1. Introduction
Cervical cancer is the fourth most common cancer among women worldwide, accounting for over 600,000 new cases and over 340,000 new deaths in 2020 [
1]. However, in much of Sub-Saharan Africa (SSA), which has high rates of HIV, it is the most common cancer among women [
1]. Malawi, a country in SSA with a HIV infection rate of 9.4% among women aged 15–49 years, has the world’s highest cervical cancer incidence and mortality rates [
1,
2].
Almost all cervical cancer is the result of an infection with high-risk human papillomavirus (hrHPV) [
3]. Therefore, primary prevention for cervical cancer can be achieved on a national level through national HPV vaccination, particularly among girls aged 9–14 years, before they are exposed to HPV through sexual activity [
4]. However, many countries only recently began rolling out HPV vaccination and as of June 2020, only 107 (55%) of the 194 World Health Organization (WHO) Member States were considered to have introduced HPV vaccination in their country [
5]. Therefore, secondary prevention of cervical cancer through screening with a high-performance test, followed by treatment for those who are screen-positive, is also critical to prevent cervical cancer [
6].
The WHO currently recommends using HPV detection as the primary screening test, rather than visual inspection with acetic acid (VIA) or cytology, starting at age 30 years for the general population of women and at age 25 years for women living with HIV (WLWH) [
6]. In some programs, women who screen HPV-positive undergo immediate treatment, known as the “HPV screen-and-treat” approach, where the decision to treat is based on the HPV test only. In contrast, other programs use the “HPV screen, triage and treat” approach, where the decision to treat an HPV-positive woman is based on the result of a positive second “triage” test, usually VIA [
6]. With either approach, ablative therapy can be performed on the same day among eligible women if the HPV test results can be given to the patient on the same day, minimizing loss-to-follow-up after screening. The GeneXpert
® HPV test (Cepheid Inc, Sunnyvale, CA, USA) is one such test that allows HPV results to be given on the same day because samples are non-batched and each take only 1–2 h to complete [
7].
HPV self-collection is an HPV-based screening strategy that is increasingly used to improve access to cervical cancer screening in at least 27 countries around the world, including both high-income and low-and-middle-income countries (LMIC) across six continents [
8]. This strategy is endorsed by the WHO, which notes that “HPV self-collection should be made available as an additional approach to sampling in cervical cancer screening services for individuals aged 30–60 years” [
9]. HPV self-collection is highly acceptable among women, regardless of age, income, or country of residence [
10], and is similarly accurate as provider-collected samples when highly sensitive hrHPV assays are used [
11].
Therefore, we designed a cluster randomized feasibility trial of two different implementation models for integrating HPV self-collection into family planning (FP) services in Malawi. Model 1 involved only clinic-based HPV self-collection, whereas Model 2 included both clinic-based and community-based HPV self-collection. Our primary hypothesis was that the Model 2 facilities would have a higher proportion of eligible women undergo cervical cancer screening (CCS) in their catchment areas during our study than in the catchment areas of the Model 1 facilities [
12]. A secondary hypothesis was that the Model 2 facilities would have a higher proportion of eligible women who received FP services in their catchment areas during the study than in the catchment areas of the Model 1 facilities.
2. Materials and Methods
2.1. Study Design and Population
This study was a hybrid type 2 cluster randomized feasibility trial [
12], with 16 health facilities from two districts in Malawi (Lilongwe and Zomba Districts) assigned to either Model 1 or Model 2 (
Table 1). In June 2019, we began working with the Malawi Ministry of Health (MoH) to modify existing MoH registers and documents for facility cervical cancer screening and preventive therapy (CCSPT), facility FP, and community FP services. We also worked with the Lilongwe and Zomba District Health Management Teams to determine which eight facilities in each of their districts should be included in the study, based on their needs and practical constraints, such as if it had a lab, and if so, if it had sufficient space to store and operate a GeneXpert machine. To evaluate implementation of our models at multiple types of facilities, each district was asked to identify one central or district hospital, one Christian Health Association of Malawi (CHAM) hospital, two Urban Health Centers, and four rural health facilities for inclusion in the study.
