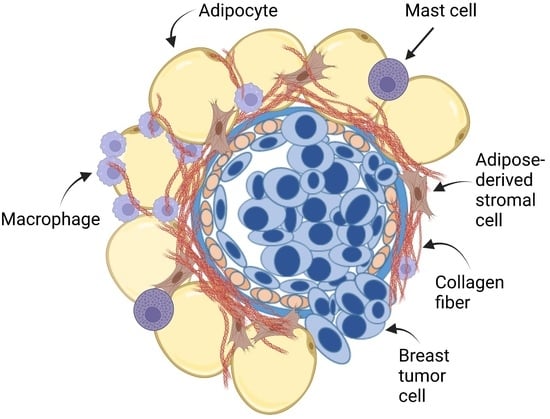
Obesity and Fibrosis: Setting the Stage for Breast Cancer
Abstract
Simple Summary
Abstract
1. Introduction
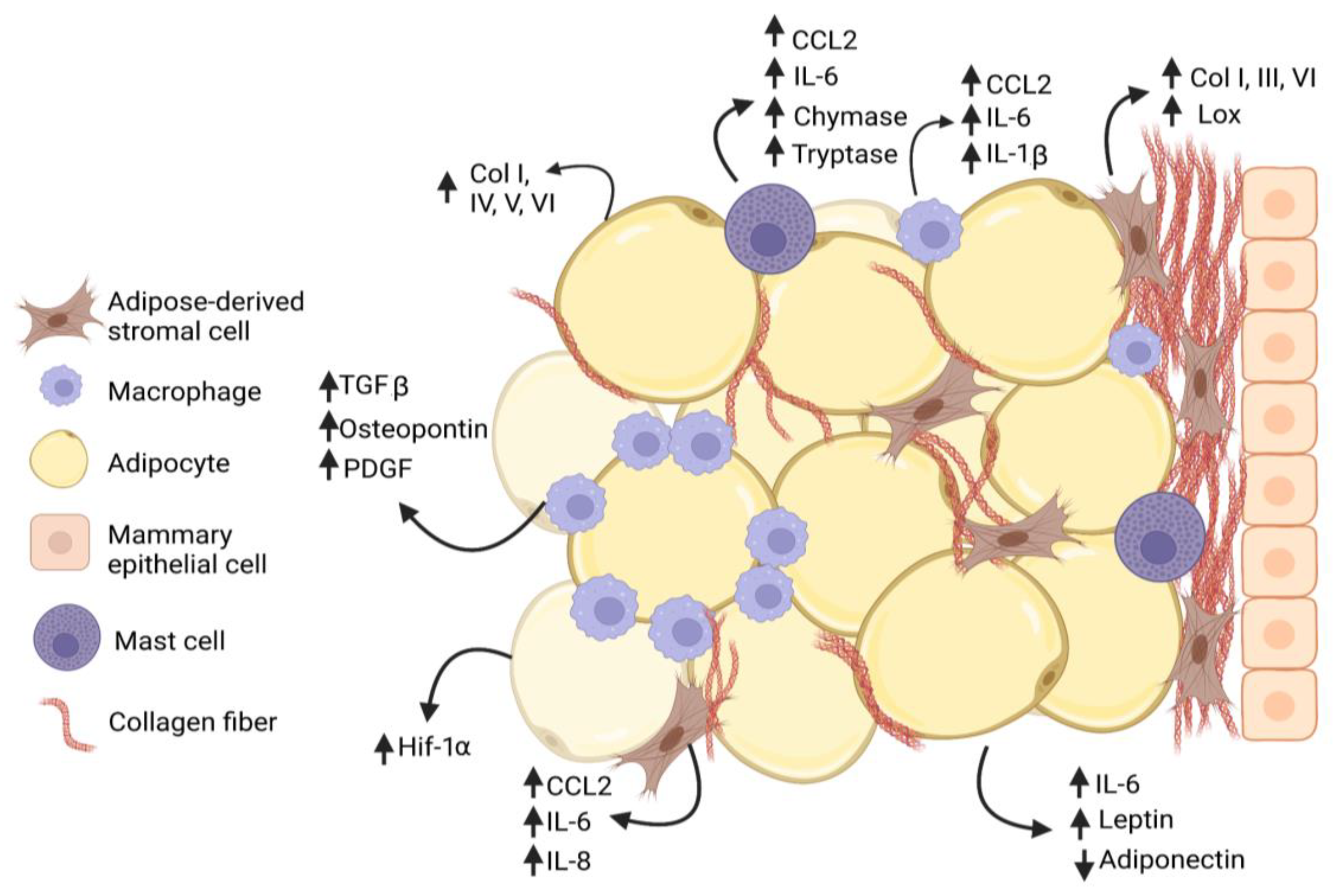
2. Obesity and Adipose Tissue Fibrosis
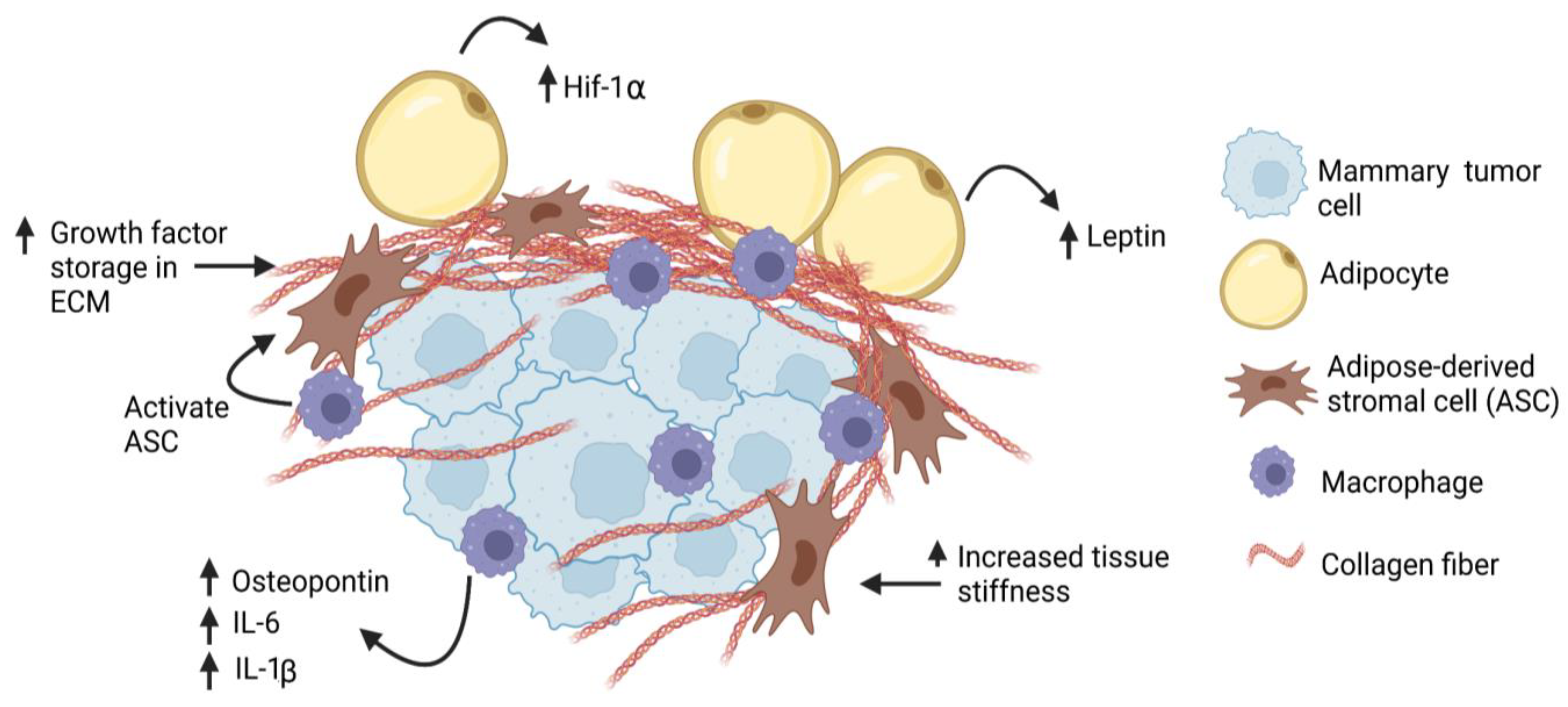
3. Obesity and Stromal Cell Function
4. Myeloid Lineage Cells and Collagen Deposition in Obesity
5. Weight Loss and Resolution of Fibrosis
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- World Health Organization, Breast Cancer Fact Sheet. 2021. Available online: https://www.who.int/news-room/fact-sheets/detail/breast-cancer (accessed on 31 January 2023).
- National Institute of Diabetes and Digestive and Kidney Diseases, Overweight and Obesity Statistics. 2021. Available online: https://www.niddk.nih.gov/health-information/health-statistics/overweight-obesity (accessed on 31 January 2023).
