594, 106–110. [Google Scholar] [CrossRef] [PubMed]
- Pavlidis, N.; Pentheroudakis, G. Cancer of Unknown Primary Site. Lancet 2012, 379, 1428–1435. [Google Scholar] [CrossRef]
- Bollen, L.; Dijkstra, S.P.D.; Bartels, R.H.M.A.; de Graeff, A.; Poelma, D.L.H.; Brouwer, T.; Algra, P.R.; Kuijlen, J.M.A.; Minnema, M.C.; Nijboer, C.; et al. Clinical Management of Spinal Metastases—The Dutch National Guideline. Eur. J. Cancer 2018, 104, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Shah, L.M.; Salzman, K.L. Imaging of Spinal Metastatic Disease. Int. J. Surg. Oncol. 2011, 2011, e769753. [Google Scholar] [CrossRef]
- Litjens, G.; Kooi, T.; Bejnordi, B.E.; Setio, A.A.A.; Ciompi, F.; Ghafoorian, M.; van der Laak, J.A.W.M.; van Ginneken, B.; Sánchez, C.I. A Survey on Deep Learning in Medical Image Analysis. Med. Image Anal. 2017, 42, 60–88. [Google Scholar] [CrossRef] [PubMed]
- Yeh, L.-R.; Zhang, Y.; Chen, J.-H.; Liu, Y.-L.; Wang, A.-C.; Yang, J.-Y.; Yeh, W.-C.; Cheng, C.-S.; Chen, L.-K.; Su, M.-Y. A Deep Learning-Based Method for the Diagnosis of Vertebral Fractures on Spine MRI: Retrospective Training and Validation of ResNet. Eur. Spine J. 2022, 31, 2022–2030. [Google Scholar] [CrossRef] [PubMed]
- Talo, M.; Yildirim, O.; Baloglu, U.B.; Aydin, G.; Acharya, U.R. Convolutional Neural Networks for Multi-Class Brain Disease Detection Using MRI Images. Comput. Med. Imaging Graph. 2019, 78, 101673. [Google Scholar] [CrossRef]
- Liu, S.; Shah, Z.; Sav, A.; Russo, C.; Berkovsky, S.; Qian, Y.; Coiera, E.; Di Ieva, A. Isocitrate Dehydrogenase (IDH) Status Prediction in Histopathology Images of Gliomas Using Deep Learning. Sci. Rep. 2020, 10, 7733. [Google Scholar] [CrossRef]
- Matsui, Y.; Maruyama, T.; Nitta, M.; Saito, T.; Tsuzuki, S.; Tamura, M.; Kusuda, K.; Fukuya, Y.; Asano, H.; Kawamata, T.; et al. Prediction of Lower-Grade Glioma Molecular Subtypes Using Deep Learning. J. Neurooncol. 2020, 146, 321–327. [Google Scholar] [CrossRef]
- He, K.; Zhang, X.; Ren, S.; Sun, J. Deep Residual Learning for Image Recognition; IEEE: Piscataway, NJ, USA, 2016; pp. 770–778. [Google Scholar]
- Lin, T.-Y.; Dollar, P.; Girshick, R.; He, K.; Hariharan, B.; Belongie, S. Feature Pyramid Networks for Object Detection. In Proceedings of the IEEE Conference on Computer Vision and Pattern Recognition, Honolulu, HI, USA, 21–26 July 2017; pp. 2117–2125. [Google Scholar]
- Bridle, J.S. Probabilistic Interpretation of Feedforward Classification Network Outputs, with Relationships to Statistical Pattern Recognition. In Neurocomputing: Algorithms, Architectures and Applications; Soulié, F.F., Hérault, J., Eds.; Springer: Berlin/Heidelberg, Germany, 1990; pp. 227–236. [Google Scholar]
- Ruder, S. An Overview of Gradient Descent Optimization Algorithms. arXiv 2017, arXiv:1609.04747. [Google Scholar] [CrossRef]
- Kingma, D.P.; Ba, J. Adam: A Method for Stochastic Optimization. arXiv 2017, arXiv:1412.6980. [Google Scholar] [CrossRef]