In November and December 2019, we conducted educational talks in the catchment areas of the 16 health facilities and held stakeholder-engagement meetings with community leaders and heath-facility staff so that they were aware of our study activities. During this time period, we purchased GeneXpert® machines for each of the facilities and installed solar panels and back-up uninterruptable power supply (UPS) systems for each machine since power outages in Malawian facilities are common. We provided training for lab technicians from our study facilities on how to perform HPV testing on the GeneXpert® machines, as well as on our study protocol and related documents. We also provided training for providers (medical officers, clinical officers, and nurses) from our study facilities in the Malawi MoH’s one-week VIA and thermal ablation course, followed by a one-day training on our HPV self-collection algorithm and procedures.
For facilities randomized to Model 2, we also trained their health surveillance assistants (HSAs) on how to offer HPV self-collection in the communities. HSAs are a cadre of Malawi’s government-employed community-health workers who are assigned to provide primary health services to specific villages that are located 5 km or more from the health facility at which they are based [
13]. HSAs typically provide services to a population of ~1200 people by visiting these communities at least once a month through outreach clinics (typically held in a local school or other public buildings with nurses from the facilities), village clinics (typically held in the HSA’s home without nurses), and occasionally, home visits. In addition to other health services, HSAs can provide the depot medroxyprogesterone acetate (DMPA) injectable, oral contraceptives, and condoms in the community if they undergo the Malawi MoH’s five-day FP training for HSAs. Since one of our project’s objectives was to improve FP access, we also provided this FP training to Model 2 HSAs who had never completed it.
Our HPV self-collection strategy was integrated into FP services at the facility-level by providing educational talks to women in the FP clinic waiting room about cervical cancer, the importance of screening for it, and the option to undergo HPV self-collection at the clinic while they waited to access FP and other services. Women who were eligible for cervical cancer screening were then offered HPV self-collection kits, which included a Viba-Brush® (Rovers Medical Devices, Amsterdam, The Netherlands) and an empty tube labeled with their name, enclosed within a plastic zipper bag. Eligibility criteria for cervical cancer screening were: (1) age 25–49 years; (2) no prior total hysterectomy, (3) no history of cervical cancer, and (4) not pregnant.
Women interested in undergoing HPV self-collection were given instructions and a pictorial guide (please see
File S1) on how to use the self-collection kit, which included 10 steps: (1) making sure that they had the brush and a small tube labeled with their name; (2) finding a private place to collect the sample and washing their hands with soap and water; (3) removing the brush from its envelope and trying not to touch the white brush tip with their hands; (4) standing, sitting, lying down, or squatting with their legs apart; (5) holding the lips of the vagina open with one hand and placing the brush into the vagina with the other hand until slight pressure was felt; (6) gently turning the brush around five times while in the vagina and then slowly pulling out the brush from the vagina; (7) holding the brush and removing the cap from the labeled tube; (8) pushing the clear plastic on the handle down so that the white brush tip falls into the tube and throwing away the blue handle; (9) capping the tube with the brush inside, screwing its lid on tightly, washing their hands again, and placing the tube in the bio-hazard bag provided; and (10) returning the plastic bag with the tube/brush to the provider.
Once a woman completed HPV self-collection, she was encouraged to wait to receive her HPV test results if possible, so that she could undergo treatment on the same day if her HPV test was positive. The tubes were transported to the lab for the woman’s facility, where HPV testing was performed on them as per GeneXpert
® instructions, on the same day whenever possible. We used the HPV-and-treat strategy [
6], i.e., if a women tested HPV-positive, she was offered VIA, and if she did not have cervical lesions or lesions ineligible for ablative therapy, she would undergo thermal ablation immediately after VIA if she consented to it. Women who underwent thermal ablation were advised to return for follow-up cervical cancer screening in one year. Eligibility criteria for thermal ablation treatment were: (1) no cervical lesions or lesions that can be seen in their entirety and do not cover >75% of the cervix; (2) no lesions that extended into the endocervix or to the vaginal wall; (3) visible squamocolumnar junction; (4) not currently pregnant or <3 months from delivery; (5) no concern for cervical cancer on exam; (6) no polyps or other cervical lesions that would prevent the probe from contacting the cervix; (7) not currently menstruating; and (8) no concern for cervicitis.