- Wolin, K.Y.; Carson, K.; Colditz, G.A. Obesity and cancer. Oncologist 2010, 15, 556–565. [Google Scholar] [CrossRef]
- Calle, E.E.; Rodriguez, C.; Walker-Thurmond, K.; Thun, M.J. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N. Engl. J. Med. 2003, 348, 1625–1638. [Google Scholar] [CrossRef] [PubMed]
- Pati, S.; Irfan, W.; Jameel, A.; Ahmed, S.; Shahid, R.K. Obesity and cancer: A current overview of epidemiology, pathogenesis, outcomes, and management. Cancers 2023, 15, 485. [Google Scholar] [CrossRef]
- Suzuki, R.; Orsini, N.; Saji, S.; Key, T.J.; Wolk, A. Body weight and incidence of breast cancer defined by estrogen and progesterone receptor status--a meta-analysis. Int. J. Cancer 2009, 124, 698–712. [Google Scholar] [CrossRef] [PubMed]
- Munsell, M.F.; Sprague, B.L.; Berry, D.A.; Chisholm, G.; Trentham-Dietz, A. Body mass index and breast cancer risk according to postmenopausal estrogen-progestin use and hormone receptor status. Epidemiol. Rev. 2014, 36, 114–136. [Google Scholar] [CrossRef]
- Brinton, L.A.; Cook, M.B.; McCormack, V.; Johnson, K.C.; Olsson, H.; Casagrande, J.T.; Cooke, R.; Falk, R.T.; Gapstur, S.M.; Gaudet, M.M.; et al. Anthropometric and hormonal risk factors for male breast cancer: Male breast cancer pooling project results. J. Natl. Cancer Inst. 2014, 106, djt465. [Google Scholar] [CrossRef] [PubMed]
- Inic, Z.; Zegarac, M.; Inic, M.; Markovic, I.; Kozomara, Z.; Djurisic, I.; Inic, I.; Pupic, G.; Jancic, S. Difference between luminal A and luminal B subtypes according to Ki-67, tumor size, and progesterone receptor negativity providing prognostic information. Clin. Med. Insights. Oncol. 2014, 8, 107–111. [Google Scholar] [CrossRef]
- Ahn, H.J.; Jung, S.J.; Kim, T.H.; Oh, M.K.; Yoon, H.K. Differences in clinical outcomes between luminal A and B type breast cancers according to the St. Gallen Consensus 2013. J. Breast Cancer 2015, 18, 149–159. [Google Scholar] [CrossRef]
- Hashmi, A.A.; Aijaz, S.; Khan, S.M.; Mahboob, R.; Irfan, M.; Zafar, N.I.; Nisar, M.; Siddiqui, M.; Edhi, M.M.; Faridi, N.; et al. Prognostic parameters of luminal A and luminal B intrinsic breast cancer subtypes of Pakistani patients. World J. Surg. Oncol. 2018, 16, 1. [Google Scholar] [CrossRef]
- Parker, J.S.; Mullins, M.; Cheang, M.C.; Leung, S.; Voduc, D.; Vickery, T.; Davies, S.; Fauron, C.; He, X.; Hu, Z.; et al. Supervised risk predictor of breast cancer based on intrinsic subtypes. J. Clin. Oncol. 2009, 27, 1160–1167. [Google Scholar] [CrossRef]
- Gaudet, M.M.; Press, M.F.; Haile, R.W.; Lynch, C.F.; Glaser, S.L.; Schildkraut, J.; Gammon, M.D.; Douglas, T.W.; Bernstein, J.L. Risk factors by molecular subtypes of breast cancer across a population-based study of women 56 years or younger. Breast Cancer Res. Treat. 2011, 130, 587–597. [Google Scholar] [CrossRef]
- Premenopausal Breast Cancer Collaborative Group. Association of body mass index and age with subsequent breast cancer risk in premenopausal women. JAMA Oncol. 2018, 4, e181771. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, C.L.; Ross, R.K.; Paganini-Hill, A.; Bernstein, L. Effect of family history, obesity and exercise on breast cancer risk among postmenopausal women. Int. J. Cancer 2003, 106, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Kotsopoulos, J.; Olopado, O.I.; Ghadirian, P.; Lubinski, J.; Lynch, H.T.; Isaacs, C.; Weber, B.; Kim-Sing, C.; Ainsworth, P.; Foulkes, W.D.; et al. Changes in body weight and the risk of breast cancer in BRCA1 and BRCA2 mutation carriers. Breast Cancer Res. 2005, 7, R833–R843. [Google Scholar] [CrossRef] [PubMed]
- Nkondjock, A.; Robidoux, A.; Paredes, Y.; Narod, S.A.; Ghadirian, P. Diet, lifestyle and BRCA-related breast cancer risk among French-Canadians. Breast Cancer Res. Treat. 2006, 98, 285–294. [Google Scholar] [CrossRef]
- Jenkins, E.O.; Deal, A.M.; Anders, C.K.; Prat, A.; Perou, C.M.; Carey, L.A.; Muss, H.B. Age-specific changes in intrinsic breast cancer subtypes: A focus on older women. Oncologist 2014, 19, 1076–1083. [Google Scholar] [CrossRef]
- Dolle, J.M.; Daling, J.R.; White, E.; Brinton, L.A.; Doody, D.R.; Porter, P.L.; Malone, K.E. Risk factors for triple-negative breast cancer in women under the age of 45 years. Cancer Epidemiol. Biomark. Prev. 2009, 18, 1157–1166. [Google Scholar] [CrossRef]
- Pierobon, M.; Frankenfeld, C.L. Obesity as a risk factor for triple-negative breast cancers: A systematic review and meta-analysis. Breast Cancer Res. Treat. 2013, 137, 307–314. [Google Scholar] [CrossRef]
- Yang, X.R.; Sherman, M.E.; Rimm, D.L.; Lissowska, J.; Brinton, L.A.; Peplonska, B.; Hewitt, S.M.; Anderson, W.F.; Szeszenia-Dabrowska, N.; Bardin-Mikolajczak, A.; et al. Differences in risk factors for breast cancer molecular subtypes in a population-based study. Cancer Epidemiol. Biomark. Prev. 2007, 16, 439–443. [Google Scholar] [CrossRef]
- Jiralerspong, S.; Goodwin, P.J. Obesity and breast cancer prognosis: Evidence, challenges, and opportunities. J. Clin. Oncol. 2016, 34, 4203–4216. [Google Scholar] [CrossRef]
- Carmichael, A.R. Obesity as a risk factor for development and poor prognosis of breast cancer. BJOG 2006, 113, 1160–1166. [Google Scholar] [CrossRef] [PubMed]
- Calle, E.E.; Kaaks, R. Overweight, obesity and cancer: Epidemiological evidence and proposed mechanisms. Nat. Rev. Cancer 2004, 4, 579–591. [Google Scholar] [CrossRef] [PubMed]
- Mazzarella, L.; Disalvatore, D.; Bagnardi, V.; Rotmensz, N.; Galbiati, D.; Caputo, S.; Curigliano, G.; Pelicci, P.G. Obesity increases the incidence of distant metastases in oestrogen receptor-negative human epidermal growth factor receptor 2-positive breast cancer patients. Eur. J. Cancer 2013, 49, 3588–3597. [Google Scholar] [CrossRef]
- Ewertz, M.; Jensen, M.B.; Gunnarsdottir, K.A.; Hojris, I.; Jakobsen, E.H.; Nielsen, D.; Stenbygaard, L.E.; Tange, U.B.; Cold, S. Effect of obesity on prognosis after early-stage breast cancer. J. Clin. Oncol. 2011, 29, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Majed, B.; Moreau, T.; Senouci, K.; Salmon, R.J.; Fourquet, A.; Asselain, B. Is obesity an independent prognosis factor in woman breast cancer? Breast Cancer Res. Treat. 2008, 111, 329–342. [Google Scholar] [CrossRef]
- Karatas, F.; Erdem, G.U.; Sahin, S.; Aytekin, A.; Yuce, D.; Sever, A.R.; Babacan, T.; Ates, O.; Ozisik, Y.; Altundag, K. Obesity is an independent prognostic factor of decreased pathological complete response to neoadjuvant chemotherapy in breast cancer patients. Breast 2017, 32, 237–244. [Google Scholar] [CrossRef]
- Ioannides, S.J.; Barlow, P.L.; Elwood, J.M.; Porter, D. Effect of obesity on aromatase inhibitor efficacy in postmenopausal, hormone receptor-positive breast cancer: A systematic review. Breast Cancer Res. Treat. 2014, 147, 237–248. [Google Scholar] [CrossRef] [PubMed]
- Seo, B.R.; Bhardwaj, P.; Choi, S.; Gonzalez, J.; Andresen Eguiluz, R.C.; Wang, K.; Mohanan, S.; Morris, P.G.; Du, B.; Zhou, X.K.; et al. Obesity-dependent changes in interstitial ECM mechanics promote breast tumorigenesis. Sci. Transl. Med. 2015, 7, 301ra130. [Google Scholar] [CrossRef]
- Beck, A.H.; Sangoi, A.R.; Leung, S.; Marinelli, R.J.; Nielsen, T.O.; van de Vijver, M.J.; West, R.B.; van de Rijn, M.; Koller, D. Systematic analysis of breast cancer morphology uncovers stromal features associated with survival. Sci. Transl. Med. 2011, 3, 108ra113. [Google Scholar] [CrossRef]
- de Kruijf, E.M.; van Nes, J.G.; van de Velde, C.J.; Putter, H.; Smit, V.T.; Liefers, G.J.; Kuppen, P.J.; Tollenaar, R.A.; Mesker, W.E. Tumor-stroma ratio in the primary tumor is a prognostic factor in early breast cancer patients, especially in triple-negative carcinoma patients. Breast Cancer Res. Treat. 2011, 125, 687–696. [Google Scholar] [CrossRef]
- Dekker, T.J.; van de Velde, C.J.; van Pelt, G.W.; Kroep, J.R.; Julien, J.P.; Smit, V.T.; Tollenaar, R.A.; Mesker, W.E. Prognostic significance of the tumor-stroma ratio: Validation study in node-negative premenopausal breast cancer patients from the EORTC perioperative chemotherapy (POP) trial (10854). Breast Cancer Res. Treat. 2013, 139, 371–379. [Google Scholar] [CrossRef]
- Moorman, A.M.; Vink, R.; Heijmans, H.J.; van der Palen, J.; Kouwenhoven, E.A. The prognostic value of tumour-stroma ratio in triple-negative breast cancer. Eur. J. Surg. Oncol. 2012, 38, 307–313. [Google Scholar] [CrossRef]
- Whatcott, C.J.; Diep, C.H.; Jiang, P.; Watanabe, A.; LoBello, J.; Sima, C.; Hostetter, G.; Shepard, H.M.; Von Hoff, D.D.; Han, H. Desmoplasia in primary tumors and metastatic lesions of pancreatic cancer. Clin. Cancer Res. 2015, 21, 3561–3568. [Google Scholar] [CrossRef]
- Friman, T.; Gustafsson, R.; Stuhr, L.B.; Chidiac, J.; Heldin, N.E.; Reed, R.K.; Oldberg, A.; Rubin, K. Increased fibrosis and interstitial fluid pressure in two different types of syngeneic murine carcinoma grown in integrin β3-subunit deficient mice. PLoS ONE 2012, 7, e34082. [Google Scholar] [CrossRef] [PubMed]
