Belantamab Mafodotin in Patients with Relapsed/Refractory Multiple Myeloma: Results of the Compassionate Use or the Expanded Access Program in Spain
Abstract
Simple Summary
Abstract
1. Introduction
2. Methods and Patients
Statistical Analysis
3. Results
3.1. Efficacy
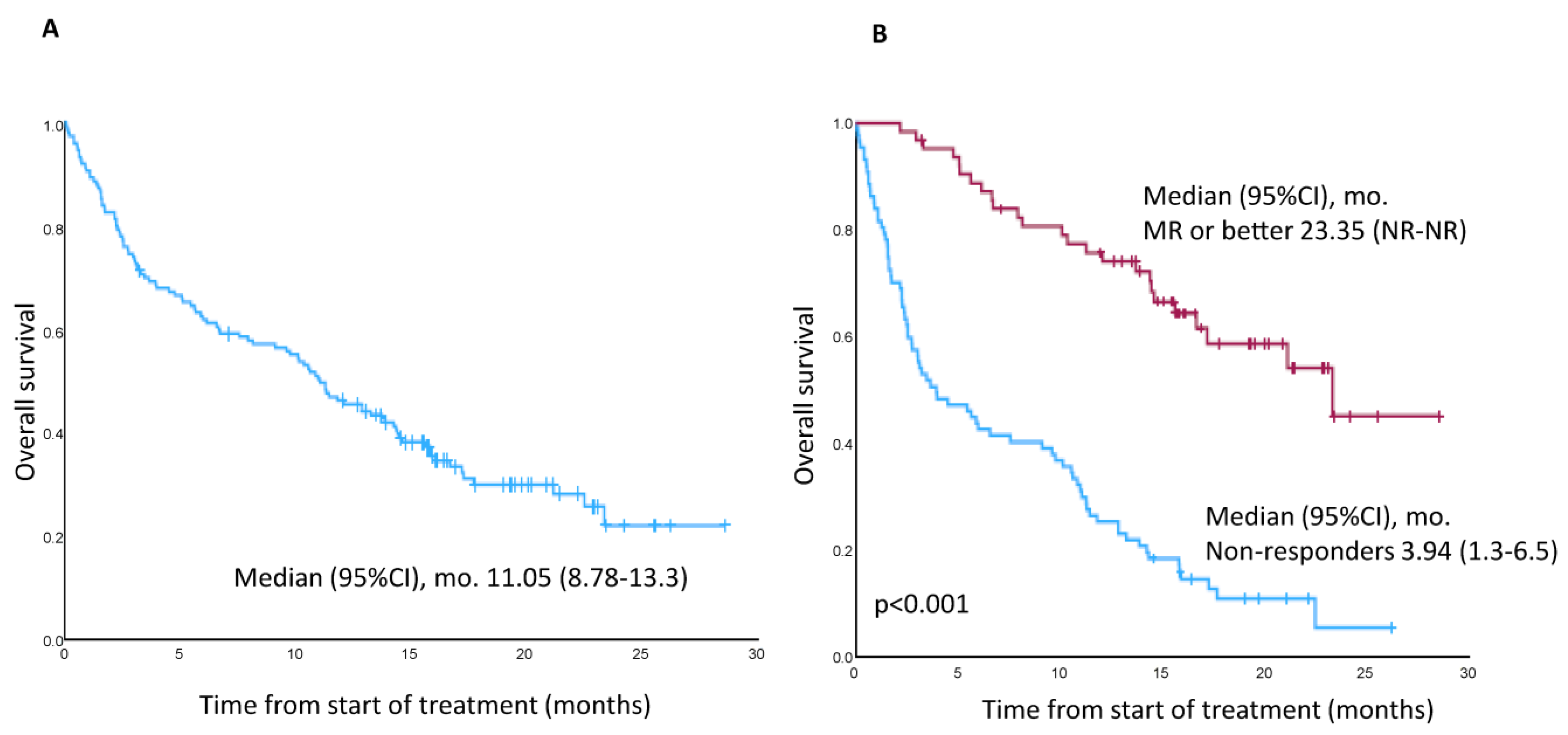
3.2. PFS, DoR, OS, and TTNT
3.3. Safety
3.3.1. Ocular Toxicity
3.3.2. Non-Ocular Toxicity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, B.; Wu, C.; Zhong, Q. Belantamab mafodotin for the treatment of multiple myeloma. Drugs Today 2021, 57, 653–663. [Google Scholar] [CrossRef] [PubMed]
- Trudel, S.; Lendvai, N.; Popat, R.; Voorhees, P.M.; Reeves, B.; Libby, E.N.; Richardson, P.G.; Hoos, A.; Gupta, I.; Bragulat, V.; et al. Antibody-drug conjugate, GSK2857916, in relapsed/refractory multiple myeloma: An update on safety and efficacy from dose expansion phase I study. Blood Cancer J. 2019, 9, 37. [Google Scholar] [CrossRef] [PubMed]
- Lonial, S.; Lee, H.C.; Badros, A.; Trudel, S.; Nooka, A.K.; Chari, A.; Abdallah, A.O.; Callander, N.; Lendvai, N.; Sborov, D.; et al. Belantamab mafodotin for relapsed or refractory multiple myeloma (DREAMM-2) study: A two-arm, randomized, open-label, phase 2 study. Lancet 2020, 21, 207–221. [Google Scholar] [CrossRef] [PubMed]
- Lonial, S.; Lee, H.C.; Badros, A.; Trudel, S.; Nooka, A.K.; Chari, A.; Abdallah, A.O.; Callander, N.; Sborov, D.; Suvannasankha, A.; et al. Long term outcomes with single-agent belantamab mafodotin in patients with relapsed or refractory multiple myeloma: 13-month follow-up from the pivotal DREAMM-2 study. Cancer 2021, 127, 4198–4212. [Google Scholar] [CrossRef] [PubMed]
- Shah, J.J.; Abonour, R.; Gasparetto, C.; Hardin, J.W.; Toomey, K.; Narang, M.; Srinivasan, S.; Kitali, A.; Zafar, F.; Flick, E.D.; et al. Analysis of common eligibility criteria of randomized controlled trials in newly diagnosed multiple myeloma patients and extrapolating outcomes. Clin. Lymphoma Myeloma Leuk. 2017, 17, 575–583.e572. [Google Scholar] [CrossRef] [PubMed]
- Chari, A.; Romanus, D.; Palumbo, A.; Blazer, M.; Farrelly, E.; Raju, A.; Huang, H.; Richardson, P. Randomized clinical trial representativeness and outcomes in real-world patients: Comparison of 6 hallmark randomized clinical trials of relapsed/refractory multiple myeloma. Clin. Lymphoma Myeloma Leuk. 2020, 20, 8–17.e16. [Google Scholar] [CrossRef]
- Fiala, M.A.; Dukeman, J.; Stockerl-Goldstein, K.; Tomasson, M.H.; Wildes, T.M.; Vij, R. The real-world characteristics and outcomes of newly diagnosed myeloma patients ineligible for clinical trials. Clin. Lymphoma Myeloma Leuk. 2017, 17, e55–e56. [Google Scholar] [CrossRef]
