Nectin-4 as a Predictive Marker for Poor Prognosis of Endometrial Cancer with Mismatch Repair Impairment
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Tissue Specimens
2.2. Tissue Microarray Construction and Immunohistochemistry
2.3. Public Databases
2.4. Statistical Analysis
3. Results
3.1. Nectin-4 Is Overexpressed in EC Tissues Compared with Normal Endometrial and EIN Tissues
3.2. Nectin-4 Is a Powerful Diagnostic Marker of EC
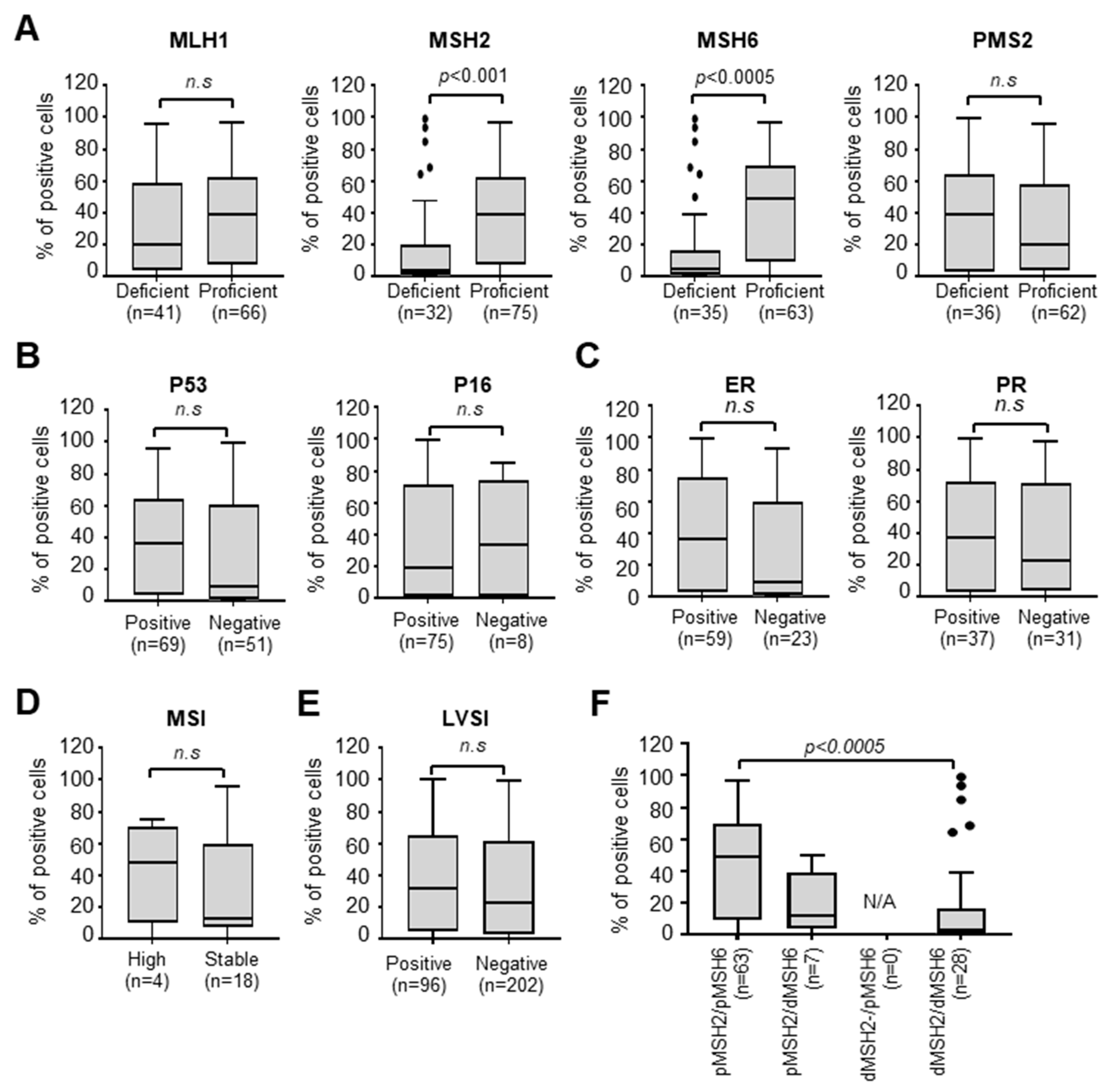
3.3. Nectin-4 Is Associated with MMR Deficiency
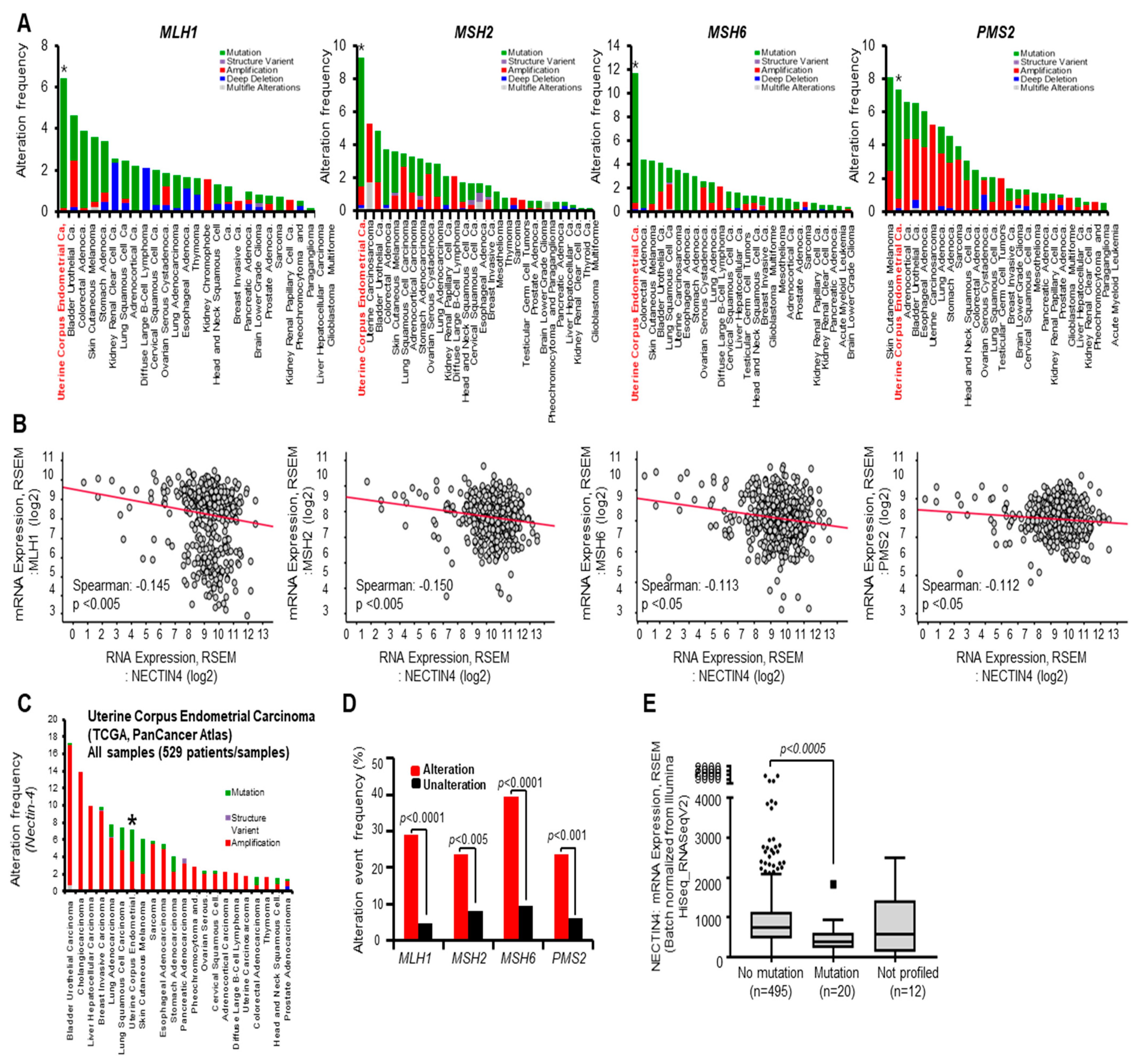
3.4. Alteration Frequency of MMR Is Associated with Nectin-4 Expression in EC
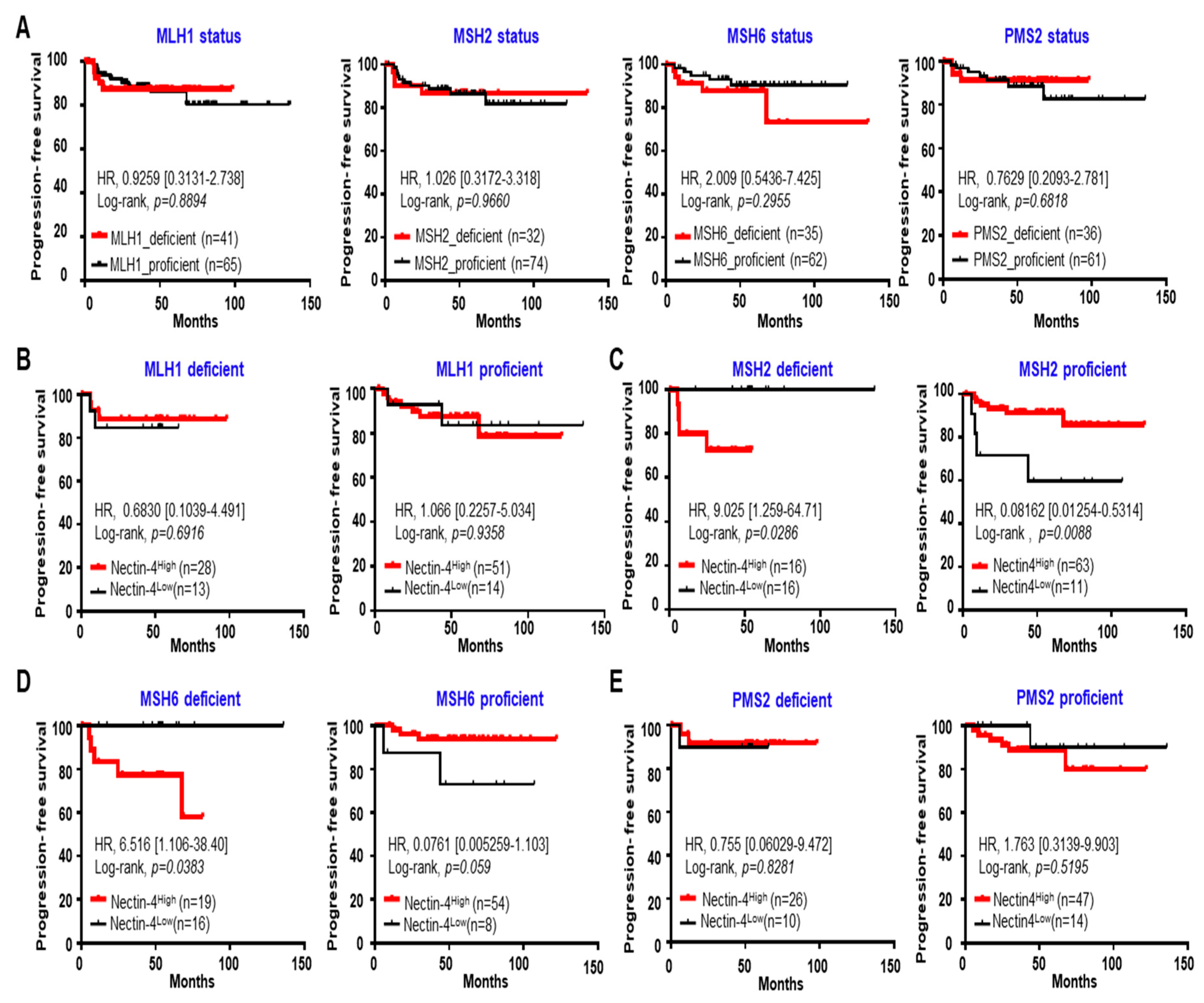
3.5. High Expression of Nectin-4 in MSH2 Deficiency Caused a Short PFS in EC
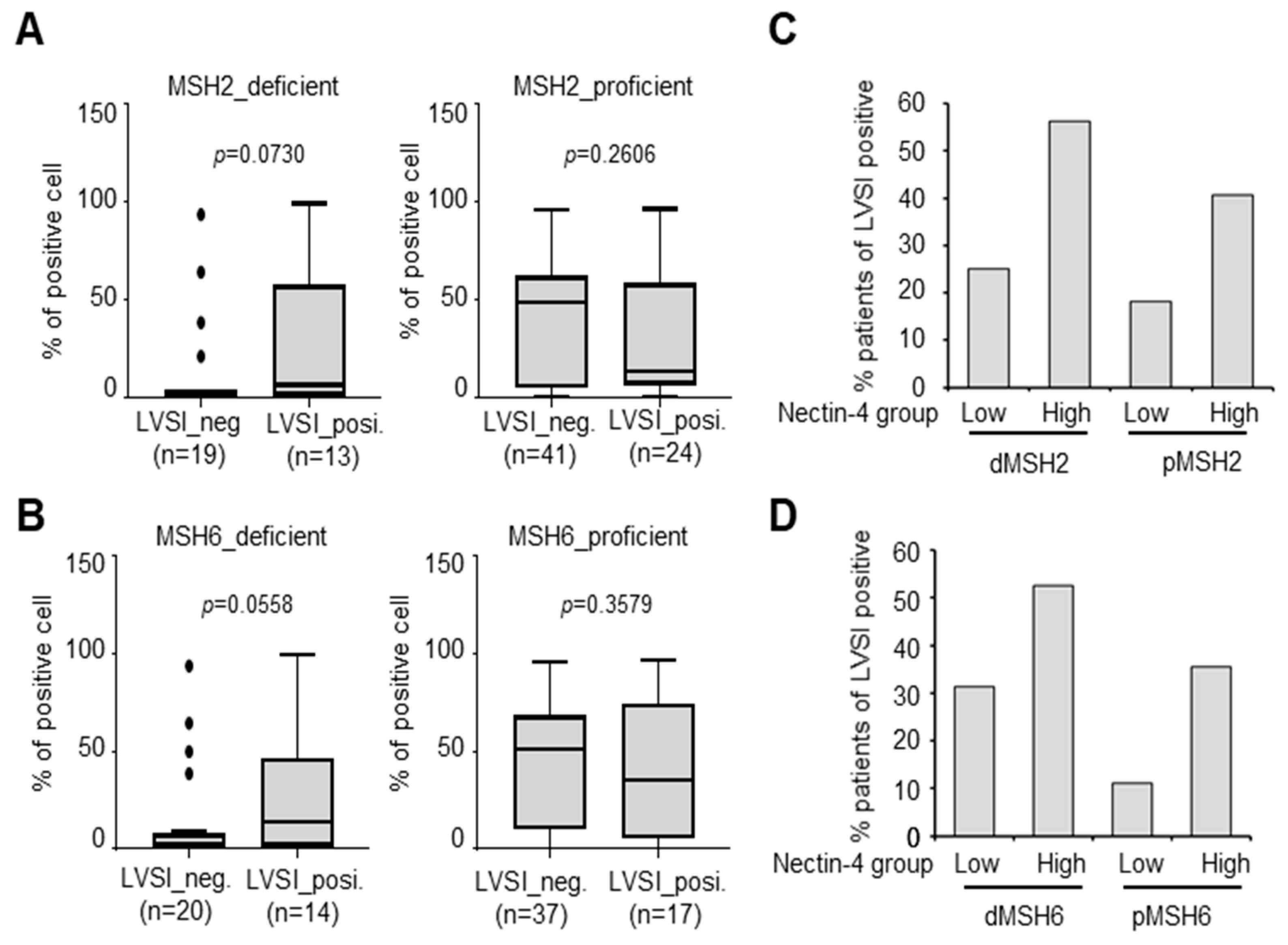
3.6. Increase in LVSI-Positive Patients in the MSH2-Deficient/Nectin-4High Group
3.7. Decrease in CD8-Positive Patients in the MSH2-Deficient/Nectin-4High Group
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Parkin, D.M.; Pisani, P.; Ferlay, J. Estimates of the worldwide incidence of 25 major cancers in 1990. Int. J. Cancer 1999, 80, 827–841. [Google Scholar] [CrossRef]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Masuda, K.; Banno, K.; Yanokura, M.; Kobayashi, Y.; Kisu, I.; Ueki, A.; Ono, A.; Asahara, N.; Nomura, H.; Hirasawa, A.; et al. Relationship between DNA Mismatch Repair Deficiency and Endometrial Cancer. Mol. Biol. Int. 2011, 2011, 256063. [Google Scholar] [CrossRef]
- Aarnio, M.; Sankila, R.; Pukkala, E.; Salovaara, R.; Aaltonen, L.A.; de la Chapelle, A.; Peltomäki, P.; Mecklin, J.P.; Järvinen, H.J. Cancer risk in mutation carriers of DNA-mismatch-repair genes. Int. J. Cancer 1999, 81, 214–218. [Google Scholar] [CrossRef]
- MacDonald, N.D.; Salvesen, H.B.; Ryan, A.; Iversen, O.E.; Akslen, L.A.; Jacobs, I.J. Frequency and prognostic impact of microsatellite instability in a large population-based study of endometrial carcinomas. Cancer Res. 2000, 60, 1750–1752. [Google Scholar]
- Bell, D.W.; Ellenson, L.H. Molecular genetics of endometrial carcinoma. Annu. Rev. Pathol. 2019, 14, 339–367. [Google Scholar] [CrossRef]
- Reitsam, N.G.; Märkl, B.; Dintner, S.; Waidhauser, J.; Vlasenko, D.; Grosser, B. Concurrent loss of MLH1, PMS2 and MSH6 immunoexpression in digestive system cancers indicating a widespread dysregulation in DNA repair processes. Front. Oncol. 2022, 12, 1019798. [Google Scholar] [CrossRef]
- McCoy, P.; Mangiola, S.; Macintyre, G.; Hutchinson, R.; Tran, B.; Pope, B.; Georgeson, P.; Hong, M.K.H.; Kurganovs, N.; Lunke, S.; et al. MSH2-deficient prostate tumours have a distinct immune response and clinical outcome compared to MSH2-deficient colorectal or endometrial cancer. Prostate Cancer Prostatic. Dis. 2021, 24, 1167–1180. [Google Scholar] [CrossRef]