- Alkhouli, N.; Mansfield, J.; Green, E.; Bell, J.; Knight, B.; Liversedge, N.; Tham, J.C.; Welbourn, R.; Shore, A.C.; Kos, K.; et al. The mechanical properties of human adipose tissues and their relationships to the structure and composition of the extracellular matrix. Am. J. Physiol. Endocrinol. Metab. 2013, 305, E1427–E1435. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, I.; Muroya, S.; Tanabe, R.; Chikuni, K. Positive effect of collagen V and VI on triglyceride accumulation during differentiation in cultures of bovine intramuscular adipocytes. Differentiation 2002, 70, 84–91. [Google Scholar] [CrossRef]
- Sun, K.; Tordjman, J.; Clement, K.; Scherer, P.E. Fibrosis and adipose tissue dysfunction. Cell. Metab. 2013, 18, 470–477. [Google Scholar] [CrossRef] [PubMed]
- Vila, I.K.; Badin, P.M.; Marques, M.A.; Monbrun, L.; Lefort, C.; Mir, L.; Louche, K.; Bourlier, V.; Roussel, B.; Gui, P.; et al. Immune cell toll-like receptor 4 mediates the development of obesity- and endotoxemia-associated adipose tissue fibrosis. Cell. Rep. 2014, 7, 1116–1129. [Google Scholar] [CrossRef]
- Divoux, A.; Tordjman, J.; Lacasa, D.; Veyrie, N.; Hugol, D.; Aissat, A.; Basdevant, A.; Guerre-Millo, M.; Poitou, C.; Zucker, J.D.; et al. Fibrosis in human adipose tissue: Composition, distribution, and link with lipid metabolism and fat mass loss. Diabetes 2010, 59, 2817–2825. [Google Scholar] [CrossRef]
- Springer, N.L.; Iyengar, N.M.; Bareja, R.; Verma, A.; Jochelson, M.S.; Giri, D.D.; Zhou, X.K.; Elemento, O.; Dannenberg, A.J.; Fischbach, C. Obesity-associated extracellular matrix remodeling promotes a macrophage phenotype similar to tumor-associated macrophages. Am. J. Pathol. 2019, 189, 2019–2035. [Google Scholar] [CrossRef]
- Kamikawa, A.; Ichii, O.; Yamaji, D.; Imao, T.; Suzuki, C.; Okamatsu-Ogura, Y.; Terao, A.; Kon, Y.; Kimura, K. Diet-induced obesity disrupts ductal development in the mammary glands of nonpregnant mice. Dev. Dyn. 2009, 238, 1092–1099. [Google Scholar] [CrossRef]
- Wolfson, B.; Zhang, Y.; Gernapudi, R.; Duru, N.; Yao, Y.; Lo, P.K.; Zhou, Q. A high-fat diet promotes mammary gland myofibroblast differentiation through microRNA 140 downregulation. Mol. Cell. Biol. 2017, 37, e00461-16. [Google Scholar] [CrossRef]
- Chamberlin, T.; Clack, M.; Silvers, C.; Kuziel, G.; Thompson, V.; Johnson, H.; Arendt, L.M. Targeting obesity-induced macrophages during preneoplastic growth promotes mammary epithelial stem/progenitor activity, DNA damage, and tumor formation. Cancer Res. 2020, 80, 4465–4475. [Google Scholar] [CrossRef]
- Marcelin, G.; Ferreira, A.; Liu, Y.; Atlan, M.; Aron-Wisnewsky, J.; Pelloux, V.; Botbol, Y.; Ambrosini, M.; Fradet, M.; Rouault, C.; et al. A PDGFRα-mediated switch toward CD9high adipocyte progenitors controls obesity-induced adipose tissue fibrosis. Cell. Metab. 2017, 25, 673–685. [Google Scholar] [CrossRef]
- Spencer, M.; Yao-Borengasser, A.; Unal, R.; Rasouli, N.; Gurley, C.M.; Zhu, B.; Peterson, C.A.; Kern, P.A. Adipose tissue macrophages in insulin-resistant subjects are associated with collagen VI and fibrosis and demonstrate alternative activation. Am. J. Physiol. Endocrinol. Metab. 2010, 299, E1016–E1027. [Google Scholar] [CrossRef]
- Khan, T.; Muise, E.S.; Iyengar, P.; Wang, Z.V.; Chandalia, M.; Abate, N.; Zhang, B.B.; Bonaldo, P.; Chua, S.; Scherer, P.E. Metabolic dysregulation and adipose tissue fibrosis: Role of collagen VI. Mol. Cell. Biol. 2009, 29, 1575–1591. [Google Scholar] [CrossRef]
- Catalán, V.; Gómez-Ambrosi, J.; Rodríguez, A.; Ramírez, B.; Rotellar, F.; Valentí, V.; Silva, C.; Gil, M.J.; Salvador, J.; Frühbeck, G. Increased tenascin C and Toll-like receptor 4 levels in visceral adipose tissue as a link between inflammation and extracellular matrix remodeling in obesity. J. Clin. Endocrinol. Metab. 2012, 97, E1880–E1889. [Google Scholar] [CrossRef]
- Bhattacharyya, S.; Wang, W.; Morales-Nebreda, L.; Feng, G.; Wu, M.; Zhou, X.; Lafyatis, R.; Lee, J.; Hinchcliff, M.; Feghali-Bostwick, C.; et al. Tenascin-C drives persistence of organ fibrosis. Nat. Commun. 2016, 7, 11703. [Google Scholar] [CrossRef]
- Kiefer, F.W.; Zeyda, M.; Todoric, J.; Huber, J.; Geyeregger, R.; Weichhart, T.; Aszmann, O.; Ludvik, B.; Silberhumer, G.R.; Prager, G.; et al. Osteopontin expression in human and murine obesity: Extensive local up-regulation in adipose tissue but minimal systemic alterations. Endocrinol 2008, 149, 1350–1357. [Google Scholar] [CrossRef]
- Abdennour, M.; Reggio, S.; Le Naour, G.; Liu, Y.; Poitou, C.; Aron-Wisnewsky, J.; Charlotte, F.; Bouillot, J.L.; Torcivia, A.; Sasso, M.; et al. Association of adipose tissue and liver fibrosis with tissue stiffness in morbid obesity: Links with diabetes and BMI loss after gastric bypass. J. Clin. Endocrinol. Metab. 2014, 99, 898–907. [Google Scholar] [CrossRef]
- Wang, T.H.; Hsia, S.M.; Shieh, T.M. Lysyl oxidase and the tumor microenvironment. Int. J. Mol. Sci. 2016, 18, 62. [Google Scholar] [CrossRef]
- Halberg, N.; Khan, T.; Trujillo, M.E.; Wernstedt-Asterholm, I.; Attie, A.D.; Sherwani, S.; Wang, Z.V.; Landskroner-Eiger, S.; Dineen, S.; Magalang, U.J.; et al. Hypoxia-inducible factor 1α induces fibrosis and insulin resistance in white adipose tissue. Mol. Cell. Biol. 2009, 29, 4467–4483. [Google Scholar] [CrossRef] [PubMed]
- Miana, M.; Galan, M.; Martinez-Martinez, E.; Varona, S.; Jurado-Lopez, R.; Bausa-Miranda, B.; Antequera, A.; Luaces, M.; Martinez-Gonzalez, J.; Rodriguez, C.; et al. The lysyl oxidase inhibitor β-aminopropionitrile reduces body weight gain and improves the metabolic profile in diet-induced obesity in rats. Dis. Model. Mech. 2015, 8, 543–551. [Google Scholar] [CrossRef] [PubMed]
- Pastel, E.; Price, E.; Sjöholm, K.; McCulloch, L.J.; Rittig, N.; Liversedge, N.; Knight, B.; Møller, N.; Svensson, P.A.; Kos, K. Lysyl oxidase and adipose tissue dysfunction. Metabolism 2018, 78, 118–127. [Google Scholar] [CrossRef] [PubMed]
- Levental, K.R.; Yu, H.; Kass, L.; Lakins, J.N.; Egeblad, M.; Erler, J.T.; Fong, S.F.; Csiszar, K.; Giaccia, A.; Weninger, W.; et al. Matrix crosslinking forces tumor progression by enhancing integrin signaling. Cell 2009, 139, 891–906. [Google Scholar] [CrossRef]
- Proudfoot, A.E.I.; Johnson, Z.; Bonvin, P.; Handel, T.M. Glycosaminoglycan interactions with chemokines add complexity to a complex system. Pharmaceuticals 2017, 10, 70. [Google Scholar] [CrossRef] [PubMed]
- Monneau, Y.; Arenzana-Seisdedos, F.; Lortat-Jacob, H. The sweet spot: How GAGs help chemokines guide migrating cells. J. Leukoc. Biol. 2016, 99, 935–953. [Google Scholar] [CrossRef] [PubMed]
- Taipale, J.; Keski-Oja, J. Growth factors in the extracellular matrix. FASEB J. 1997, 11, 51–59. [Google Scholar] [CrossRef]
- Samad, F.; Yamamoto, K.; Pandey, M.; Loskutoff, D.J. Elevated expression of transforming growth factor-beta in adipose tissue from obese mice. Mol. Med. 1997, 3, 37–48. [Google Scholar] [CrossRef]
- Alessi, M.C.; Bastelica, D.; Morange, P.; Berthet, B.; Leduc, I.; Verdier, M.; Geel, O.; Juhan-Vague, I. Plasminogen activator inhibitor 1, transforming growth factor-beta1, and BMI are closely associated in human adipose tissue during morbid obesity. Diabetes 2000, 49, 1374–1380. [Google Scholar] [CrossRef]
- Fain, J.N.; Tichansky, D.S.; Madan, A.K. Transforming growth factor beta1 release by human adipose tissue is enhanced in obesity. Metabolism 2005, 54, 1546–1551. [Google Scholar] [CrossRef]
- Moses, H.L.; Coffey, R.J., Jr.; Leof, E.B.; Lyons, R.M.; Keski-Oja, J. Transforming growth factor beta regulation of cell proliferation. J. Cell. Physiol. Suppl. 1987, 133 (Suppl. S5), 1–7. [Google Scholar] [CrossRef] [PubMed]
- Horiguchi, M.; Ota, M.; Rifkin, D.B. Matrix control of transforming growth factor-β function. J. Biochem. 2012, 152, 321–329. [Google Scholar] [CrossRef]
- Chamberlin, T.; Thompson, V.; Hillers-Ziemer, L.E.; Walton, B.N.; Arendt, L.M. Obesity reduces mammary epithelial cell TGFβ1 activity through macrophage-mediated extracellular matrix remodeling. FASEB J. 2020, 34, 8611–8624. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Lamouille, S.; Derynck, R. TGF-β-induced epithelial to mesenchymal transition. Cell. Res. 2009, 19, 156–172. [Google Scholar] [CrossRef] [PubMed]
- Biffi, G.; Tuveson, D.A. Diversity and biology of cancer-associated fibroblasts. Physiol. Rev. 2021, 101, 147–176. [Google Scholar] [CrossRef] [PubMed]
- Rausch, M.E.; Weisberg, S.; Vardhana, P.; Tortoriello, D.V. Obesity in C57BL/6J mice is characterized by adipose tissue hypoxia and cytotoxic T-cell infiltration. Int. J. Obes. 2008, 32, 451–463. [Google Scholar] [CrossRef]
- Sun, K.; Halberg, N.; Khan, M.; Magalang, U.J.; Scherer, P.E. Selective inhibition of hypoxia-inducible factor 1α ameliorates adipose tissue dysfunction. Mol. Cell. Biol. 2013, 33, 904–917. [Google Scholar] [CrossRef]
- Yong, L.; Tang, S.; Yu, H.; Zhang, H.; Zhang, Y.; Wan, Y.; Cai, F. The role of hypoxia-inducible factor-1 alpha in multidrug-resistant breast cancer. Front. Oncol. 2022, 12, 964934. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, S.; Manabe, I.; Nagasaki, M.; Seo, K.; Yamashita, H.; Hosoya, Y.; Ohsugi, M.; Tobe, K.; Kadowaki, T.; Nagai, R.; et al. In vivo imaging in mice reveals local cell dynamics in inflammation in obese adipose tissue. J. Clin. Investig. 2008, 11, 710–721. [Google Scholar] [CrossRef]