- Bergin, K.; McQuilten, Z.; Moore, E.; Wood, E.; Spencer, A. Myeloma in the real world: What is really happening? Clin. Lymphoma Myeloma Leuk. 2017, 17, 133–144.e1. [Google Scholar] [CrossRef]
- Vaxman, I.; Abeykoon, J.; Dispenzieri, A.; Kumar, S.K.; Buadi, F.; Lacy, M.Q.; Dingli, D.; Hwa, Y.; Fonder, A.; Hobbs, M.; et al. “Real-life” data of the efficacy and safety of belantamab mafodotin in relapsed multiple myeloma—The Mayo Clinic experience. Blood Cancer J. 2021, 11, 196. [Google Scholar] [CrossRef]
- Iula, R.; De Novellis, D.; Trastulli, F.; Della Pepa, R.; Fontana, R.; Carobene, A.; Di Perna, M.; D’Ambrosio, A.; Romano, M.; Leone, A.; et al. Efficacy and safety of belantamab mafodotin in triple-refractory multiple myeloma patients. Front. Oncol. 2022, 12, 1026251. [Google Scholar] [CrossRef]
- Shragai, T.; Magen, H.; Lavi, N.; Gatt, M.; Trestman, S.; Zektser, M.; Ganzel, C.; Jarchowsky, O.; Berger, T.; Tadmor, T.; et al. Real-world experience with belantamab mafodotin therapy for relapsed/refractory multiple myeloma: A multicenter retrospective study. Br. J. Haematol. 2022, 200, 45–53. [Google Scholar] [CrossRef]
- Atieh, T.; Atrash, S.; Mohan, M.; Shune, L.; Mahmoudjafari, Z.; Quick, J.; Riffel, J.; McGuirk, J.P.; Mohyuddin, G.R.; Abdallah, A.O.; et al. Belantamab in combination with dexamethasone in patients with triple-class relapsed/refractory multiple myeloma. Blood 2021, 138 (Suppl. S1). [Google Scholar] [CrossRef]
- Roussel, M.; Texier, N.; Germain, R.; Sapra, S.; Paka, P.; Kerbouche, N.; Colin, X.; Leleu, X. Effectiveness and safety of belantamab mafodotin in patients with relapsed or refractory multiple myeloma in real-life setting: The ALFA study. Blood 2022, 140 (Suppl. S1), 1856. [Google Scholar] [CrossRef]
- Offidani, M.; Cavo, M.; Derudas, D.; Di Raimondo, F.; Cuneo, A.; Baldini, L.; Della Pepa, R.; Musso, M.; Boccadoro, M.; Musto, P.; et al. Belantamab mafodotin in patients with relapsed and refractory multiple myeloma who have received at least one proteasome inhibitor, one immunomodulatory agent and one anti-CD38 monoclonal antibody: A retrospective Italian observational study. Blood 2022, 140 (Suppl. S1), 7222–7223. [Google Scholar] [CrossRef]
- Hultcrantz, M.; Orozco, J.; Peterson, T.J.; Derkach, A.; Hassoun, H.; Korde, N.; Lu, S.X.; Mailankody, S.; Patel, D.; Shah, U.A.; et al. Belantamab mafodotin in patients with relapsed/refractory multiple myeloma, a real-world single-center experience. Blood 2022, 140 (Suppl. S1), 3225. [Google Scholar] [CrossRef]