- McMeekin, D.S.; Tritchler, D.L.; Cohn, D.E.; Mutch, D.G.; Lankes, H.A.; Geller, M.A.; Powell, M.A.; Backes, F.J.; Landrum, L.M.; Zaino, R.; et al. Clinicopathologic Significance of Mismatch Repair Defects in Endometrial Cancer: An NRG Oncology/Gynecologic Oncology Group Study. J. Clin. Oncol. 2016, 34, 3062–3068. [Google Scholar] [CrossRef]
- Shikama, A.; Minaguchi, T.; Matsumoto, K.; Akiyama-Abe, A.; Nakamura, Y.; Michikami, H.; Nakao, S.; Sakurai, M.; Ochi, H.; Onuki, M.; et al. Clinicopathologic implications of DNA mismatch repair status in endometrial carcinomas. Gynecol. Oncol. 2016, 140, 226–233. [Google Scholar] [CrossRef]
- Cosgrove, C.M.; Cohn, D.E.; Hampel, H.; Frankel, W.L.; Jones, D.; McElroy, J.P.; Suarez, A.A.; Zhao, W.; Chen, W.; Salani, R.; et al. Epigenetic silencing of MLH1 in endometrial cancers is associated with larger tumor volume, increased rate of lymph node positivity and reduced recurrence-free survival. Gynecol. Oncol. 2017, 146, 588–595. [Google Scholar] [CrossRef]
- Carr, C.; Son, J.; Yao, M.; Priyadarshini, A.; Marquard, J.; Vargas, R.; Michener, C.; AlHilli, M.M. Clinicopathologic characteristics and outcomes of endometrial Cancer patients with mismatch repair deficiency in the era of universal Lynch syndrome screening. Gynecol. Oncol. 2020, 159, 712–720. [Google Scholar] [CrossRef]
- Pasanen, A.; Loukovaara, M.; Bützow, R. Clinicopathological significance of deficient DNA mismatch repair and MLH1 promoter methylation in endometrioid endometrial carcinoma. Mod. Pathol. 2020, 33, 1443–1452. [Google Scholar] [CrossRef]
- Kim, S.R.; Tone, A.; Kim, R.H.; Cesari, M.; Clarke, B.A.; Eiriksson, L.; Hart, T.; Aronson, M.; Holter, S.; Lytwyn, A.; et al. Understanding the clinical implication of mismatch repair deficiency in endometrioid endometrial cancer through a prospective study. Gynecol. Oncol. 2021, 161, 221–227. [Google Scholar] [CrossRef]
- Diaz-Padilla, I.; Romero, N.; Amir, E.; Matias-Guiu, X.; Vilar, E.; Muggia, F.; Garcia-Donas, J. Mismatch repair status and clinical outcome in endometrial cancer: A systematic review and meta-analysis. Crit. Rev. Oncol. Hematol. 2013, 88, 154–167. [Google Scholar] [CrossRef]
- Nagle, C.M.; O’Mara, T.A.; Tan, Y.; Buchanan, D.D.; Obermair, A.; Blomfield, P.; Quinn, M.A.; Webb, P.M.; Spurdle, A.B. Australian Endometrial Cancer Study Group. Endometrial cancer risk and survival by tumor MMR status. J. Gynecol. Oncol. 2018, 29, e39. [Google Scholar] [CrossRef]