- Provenzano, P.P.; Inman, D.R.; Eliceiri, K.W.; Knittel, J.G.; Yan, L.; Rueden, C.T.; White, J.G.; Keely, P.J. Collagen density promotes mammary tumor initiation and progression. BMC Med. 2008, 6, 11. [Google Scholar] [CrossRef]
- Nallanthighal, S.; Heiserman, J.P.; Cheon, D.-J. The role of the extracellular matrix in cancer stemness. Front. Cell. Dev. Biol. 2019, 7, 86. [Google Scholar] [CrossRef] [PubMed]
- Shan, N.L.; Shin, Y.; Yang, G.; Furmanski, P.; Suh, N. Breast cancer stem cells: A review of their characteristics and the agents that affect them. Mol. Carcinog. 2021, 60, 73–100. [Google Scholar] [CrossRef] [PubMed]
- Song, K.; Farzaneh, M. Signaling pathways governing breast cancer cells behavior. Stem. Cell. Res. Ther. 2021, 12, 245. [Google Scholar] [CrossRef] [PubMed]
- Barcus, C.E.; O’Leary, K.A.; Brockman, J.L.; Rugowski, D.E.; Liu, Y.; Garcia, N.; Yu, M.; Keely, P.J.; Eliceiri, K.W.; Schuler, L.A. Elevated collagen-I augments tumor progressive signals, intravasation and metastasis of prolactin-induced estrogen receptor alpha positive mammary tumor cells. Breast Cancer Res. 2017, 19, 9. [Google Scholar] [CrossRef]
- Shea, M.P.; O’Leary, K.A.; Wegner, K.A.; Vezina, C.M.; Schuler, L.A. High collagen density augments mTOR-dependent cancer stem cells in ERα+ mammary carcinomas and increases mTOR-independent lung metastases. Cancer Lett. 2018, 433, 1–9. [Google Scholar] [CrossRef]
- Zanotelli, M.R.; Reinhart-King, C.A. Mechanical forces in tumor angiogenesis. Adv. Exp. Med. Biol. 2018, 1092, 91–112. [Google Scholar] [CrossRef]
- Nakajima, I.; Yamaguchi, T.; Ozutsumi, K.; Aso, H. Adipose tissue extracellular matrix: Newly organized by adipocytes during differentiation. Differentiation 1998, 63, 193–200. [Google Scholar] [CrossRef]
- Baptista, L.S. Adipose stromal/stem cells in regenerative medicine: Potentials and limitations. World J. Stem. Cell. 2020, 12, 1–7. [Google Scholar] [CrossRef]
- Bourin, P.; Bunnell, B.A.; Casteilla, L.; Dominici, M.; Katz, A.J.; March, K.L.; Redl, H.; Rubin, J.P.; Yoshimura, K.; Gimble, J.M. Stromal cells from the adipose tissue-derived stromal vascular fraction and culture expanded adipose tissue-derived stromal/stem cells: A joint statement of the International Federation for Adipose Therapeutics and Science (IFATS) and the International Society for Cellular Therapy (ISCT). Cytother 2013, 15, 641–648. [Google Scholar] [CrossRef]
- Zhang, Y.; Daquinag, A.C.; Amaya-Manzanares, F.; Sirin, O.; Tseng, C.; Kolonin, M.G. Stromal progenitor cells from endogenous adipose tissue contribute to pericytes and adipocytes that populate the tumor microenvironment. Cancer Res. 2012, 72, 5198–5208. [Google Scholar] [CrossRef] [PubMed]
- Avagliano, A.; Fiume, G.; Ruocco, M.R.; Martucci, N.; Vecchio, E.; Insabato, L.; Russo, D.; Accurso, A.; Masone, S.; Montagnani, S.; et al. Influence of fibroblasts on mammary gland development, breast cancer microenvironment remodeling, and cancer cell dissemination. Cancers 2020, 12, 1697. [Google Scholar] [CrossRef] [PubMed]
- Hu, D.; Li, Z.; Zheng, B.; Lin, X.; Pan, Y.; Gong, P.; Zhuo, W.; Hu, Y.; Chen, C.; Chen, L.; et al. Cancer-associated fibroblasts in breast cancer: Challenges and opportunities. Cancer Commun. 2022, 42, 401–434. [Google Scholar] [CrossRef]
- Morsing, M.; Klitgaard, M.C.; Jafari, A.; Villadsen, R.; Kassem, M.; Petersen, O.W.; Ronnov-Jessen, L. Evidence of two distinct functionally specialized fibroblast lineages in breast stroma. Breast Cancer Res. 2016, 18, 108. [Google Scholar] [CrossRef]
- Morsing, M.; Kim, J.; Villadsen, R.; Goldhammer, N.; Jafari, A.; Kassem, M.; Petersen, O.W.; Ronnov-Jessen, L. Fibroblasts direct differentiation of human breast epithelial progenitors. Breast Cancer Res. 2020, 22, 102. [Google Scholar] [CrossRef] [PubMed]
- Ling, L.; Mulligan, J.A.; Ouyang, Y.; Shimpi, A.A.; Williams, R.M.; Beeghly, G.F.; Hopkins, B.D.; Spector, J.A.; Adie, S.G.; Fischbach, C. Obesity-associated adipose stromal cells promote breast cancer invasion through direct cell contact and ECM remodeling. Adv. Funct. Mater. 2020, 30, 1910650. [Google Scholar] [CrossRef]
- Hillers, L.E.; D’Amato, J.V.; Chamberlin, T.; Paderta, G.; Arendt, L.M. Obesity-activated adipose-derived stromal cells promote breast cancer growth and invasion. Neoplasia 2018, 20, 1161–1174. [Google Scholar] [CrossRef]
- Park, J.; Scherer, P.E. Leptin and cancer: From cancer stem cells to metastasis. Endocr. Relat. Cancer 2011, 18, C25–C29. [Google Scholar] [CrossRef]
- Russell, C.D.; Petersen, R.N.; Rao, S.P.; Ricci, M.R.; Prasad, A.; Zhang, Y.; Brolin, R.E.; Fried, S.K. Leptin expression in adipose tissue from obese humans: Depot-specific regulation by insulin and dexamethasone. Am. J. Physiol. Endocrinol. Metab. 1998, 275, E507–E515. [Google Scholar] [CrossRef]
- Arita, Y.; Kihara, S.; Ouchi, N.; Takahashi, M.; Maeda, K.; Miyagawa, J.-I.; Hotta, K.; Shimomura, I.; Nakamura, T.; Miyaoka, K.; et al. Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. Biochem. Biophys. Res. Commun. 1999, 257, 79–83. [Google Scholar] [CrossRef]
- Pham, D.-V.; Park, P.-H. Adiponectin triggers breast cancer cell death via fatty acid metabolic reprogramming. J. Exp. Clin. Cancer Res. 2022, 41, 9. [Google Scholar] [CrossRef]
- Coppack, S.W. Pro-inflammatory cytokines and adipose tissue. Proc. Nutr. Soc. 2001, 60, 349–356. [Google Scholar] [CrossRef] [PubMed]
- Onate, B.; Vilahur, G.; Camino-Lopez, S.; Diez-Caballero, A.; Ballesta-Lopez, C.; Ybarra, J.; Moscatiello, F.; Herrero, J.; Badimon, L. Stem cells isolated from adipose tissue of obese patients show changes in their transcriptomic profile that indicate loss in stemcellness and increased commitment to an adipocyte-like phenotype. BMC Genom. 2013, 14, 625. [Google Scholar] [CrossRef] [PubMed]
- Onate, B.; Vilahur, G.; Ferrer-Lorente, R.; Ybarra, J.; Diez-Caballero, A.; Ballesta-Lopez, C.; Moscatiello, F.; Herrero, J.; Badimon, L. The subcutaneous adipose tissue reservoir of functionally active stem cells is reduced in obese patients. FASEB J. 2012, 26, 4327–4336. [Google Scholar] [CrossRef]
- Silva, K.R.; Liechocki, S.; Carneiro, J.R.; Claudio-da-Silva, C.; Maya-Monteiro, C.M.; Borojevic, R.; Baptista, L.S. Stromal-vascular fraction content and adipose stem cell behavior are altered in morbid obese and post bariatric surgery ex-obese women. Stem. Cell. Res. Ther. 2015, 6, 72. [Google Scholar] [CrossRef] [PubMed]
- Bullwinkle, E.M.; Parker, M.D.; Bonan, N.F.; Falkenberg, L.G.; Davison, S.P.; DeCicco-Skinner, K.L. Adipocytes contribute to the growth and progression of multiple myeloma: Unraveling obesity related differences in adipocyte signaling. Cancer Lett. 2016, 380, 114–121. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.J.; Kim, J.; Kim, M.Y.; Bae, Y.S.; Ryu, S.H.; Lee, T.G.; Kim, J.H. Proteomic analysis of tumor necrosis factor-alpha-induced secretome of human adipose tissue-derived mesenchymal stem cells. J. Proteome Res. 2010, 9, 1754–1762. [Google Scholar] [CrossRef]
- Kilroy, G.E.; Foster, S.J.; Wu, X.; Ruiz, J.; Sherwood, S.; Heifetz, A.; Ludlow, J.W.; Stricker, D.M.; Potiny, S.; Green, P.; et al. Cytokine profile of human adipose-derived stem cells: Expression of angiogenic, hematopoietic, and pro-inflammatory factors. J. Cell. Physiol. 2007, 212, 702–709. [Google Scholar] [CrossRef] [PubMed]
- Harrison, M.A.A.; Wise, R.M.; Benjamin, B.P.; Hochreiner, E.M.; Mohiuddin, O.A.; Bunnell, B.A. Adipose-derived stem cells from obese donors polarize macrophages and microglia toward a pro-inflammatory phenotype. Cells 2020, 10, 26. [Google Scholar] [CrossRef]
- Puissant, B.; Barreau, C.; Bourin, P.; Clavel, C.; Corre, J.; Bousquet, C.; Taureau, C.; Cousin, B.; Abbal, M.; Laharrague, P.; et al. Immunomodulatory effect of human adipose tissue-derived adult stem cells: Comparison with bone marrow mesenchymal stem cells. Br. J. Haematol. 2005, 129, 118–129. [Google Scholar] [CrossRef]
- McIntosh, K.; Zvonic, S.; Garrett, S.; Mitchell, J.B.; Floyd, Z.E.; Hammill, L.; Kloster, A.; Di Halvorsen, Y.; Ting, J.P.; Storms, R.W.; et al. The immunogenicity of human adipose-derived cells: Temporal changes in vitro. Stem Cells 2006, 24, 1246–1253. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.Y.; Klomjit, N.; Conley, S.M.; Ostlie, M.M.; Jordan, K.L.; Lerman, A.; Lerman, L.O. Impaired immunomodulatory capacity in adipose tissue-derived mesenchymal stem/stromal cells isolated from obese patients. J. Cell. Mol. Med. 2021, 25, 9051–9059. [Google Scholar] [CrossRef] [PubMed]
- Costa, A.; Kieffer, Y.; Scholer-Dahirel, A.; Pelon, F.; Bourachot, B.; Cardon, M.; Sirven, P.; Magagna, I.; Fuhrmann, L.; Bernard, C.; et al. Fibroblast heterogeneity and immunosuppressive environment in human breast cancer. Cancer Cell. 2018, 33, 463–479. [Google Scholar] [CrossRef] [PubMed]
