Actinic Keratoses: A Prospective Pilot Study on a Novel Formulation of 4% 5-Fluorouracil Cream and a Review of Other Current Topical Treatment Options
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- de Oliveira, E.C.V.; da Motta, V.R.V.; Pantoja, P.C.; Ilha, d.O.C.S.; Magalhães, R.F.; Galadari, H.; Leonardi, G.R. Actinic Keratosis—Review for Clinical Practice. Int. J. Dermatol. 2019, 58, 400–407. [Google Scholar] [CrossRef] [PubMed]
- Olsen, E.A.; Lisa Abernethy, M.; Kulp-Shorten, C.; Callen, J.P.; Glazer, S.D.; Huntley, A.; McCray, M.; Monroe, A.B.; Tschen, E.; Wolf, J.E. A Double-Blind, Vehicle-Controlled Study Evaluating Masoprocol Cream in the Treatment of Actinic Keratoses on the Head and Neck. J. Am. Acad. Dermatol. 1991, 24, 738–743. [Google Scholar] [CrossRef] [PubMed]
- Del Regno, L.; Catapano, S.; Di Stefani, A.; Cappilli, S.; Peris, K. A Review of Existing Therapies for Actinic Keratosis: Current Status and Future Directions. Am. J. Clin. Dermatol. 2022, 23, 339–352. [Google Scholar] [CrossRef] [PubMed]
- Conforti, C.; Giuffrida, R.; Dianzani, C.; Guarneri, F.; Marangi, G.F.; Neagu, N.; Persichetti, P.; Zalaudek, I.; di Meo, N. Effectiveness and Tolerability of Treatment for Isolated Actinic Keratoses: A Retrospective Comparison between Cryotherapy, CO2 Laser and 5-Fluorouracil 0.5%/Salicylic Acid 10%. Dermatol. Ther. 2021, 34, e14846. [Google Scholar] [CrossRef] [PubMed]
- Dianzani, C.; Conforti, C.; Giuffrida, R.; Corneli, P.; di Meo, N.; Farinazzo, E.; Moret, A.; Magaton Rizzi, G.; Zalaudek, I. Current Therapies for Actinic Keratosis. Int. J. Dermatol. 2020, 59, 677–684. [Google Scholar] [CrossRef] [PubMed]
- Mittelbronn, M.A.; Mullins, D.L.; Ramos-Caro, F.A.; Flowers, F.P. Frequency of pre-Existing Actinic Keratosis in Cutaneous Squamous Cell Carcinoma. Int. J. Dermatol. 1998, 37, 677–681. [Google Scholar] [CrossRef]
- Fernández-Figueras, M.T.; Carrato, C.; Sáenz, X.; Puig, L.; Musulen, E.; Ferrándiz, C.; Ariza, A. Actinic Keratosis with Atypical Basal Cells (AK I) Is the Most Common Lesion Associated with Invasive Squamous Cell Carcinoma of the Skin. J. Eur. Acad. Dermatol. Venereol. 2015, 29, 991–997. [Google Scholar] [CrossRef]