- Noyce, R.S.; Richardson, C.D. Nectin 4 is the epithelial cell receptor for measles virus. Trends Microbiol. 2012, 20, 429–439. [Google Scholar] [CrossRef]
- Mühlebach, M.D.; Mateo, M.; Sinn, P.L.; Prüfer, S.; Uhlig, K.M.; Leonard, V.H.; Navaratnarajah, C.K.; Frenzke, M.; Wong, X.X.; Sawatsky, B.; et al. Adherens junction protein nectin-4 is the epithelial receptor for measles virus. Nature 2011, 480, 530–533. [Google Scholar] [CrossRef]
- Bouleftour, W.; Guillot, A.; Magne, N. The Anti-Nectin 4: A Promising Tumor Cells Target. A Systematic Review. Mol. Cancer Ther. 2022, 21, 493–501. [Google Scholar] [CrossRef]
- Tomiyama, E.; Fujita, K.; Rodriguez Pena, M.D.C.; Taheri, D.; Banno, E.; Kato, T.; Hatano, K.; Kawashima, A.; Ujike, T.; Uemura, M.; et al. Expression of Nectin-4 and PD-L1 in Upper Tract Urothelial Carcinoma. Int. J. Mol. Sci. 2020, 21, 5390. [Google Scholar] [CrossRef]
- Takano, A.; Ishikawa, N.; Nishino, R.; Masuda, K.; Yasui, W.; Inai, K.; Nishimura, H.; Ito, H.; Nakayama, H.; Miyagi, Y.; et al. Identification of nectin-4 oncoprotein as a diagnostic and therapeutic target for lung cancer. Cancer Res. 2009, 69, 6694–6703. [Google Scholar] [CrossRef]
- Derycke, M.S.; Pambuccian, S.E.; Gilks, C.B.; Kalloger, S.E.; Ghidouche, A.; Lopez, M.; Bliss, R.L.; Geller, M.A.; Argenta, P.A.; Harrington, K.M.; et al. Nectin 4 overexpression in ovarian cancer tissues and serum: Potential role as a serum biomarker. Am. J. Clin. Pathol. 2010, 134, 835–845. [Google Scholar] [CrossRef]
- Nishiwada, S.; Sho, M.; Yasuda, S.; Shimada, K.; Yamato, I.; Akahori, T.; Kinoshita, S.; Nagai, M.; Konishi, N.; Nakajima, Y. Nectin-4 expression contributes to tumor proliferation, angiogenesis and patient prognosis in human pancreatic cancer. J. Exp. Clin. Cancer Res. 2015, 34, 30. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, J.; Shen, Q.; Yin, W.; Huang, H.; Liu, Y.; Ni, Q. High expression of Nectin-4 is associated with unfavorable prognosis in gastric cancer. Oncol. Lett. 2018, 15, 8789–8795. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, S.; Wang, L.; Wu, Y.; Hao, J.; Wang, Z.; Lu, W.; Wang, X.A.; Zhang, F.; Cao, Y.; et al. A novel PI3K/AKT signaling axis mediates Nectin-4-induced gallbladder cancer cell proliferation, metastasis and tumor growth. Cancer Lett. 2016, 375, 179–189. [Google Scholar] [CrossRef]
- Pavlova, N.N.; Pallasch, C.; Elia, A.E.; Braun, C.J.; Westbrook, T.F.; Hemann, M.; Elledge, S.J. A role for PVRL4-driven cell-cell interactions in tumorigenesis. eLife 2013, 2, e00358. [Google Scholar] [CrossRef]