- Erez, N.; Glanz, S.; Raz, Y.; Avivi, C.; Barshack, I. Cancer Associated Fibroblasts express pro-inflammatory factors in human breast and ovarian tumors. Biochem. Biophys. Res. Commun. 2013, 437, 397–402. [Google Scholar] [CrossRef]
- Lockwood, D.S.; Yeadon, T.M.; Clouston, A.D.; Crawford, D.G.; Fawcett, J.; Callaghan, S.A.; Gotley, D.C. Tumor progression in hepatocellular carcinoma: Relationship with tumor stroma and parenchymal disease. J. Gastroenterol. Hepatol. 2003, 18, 666–672. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Kim, H.M.; Koo, J.S. Differential expression of cancer-associated fibroblast-related proteins in ductal carcinoma in situ according to molecular subtype and stromal histology. Pathobiology 2018, 85, 311–321. [Google Scholar] [CrossRef]
- Tchou, J.; Kossenkov, A.V.; Chang, L.; Satija, C.; Herlyn, M.; Showe, L.C.; Puré, E. Human breast cancer associated fibroblasts exhibit subtype specific gene expression profiles. BMC Med. Genom. 2012, 5, 39. [Google Scholar] [CrossRef]
- De Wever, O.; Demetter, P.; Mareel, M.; Bracke, M. Stromal myofibroblasts are drivers of invasive cancer growth. Int. J. Cancer 2008, 123, 2229–2238. [Google Scholar] [CrossRef]
- Sugimoto, H.; Mundel, T.M.; Kieran, M.W.; Kalluri, R. Identification of fibroblast heterogeneity in the tumor microenvironment. Cancer Biol. Ther. 2006, 5, 1640–1646. [Google Scholar] [CrossRef]
- Belhabib, I.; Zaghdoudi, S.; Lac, C.; Bousquet, C.; Jean, C. Extracellular matrices and cancer-associated fibroblasts: Targets for cancer diagnosis and therapy? Cancers 2021, 13, 3466. [Google Scholar] [CrossRef]
- Elwakeel, E.; Weigert, A. Breast Cancer CAFs: Spectrum of phenotypes and promising targeting avenues. Int. J. Mol. Sci. 2021, 22, 11636. [Google Scholar] [CrossRef]
- Bartoschek, M.; Oskolkov, N.; Bocci, M.; Lovrot, J.; Larsson, C.; Sommarin, M.; Madsen, C.D.; Lindgren, D.; Pekar, G.; Karlsson, G.; et al. Spatially and functionally distinct subclasses of breast cancer associated fibroblasts revealed by single cell RNA sequencing. Nat. Commun. 2018, 9, 5150. [Google Scholar] [CrossRef] [PubMed]
- Bochet, L.; Lehuede, C.; Dauvillier, S.; Wang, Y.Y.; Dirat, B.; Laurent, V.; Dray, C.; Guiet, R.; Maridonneau-Parini, I.; Le Gonidec, S.; et al. Adipocyte-derived fibroblasts promote tumor progression and contribute to the desmoplastic reaction in breast cancer. Cancer Res. 2013, 73, 5657–5668. [Google Scholar] [CrossRef] [PubMed]
- Strong, A.L.; Pei, D.T.; Hurst, C.G.; Gimble, J.M.; Burow, M.E.; Bunnell, B.A. Obesity enhances the conversion of adipose-derived stromal/stem cells into carcinoma-associated fibroblast leading to cancer cell proliferation and progression to an invasive phenotype. Stem Cells Int. 2017, 2017, 9216502. [Google Scholar] [CrossRef]
- Strong, A.L.; Ohlstein, J.F.; Biagas, B.A.; Rhodes, L.V.; Pei, D.T.; Tucker, H.A.; Llamas, C.; Bowles, A.C.; Dutreil, M.F.; Zhang, S.; et al. Leptin produced by obese adipose stromal/stem cells enhances proliferation and metastasis of estrogen receptor positive breast cancers. Breast Cancer Res. 2015, 17, 112. [Google Scholar] [CrossRef] [PubMed]
- Cinkajzlová, A.; Mráz, M.; Haluzík, M. Lymphocytes and macrophages in adipose tissue in obesity: Markers or makers of subclinical inflammation? Protoplasma 2017, 254, 1219–1232. [Google Scholar] [CrossRef]
- Weisberg, S.P.; McCann, D.; Desai, M.; Rosenbaum, M.; Leibel, R.L.; Ferrante, A.W. Obesity is associated with macrophage accumulation in adipose tissue. J. Clin. Investig. 2003, 112, 1796–1808. [Google Scholar] [CrossRef]
- Xu, H.; Barnes, G.T.; Yang, Q.; Tan, G.; Yang, D.; Chou, C.J.; Sole, J.; Nichols, A.; Ross, J.S.; Tartaglia, L.A.; et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J. Clin. Investig. 2003, 112, 1821–1830. [Google Scholar] [CrossRef]
- Sartipy, P.; Loskutoff, D.J. Monocyte chemoattractant protein 1 in obesity and insulin resistance. Proc. Natl. Acad. Sci. USA 2003, 100, 7265–7270. [Google Scholar] [CrossRef]
- Arendt, L.M.; McCready, J.; Keller, P.J.; Baker, D.D.; Naber, S.P.; Seewaldt, V.; Kuperwasser, C. Obesity promotes breast cancer by CCL2-mediated macrophage recruitment and angiogenesis. Cancer Res. 2013, 73, 6080–6093. [Google Scholar] [CrossRef]
- Gschwandtner, M.; Derler, R.; Midwood, K.S. More than just attractive: How CCL2 influences myeloid cell behavior beyond chemotaxis. Front. Immunol. 2019, 10, 2759. [Google Scholar] [CrossRef] [PubMed]
- Nagarsheth, N.; Wicha, M.S.; Zou, W. Chemokines in the cancer microenvironment and their relevance in cancer immunotherapy. Nat. Rev. Immunol. 2017, 17, 559–572. [Google Scholar] [CrossRef]
- Zhu, J.; Yong, W.; Wu, X.; Yu, Y.; Lv, J.; Liu, C.; Mao, X.; Zhu, Y.; Xu, K.; Han, X.; et al. Anti-inflammatory effect of resveratrol on TNF-α-induced MCP-1 expression in adipocytes. Biochem. Biophys. Res. Commun. 2008, 369, 471–477. [Google Scholar] [CrossRef]
- Kanda, H.; Tateya, S.; Tamori, Y.; Kotani, K.; Hiasa, K.; Kitazawa, R.; Kitazawa, S.; Miyachi, H.; Maeda, S.; Egashira, K.; et al. MCP-1 contributes to macrophage infiltration into adipose tissue, insulin resistance, and hepatic steatosis in obesity. J. Clin. Investig. 2006, 116, 1494–1505. [Google Scholar] [CrossRef] [PubMed]
- Weisberg, S.P.; Hunter, D.; Huber, R.; Lemieux, J.; Slaymaker, S.; Vaddi, K.; Charo, I.; Leibel, R.L.; Ferrante, A.W., Jr. CCR2 modulates inflammatory and metabolic effects of high-fat feeding. J. Clin. Investig. 2006, 116, 115–124. [Google Scholar] [CrossRef]
- Fujimura, N.; Xu, B.; Dalman, J.; Deng, H.; Aoyama, K.; Dalman, R.L. CCR2 inhibition sequesters multiple subsets of leukocytes in the bone marrow. Sci. Rep. 2015, 5, 11664. [Google Scholar] [CrossRef] [PubMed]
- Strissel, K.J.; Stancheva, Z.; Miyoshi, H.; Perfield, J.W.; Defuria, J.; Jick, Z.; Greenberg, A.S.; Obin, M.S. Adipocyte death, adipose tissue remodeling, and obesity complications. Diabetes 2007, 56, 2910–2918. [Google Scholar] [CrossRef]
- Kraakman, M.J.; Murphy, A.J.; Jandeleit-Dahm, K.; Kammoun, H.L. Macrophage polarization in obesity and type 2 diabetes: Weighing down our understanding of macrophage function? Front. Immunol. 2014, 5, 470. [Google Scholar] [CrossRef]
- Lumeng, C.N.; Bodzin, J.L.; Saltiel, A.R. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J. Clin. Investig. 2007, 117, 175–184. [Google Scholar] [CrossRef]
- Lumeng, C.N.; DeYoung, S.M.; Bodzin, J.L.; Saltiel, A.R. Increased inflammatory properties of adipose tissue macrophages recruited during diet-induced obesity. Diabetes 2007, 56, 16–23. [Google Scholar] [CrossRef]
- Shaul, M.E.; Bennett, G.; Strissel, K.J.; Greenberg, A.S.; Obin, M.S. Dynamic, M2-like remodeling phenotypes of CD11c+ adipose tissue macrophages during high-fat diet--induced obesity in mice. Diabetes 2010, 59, 1171–1181. [Google Scholar] [CrossRef]
- Wentworth, J.M.; Naselli, G.; Brown, W.A.; Doyle, L.; Phipson, B.; Smyth, G.K.; Wabitsch, M.; O’Brien, P.E.; Harrison, L.C. Pro-Inflammatory CD11c+CD206+ adipose tissue macrophages are associated with insulin resistance in human obesity. Diabetes 2010, 59, 1648–1656. [Google Scholar] [CrossRef]
- Cinti, S.; Mitchell, G.; Barbatelli, G.; Murano, I.; Ceresi, E.; Faloia, E.; Wang, S.; Fortier, M.; Greenberg, A.S.; Obin, M.S. Adipocyte death defines macrophage localization and function in adipose tissue of obese mice and humans. J. Lipid Res. 2005, 46, 2347–2355. [Google Scholar] [CrossRef]
- Sun, K.; Kusminski, C.M.; Scherer, P.E. Adipose tissue remodeling and obesity. J. Clin. Investig. 2011, 121, 2094–2101. [Google Scholar] [CrossRef]
- Hill, D.A.; Lim, H.W.; Kim, Y.H.; Ho, W.Y.; Foong, Y.H.; Nelson, V.L.; Nguyen, H.C.B.; Chegireddy, K.; Kim, J.; Habertheuer, A.; et al. Distinct macrophage populations direct inflammatory versus physiological changes in adipose tissue. Proc. Natl. Acad. Sci. USA 2018, 115, E5096–E5105. [Google Scholar] [CrossRef]
- Xu, X.; Grijalva, A.; Skowronski, A.; van Eijk, M.; Serlie, M.J.; Ferrante, A.W., Jr. Obesity activates a program of lysosomal-dependent lipid metabolism in adipose tissue macrophages independently of classic activation. Cell. Metab. 2013, 18, 816–830. [Google Scholar] [CrossRef] [PubMed]
- Kratz, M.; Coats, B.R.; Hisert, K.B.; Hagman, D.; Mutskov, V.; Peris, E.; Schoenfelt, K.Q.; Kuzma, J.N.; Larson, I.; Billing, P.S.; et al. Metabolic dysfunction drives a mechanistically distinct proinflammatory phenotype in adipose tissue macrophages. Cell. Metab. 2014, 20, 614–625. [Google Scholar] [CrossRef] [PubMed]
- Coats, B.R.; Schoenfelt, K.Q.; Barbosa-Lorenzi, V.C.; Peris, E.; Cui, C.; Hoffman, A.; Zhou, G.; Fernandez, S.; Zhai, L.; Hall, B.A.; et al. Metabolically activated adipose tissue macrophages perform detrimental and beneficial functions during diet-induced obesity. Cell. Rep. 2017, 20, 3149–3161. [Google Scholar] [CrossRef]
- Morris, P.G.; Hudis, C.A.; Giri, D.; Morrow, M.; Falcone, D.J.; Zhou, X.K.; Du, B.; Brogi, E.; Crawford, C.B.; Kopelovich, L.; et al. Inflammation and increased aromatase expression occur in the breast tissue of obese women with breast cancer. Cancer Prev. Res. 2011, 4, 1021–1029. [Google Scholar] [CrossRef] [PubMed]