- Dirschka, T.; Gupta, G.; Micali, G.; Stockfleth, E.; Basset-Séguin, N.; Del Marmol, V.; Dummer, R.; Jemec, G.B.E.; Malvehy, J.; Peris, K.; et al. Real-World Approach to Actinic Keratosis Management: Practical Treatment Algorithm for Office-Based Dermatology. J. Dermatol. Treat. 2017, 28, 431–442. [Google Scholar] [CrossRef]
- Philipp-Dormston, W.G.; Battistella, M.; Boussemart, L.; Di Stefani, A.; Broganelli, P.; Thoms, K.M. Patient-Centered Management of Actinic Keratosis. Results of a Multi-Center Clinical Consensus Analyzing Non-Melanoma Skin Cancer Patient Profiles and Field-Treatment Strategies. J. Dermatol. Treat. 2020, 31, 576–582. [Google Scholar] [CrossRef]
- Stockfleth, E.; Kerl, H.; Zwingers, T.; Willers, C. Low-Dose 5-Fluorouracil in Combination with Salicylic Acid as a New Lesion-Directed Option to Treat Topically Actinic Keratoses: Histological and Clinical Study Results. Br. J. Dermatol. 2011, 165, 1101–1108. [Google Scholar] [CrossRef]
- Jansen, M.H.E.; Kessels, J.P.H.M.; Nelemans, P.J.; Kouloubis, N.; Arits, A.H.M.M.; van Pelt, H.P.A.; Quaedvlieg, P.J.F.; Essers, B.A.B.; Steijlen, P.M.; Kelleners-Smeets, N.W.J.; et al. Randomized Trial of Four Treatment Approaches for Actinic Keratosis. N. Engl. J. Med. 2019, 380, 935–946. [Google Scholar] [CrossRef]
- Magdalene, A. Dohil MD Efficacy, Safety, and Tolerability of 4% 5-Fluorouracil Cream in a Novel Patented Aqueous Cream Containing Peanut Oil Once Daily Compared with 5% 5-Fluorouracil Cream Twice Daily: Meeting the Challenge in the Treatment of Actinic Keratosis. J. Drugs Dermatol. 2016, 15, 1218–1224. [Google Scholar]
- Zalaudek, I.; Piana, S.; Moscarella, E.; Longo, C.; Zendri, E.; Castagnetti, F.; Pellacani, G.; Lallas, A.; Argenziano, G. Morphologic Grading and Treatment of Facial Actinic Keratosis. Clin. Dermatol. 2014, 32, 80–87. [Google Scholar] [CrossRef]
- Zalaudek, I.; Argenziano, G. Dermoscopy of Actinic Keratosis, Intraepidermal Carcinoma and Squamous Cell Carcinoma. Curr. Probl. Dermatol. 2015, 46, 70–76. [Google Scholar] [CrossRef]