- Vanel, D.; Casadei, R.; Alberghini, M.; Razgallah, M.; Busacca, M.; Albisinni, U. MR Imaging of Bone Metastases and Choice of Sequence: Spin Echo, In-Phase Gradient Echo, Diffusion, and Contrast Medium. Semin. Musculoskelet. Radiol. 2009, 13, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Filograna, L.; Lenkowicz, J.; Cellini, F.; Dinapoli, N.; Manfrida, S.; Magarelli, N.; Leone, A.; Colosimo, C.; Valentini, V. Identification of the Most Significant Magnetic Resonance Imaging (MRI) Radiomic Features in Oncological Patients with Vertebral Bone Marrow Metastatic Disease: A Feasibility Study. Radiol. Med. 2019, 124, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Ben-Cohen, A.; Klang, E.; Diamant, I.; Rozendorn, N.; Raskin, S.P.; Konen, E.; Amitai, M.M.; Greenspan, H. CT Image-Based Decision Support System for Categorization of Liver Metastases into Primary Cancer Sites. Acad. Radiol. 2017, 24, 1501–1509. [Google Scholar] [CrossRef] [PubMed]
- Kniep, H.C.; Madesta, F.; Schneider, T.; Hanning, U.; Schönfeld, M.H.; Schön, G.; Fiehler, J.; Gauer, T.; Werner, R.; Gellissen, S. Radiomics of Brain MRI: Utility in Prediction of Metastatic Tumor Type. Radiology 2019, 290, 479–487. [Google Scholar] [CrossRef] [PubMed]
- Hua, W.; Xiao, T.; Jiang, X.; Liu, Z.; Wang, M.; Zheng, H.; Wang, S. Lymph-Vascular Space Invasion Prediction in Cervical Cancer: Exploring Radiomics and Deep Learning Multilevel Features of Tumor and Peritumor Tissue on Multiparametric MRI. Biomed. Signal Process. Control 2020, 58, 101869. [Google Scholar] [CrossRef]
- Jiang, X.; Li, J.; Kan, Y.; Yu, T.; Chang, S.; Sha, X.; Zheng, H.; Luo, Y.; Wang, S. MRI Based Radiomics Approach With Deep Learning for Prediction of Vessel Invasion in Early-Stage Cervical Cancer. IEEE/ACM Trans. Comput. Biol. Bioinform. 2021, 18, 995–1002. [Google Scholar] [CrossRef]
- Chlap, P.; Min, H.; Vandenberg, N.; Dowling, J.; Holloway, L.; Haworth, A. A Review of Medical Image Data Augmentation Techniques for Deep Learning Applications. J. Med. Imaging Radiat. Oncol. 2021, 65, 545–563. [Google Scholar] [CrossRef]
- Frid-Adar, M.; Diamant, I.; Klang, E.; Amitai, M.; Goldberger, J.; Greenspan, H. GAN-Based Synthetic Medical Image Augmentation for Increased CNN Performance in Liver Lesion Classification. Neurocomputing 2018, 321, 321–331. [Google Scholar] [CrossRef]
- Hashemi, S.R.; Mohseni Salehi, S.S.; Erdogmus, D.; Prabhu, S.P.; Warfield, S.K.; Gholipour, A. Asymmetric Loss Functions and Deep Densely-Connected Networks for Highly-Imbalanced Medical Image Segmentation: Application to Multiple Sclerosis Lesion Detection. IEEE Access 2019, 7, 1721–1735. [Google Scholar] [CrossRef]
- Herzog, L.; Murina, E.; Dürr, O.; Wegener, S.; Sick, B. Integrating Uncertainty in Deep Neural Networks for MRI Based Stroke Analysis. Med. Image Anal. 2020, 65, 101790. [Google Scholar] [CrossRef] [PubMed]
| Lung Cancer (n = 142) | Kidney Cancer (n = 50) | Mammary Cancer (n = 41) | Thyroid Cancer (n = 34) | Prostate Cancer (n = 28) | |
|---|---|---|---|---|---|
| Sex | |||||
| M | 76 | 38 | 0 | 12 | 28 |
| F | 66 | 12 | 41 | 22 | 0 |
| Mean age (y) * | |||||
| M | 61.4 ± 9.7 (38–81) | 58.6 ± 9.0 (37–72) | - | 60.8 ± 6.8 (46–72) | 68.0 ± 11.0 (39–84) |
| F | 62.4 ± 9.5 (37–82) | 56.5 ± 12.6 (30–75) | 52.1 ± 8.7 (34–73) | 55.3 ± 13.3 (27–76) | - |