- Mullooly, M.; Yang, H.P.; Falk, R.T.; Nyante, S.J.; Cora, R.; Pfeiffer, R.M.; Radisky, D.C.; Visscher, D.W.; Hartmann, L.C.; Carter, J.M.; et al. Relationship between crown-like structures and sex-steroid hormones in breast adipose tissue and serum among postmenopausal breast cancer patients. Breast Cancer Res. 2017, 19, 8. [Google Scholar] [CrossRef] [PubMed]
- Wahl, S.M.; McCartney-Francis, N.; Allen, J.B.; Dougherty, E.B.; Dougherty, S.F. Macrophage production of TGF-beta and regulation by TGF-beta. Ann. N. Y. Acad. Sci. 1990, 593, 188–196. [Google Scholar] [CrossRef]
- Lacasa, D.; Taleb, S.; Keophiphath, M.; Miranville, A.; Clement, K. Macrophage-secreted factors impair human adipogenesis: Involvement of proinflammatory state in preadipocytes. Endocrinology 2007, 148, 868–877. [Google Scholar] [CrossRef] [PubMed]
- Keophiphath, M.; Achard, V.; Henegar, C.; Rouault, C.; Clement, K.; Lacasa, D. Macrophage-secreted factors promote a profibrotic phenotype in human preadipocytes. Mol. Endocrinol. 2009, 23, 11–24. [Google Scholar] [CrossRef]
- Hotchkiss, R.S.; Strasser, A.; McDunn, J.E.; Swanson, P.E. Cell Death. N. Engl. J. Med. 2009, 361, 1570–1583. [Google Scholar] [CrossRef] [PubMed]
- Rock, K.L.; Kono, H. The inflammatory response to cell death. Annu. Rev. Pathol. 2008, 3, 99–126. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Ikeda, K.; Suganami, T.; Komiya, C.; Ochi, K.; Shirakawa, I.; Hamaguchi, M.; Nishimura, S.; Manabe, I.; Matsuda, T.; et al. Macrophage-inducible C-type lectin underlies obesity-induced adipose tissue fibrosis. Nat. Commun. 2014, 5, 4982. [Google Scholar] [CrossRef]
- Bonner, J.C. Regulation of PDGF and its receptors in fibrotic diseases. Cytokine Growth Factor Rev. 2004, 15, 255–273. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Lee, T.C.; Guillemin, B.; Yu, M.C.; Rom, W.N. Enhanced IL-1 beta and tumor necrosis factor-alpha release and messenger RNA expression in macrophages from idiopathic pulmonary fibrosis or after asbestos exposure. J. Immunol. 1993, 150, 4188–4196. [Google Scholar] [CrossRef]
- Kolb, M.; Margetts, P.J.; Anthony, D.C.; Pitossi, F.; Gauldie, J. Transient expression of IL-1beta induces acute lung injury and chronic repair leading to pulmonary fibrosis. J. Clin. Investig. 2001, 107, 1529–1536. [Google Scholar] [CrossRef]
- Lee, Y.H.; Petkova, A.P.; Granneman, J.G. Identification of an adipogenic niche for adipose tissue remodeling and restoration. Cell. Metab. 2013, 18, 355–367. [Google Scholar] [CrossRef]
- Deligne, C.; Midwood, K.S. Macrophages and extracellular matrix in breast cancer: Partners in crime or protective allies? Front. Oncol. 2021, 11, 620773. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Glynn, D.J.; Hodson, L.J.; Huo, C.; Britt, K.; Thompson, E.W.; Woolford, L.; Evdokiou, A.; Pollard, J.W.; Robertson, S.A.; et al. CCL2-driven inflammation increases mammary gland stromal density and cancer susceptibility in a transgenic mouse model. Breast Cancer Res. 2017, 19, 4. [Google Scholar] [CrossRef] [PubMed]
- Cottam, M.A.; Caslin, H.L.; Winn, N.C.; Hasty, A.H. Multiomics reveals persistence of obesity-associated immune cell phenotypes in adipose tissue during weight loss and weight regain in mice. Nat. Commun. 2022, 13, 2950. [Google Scholar] [CrossRef]
- Weinstock, A.; Brown, E.J.; Garabedian, M.L.; Pena, S.; Sharma, M.; Lafaille, J.; Moore, K.J.; Fisher, E.A. Single-cell RNA sequencing of visceral adipose tissue leukocytes reveals that caloric restriction following obesity promotes the accumulation of a distinct macrophage population with features of phagocytic cells. Immunometab 2019, 1, e190008. [Google Scholar] [CrossRef]
- Jappinen, N.; Felix, I.; Lokka, E.; Tyystjarvi, S.; Pynttari, A.; Lahtela, T.; Gerke, H.; Elima, K.; Rantakari, P.; Salmi, M. Fetal-derived macrophages dominate in adult mammary glands. Nat. Commun. 2019, 10, 281. [Google Scholar] [CrossRef]
- Wang, Y.; Chaffee, T.S.; LaRue, R.S.; Huggins, D.N.; Witschen, P.M.; Ibrahim, A.M.; Nelson, A.C.; Machado, H.L.; Schwertfeger, K.L. Tissue-resident macrophages promote extracellular matrix homeostasis in the mammary gland stroma of nulliparous mice. Elife 2020, 9, e57438. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Lai, S.M.; Xu, S.; Tan, Y.; Leong, K.; Liu, D.; Tan, J.C.; Naik, R.R.; Barron, A.M.; Adav, S.S.; et al. Resident macrophages restrain pathological adipose tissue remodeling and protect vascular integrity in obese mice. EMBO Rep. 2021, 22, e52835. [Google Scholar] [CrossRef]
- Zhou, Z.; Zhang, H.; Tao, Y.; Jie, H.; Zhao, J.; Zang, J.; Li, H.; Wang, Y.; Wang, T.; Zhao, H.; et al. CX3CR1(hi) macrophages sustain metabolic adaptation by relieving adipose-derived stem cell senescence in visceral adipose tissue. Cell. Rep. 2023, 42, 112424. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Meng, X.M.; Ng, Y.Y.; Ma, F.Y.; Zhou, S.; Zhang, Y.; Yang, C.; Huang, X.R.; Xiao, J.; Wang, Y.Y.; et al. TGF-β/Smad3 signalling regulates the transition of bone marrow-derived macrophages into myofibroblasts during tissue fibrosis. Oncotarget 2015, 7, 8809–8822. [Google Scholar] [CrossRef]
- Meng, X.M.; Wang, S.; Huang, X.R.; Yang, C.; Xiao, J.; Zhang, Y.; To, K.F.; Nikolic-Paterson, D.J.; Lan, H.Y. Inflammatory macrophages can transdifferentiate into myofibroblasts during renal fibrosis. Cell. Death Dis. 2016, 7, e2495. [Google Scholar] [CrossRef]
- Wang, Y.Y.; Jiang, H.; Pan, J.; Huang, X.R.; Wang, Y.C.; Huang, H.F.; To, K.F.; Nikolic-Paterson, D.J.; Lan, H.Y.; Chen, J.H. Macrophage-to-myofibroblast transition contributes to interstitial fibrosis in chronic renal allograft injury. J. Am. Soc. Nephrol. 2017, 28, 2053–2067. [Google Scholar] [CrossRef]
- Maller, O.; Drain, A.P.; Barrett, A.S.; Borgquist, S.; Ruffell, B.; Zakharevich, I.; Pham, T.T.; Gruosso, T.; Kuasne, H.; Lakins, J.N.; et al. Tumour-associated macrophages drive stromal cell-dependent collagen crosslinking and stiffening to promote breast cancer aggression. Nat. Mat. 2021, 20, 548–559. [Google Scholar] [CrossRef]
- Burke, R.M.; Madden, K.S.; Perry, S.W.; Zettel, M.L.; Brown, E.B., 3rd. Tumor-associated macrophages and stromal TNF-α regulate collagen structure in a breast tumor model as visualized by second harmonic generation. J. Biomed. Opt. 2013, 18, 086003. [Google Scholar] [CrossRef]
- Kuziel, G.; Thompson, V.; D’Amato, J.V.; Arendt, L.M. Stromal CCL2 signaling promotes mammary tumor fibrosis through recruitment of myeloid-lineage cells. Cancers 2020, 12, 2083. [Google Scholar] [CrossRef] [PubMed]
- Acerbi, I.; Cassereau, L.; Dean, I.; Shi, Q.; Au, A.; Park, C.; Chen, Y.Y.; Liphardt, J.; Hwang, E.S.; Weaver, V.M. Human breast cancer invasion and aggression correlates with ECM stiffening and immune cell infiltration. Integr. Biol. 2015, 7, 1120–1134. [Google Scholar] [CrossRef]
- Zhang, Y.; Baloglu, F.K.; Ziemer, L.E.H.; Liu, Z.; Lyu, B.; Arendt, L.M.; Georgakoudi, I. Factors associated with obesity alter matrix remodeling in breast cancer tissues. J. Biomed. Opt. 2020, 25, 014513. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, R. Immunotherapy targeting tumor-associated macrophages. Front. Med. 2020, 7, 583708. [Google Scholar] [CrossRef] [PubMed]
- Tian, L.; Lei, A.; Tan, T.; Zhu, M.; Zhang, L.; Mou, H.; Zhang, J. Macrophage-based combination therapies as a new strategy for cancer immunotherapy. Kidney Dis. 2022, 8, 26–43. [Google Scholar] [CrossRef] [PubMed]
- Adler, B.J.; Kaushansky, K.; Rubin, C.T. Obesity-driven disruption of haematopoiesis and the bone marrow niche. Nat. Rev. Endocrinol. 2014, 10, 737–748. [Google Scholar] [CrossRef]
- Divoux, A.; Moutel, S.; Poitou, C.; Lacasa, D.; Veyrie, N.; Aissat, A.; Arock, M.; Guerre-Millo, M.; Clément, K. Mast cells in human adipose tissue: Link with morbid obesity, inflammatory status, and diabetes. J. Clin. Endocrinol. Metab. 2012, 97, E1677–E1685. [Google Scholar] [CrossRef]
- Liu, J.; Divoux, A.; Sun, J.; Zhang, J.; Clément, K.; Glickman, J.N.; Sukhova, G.K.; Wolters, P.J.; Du, J.; Gorgun, C.Z.; et al. Genetic deficiency and pharmacological stabilization of mast cells reduce diet-induced obesity and diabetes in mice. Nat. Med. 2009, 15, 940–945. [Google Scholar] [CrossRef]
- Altintas, M.M.; Rossetti, M.A.; Nayer, B.; Puig, A.; Zagallo, P.; Ortega, L.M.; Johnson, K.B.; McNamara, G.; Reiser, J.; Mendez, A.J.; et al. Apoptosis, mastocytosis, and diminished adipocytokine gene expression accompany reduced epididymal fat mass in long-standing diet-induced obese mice. Lipids Health Dis. 2011, 10, 198. [Google Scholar] [CrossRef] [PubMed]
- Gurung, P.; Moussa, K.; Adams-Huet, B.; Devaraj, S.; Jialal, I. Increased mast cell abundance in adipose tissue of metabolic syndrome: Relevance to the proinflammatory state and increased adipose tissue fibrosis. Am. J. Physiol. Endocrinol. Metab. 2019, 316, E504–E509. [Google Scholar] [CrossRef] [PubMed]
- Metcalfe, D.D.; Baram, D.; Mekori, Y.A. Mast cells. Physiol. Rev. 1997, 77, 1033–1079. [Google Scholar] [CrossRef] [PubMed]