- Siddharth, S.; Goutam, K.; Das, S.; Nayak, A.; Nayak, D.; Sethy, C.; Wyatt, M.D.; Kundu, C.N. Nectin-4 is a breast cancer stem cell marker that induces WNT/β-catenin signaling via Pi3k/Akt axis. Int. J. Biochem. Cell Biol. 2017, 89, 85–94. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, P.; Yin, W.; Ji, Y.; Shen, Q.; Ni, Q. Nectin-4 promotes gastric cancer progression via the PI3K/AKT signaling pathway. Hum. Pathol. 2018, 72, 107–116. [Google Scholar] [CrossRef]
- Heath, E.I.; Rosenberg, J.E. The biology and rationale of targeting nectin-4 in urothelial carcinoma. Nat. Rev. Urol. 2021, 18, 93–103. [Google Scholar] [CrossRef]
- Fabre-Lafay, S.; Monville, F.; Garrido-Urbani, S.; Berruyer-Pouyet, C.; Ginestier, C.; Reymond, N.; Finetti, P.; Sauvan, R.; Adélaïde, J.; Geneix, J.; et al. Nectin-4 is a new histological and serological tumor associated marker for breast cancer. BMC Cancer 2007, 7, 73. [Google Scholar] [CrossRef]
- Challita-Eid, P.M.; Satpayev, D.; Yang, P.; An, Z.; Morrison, K.; Shostak, Y.; Raitano, A.; Nadell, R.; Liu, W.; Lortie, D.R.; et al. Enfortumab Vedotin Antibody-Drug Conjugate Targeting Nectin-4 Is a Highly Potent Therapeutic Agent in Multiple Preclinical Cancer Models. Cancer Res. 2016, 76, 3003–3013. [Google Scholar] [CrossRef]
- Siegel, R.; Ma, J.; Zou, Z.; Jemal, A. Cancer statistics, 2014. CA Cancer J. Clin. 2014, 64, 9–29. [Google Scholar] [CrossRef]
- Moore, M.J.; Goldstein, D.; Hamm, J.; Figer, A.; Hecht, J.R.; Gallinger, S.; Au, H.J.; Murawa, P.; Walde, D.; Wolff, R.A.; et al. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: A phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J. Clin. Oncol. 2007, 25, 1960–1966. [Google Scholar] [CrossRef]
- Rajc, J.; Gugić, D.; Fröhlich, I.; Marjanović, K.; Dumenčić, B. Prognostic role of Nectin-4 expression in luminal B (HER2 negative) breast cancer. Pathol. Res. Pract. 2017, 213, 1102–1108. [Google Scholar] [CrossRef]
- Siddharth, S.; Nayak, A.; Das, S.; Nayak, D.; Panda, J.; Wyatt, M.D.; Kundu, C.N. The soluble nectin-4 ecto-domain promotes breast cancer induced angiogenesis via endothelial Integrin-β4. Int. J. Biochem. Cell Biol. 2018, 102, 151–160. [Google Scholar] [CrossRef]
- Zeindler, J.; Soysal, S.D.; Piscuoglio, S.; Ng, C.K.Y.; Mechera, R.; Isaak, A.; Weber, W.P.; Muenst, S.; Kurzeder, C. Nectin-4 Expression Is an Independent Prognostic Biomarker and Associated with Better Survival in Triple-Negative Breast Cancer. Front. Med. 2019, 6, 200. [Google Scholar] [CrossRef]
- Liu, Y.; Li, G.; Zhang, Y.; Li, L.; Zhang, Y.; Huang, X.; Wei, X.; Zhou, P.; Liu, M.; Zhao, G.; et al. Nectin-4 promotes osteosarcoma progression and metastasis through activating PI3K/AKT/NF-κB signaling by down-regulation of miR-520c-3p. Cancer Cell Int. 2022, 22, 252. [Google Scholar] [CrossRef]