- Pellacani, G.; Gupta, G.; Micali, G.; Malvehy, J.; Stratigos, A.J.; Casari, A.; Chester, J.; Kaleci, S.; Dirschka, T. Actinic Keratosis Area Severity Index (AKASI): Reproducibility Study and Comparison with Total Lesion Count. Br. J. Dermatol. 2018, 179, 763–764. [Google Scholar] [CrossRef]
- Calzavara-Pinton, P.; Calzavara-Pinton, I.; Rovati, C.; Rossi, M. Topical Pharmacotherapy for Actinic Keratoses in Older Adults. Drugs Aging 2022, 39, 143–152. [Google Scholar] [CrossRef]
- Kaur, R.R.; Alikhan, A.; Maibach, H.I. Comparison of Topical 5-Fluorouracil Formulations in Actinic Keratosis Treatment. J. Dermatol. Treat. 2010, 21, 267–271. [Google Scholar] [CrossRef]
- Loven, K.; Stein, L.; Furst, K.; Levy, S. Evaluation of the Efficacy and Tolerability of 0.5% Fluorouracil Cream and 5% Fluorouracil Cream Applied to Each Side of the Face in Patients with Actinic Keratosis. Clin. Ther. 2002, 24, 990–1000. [Google Scholar] [CrossRef]
- Stockfleth, E.; Bégeault, N.; Delarue, A. Intensity of Local Skin Reactions During 5-Fluorouracil Treatment Related to the Number of Actinic Keratosis Lesions: A Post Hoc, Exploratory Analysis. Dermatol. Ther. 2022, 12, 467–479. [Google Scholar] [CrossRef]
- Lallas, A.; Argenziano, G.; Zendri, E.; Moscarella, E.; Longo, C.; Grenzi, L.; Pellacani, G.; Zalaudek, I. Update on Non-Melanoma Skin Cancer and the Value of Dermoscopy in Its Diagnosis and Treatment Monitoring. Expert Rev. Anticancer Ther. 2013, 13, 541–558. [Google Scholar] [CrossRef]
- Huerta-Brogeras, M.; Olmos, O.; Borbujo, J.; Hernández-Núñez, A.; Castaño, E.; Romero-Maté, A.; Martínez-Sánchez, D.; Martínez-Morán, C. Validation of Dermoscopy as a Real-Time Noninvasive Diagnostic Imaging Technique for Actinic Keratosis. Arch. Dermatol. 2012, 148, 1159–1164. [Google Scholar] [CrossRef]
- Kaçar, N.; Sanli, B.; Zalaudek, I.; Yildiz, N.; Ergin, S. Dermatoscopy for Monitoring Treatment of Actinic Keratosis with Imiquimod. Clin. Exp. Dermatol. 2012, 37, 567–569. [Google Scholar] [CrossRef] [PubMed]