- Staser, K.; Yang, F.C.; Clapp, D.W. Mast cells and the neurofibroma microenvironment. Blood 2010, 116, 157–164. [Google Scholar] [CrossRef]
- Lilla, J.N.; Werb, Z. Mast cells contribute to the stromal microenvironment in mammary gland branching morphogenesis. Dev. Biol. 2010, 337, 124–133. [Google Scholar] [CrossRef]
- Hirai, S.; Ohyane, C.; Kim, Y.I.; Lin, S.; Goto, T.; Takahashi, N.; Kim, C.S.; Kang, J.; Yu, R.; Kawada, T. Involvement of mast cells in adipose tissue fibrosis. Am. J. Physiol. Endocrinol. Metab. 2014, 306, E247–E255. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.; Pandya, S.K.; Varshney, S.; Shankar, K.; Rajan, S.; Srivastava, A.; Gupta, A.; Gupta, S.; Vishwakarma, A.L.; Misra, A.; et al. Temporal immmunometabolic profiling of adipose tissue in HFD-induced obesity: Manifestations of mast cells in fibrosis and senescence. Int. J. Obes. 2019, 43, 1281–1294. [Google Scholar] [CrossRef]
- Reilkoff, R.A.; Bucala, R.; Herzog, E.L. Fibrocytes: Emerging effector cells in chronic inflammation. Nat. Rev. Immunol. 2011, 11, 427–435. [Google Scholar] [CrossRef]
- Bucala, R.; Spiegel, L.A.; Chesney, J.; Hogan, M.; Cerami, A. Circulating fibrocytes define a new leukocyte subpopulation that mediates tissue repair. Mol. Med. 1994, 1, 71–81. [Google Scholar] [CrossRef]
- Wada, T.; Sakai, N.; Matsushima, K.; Kaneko, S. Fibrocytes: A new insight into kidney fibrosis. Kidney Int. 2007, 72, 269–273. [Google Scholar] [CrossRef] [PubMed]
- Keeley, E.C.; Mehrad, B.; Strieter, R.M. The role of fibrocytes in fibrotic diseases of the lungs and heart. Fibrogenesis Tissue Repair 2011, 4, 2. [Google Scholar] [CrossRef] [PubMed]
- Maharaj, S.; Shimbori, C.; Kolb, M. Fibrocytes in pulmonary fibrosis: A brief synopsis. Eur. Respir. Rev. 2013, 22, 552–557. [Google Scholar] [CrossRef] [PubMed]
- Kisseleva, T.; Uchinami, H.; Feirt, N.; Quintana-Bustamante, O.; Segovia, J.C.; Schwabe, R.F.; Brenner, D.A. Bone marrow-derived fibrocytes participate in pathogenesis of liver fibrosis. J. Hepatol. 2006, 45, 429–438. [Google Scholar] [CrossRef]
- Niedermeier, M.; Reich, B.; Rodriguez Gomez, M.; Denzel, A.; Schmidbauer, K.; Gobel, N.; Talke, Y.; Schweda, F.; Mack, M. CD4+ T cells control the differentiation of Gr1+ monocytes into fibrocytes. Proc. Natl. Acad. Sci. USA 2009, 106, 17892–17897. [Google Scholar] [CrossRef]
- Aono, Y.; Kishi, M.; Yokota, Y.; Azuma, M.; Kinoshita, K.; Takezaki, A.; Sato, S.; Kawano, H.; Kishi, J.; Goto, H.; et al. Role of Platelet-Derived Growth Factor/Platelet-Derived Growth Factor Receptor Axis in the Trafficking of Circulating Fibrocytes in Pulmonary Fibrosis. Am. J. Respir. Cell. Mol. Biol. 2014, 51, 793–801. [Google Scholar] [CrossRef]
- Sato, S.; Shinohara, S.; Hayashi, S.; Morizumi, S.; Abe, S.; Okazaki, H.; Chen, Y.; Goto, H.; Aono, Y.; Ogawa, H.; et al. Anti-fibrotic efficacy of nintedanib in pulmonary fibrosis via the inhibition of fibrocyte activity. Respir. Res. 2017, 18, 172. [Google Scholar] [CrossRef]
- Reich, B.; Schmidbauer, K.; Rodriguez Gomez, M.; Johannes Hermann, F.; Gobel, N.; Bruhl, H.; Ketelsen, I.; Talke, Y.; Mack, M. Fibrocytes develop outside the kidney but contribute to renal fibrosis in a mouse model. Kidney Int. 2013, 84, 78–89. [Google Scholar] [CrossRef]
- Moore, B.B.; Murray, L.; Das, A.; Wilke, C.A.; Herrygers, A.B.; Toews, G.B. The role of CCL12 in the recruitment of fibrocytes and lung fibrosis. Am. J. Respir. Cell. Mol. Biol. 2006, 35, 175–181. [Google Scholar] [CrossRef]
- Hong, K.M.; Burdick, M.D.; Phillips, R.J.; Heber, D.; Strieter, R.M. Characterization of human fibrocytes as circulating adipocyte progenitors and the formation of human adipose tissue in SCID mice. FASEB J. 2005, 19, 2029–2031. [Google Scholar] [CrossRef]
- Hong, K.M.; Belperio, J.A.; Keane, M.P.; Burdick, M.D.; Strieter, R.M. Differentiation of human circulating fibrocytes as mediated by transforming growth factor-β and peroxisome proliferator-activated receptor γ. J. Biol. Chem. 2007, 282, 22910–22920. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.H.; Burdick, M.D.; Strieter, R.M. Human circulating fibrocytes have the capacity to differentiate osteoblasts and chondrocytes. Int. J. Biochem. Cell. Biol. 2010, 42, 662–671. [Google Scholar] [CrossRef] [PubMed]
- Eto, H.; Ishimine, H.; Kinoshita, K.; Watanabe-Susaki, K.; Kato, H.; Doi, K.; Kuno, S.; Kurisaki, A.; Yoshimura, K. Characterization of human adipose tissue-resident hematopoietic cell populations reveals a novel macrophage subpopulation with CD34 expression and mesenchymal multipotency. Stem Cells Dev. 2013, 22, 985–997. [Google Scholar] [CrossRef]
- Abe, R.; Donnelly, S.C.; Peng, T.; Bucala, R.; Metz, C.N. Peripheral blood fibrocytes: Differentiation pathway and migration to wound sites. J. Immunol. 2001, 166, 7556–7562. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, M.; Sun, G.; Stacey, M.A.; Moi, L.; Mattoli, S. Identification of circulating fibrocytes as precursors of bronchial myofibroblasts in asthma. J. Immunol. 2003, 171, 380–389. [Google Scholar] [CrossRef]
- Mori, L.; Bellini, A.; Stacey, M.A.; Schmidt, M.; Mattoli, S. Fibrocytes contribute to the myofibroblast population in wounded skin and originate from the bone marrow. Exp. Cell. Res. 2005, 304, 81–90. [Google Scholar] [CrossRef]
- Suga, H.; Rennert, R.C.; Rodrigues, M.; Sorkin, M.; Glotzbach, J.P.; Januszyk, M.; Fujiwara, T.; Longaker, M.T.; Gurtner, G.C. Tracking the elusive fibrocyte: Identification and characterization of collagen-producing hematopoietic lineage cells during murine wound healing. Stem Cells 2014, 32, 1347–1360. [Google Scholar] [CrossRef]
- Terai, S.; Fushida, S.; Tsukada, T.; Kinoshita, J.; Oyama, K.; Okamoto, K.; Makino, I.; Tajima, H.; Ninomiya, I.; Fujimura, T.; et al. Bone marrow derived “fibrocytes” contribute to tumor proliferation and fibrosis in gastric cancer. Gastric Cancer 2015, 18, 306–313. [Google Scholar] [CrossRef]
- Yang, L.; Scott, P.G.; Giuffre, J.; Shankowsky, H.A.; Ghahary, A.; Tredget, E.E. Peripheral blood fibrocytes from burn patients: Identification and quantification of fibrocytes in adherent cells cultured from peripheral blood mononuclear Cells. Lab. Investig. 2002, 82, 1183–1192. [Google Scholar] [CrossRef]
- Garcia de Alba, C.; Buendia-Roldan, I.; Salgado, A.; Becerril, C.; Ramirez, R.; Gonzalez, Y.; Checa, M.; Navarro, C.; Ruiz, V.; Pardo, A.; et al. Fibrocytes contribute to inflammation and fibrosis in chronic hypersensitivity pneumonitis through paracrine effects. Am. J. Respir. Crit. Care Med. 2015, 191, 427–436. [Google Scholar] [CrossRef]
- Wang, J.F.; Jiao, H.; Stewart, T.L.; Shankowsky, H.A.; Scott, P.G.; Tredget, E.E. Fibrocytes from burn patients regulate the activities of fibroblasts. Wound Repair Regen. 2007, 15, 113–121. [Google Scholar] [CrossRef]
- Picon-Ruiz, M.; Morata-Tarifa, C.; Valle-Goffin, J.J.; Friedman, E.R.; Slingerland, J.M. Obesity and adverse breast cancer risk and outcome: Mechanistic insights and strategies for intervention. CA Cancer J. Clin. 2017, 67, 378–397. [Google Scholar] [CrossRef] [PubMed]
- Schauer, D.P.; Feigelson, H.S.; Koebnick, C.; Caan, B.; Weinmann, S.; Leonard, A.C.; Powers, J.D.; Yenumula, P.R.; Arterburn, D.E. Bariatric surgery and the risk of cancer in a large multisite cohort. Ann. Surg. 2019, 269, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Winder, A.A.; Kularatna, M.; MacCormick, A.D. Does bariatric surgery affect the incidence of breast cancer development? A systematic review. Obes. Surg. 2017, 27, 3014–3020. [Google Scholar] [CrossRef] [PubMed]
- Schauer, D.P.; Feigelson, H.S.; Koebnick, C.; Caan, B.; Weinmann, S.; Leonard, A.C.; Powers, J.D.; Yenumula, P.R.; Arterburn, D.E. Association between weight loss and the risk of cancer after bariatric surgery. Obesity 2017, 25, S52–S57. [Google Scholar] [CrossRef]
- Mackenzie, H.; Markar, S.R.; Askari, A.; Faiz, O.; Hull, M.; Purkayastha, S.; Møller, H.; Lagergren, J. Obesity surgery and risk of cancer. Br. J. Surg. 2018, 105, 1650–1657. [Google Scholar] [CrossRef]
- Christou, N.V.; Lieberman, M.; Sampalis, F.; Sampalis, J.S. Bariatric surgery reduces cancer risk in morbidly obese patients. Surg. Obes. Relat. Dis. 2008, 4, 691–695. [Google Scholar] [CrossRef]
- Feigelson, H.S.; Caan, B.; Weinmann, S.; Leonard, A.C.; Powers, J.D.; Yenumula, P.R.; Arterburn, D.E.; Koebnick, C.; Altaye, M.; Schauer, D.P. Bariatric surgery is associated with reduced risk of breast cancer in both premenopausal and postmenopausal women. Ann. Surg. 2020, 272, 1053–1059. [Google Scholar] [CrossRef]
- Bandera, E.V.; Maskarinec, G.; Romieu, I.; John, E.M. Racial and ethnic disparities in the impact of obesity on breast cancer risk and survival: A global perspective. Adv. Nutr. 2015, 6, 803–819. [Google Scholar] [CrossRef]
- Laudisio, D.; Muscogiuri, G.; Barrea, L.; Savastano, S.; Colao, A. Obesity and breast cancer in premenopausal women: Current evidence and future perspectives. Eur. J. Obstet. Gynecol. Reprod. Biol. 2018, 230, 217–221. [Google Scholar] [CrossRef]