- Hashimoto, H.; Tanaka, Y.; Murata, M.; Ito, T. Nectin-4: A Novel Therapeutic Target for Skin Cancers. Curr. Treat Options Oncol. 2022, 23, 578–593. [Google Scholar] [CrossRef]
- Kedashiro, S.; Kameyama, T.; Mizutani, K.; Takai, Y. Nectin-4 and p95-ErbB2 cooperatively regulate Hippo signaling-dependent SOX2 gene expression, enhancing anchorage-independent T47D cell proliferation. Sci. Rep. 2021, 11, 7344. [Google Scholar] [CrossRef]
- Sethy, C.; Goutam, K.; Das, B.; Dash, S.R.; Kundu, C.N. Nectin-4 promotes lymphangiogenesis and lymphatic metastasis in breast cancer by regulating CXCR4-LYVE-1 axis. Vascul. Pharmacol. 2021, 140, 106865. [Google Scholar] [CrossRef]
- Murali, R.; Soslow, R.A.; Weigelt, B. Classification of endometrial carcinoma: More than two types. Lancet Oncol. 2014, 15, e268–e278. [Google Scholar] [CrossRef]
- Alexa, M.; Hasenburg, A.; Battista, M.J. The TCGA Molecular Classification of Endometrial Cancer and Its Possible Impact on Adjuvant Treatment Decisions. Cancers 2021, 13, 1478. [Google Scholar] [CrossRef]
- Talhouk, A.; McConechy, M.K.; Leung, S.; Yang, W.; Lum, A.; Senz, J.; Boyd, N.; Pike, J.; Anglesio, M.; Kwon, J.S.; et al. Confirmation of ProMisE: A simple, genomics-based clinical classifier for endometrial cancer. Cancer 2017, 123, 802–813. [Google Scholar] [CrossRef]
- Li, K.; Luo, H.; Huang, L.; Luo, H.; Zhu, X. Microsatellite instability: A review of what the oncologist should know. Cancer Cell Int. 2020, 20, 16. [Google Scholar] [CrossRef]
- Kurnit, K.C.; Westin, S.N.; Coleman, R.L. Microsatellite instability in endometrial cancer: New purpose for an old test. Cancer 2019, 125, 2154–2163. [Google Scholar] [CrossRef]
- Zhang, X.; Wu, T.; Cai, X.; Dong, J.; Xia, C.; Zhou, Y.; Ding, R.; Yang, R.; Tan, J.; Zhang, L.; et al. Neoadjuvant Immunotherapy for MSI-H/dMMR Locally Advanced Colorectal Cancer: New Strategies and Unveiled Opportunities. Front. Immunol. 2022, 13, 795972. [Google Scholar] [CrossRef]
- Di Dio, C.; Bogani, G.; Di Donato, V.; Cuccu, I.; Muzii, L.; Musacchio, L.; Scambia, G.; Lorusso, D. The role of immunotherapy in advanced and recurrent MMR deficient and proficient endometrial carcinoma. Gynecol. Oncol. 2023, 169, 27–33. [Google Scholar] [CrossRef]
- Gelsomino, F.; Barbolini, M.; Spallanzani, A.; Pugliese, G.; Cascinu, S. The evolving role of microsatellite instability in colorectal cancer: A review. Cancer Treat Rev. 2016, 51, 19–26. [Google Scholar] [CrossRef]
- Wang, L.; Yang, M.; Guo, X.; Yang, Z.; Liu, S.; Ji, Y.; Jin, H. Estrogen-related receptor-α promotes gallbladder cancer development by enhancing the transcription of Nectin-4. Cancer Sci. 2020, 111, 1514–1527. [Google Scholar] [CrossRef]