For facilities randomized to Model 2, we also integrated HPV self-collection into the HSAs’ community-based clinics, so that they would offer both FP services and HPV self-collection to eligible women in the community. The HSAs would bring HPV self-collection kits into the community and then return the collected tubes/brushes within seven days of self-collection to their facility, where HPV testing would be performed on the samples. Women who underwent HPV self-collection in the community were given the option to go to their local health facility to get their HPV results or have the HSA bring the result to their next monthly visit to their village. Women who received an HPV-positive result were referred to the facility for VIA and treatment upon indication. For further details about other study components that will be analyzed in separate manuscripts, please refer to the study protocol paper [
12].
2.2. Study Outcomes and Variables
Our primary and secondary outcomes were originally as follows: (a) proportion of eligible women aged 25–50 years who received CCS during project implementation (Primary Outcome); (b) proportion of treatment-eligible women who received thermocoagulation during project implementation (Secondary Outcome #1); and (c) proportion of women aged 15–50 years using a modern FP method (Secondary Outcome #2).
However, due to the difficulty that participants had with remembering the approximate date of their last CCS, we were not able to calculate the proportions of women who received CCS during study implementation for our primary outcome. Since women were able to recall if they had ever been screened for CCS, we used this response as our primary outcome instead.
The study outcomes were evaluated through an endline household survey (EHS), which was performed between October to December 2021, approximately 12 months after all facilities had begun implementing our study. The EHS was administered to women living in the catchment areas of all 16 facilities except for Zomba Central Hospital because its catchment area encompassed other districts in southern Malawi not included in our study. Inclusion criteria for the EHS were: (1) women between the ages of 15–50 years, (2) no history of total hysterectomy prior the initial study implementation in March 2020, and (3) ability to give informed consent in English or Chichewa (the local language).
The EHS included the following study variables: basic demographics, reproductive health history, HIV status, distance to the nearest health facility, prior CCS (Primary Outcome), CCS since March 2020, ever use of HPV self-collection, ever receipt of thermal ablation (Secondary Outcome #1), prior FP use, FP method use since March 2020, and current FP method use (Secondary Outcome #2). In Malawi, all medical information is recorded in an individual’s health passport. If a participant had her health passport available and was willing to allow the study research assistant to review it, her FP-method use and prior CCSPT responses were verified with the health passport.
2.3. Study Sampling for the Endline Household Survey
The target sample size was 8000 women from 400 villages selected proportionally from the 16 facility catchment areas. To develop our sampling strategy for the EHS, village data and enumeration maps for the 16 facility catchment areas were gathered from Malawi National Statistics Office, the Lilongwe and Zomba District Health Offices, and the HSAs from each facility. A two-stage sampling plan was then followed. For Stage 1, our biostatistician (MC) selected a random sample of 400 villages to be interviewed from each facility’s catchment area, which was proportional to the size of the catchment area of each facility. Household lists for each randomly sampled village were then obtained from their HSAs and village leaders. If a village could not be interviewed (because it was found to not actually be in the catchment area of the facility, it no longer existed and/or had merged with another village, it was inaccessible because it required a boat to reach, or because its leaders did not allow us to interview there), a village was replaced with another similar village nearby.
Then, for Stage 2, our EHS research assistants (RAs) randomly selected 20 households per village to interview. Specifically, on each day of data collection, teams of two RAs arrived at their assigned village and verified the household list with the village HSA. A sampling fraction (h) was calculated by dividing the total number of households in the village by the number of households to survey (n = 20). The first household was identified by selecting a number between 1 and h. Random number selection was done in the field by writing numbers on pieces of paper, folding them up, placing them in a container and mixing before drawing one out at random. The second household to sample was determined by the initial number + h and sampling was proceeded in this manner with every hth household being sampled. When individual(s) in selected households were unavailable or not eligible to participate, that household is replaced with the next household in the direction of travel. HSAs were compensated for their field assistance at the equivalent of about USD$5.