- Kumar, S.; Paiva, B.; Anderson, K.C.; Durie, B.; Landgren, O.; Moreau, P.; Munshi, N.; Lonial, S.; Bladé, J.; Mateos, M.-V.; et al. International myeloma working group consensus criteria for response and minimal residual disease assessment in multiple myeloma. Lancet Oncol. 2016, 17, e228–e346. [Google Scholar] [CrossRef]
- Talbot, A.; Bobin, A.; Tabone, L.; Lambert, J.; Boccaccio, C.; Deal, C.; Petillon, M.O.; Allangba, O.; Agape, P.; Arnautou, P.; et al. Real-world study of the efficacy and safety of belantamab mafodotin (GSK2857916) in relapsed or refractory multiple myeloma based on data from the nominative ATU in France: IFM 2020-04 study. Haematologica, 2023; ahead of print. [Google Scholar] [CrossRef]
- Masters, J.C.; Nickens, D.J.; Xuan, D.; Shazer, R.L.; Amantea, M. Clinical toxicity of antibody drug conjugates: A meta-analysis of payloads. Investig. New Drugs 2018, 36, 121–135. [Google Scholar] [CrossRef]
- Farooq, A.V.; Degli Esposti, S.; Popat, R.; Thulasi, P.; Lonial, S.; Nooka, A.K.; Jakubowiak, A.; Sborov, D.; Zaugg, B.E.; Badros, A.Z. Corneal epithelial findings in patients with multiple myeloma treated with antibody-drug conjugate belantamab mafodotin in the pivotal, randomized, DREAMM-2 study. Ophthalmol. Ther. 2020, 9, 889–911. [Google Scholar] [CrossRef]
- Abeykoon, J.P.; Vaxman, J.; Patel, S.V.; Kumar, S.; Malave, G.C.; Young, K.S.; Ailawadhi, S.; Larsen, J.T.; Dispenzieri, A.; Muchtar, E.; et al. Impact of belantamab mafodotin-induced ocular toxicity on outcomes of patients with advanced multiple myeloma. Br. J. Haematol. 2022, 199, 95–99. [Google Scholar] [CrossRef]
- Terpos, E.; Kastritis, E.; Gavriatopoulou, M.; Stathopoulos, I.; Malandrakis, P.; Fotiou, D.; Kanellias, N.; Gkolfinopoulos, S.; Manousou, K.; Kastritis, E.; et al. A Phase I/II, Dose and Schedule Evaluation Study to Investigate the Safety and Clinical Activity of Belantamab Mafodotin Administered in Combination with Lenalidomide and Dexamethasone in Transplant-Ineligible NDMM. Blood 2022, 140 (Suppl. S1), 12616–12617. [Google Scholar] [CrossRef]
- Xing, L.; Liu, Y.; Liu, J. Targeting BCMA in Multiple Myeloma: Advances in Antibody-Drug Conjugate Therapy. Cancers 2023, 15, 2240. [Google Scholar] [CrossRef] [PubMed]
- Hultcrantz, M.; Kleinman, D.; Ghataorhe, P.; McKeown, A.; He, W.; Ling, T.; Jewell, R.C.; Byrne, J.; Eliason, L.; Scott, E.C.; et al. Exploring alternative dosing regimens of single-agent belantamab mafodotin on safety and efficacy in patients with relapsed or refractory multiple myeloma: DREAMM-14. Blood 2021, 138 (Suppl. S1), 1645. [Google Scholar] [CrossRef]