- Chen, J.; Bhandari, A.; Hirachan, S.; Lv, S.; Mainali, S.; Zheng, C.; Hao, R. A Specificity Protein 1 assists the progression of the papillary thyroid cell line by initiating NECTIN4. Endocr. Metab. Immune Disord. Drug Targets 2023. [Google Scholar] [CrossRef]
- Mandai, K.; Rikitake, Y.; Mori, M.; Takai, Y. Nectins and nectin-like molecules in development and disease. Curr. Top. Dev. Biol. 2015, 112, 197–231. [Google Scholar]
- Mizutani, K.; Kedashiro, S.; Maruoka, M.; Ueda, Y.; Takai, Y. Nectin-like molecule-4/cell adhesion molecule 4 inhibits the ligand-induced dimerization of ErbB3 with ErbB2. Sci. Rep. 2017, 7, 11375. [Google Scholar] [CrossRef]
- Maruoka, M.; Kedashiro, S.; Ueda, Y.; Mizutani, K.; Takai, Y. Nectin-4 co-stimulates the prolactin receptor by interacting with SOCS1 and inhibiting its activity on the JAK2-STAT5a signaling pathway. J. Biol. Chem. 2017, 292, 6895–6909. [Google Scholar] [CrossRef]
- Sakamoto, Y.; Ogita, H.; Hirota, T.; Kawakatsu, T.; Fukuyama, T.; Yasumi, M.; Kanzaki, N.; Ozaki, M.; Takai, Y. Interaction of integrin alpha(v)beta3 with nectin. Implication in cross-talk between cell-matrix and cell-cell junctions. J. Biol. Chem. 2006, 281, 19631–19644. [Google Scholar] [CrossRef]
- Kedashiro, S.; Sugiura, A.; Mizutani, K.; Takai, Y. Nectin-4 cis-interacts with ErbB2 and its trastuzumab-resistant splice variants, enhancing their activation and DNA synthesis. Sci. Rep. 2019, 9, 18997. [Google Scholar] [CrossRef]


| Characteristic | Nectin-4 Expression | ||
|---|---|---|---|
| Nectin-4High | Nectin-4Low | p-Value | |
| n (%) | n (%) | ||
| Diagnosis | 0.000 * | ||
| Nonadjacent normal tissue | 2 (2.3%) | 85 (97.7%) | |
| EIN | 28 (51.9%) | 26 (48.1%) | |
| Cancer | 240 (75.0%) | 80 (25.0%) | |
| Age | 0.098 | ||
| ≥50 | 179 (74.6%) | 52 (65.0%) | |
| 50< | 61 (25.4%) | 28 (35.0%) | |
| FIGO stage | 0.900 | ||
| I | 173 (72.1%) | 55 (68.8%) | |
| II | 14 (5.8%) | 6 (7.5%) | |
| III | 40 (16.7%) | 15 (18.8%) | |
| IV | 6 (2.5%) | 3 (3.8%) | |
| Recurrence | 6 (2.5%) | 1 (1.3%) | |
| Unclassified | 1 (0.4%) | 0 (0%) | |
| Differentiation | 0.059 | ||
| Grade I | 97 (40.4%) | 37 (46.3%) | |
| Grade II | 83 (34.6%) | 21 (26.3%) | |
| Grade III | 56 (23.3%) | 17 (21.3%) | |
| Mix | 0 (0%) | 2 (2.5%) | |
| Unclassified | 4 (1.7%) | 3 (3.8%) | |
| Histology | 0.076 | ||
| Endometrioid | 206 (85.8%) | 68 (85.0%) | |
| Serous | 10 (4.2%) | 1 (1.3%) | |
| Clear | 2 (0.8%) | 5 (6.3%) | |
| Mix | 14 (5.8%) | 4 (5.0%) | |
| ETS | 7 (2.9%) | 2 (2.5%) | |
| Unclassified | 1 (0.4%) | 0 (0.0%) | |
| CA125 (U/mL) | 0.559 | ||
| ≥35 | 167 (69.6%) | 58 (72.5%) | |
| 35< | 60 (25.0%) | 20 (25.0%) | |
| Unclassified | 13 (5.4%) | 2 (2.5%) | |