At each selected household, all potential participants were counseled about the purpose of the study and its procedures. Those interested were screened for eligibility and if eligible, two consent forms were completed for each participant, one of which was given to the participant. Study staff read the informed consent form aloud and assessed potential participants’ comprehension of the study throughout the consent process by asking questions to gauge understanding of the study. Consents were then signed by the participant and RA if the participants still wanted to enroll. Illiterate participants used their fingerprint as their signature and had another person not involved in the study sign the consent as their witness. Parental consents and pediatric assents were obtained for participants aged 14–17 years of age, who were included so that we could evaluate their FP use. Participants received an equivalent of about USD$2 for completing the survey.
Surveys were conducted by RAs and entered directly into electronic tablets using Open Data Kit (ODK) Aggregate software (version 2.0). Data collected were uploaded from the tablet to a secure and password-protected UNC Project-Malawi ODK server and study ODK database daily in real-time by the RAs and cross-checked by our data managers for accuracy and completeness. Data from the ODK database were exported to Stata for data cleaning and analysis. The ODK tablets, ODK database, and Stata database only include de-identified data and can only be accessed by study staff members.
Prior to EHS data collection, two community sensitization strategies were employed. First, village leaders from the selected villages were invited for an information session on the purpose and procedure of EHS, with the plan that the leaders would then sensitize their villages. Travel costs to information sessions were reimbursed. Second, HSAs conducted sensitization in the villages prior to the team arriving in each village. These measures helped to ensure that the community was aware and welcoming of the RAs.
2.4. Study Period
Implementation of this study was originally planned to occur over 12 months (March 2020–February 2021). However, this time period was ultimately extended to 22 months because the study had to pause activities in April 2020 due to COVID-19 restrictions implemented by the Malawi MoH, after study implementation had started at six health facilities (Chileka, Kabudula, and Nkhoma in Lilongwe District, and Matawale, Namasalima, and St. Luke’s in Zomba District). Three of these facilities were assigned to Model 1 (St. Luke’s, Kabudula, and Namasalima), and the other three (Chileka, Matawale, and Nkhoma) to Model 2. During this COVID-19 pause, the six facilities were asked to stop community-based screening (if randomized to Model 2) and revert back to VIA screening at the facility; however, a few facilities still continued to offer HPV self-collection and thermal ablation when possible. We restarted implementation of the study in August 2020 when COVID-19 restricted were eased. We conducted refresher trainings in the study procedures at all 16 health facilities, first at the six facilities that had already started implementation in August 2020. By November 2020, all facilities were implementing study activities.
2.5. Study Randomization
The 16 selected facilities were each assigned a code and randomized 1:1 by our study biostatistician (MC) within each of the four strata (
Table 1) using the codes [
12]. This randomization resulted in four facilities from each district assigned to Model 1 and the other four facilities to Model 2. However, after randomization, we learned that neither facility in the first strata (Bwaila District Hospital and Zomba Central Hospital) could implement Model 2 because their HSAs were based at the hospital and did not have the capacity to perform community-based work. Therefore, they were dropped from the analysis for this manuscript, but kept in the study so that we could evaluate our implementation outcomes (acceptability, feasibility, etc.) at their sites for other study-related analyses. Of the 14 remaining facilities for this analysis, seven were assigned to Model 1 (three in Lilongwe, four in Zomba) and seven to Model 2 (four in Lilongwe, three in Zomba).
2.6. Power Calculations and Statistical Analyses
A study performed between 2011–2015 found that only 26.5% of Malawian women had ever undergone VIA for primary cervical cancer screening [
14]. We estimated that with the introduction of clinic-based HPV self-collection in Model 1, at least 40% of eligible women in its catchment areas could be screened during study implementation. We then estimated that with the addition of community-based HPV self-collection to reach unscreened women, we could approach Malawi’s target rate of 80% of eligible women ever screened in Model 2 [
15]. We collected data from potential facilities in Lilongwe and Zomba Districts and were able to estimate an average cluster size of 8000 women eligible for cervical cancer screening per health facility, with a coefficient of variation of the cluster sizes of 0.35. To estimate our intra-cluster correlation coefficient (ICC), we reviewed other similar studies from Malawi and found that their ICC ranged from 0.004–0.20 [
12,
16,
17]. We therefore estimated that our ICC would range from 0.12 to 0.19.