- Pampena, R.; Condorelli, A.; Cornacchia, L.; Guiducci, L.; Raucci, M.; Benati, E.; Mirra, M.; Peris, K.; Lai, M.; Pellacani, G.; et al. Treatment Monitoring of 5-Fluorouracil 0.5%/Salicylic Acid 10% Lesion-Directed Therapy for Actinic Keratosis Using Dermoscopy and in-Vivo Reflectance Confocal Microscopy. Dermatol. Ther. 2020, 33, e13744. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Won, C.Y.; Kim, G.M.; Kim, S.Y. Dermoscopic Features of Actinic Keratosis and Follow up with Dermoscopy: A Pilot Study. J. Dermatol. 2014, 41, 487–493. [Google Scholar] [CrossRef] [PubMed]
- Garofalo, V.; Geraci, F.; Di Prete, M.; Lanna, C.; Lozzi, F.; Cosio, T.; Lambiase, S.; Gaeta Shumak, R.; Di Raimondo, C.; Diluvio, L.; et al. Early Clinical Response to 5-Fluorouracil 0.5% and Salicylic Acid 10% Topical Solution in the Treatment of Actinic Keratoses of the Head: An Observational Study. J. Dermatol. Treat. 2022, 33, 2664–2669. [Google Scholar] [CrossRef]
- Schmitz, L.; Gambichler, T.; Gupta, G.; Stücker, M.; Dirschka, T. Actinic Keratosis Area and Severity Index (AKASI) Is Associated with the Incidence of Squamous Cell Carcinoma. J. Eur. Acad. Dermatol. Venereol. 2018, 32, 752–756. [Google Scholar] [CrossRef]
- Acar, A.; Karaarslan, I. Comparison of Actinic Keratosis and Severity Index with Physician Global Assessment and Total Lesion Count and the Ability to Predict Skin Cancer. Dermatol. Pract. Concept. 2022, 12, 5–10. [Google Scholar] [CrossRef]
- Weinstock, M.A.; Thwin, S.S.; Siegel, J.A.; Marcolivio, K.; Means, A.D.; Leader, N.F.; Shaw, F.M.; Hogan, D.; Eilers, D.; Swetter, S.M.; et al. Chemoprevention of Basal and Squamous Cell Carcinoma with a Single Course of Fluorouracil, 5%, Cream: A Randomized Clinical Trial. JAMA Dermatol. 2018, 154, 167–174. [Google Scholar] [CrossRef]
- Keshavarz-Fathi, M.; Rezaei, N. Cancer Immunoprevention: Current Status and Future Directions. Arch. Immunol. Ther. Exp. 2021, 69, 3. [Google Scholar] [CrossRef]