| MSH2 status | 0.001 * | ||
| Deficient | 16 (6.7%) | 16 (20.0%) | |
| Proficient | 63 (26.3%) | 12 (15.0%) | |
| Unclassified | 161 (67.1%) | 52 (65.0%) | |
| MSH6 status | 0.003 * | ||
| Deficient | 19 (7.90%) | 16 (20.0%) | |
| Proficient | 54 (22.5%) | 9 (11.3%) | |
| Unclassified | 167 (69.6%) | 55 (68.8%) | |
| Group | Youden Index J | ROC–AUC (95%CI) | Sensitivity (95%CI) | Specificity (95%CI) | PPV (95%CI) | NPV (95%CI) |
|---|---|---|---|---|---|---|
| Nectin-4 | 0.7821 | 0.922 (0.892–0.946) | 82.81 (78.2–86.8) | 95.40 (88.6–98.7) | 98.5 (96.2–99.4) | 60.1 (54.2–65.8) |
| Group | Odds Ratio | (95% CI) | p-Value |
|---|---|---|---|
| Nectin-4 > 0.7821% | 79.01 | (30.60–203.99) | <0.0001 |
| Gene | Altered Group | Unaltered Group | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Max. | 75% | Median | 25% | Min. | Max. | 75% | Median | 25% | Min. | p-Value | ||
| Nectin-4 | Tumor mutation burden (nonsynonymous) | 748.4 | 300.6 | 15.4 | 2.1 | 0.9 | 38.7 | 16.4 | 2.4 | 1.5 | 0 | <0.0001 |
| MSIsensor Score | 23.17 | 9.49 | 0.97 | 0.37 | 0 | 26.4 | 10.65 | 0.41 | 0.09 | 0 | 0.0431 | |
| MSH2 | Tumor mutation burden (nonsynonymous) | 758.8 | 325.1 | 166.9 | 36.1 | 1.1 | 33.0 | 14.1 | 2.3 | 1.5 | 0 | <0.0001 |
| MSIsensor Score | 35.7 | 16.0 | 2.1 | 0.35 | 0.0 | 24.4 | 9.83 | 0.4 | 0.09 | 0 | <0.0001 | |
| MSH6 | Tumor mutation burden (nonsynonymous) | 763.9 | 325.1 | 192.3 | 32.6 | 1.1 | 27.1 | 11.7 | 2.2 | 1.5 | 0 | <0.0001 |
| MSIsensor Score | 34.8 | 14.3 | 2.6 | 0.7 | 0 | 22.8 | 9.1 | 0.3 | 0.0 | 0 | <0.0001 | |
| Clinicopathologic Variables | MSH2 Status | MSH6 Status | ||||
|---|---|---|---|---|---|---|
| Deficiency | Proficiency | p-Value | Deficiency | Proficiency | p-Value | |
| n = 32 | n = 75 | n = 35 | n = 63 | |||
| Age | 0.091 | 0.604 | ||||
| ≥50 | 20 (62.5%) | 58 (77.3%) | 25 (71.4%) | 48 (76.2%) | ||
| <50 | 12 (37.5%) | 17 (22.7%) | 10 (28.6%) | 15 (23.8%) | ||
| FIGO stage | 0.077 | 0.317 | ||||
| I/II | 23 (71.9%) | 54 (72.0%) | 23 (65.7%) | 50 (79.4%) | ||
| III/IV | 9 (28.1%) | 14 (18.7%) | 10 (28.6%) | 9 (14.3%) | ||
| Recurrence | 0 (0%) | 6 (8.0%) | 2 (5.7%) | 3 (4.8 %) | ||
| Unclassified | 0 (0%) | 1 (1.3 %) | 0 (0 %) | 1 (1.6%) | ||
| Differentiation | 0.580 | 0.335 | ||||
| Grade I | 10 (31.3%) | 28 (37.3%) | 11(31.4%) | 27 (42.9%) | ||
| Grade II/III | 21 (65.6%) | 42 (56.0%) | 21 (60.0 %) | 34 (54.0%) | ||
| Unclassified | 1 (3.1 %) | 5 (6.7%) | 3 (8.6 %) | 2 (3.2%) | ||
| CA125 (U/mL) | 0.493 | 0.739 | ||||
| ≥35 | 11 (34.4%) | 27 (36.0%) | 13 (37.1 %) | 20 (31.7%) | ||
| <35 | 20 (62.5%) | 41 (54.7%) | 20 (57.1%) | 37 (58.7%) | ||
| Unclassified | 1 (3.1%) | 7 (9.3%) | 2 (5.7%) | 6 (9.5%) | ||