We initially calculated ed that with 16 randomized facilities (8 per Model), we would have at least 80% power to detect a difference of at least 25–30% between our 2 Models, even if the proportion of women screened in Model 1 was as low as 40%. For example, if Model 1 screened 40% of eligible women, there would be 80% power to detect a difference if Model 2 screened at least 70% of women if the ICC was 0.18. If the ICC was 0.12, we would have 80% power to detect a 25% difference between Model 1 and Model 2 if Model 1 screened 40% of women.
However, after learning that Bwaila District Hospital and Zomba Central Hospital could not be assigned to Model 2, we dropped them from our sample size calculations, which decreased our average cluster size to 3400 eligible women. Assuming an ICC of 0.15, a coefficient of variation of cluster sizes of 0.35, and seven randomized facilities per Model, we would have at least 83% power to detect a difference of at least 30% between our 2 Models, even if the proportion of women screened in Model 1 was as low as 40%. All power calculations were calculated in R (Version 3.5.1, clusterPower package, Vienna, Austria).
The weighted proportions for the primary and secondary outcomes were based on the probability of selecting a village among villages in a facility catchment area using simple random sampling and selecting a household within a selected village using simple random sampling to account for the two-stage sampling method used in selecting the households. Our weights were therefore the inverse of the joint probability of selecting a village within a facility catchment area and selecting a household within a village.
The effect of the Model 1 compared to the Model 2 was assessed via logistic regression, adjusted for district, facility size, and facility location. The regression model accounted for health facility clustering by stratification, and the small number of clusters using the weights. Because of the small numbers for secondary outcome #1 (treatment-eligible women who received thermocoagulation), logistic regression was not performed for that comparison.
3. Results
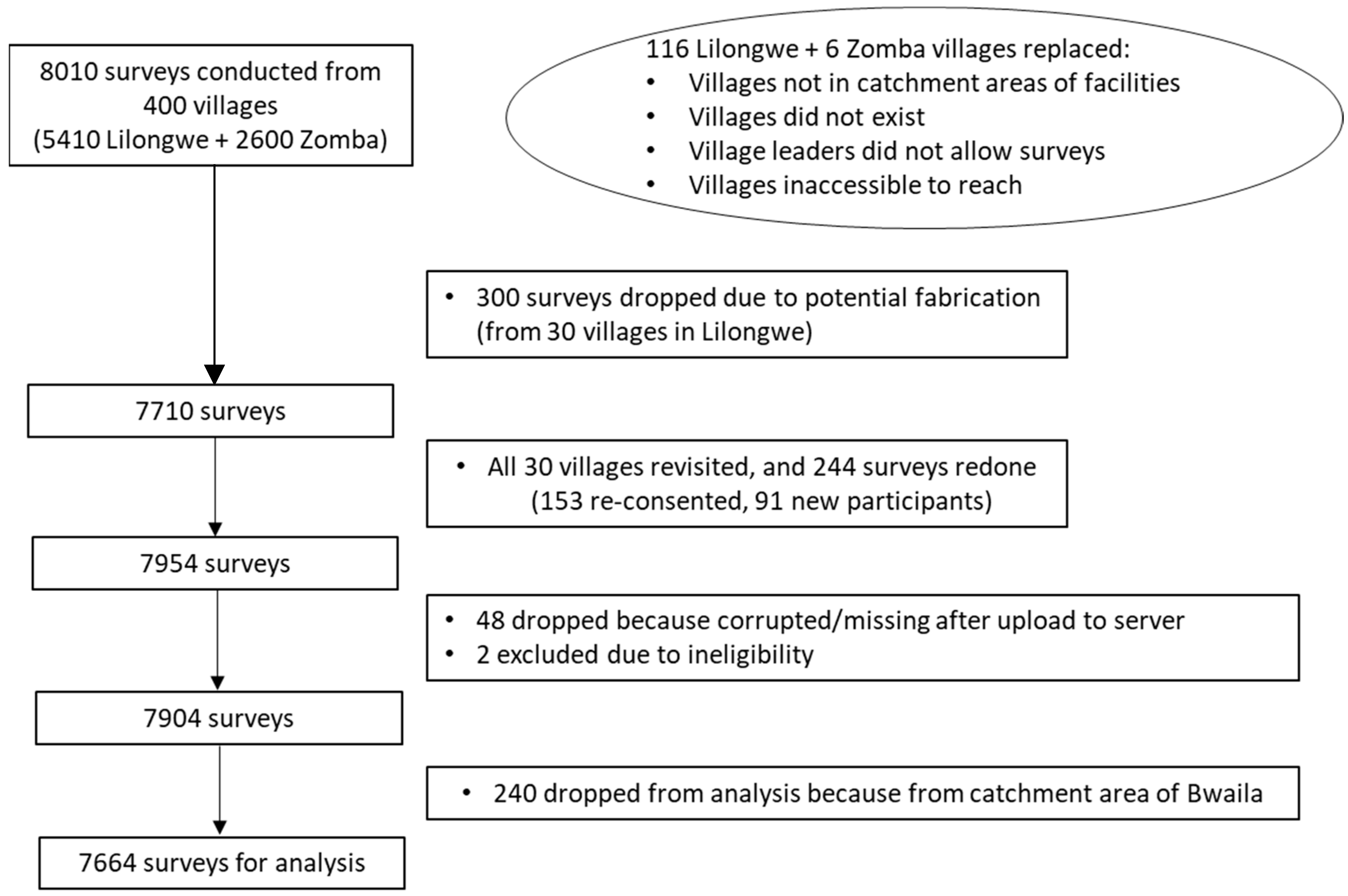
Of the 400 villages initially selected for interview, 116 villages in Lilongwe and six villages in Zomba had to be replaced once surveying was initiated (