- Reiter, M.J.; Testerman, T.L.; Miller, R.L.; Weeks, C.E.; Tomai, M.A. Cytokine Induction in Mice by the Immunomodulator Imiquimod. J. Leukoc. Biol. 1994, 55, 234–240. [Google Scholar] [CrossRef]
- Megyeri, K.; Au, W.C.; Rosztoczy, I.; Raj, N.B.; Miller, R.L.; Tomai, M.A.; Pitha, P.M. Stimulation of Interferon and Cytokine Gene Expression by Imiquimod and Stimulation by Sendai Virus Utilize Similar Signal Transduction Pathways. Mol. Cell. Biol. 1995, 15, 2207–2218. [Google Scholar] [CrossRef]
- Schon, M.; Schon, M. The Antitumoral Mode of Action of Imiquimod and Other Imidazoquinolines. Curr. Med. Chem. 2007, 14, 681–687. [Google Scholar] [CrossRef]
- Hanna, E.; Abadi, R.; Abbas, O. Imiquimod in Dermatology: An Overview. Int. J. Dermatol. 2016, 55, 831–844. [Google Scholar] [CrossRef]
- Weber, A.; Zimmermann, C.; Mausberg, A.K.; Kieseier, B.C.; Hartung, H.P.; Hofstetter, H.H. Induction of Pro-Inflammatory Cytokine Production in Thymocytes by the Immune Response Modifiers Imiquimod and GardiquimodTM. Int. Immunopharmacol. 2013, 17, 427–431. [Google Scholar] [CrossRef]
- Zavattaro, E.; Veronese, F.; Landucci, G.; Tarantino, V.; Savoia, P. Efficacy of Topical Imiquimod 3.75% in the Treatment of Actinic Keratosis of the Scalp in Immunosuppressed Patients: Our Case Series. J. Dermatol. Treat. 2020, 31, 285–289. [Google Scholar] [CrossRef]
- Rosenberg, A.R.; Tabacchi, M.; Ngo, K.H.; Wallendorf, M.; Rosman, I.S.; Cornelius, L.A.; Demehri, S. Skin Cancer Precursor Immunotherapy for Squamous Cell Carcinoma Prevention. JCI Insight 2019, 4, e125476. [Google Scholar] [CrossRef]
- Cunningham, T.J.; Tabacchi, M.; Eliane, J.P.; Tuchayi, S.M.; Manivasagam, S.; Mirzaalian, H.; Turkoz, A.; Kopan, R.; Schaffer, A.; Saavedra, A.P.; et al. Randomized Trial of Calcipotriol Combined with 5-Fluorouracil for Skin Cancer Precursor Immunotherapy. J. Clin. Investig. 2017, 127, 106–116. [Google Scholar] [CrossRef]
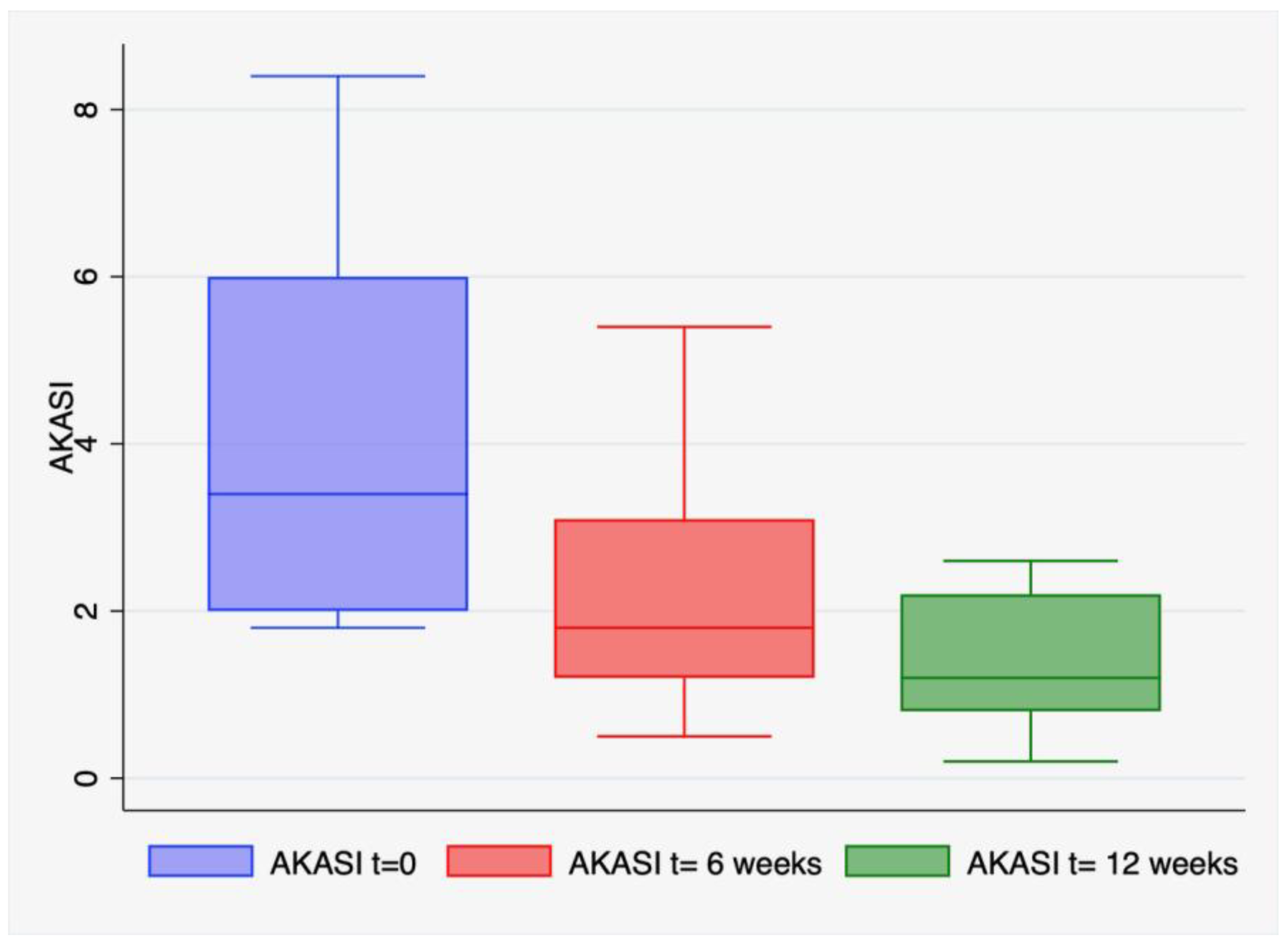

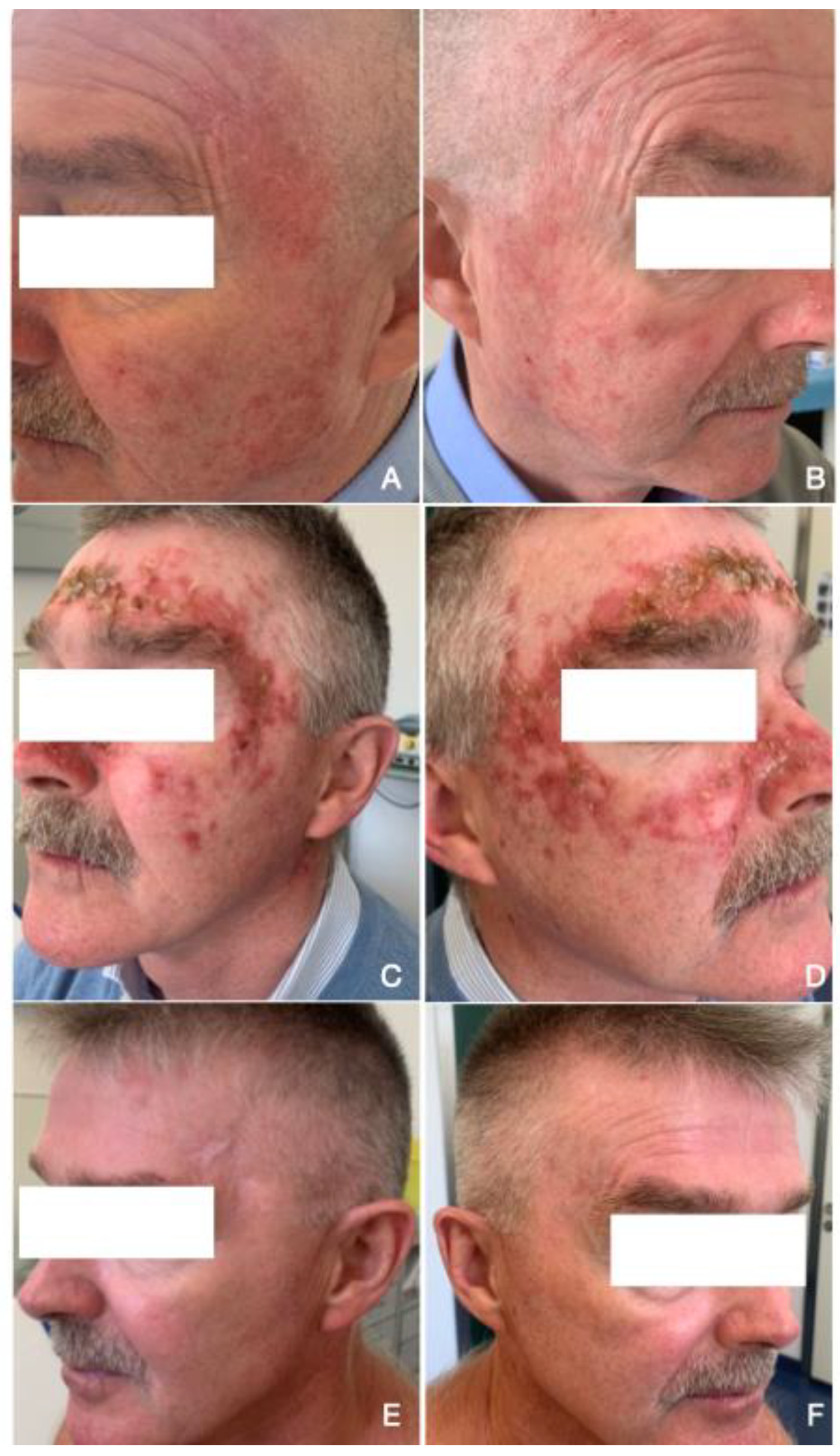
| Perifollicular Vascular Pseudonetwork (%) | White/Yellowish Scales (%) | Erythematous Background/Enlarged Follicular Openings (%) | |
|---|---|---|---|