| Lymphovascular invasion | 0.089 | 0.129 | ||||
| Absent | 19 (59.4%) | 41 (54.7%) | 20 (57.1%) | 37 (58.7%) | ||
| Present | 13 (40.6 %) | 24 (32.0 %) | 14 (40.0%) | 17 (27.0%) | ||
| Unclassified | 0 (0%) | 10 (13.3%) | 1 (2.9%) | 9 (14.3%) | ||
| Nectin-4 expression | 0.000 * | 0.001 * | ||||
| High | 16 (50%) | 63 (84.0%) | 19 (54.3%) | 54 (85.7%) | ||
| Low | 16 (50%) | 12 (16.0%) | 16 (45.7%) | 9 (14.3%) | ||
| Groups | LVSI-Positive % | LVSI-Negative % | Total | p-Value | |
|---|---|---|---|---|---|
| MSH2_deficient | Nectin-4High | 56.3% (9/16) | 43.8% (7/16) | 16 | 0.072 |
| Nectin-4Low | 25% (4/16) | 75% (12/16) | 16 | ||
| MSH2_proficient | Nectin-4High | 40.7% (22/54) | 59.3% (32/54) | 54 | 0.158 |
| Nectin-4Low | 18.2% (2/11) | 81.8% (9/11) | 11 | ||
| MSH6_deficient | Nectin-4High | 52.6% (10/19) | 47.4% (9/19) | 19 | 0.203 |
| Nectin-4Low | 31.3% (5/16) | 68.8% (11/16) | 16 | ||
| MSH6_proficient | Nectin-4High | 35.6% (16/45) | 64.4% (29/45) | 45 | 0.149 |
| Nectin-4Low | 11.1% (1/9) | 88.9% (8/9) | 9 | ||
| Clinicopathologic Variables | MSH2 Deficient | MSH2 Proficient | ||||
|---|---|---|---|---|---|---|
| Nectin-4High | Nectin-4Low | p-Value | Nectin-4High | Nectin-4Low | p-Value | |
| n = 16 | n = 16 | n = 57 | n = 11 | |||
| FIGO stage | 0.694 | 0.158 | ||||
| I/II | 11 (68.8%) | 12 (75.0%) | 47 (82.5%) | 7 (63.6%) | ||
| III/IV | 5 (31.3%) | 4 (25.0%) | 10 (17.5%) | 4 (36.4%) | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, H.K.; Park, Y.H.; Choi, J.-A.; Kim, J.W.; Kim, J.; Kim, H.S.; Lee, H.N.; Cho, H.; Chung, J.-Y.; Kim, J.-H. Nectin-4 as a Predictive Marker for Poor Prognosis of Endometrial Cancer with Mismatch Repair Impairment. Cancers 2023, 15, 2865. https://doi.org/10.3390/cancers15102865
Chang HK, Park YH, Choi J-A, Kim JW, Kim J, Kim HS, Lee HN, Cho H, Chung J-Y, Kim J-H. Nectin-4 as a Predictive Marker for Poor Prognosis of Endometrial Cancer with Mismatch Repair Impairment. Cancers. 2023; 15(10):2865. https://doi.org/10.3390/cancers15102865
Chicago/Turabian StyleChang, Ha Kyun, Young Hoon Park, Jung-A Choi, Jeong Won Kim, Jisup Kim, Hyo Sun Kim, Hae Nam Lee, Hanbyoul Cho, Joon-Yong Chung, and Jae-Hoon Kim. 2023. "Nectin-4 as a Predictive Marker for Poor Prognosis of Endometrial Cancer with Mismatch Repair Impairment" Cancers 15, no. 10: 2865. https://doi.org/10.3390/cancers15102865
APA StyleChang, H. K., Park, Y. H., Choi, J.-A., Kim, J. W., Kim, J., Kim, H. S., Lee, H. N., Cho, H., Chung, J.-Y., & Kim, J.-H. (2023). Nectin-4 as a Predictive Marker for Poor Prognosis of Endometrial Cancer with Mismatch Repair Impairment. Cancers, 15(10), 2865. https://doi.org/10.3390/cancers15102865