- Dibaba, D.T.; Ogunsina, K.; Braithwaite, D.; Akinyemiju, T. Metabolic syndrome and risk of breast cancer mortality by menopause, obesity, and subtype. Breast Cancer Res. Treat. 2019, 174, 209–218. [Google Scholar] [CrossRef]
- Reeves, M.M.; Terranova, C.O.; Eakin, E.G.; Demark-Wahnefried, W. Weight loss intervention trials in women with breast cancer: A systematic review. Obes. Rev. 2014, 15, 749–768. [Google Scholar] [CrossRef] [PubMed]
- Cancello, R.; Henegar, C.; Viguerie, N.; Taleb, S.; Poitou, C.; Rouault, C.; Coupaye, M.; Pelloux, V.; Hugol, D.; Bouillot, J.-L.; et al. Reduction of macrophage infiltration and chemoattractant gene expression changes in white adipose tissue of morbidly obese subjects after surgery-induced weight loss. Diabetes 2005, 54, 2277–2286. [Google Scholar] [CrossRef] [PubMed]
- Aron-Wisnewsky, J.; Tordjman, J.; Poitou, C.; Darakhshan, F.; Hugol, D.; Basdevant, A.; Aissat, A.; Guerre-Millo, M.; Clement, K. Human adipose tissue macrophages: M1 and M2 cell surface markers in subcutaneous and omental depots and after weight loss. J. Clin. Endocrinol. Metabol. 2009, 94, 4619–4623. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Aron-Wisnewsky, J.; Marcelin, G.; Genser, L.; Le Naour, G.; Torcivia, A.; Bauvois, B.; Bouchet, S.; Pelloux, V.; Sasso, M.; et al. Accumulation and changes in composition of collagens in subcutaneous adipose tissue after bariatric surgery. J. Clin. Endocrinol. Metabol. 2016, 101, 293–304. [Google Scholar] [CrossRef]
- García-Rubio, J.; León, J.; Redruello-Romero, A.; Pavón, E.; Cozar, A.; Tamayo, F.; Caba-Molina, M.; Salmerón, J.; Carazo, Á. Cytometric analysis of adipose tissue reveals increments of adipocyte progenitor cells after weight loss induced by bariatric surgery. Sci. Rep. 2018, 8, 15203. [Google Scholar] [CrossRef]
- Ara, I.; Auerbach, P.; Larsen, S.; Mata, E.; Stallknecht, B.; Ploug, T.; Prats, C.; Helge, J.W. Low-grade inflammation is not present in former obese males but adipose tissue macrophage infiltration persists. Biomedicines 2020, 8, 123. [Google Scholar] [CrossRef]
- Kovacikova, M.; Sengenes, C.; Kovacova, Z.; Siklova-Vitkova, M.; Klimcakova, E.; Polak, J.; Rossmeislova, L.; Bajzova, M.; Hejnova, J.; Hnevkovska, Z.; et al. Dietary intervention-induced weight loss decreases macrophage content in adipose tissue of obese women. Int. J. Obes. 2011, 35, 91–98. [Google Scholar] [CrossRef]
- Ruggiero, A.D.; Key, C.-C.C.; Kavanagh, K. Adipose tissue macrophage polarization in healthy and unhealthy obesity. Front. Nutr. 2021, 8, 625331. [Google Scholar] [CrossRef]
- Cancello, R.; Zulian, A.; Gentilini, D.; Mencarelli, M.; Della Barba, A.; Maffei, M.; Vitti, P.; Invitti, C.; Liuzzi, A.; Di Blasio, A.M. Permanence of molecular features of obesity in subcutaneous adipose tissue of ex-obese subjects. Int. J. Obes. 2013, 37, 867–873. [Google Scholar] [CrossRef]
- Kosteli, A.; Sugaru, E.; Haemmerle, G.; Martin, J.F.; Lei, J.; Zechner, R.; Ferrante, A.W., Jr. Weight loss and lipolysis promote a dynamic immune response in murine adipose tissue. J. Clin. Investig. 2010, 120, 3466–3479. [Google Scholar] [CrossRef] [PubMed]
- Zamarron, B.F.; Mergian, T.A.; Cho, K.W.; Martinez-Santibanez, G.; Luan, D.; Singer, K.; DelProposto, J.L.; Geletka, L.M.; Muir, L.A.; Lumeng, C.N. Macrophage proliferation sustains adipose tissue inflammation in formerly obese mice. Diabetes 2017, 66, 392–406. [Google Scholar] [CrossRef] [PubMed]
- Folie, S.; Radlinger, B.; Goebel, G.; Salzmann, K.; Staudacher, G.; Ress, C.; Tilg, H.; Kaser, S. Changing the dietary composition improves inflammation but not adipocyte thermogenesis in diet-induced obese mice. J. Nutr. Biochem. 2022, 99, 108837. [Google Scholar] [CrossRef] [PubMed]
- Blaszczak, A.M.; Bernier, M.; Wright, V.P.; Gebhardt, G.; Anandani, K.; Liu, J.; Jalilvand, A.; Bergin, S.; Wysocki, V.; Somogyi, A.; et al. Obesogenic memory maintains adipose tissue inflammation and insulin resistance. Immunometabolism 2020, 2, e200023. [Google Scholar] [CrossRef]
- Hoevenaars, F.P.M.; Keijer, J.; Herreman, L.; Palm, I.; Hegeman, M.A.; Swarts, H.J.M.; van Schothorst, E.M. Adipose tissue metabolism and inflammation are differently affected by weight loss in obese mice due to either a high-fat diet restriction or change to a low-fat diet. Genes Nutr. 2014, 9, 391. [Google Scholar] [CrossRef]
- Bel Lassen, P.; Charlotte, F.; Liu, Y.; Bedossa, P.; Le Naour, G.; Tordjman, J.; Poitou, C.; Bouillot, J.L.; Genser, L.; Zucker, J.D.; et al. The FAT score, a fibrosis score of adipose tissue: Predicting weight-loss outcome after gastric bypass. J. Clin. Endocrinol. Metab. 2017, 102, 2443–2453. [Google Scholar] [CrossRef]
- Magkos, F.; Fraterrigo, G.; Yoshino, J.; Luecking, C.; Kirbach, K.; Kelly, S.C.; de Las Fuentes, L.; He, S.; Okunade, A.L.; Patterson, B.W.; et al. Effects of moderate and subsequent progressive weight loss on metabolic function and adipose tissue biology in humans with obesity. Cell. Metab. 2016, 23, 591–601. [Google Scholar] [CrossRef]
- Jung, D.Y.; Ko, H.J.; Lichtman, E.I.; Lee, E.; Lawton, E.; Ong, H.; Yu, K.; Azuma, Y.; Friedline, R.H.; Lee, K.W.; et al. Short-term weight loss attenuates local tissue inflammation and improves insulin sensitivity without affecting adipose inflammation in obese mice. Am. J. Physiol. Endocrinol. Metab. 2013, 304, E964–E976. [Google Scholar] [CrossRef]
- Li, L.; Wei, Y.; Fang, C.; Liu, S.; Zhou, F.; Zhao, G.; Li, Y.; Luo, Y.; Guo, Z.; Lin, W.; et al. Exercise retards ongoing adipose tissue fibrosis in diet-induced obese mice. Endocr. Connect. 2021, 10, 325–335. [Google Scholar] [CrossRef]
- Sipe, L.M.; Chaib, M.; Korba, E.B.; Jo, H.; Lovely, M.C.; Counts, B.R.; Tanveer, U.; Holt, J.R.; Clements, J.C.; John, N.A.; et al. Response to immune checkpoint blockade improved in pre-clinical model of breast cancer after bariatric surgery. Elife 2022, 11, e79143. [Google Scholar] [CrossRef]
- Tiwari, P.; Blank, A.; Cui, C.; Schoenfelt, K.Q.; Zhou, G.; Xu, Y.; Khramtsova, G.; Olopade, F.; Shah, A.M.; Khan, S.A.; et al. Metabolically activated adipose tissue macrophages link obesity to triple-negative breast cancer. J. Exp. Med. 2019, 216, 1345–1358. [Google Scholar] [CrossRef]
- Sundaram, S.; Le, T.L.; Essaid, L.; Freemerman, A.J.; Huang, M.J.; Galanko, J.A.; McNaughton, K.K.; Bendt, K.M.; Darr, D.B.; Troester, M.A.; et al. Weight loss reversed obesity-induced HGF/c-Met pathway and basal-like breast cancer progression. Front. Oncol. 2014, 4, 175. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Sundaram, S.; Essaid, L.; Chen, X.; Miller, S.M.; Yan, F.; Darr, D.B.; Galanko, J.A.; Montgomery, S.A.; Major, M.B.; et al. Weight loss reduces basal-like breast cancer through kinome reprogramming. Cancer. Cell. Int. 2016, 16, 26. [Google Scholar] [CrossRef] [PubMed]
- De Angel, R.E.; Conti, C.J.; Wheatley, K.E.; Brenner, A.J.; Otto, G.; Degraffenried, L.A.; Hursting, S.D. The enhancing effects of obesity on mammary tumor growth and Akt/mTOR pathway activation persist after weight loss and are reversed by RAD001. Mol. Carcinog. 2013, 52, 446–458. [Google Scholar] [CrossRef] [PubMed]
- Adhikary, S.; Hoskin, T.L.; Stallings-Mann, M.L.; Arshad, M.; Frost, M.H.; Winham, S.J.; Pena, A.; Lee, D.J.; Murphy, L.M.; Rakoff, M.; et al. Cytotoxic T cell depletion with increasing epithelial abnormality in women with benign breast disease. Breast Cancer Res. Treat. 2020, 180, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Bowers, L.W.; Doerstling, S.S.; Shamsunder, M.G.; Lineberger, C.G.; Rossi, E.L.; Montgomery, S.A.; Coleman, M.F.; Gong, W.; Parker, J.S.; Howell, A.; et al. Reversing the genomic, epigenetic, and triple-negative breast cancer-enhancing effects of obesity. Cancer Prev. Res. 2022, 15, 581–594. [Google Scholar] [CrossRef]
- Rossi, E.L.; de Angel, R.E.; Bowers, L.W.; Khatib, S.A.; Smith, L.A.; Van Buren, E.; Bhardwaj, P.; Giri, D.; Estecio, M.R.; Troester, M.A.; et al. Obesity-associated alterations in inflammation, epigenetics, and mammary tumor growth persist in formerly obese mice. Cancer Prev. Res. 2016, 9, 339–348. [Google Scholar] [CrossRef]
- Stowers, R.S.; Allen, S.C.; Sanchez, K.; Davis, C.L.; Ebelt, N.D.; Van Den Berg, C.; Suggs, L.J. Extracellular matrix stiffening induces a malignant phenotypic transition in breast epithelial Cells. Cell. Mol. Bioeng. 2017, 10, 114–123. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuziel, G.; Moore, B.N.; Arendt, L.M. Obesity and Fibrosis: Setting the Stage for Breast Cancer. Cancers 2023, 15, 2929. https://doi.org/10.3390/cancers15112929
Kuziel G, Moore BN, Arendt LM. Obesity and Fibrosis: Setting the Stage for Breast Cancer. Cancers. 2023; 15(11):2929. https://doi.org/10.3390/cancers15112929
Chicago/Turabian StyleKuziel, Genevra, Brittney N. Moore, and Lisa M. Arendt. 2023. "Obesity and Fibrosis: Setting the Stage for Breast Cancer" Cancers 15, no. 11: 2929. https://doi.org/10.3390/cancers15112929
APA StyleKuziel, G., Moore, B. N., & Arendt, L. M. (2023). Obesity and Fibrosis: Setting the Stage for Breast Cancer. Cancers, 15(11), 2929. https://doi.org/10.3390/cancers15112929