- GSK Provides Update on DREAMM-3 Phase III Trial for Blenrep in Relapsed/Refractory Multiple Myeloma. News Release. GSK. 7 November 2022. Available online: https://bit.ly/3FYNEnB (accessed on 8 November 2022).
- Gandhi, U.H.; Cornell, R.F.; Lakshman, A.; Gahvari, Z.J.; McGehee, E.; Jagosky, M.H.; Gupta, R.; Varnado, W.; Fiala, M.A.; Chhabra, S.; et al. Outcomes of patients with multiple myeloma refractory to CD38-targeted monoclonal antibody therapy. Leukemia 2019, 33, 2266–2675. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Ailawadhi, M.; Dutta, N.; Abdulazeez, M.; Aggarwal, C.S.; Quintero, G.; Baksh, M.; Roy, V.; Sher, T.; Alegria, V.; et al. Trends in early mortality from multiple myeloma: A population-based analysis. Clin. Lymphoma Myeloma Leuk. 2021, 21, e449–e455. [Google Scholar] [CrossRef]
- Robak, P.; Drozdz, I.; Szemraj, J.; Robak, T. Drug resistance in multiple myeloma. Cancer Treat Rev. 2018, 70, 199–208. [Google Scholar] [CrossRef]
- Moreau, P.; Touzeau, C. T-cell-redirecting bispecific antibodies in multiple myeloma: A revolution? Blood 2022, 139, 3681–3687. [Google Scholar] [CrossRef]
- Moreau, P.; Garfall, A.L.; van de Donk, N.W.; Nahi, H.; San-Miguel, J.F.; Oriol, A.; Nooka, A.K.; Martin, T.; Rosinol, L.; Chari, A.; et al. Teclistamab in Relapsed or Refractory Multiple Myeloma. N. Engl. J. Med. 2022, 387, 495–505. [Google Scholar] [CrossRef]
- Moreau, P.; van de Donk, N.W.; Delforge, M.; Einsele, H.; De Stefano, V.; Perrot, A.; Besemer, B.; Pawlyn, C.; Karlin, L.; Manier, S.; et al. Comparative Efficacy of Teclistamab Versus Current Treatments in Real-World Clinical Practice in the Prospective LocoMMotion Study in Patients with Triple-Class-Exposed Relapsed and/or Refractory Multiple Myeloma. Adv. Ther. 2023, 40, 2412–2425. [Google Scholar] [CrossRef]


| Characteristics | n = 156 | |
|---|---|---|
| Age at diagnosis, median (IQR) | 70 (64–76.8) | |
| Sex (female), no. (%) | 84 (53.8) | |
| Time from diagnosis, years, median (IQR) | 6.4 (3.7–8.3) | |
| Creatinine clearance (mL/min), no./total no. (%) | ||
| ≥30/<30 | 130 (83.3)/19 (12.2) | |
| Type of MM, no. (%) | ||
| IgG/Non-IgG | 84 (53.9)/72 (46.1) | |
| ECOG performance status score, no./total no. (%) | ||
| 0/≥1 | 34 (21.8)/104 (66.7) | |
| ISS at diagnosis, no./total no. (%) | ||
| I/II/III | 45 (28.8)/49 (31.4)/52 (33.3) | |
| R-ISS at diagnosis, no./total no. (%) | ||
| I/II/III | 33 (21.2)/53 (34.0)/32 (20.5) | |
| EMD, no./total no. (%) | 49 (31.4) | |
| High-risk cytogenetics, no./total no. (%) | ||
| del(17p) | 17/73 (23.3) | |
| t(4;14) | 15/69 (21.7) | |
| t(14;20) | 1/51 (2) | |