| Baseline | 26 (87) | 23 (77) | 15 (50) |
| Week 6 | 10 (33) | 9 (30) | 7 (23) |
| Week 12 | 3 (10) | 2 (7) | 2 (7) |
| Grade of Reactions * | Erythema (%) | Scabs (%) | Erosion (%) | Bleeding (%) | Scales (%) | Pain (%) | Burning (%) |
|---|---|---|---|---|---|---|---|
| Severe | 6 (20) | 2 (7) | 2 (7) | 1 (3) | 1 (3) | 2 (7) | 2 (7) |
| Moderate | 4 (13) | 4 (13) | 5 (17) | 1 (3) | 1 (3) | 0 (0) | 1 (3) |
| Mild | 6 (20) | 7 (23) | 4 (13) | 4 (13) | 0 (0) | 0 (0) | 1 (3) |
| None | 14 (47) | 17 (57) | 19 (63) | 24 (80) | 28 (93) | 28 (93) | 26 (87) |
| Total | 16 (53) | 13 (43) | 11 (37) | 6 (20) | 2 (7) | 2 (7) | 4 (13) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Toffoli, L.; Dianzani, C.; Bonin, S.; Guarneri, C.; Guarneri, F.; Giuffrida, R.; Zalaudek, I.; Conforti, C. Actinic Keratoses: A Prospective Pilot Study on a Novel Formulation of 4% 5-Fluorouracil Cream and a Review of Other Current Topical Treatment Options. Cancers 2023, 15, 2956. https://doi.org/10.3390/cancers15112956
Toffoli L, Dianzani C, Bonin S, Guarneri C, Guarneri F, Giuffrida R, Zalaudek I, Conforti C. Actinic Keratoses: A Prospective Pilot Study on a Novel Formulation of 4% 5-Fluorouracil Cream and a Review of Other Current Topical Treatment Options. Cancers. 2023; 15(11):2956. https://doi.org/10.3390/cancers15112956
Chicago/Turabian StyleToffoli, Ludovica, Caterina Dianzani, Serena Bonin, Claudio Guarneri, Fabrizio Guarneri, Roberta Giuffrida, Iris Zalaudek, and Claudio Conforti. 2023. "Actinic Keratoses: A Prospective Pilot Study on a Novel Formulation of 4% 5-Fluorouracil Cream and a Review of Other Current Topical Treatment Options" Cancers 15, no. 11: 2956. https://doi.org/10.3390/cancers15112956
APA StyleToffoli, L., Dianzani, C., Bonin, S., Guarneri, C., Guarneri, F., Giuffrida, R., Zalaudek, I., & Conforti, C. (2023). Actinic Keratoses: A Prospective Pilot Study on a Novel Formulation of 4% 5-Fluorouracil Cream and a Review of Other Current Topical Treatment Options. Cancers, 15(11), 2956. https://doi.org/10.3390/cancers15112956