| Scan location | |||||
| Cervical spine | 49 | 24 | 13 | 20 | 7 |
| Thoracic spine | 25 | 12 | 10 | 4 | 11 |
| Lumbar spine | 65 | 14 | 18 | 10 | 10 |
| Sacral spine | 3 | 0 | 0 | 0 | 0 |
| Model | Top-1 Accuracy (%) | Precision (%) | Sensitivity (%) | Specificity (%) | AUC-ROC | F1 Score |
|---|---|---|---|---|---|---|
| 5-class | 52.97 (52.08~53.86) | 59.84 (59.18~60.50) | 48.56 (47.95~49.17) | 61.81 (57.23~66.39) | 0.77 (0.76~0.77) | 0.54 (0.53~0.54) |
| T1WS | 49.05 (48.51~49.59) | 100.00 (99.99~100) | 23.09 (22.30~23.88) | 65.50 (62.78~68.22) | 0.74 (0.73~0.75) | 0.38 (0.37~0.39) |
| T2WS | 41.83 (41.09~42.57) | 43.73 (42.54~44.92) | 50.00 (49.27~50.73) | 49.77 (47.28~52.06) | 0.75 (0.74~0.76) | 0.47 (0.46~0.47) |
| T2WS-FS | 36.32 (35.36~37.28) | 45.83 (45.28~46.38) | 40.01 (39.11~40.91) | 68.00 (65.87~70.13) | 0.70 (0.69~0.71) | 0.43 (0.42~0.44) |
| 4-class | 58.46 (57.78~59.14) | 61.82 (61.33~62.31) | 57.13 (56.67~57.59) | 80.77 (75.69~85.85) | 0.81 (0.80~0.82) | 0.59 (0.59~0.59) |
| 3-class | 67.16 (66.22~68.12) | 68.99 (68.04~68.12) | 66.91 (66.37~67.45) | 83.97 (77.42~90.52) | 0.85 (0.84~0.86) | 0.68 (0.67~0.69) |
| Model | Top-1 Accuracy (%) | Precision (%) | Sensitivity (%) | Specificity (%) | AUC-ROC | F1 Score |
|---|---|---|---|---|---|---|
| Fold 1 | 58.68 | 61.27 | 53.42 | 67.14 | 0.80 | 0.56 |
| Fold 2 | 56.06 | 60.15 | 48.98 | 61.81 | 0.76 | 0.51 |
| Fold 3 | 57.23 | 57.97 | 52.68 | 62.81 | 0.79 | 0.54 |
| Fold 4 | 49.59 | 58.36 | 41.22 | 53.20 | 0.74 | 0.42 |
| Fold 5 | 57.23 | 56.80 | 51.09 | 63.02 | 0.78 | 0.53 |
| Average | 55.76 | 58.91 | 49.48 | 61.60 | 0.77 | 0.51 |
| Method of Gender and Age Classifier | Top-1 Accuracy (%) |
|---|---|
| Naive Bayes | 37.96 (35.87~40.05) |
| Logistic Regression | 32.05 (20.16~33.94) |
| Support Vector Classifier | 33.90 (31.92~35.88) |
| K-Nearest Neighbors | 23.06 (21.35~25.77) |
| Random Forest | 22.76 (21.98~23.62) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, K.; Qin, S.; Ning, J.; Xin, P.; Wang, Q.; Chen, Y.; Zhao, W.; Zhang, E.; Lang, N. Prediction of Primary Tumor Sites in Spinal Metastases Using a ResNet-50 Convolutional Neural Network Based on MRI. Cancers 2023, 15, 2974. https://doi.org/10.3390/cancers15112974
Liu K, Qin S, Ning J, Xin P, Wang Q, Chen Y, Zhao W, Zhang E, Lang N. Prediction of Primary Tumor Sites in Spinal Metastases Using a ResNet-50 Convolutional Neural Network Based on MRI. Cancers. 2023; 15(11):2974. https://doi.org/10.3390/cancers15112974
Chicago/Turabian StyleLiu, Ke, Siyuan Qin, Jinlai Ning, Peijin Xin, Qizheng Wang, Yongye Chen, Weili Zhao, Enlong Zhang, and Ning Lang. 2023. "Prediction of Primary Tumor Sites in Spinal Metastases Using a ResNet-50 Convolutional Neural Network Based on MRI" Cancers 15, no. 11: 2974. https://doi.org/10.3390/cancers15112974
APA StyleLiu, K., Qin, S., Ning, J., Xin, P., Wang, Q., Chen, Y., Zhao, W., Zhang, E., & Lang, N. (2023). Prediction of Primary Tumor Sites in Spinal Metastases Using a ResNet-50 Convolutional Neural Network Based on MRI. Cancers, 15(11), 2974. https://doi.org/10.3390/cancers15112974