| 1q21+ | 28/60 (46.7) | |
| Prior treatments, median (IQR) | 5 (4–6) | |
| Refractory status, no. (%) | ||
| Refractory to ≥1 line of treatment, no. (%) | 146 (93.6%) | |
| To IMiDs | 133 (85.3) | |
| To PI | 131 (84) | |
| To anti-CD38 MoAbs | 129 (82.7) | |
| Triple-refractory | 125 (80.1) | |
| Penta-refractory | 54 (34.6) | |
| Refractory to last line of therapy | 123 (83.1) | |
| Previous HSCT, no. (%) | 101 (64.7) a | |
| Subgroup, No. | ORR, % | PFS (95% CI), mo. | OS (95% CI), mo. | |
|---|---|---|---|---|
| Refractoriness | ||||
| Triple-refractory, 125 | 60.8 | 2.6 (1.5–3.8) | 10.62 (7.4–13.8) | |
| Non-triple refractory, 23 | 69.6 | 6.9 (1.1–12.6) | 11.4 (8.2–14.7) | |
| Penta-refractory, 54 | 62.8 | 2.2 (0.5–3.8) | 9.77 (0.6–18.8) | |
| Non-penta refractory, 94 | 61.1 | 4.9 (2.3–7.4) | 11.05 (9.4–12.6) | |
| Age | ||||
| ≤70 years, 71 | 62 | 2.6 (0.7–4.5) | 10.01 (5.4–14.7) | |
| >70 years, 83 | 62.7 | 3.6 (1.9–5.3) | 12.07 (9.1–14.9) | |
| CrCl | ||||
| <30 mL/min, 17 | 41.2 | 2.07 (1.7–2.3) | 5.09 (0–21.1) | |
| ≥30 mL/min, 130 | 43.8 | 4.1 (2.7–5.5) | 11.05 (8.7–13.3) | |
| EMD | ||||
| No, 106 | 44.3 | 4.67 (2.4–6.8) | 13.2 (10.3–16.1) | |
| Yes, 49 | 37.5 | 2.1 (1.3–2.8) | 4.7 (0–10.3) | |
| All Grades, No. (%) | ≥Grade 3, No. (%) | ||
|---|---|---|---|
| Hematologic | |||
| Thrombocytopenia | 24 (15.4) | 17 (10.9) | |
| Neutropenia | 7 (3.8) | 5 (3.1) | |
| Anemia | 6 (3.9) | 2 (1.3) | |
| Non-hematologic | |||
| Infections | 25 (15) | 10 (5.6) | |
| Gastrointestinal | 9 (5.8) | 2 (1.2) | |
| Hepatobiliary | 8 (5.1) | 3 (1.8) | |
| Neurological | 6 (3.9) | 2 (1.2) | |
| Fatigue | 5 (3.2) | 0 | |
| Cardiac & Vascular | 5 (3.2) | 1 (0.6) | |
| Respiratory | 4 (2.4) | 2 (1.2) | |
| Metabolic | 3 (1.9) | 0 | |
| Renal | 3 (1.9) | 2 (1.2) | |
| Other | 10 (6.2) | 1 (0.6) | |
| Ocular a | |||
| Corneal events | 73 (87.9) | 28 (33.7) | |
| Reduced visual acuity | 50 (60.2) | 7 (8.4) | |
| Blurry vision | 31 (37.3) | 0 | |
| Dry eye | 27 (32.5) | 0 | |
| Foreign body sensation | 16 (19.2) | 0 | |
| Ocular discomfort | 15 (18.1) | 0 | |
| Photophobia | 10 (12) | 0 | |
| Other | 7 (8.4) | 0 | |
| Author, Year | No. of Patients | Age, Years (Range) | No. of Lines (Range) | Triple-ref, % | Penta-refr, % | ORR, % a | ≥PR, % | PFS (95% CI), mo. | DoR (95% CI), mo. | OS (95% CI), mo. |
|---|---|---|---|---|---|---|---|---|---|---|
| Lonial, 2020 [3] | 97 | 65 (60–70) | 7 (3–21) | 100 | NA | 35 | 31 b | 2.8 (1.6–3.6) | 11 (4.2–NR) | 13.7 (9.9–NR) |
| Vaxman, 2021 [9] | 36 | 61 (37–83) | 8 (7–11) | 100 | 100 | NA | 33 | 2 (NA) | NA | 6.5 (NA) |
| Iula, 2022 [10] | 28 | 67.5 (51–83) | 6 (3–14) | 100 | NA | NA | 40 | 3 (0–23)c | NR (2–23) | 8 (0–23) |
| Shragai, 2022 [11] | 106 | 69.4 (36.3–80) | 6 (2–11) | 72.6 | 32 | NA | 45.5 | 4.7 (3.5–5.9) | 8.1 (5.7–10.5) | 14.5 (9.5–19.6) |
| Atieh, 2021 12] | 28 | 67 (24–85) | 5 (3–15) | 100 | 54 | 46 | 46 | 4.9 (NA) | NA | 7.4 (NA) |
| Rousell, 2022 [13] | 184 | 70.3 (63.3–75.9) | 5 (2->5) | NA | NA | 36.4 | 32.7 | 2.4 (1.9–3.2) | NA | 8.8 (6.3–11.6) |
| Offidani, 2022 [14] | 67 | 66 (42–82) | 5 (2–10) | NA | NA | 37 | 31 | 3.7 (NA) | 13.8 (NA) | 12.9 (NA) |
| Hultcrantz, 2022 [15] | 90 | 66 (37–88) | 6 (2–14) | NA | NA | 63 d | 42 | 4 (NA) | 13.1 (NA) | 20.5 (NA) |
| Talbot, 2023 [17] | 106 | 66 (37–82) | 5 (3–12) | 56.7 | 11.3 | 38.1 | NA | 3.5 (1.9–4.7) | 9 (4.65–10.4) c | 9.3 (5.9.15.3) |
| This series, 2023 | 156 | 72.5 (40–89) | 5 (2–10) | 80.1 | 34.6 | 41.8 | 39.8 | 3.6 (2.1–5.1) | 13.9 (8.3–19.4) | 11.05 (8.7–13.3) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de la Rubia, J.; Alonso, R.; Clavero, M.E.; Askari, E.; García, A.; Antón, C.; Fernández, M.; Escalante, F.; García, A.; Rios-Tamayo, R.; et al. Belantamab Mafodotin in Patients with Relapsed/Refractory Multiple Myeloma: Results of the Compassionate Use or the Expanded Access Program in Spain. Cancers 2023, 15, 2964. https://doi.org/10.3390/cancers15112964
de la Rubia J, Alonso R, Clavero ME, Askari E, García A, Antón C, Fernández M, Escalante F, García A, Rios-Tamayo R, et al. Belantamab Mafodotin in Patients with Relapsed/Refractory Multiple Myeloma: Results of the Compassionate Use or the Expanded Access Program in Spain. Cancers. 2023; 15(11):2964. https://doi.org/10.3390/cancers15112964
Chicago/Turabian Stylede la Rubia, Javier, Rafael Alonso, María Esther Clavero, Elham Askari, Alfonso García, Cristina Antón, Margarita Fernández, Fernando Escalante, Ana García, Rafael Rios-Tamayo, and et al. 2023. "Belantamab Mafodotin in Patients with Relapsed/Refractory Multiple Myeloma: Results of the Compassionate Use or the Expanded Access Program in Spain" Cancers 15, no. 11: 2964. https://doi.org/10.3390/cancers15112964
APA Stylede la Rubia, J., Alonso, R., Clavero, M. E., Askari, E., García, A., Antón, C., Fernández, M., Escalante, F., García, A., Rios-Tamayo, R., Conesa, V., Bermúdez, M. A., Merchán, B., Velasco, A. E., Blanchard, M. J., Sampol, A., Gainza, E., Hernández, P. M., & Alegre, A. (2023). Belantamab Mafodotin in Patients with Relapsed/Refractory Multiple Myeloma: Results of the Compassionate Use or the Expanded Access Program in Spain. Cancers, 15(11), 2964. https://doi.org/10.3390/cancers15112964