Figure 1). Twenty surveys were conducted in each village, with the exception of one village in Lilongwe, which had 30 surveys completed due to field error (Chinthankhwa village in Chiwamba), resulting in 8010 surveys in total. Towards the end of data collection, it was discovered that a RA in Lilongwe had not collected data as per study procedures. Therefore, the study team dropped all data collected from this RA, which included 300 surveys from 30 villages in Lilongwe district. Repeat sampling of these 30 villages was conducted, and we collected 244 replacement surveys, resulting in a total of 7954 surveys. Upon data cleaning, 48 surveys were noted to be corrupted or missing after upload, and two surveys were excluded due to ineligibility discovered after data collection (participants had hysterectomies prior to program start), resulting in 7904 surveys. Because Bwaila Hospital could not be randomized, the 240 surveys from its catchment area were not included in this analysis, resulting in a total of 7664 surveys for analysis.
3.1. Respondent Characteristics for the Endline Household Survey
Respondents of the EHS were nearly evenly split between the three age groups of 15–24 years, 25–34 years, and 35–50 years (
Table 2). Most (91.8%) had attended some school and been pregnant (93.0%), and 34.1% had only a grass roof on their house, indicating low socioeconomic status. Very few (1.9%) had ever smoked tobacco, and 9.5% were known to be HIV-infected. The only sociodemographic differences between respondents from the catchment areas of Models 1 and 2 were that Model 1 participants were more likely to be single/never married (13.7% vs. 7.2%,
p = 0.029) and were able to get to the nearest health facility in less than 1 h (41.7% vs. 26.5%,
p = 0.049).
3.2. Endline Household Survey Responses for Cervical Cancer Screening (Primary Outcome) and Thermocoagulation Treatment (Secondary Outcome #1)
During our survey, only the 5138 respondents who were at least 25 years of age were asked questions about cervical cancer screening services, since Malawi MoH guidelines do not recommend such screening until that age (
Table 3). We found that 33.1% of respondents in Model 1 had ever been screened for cervical cancer (Primary Outcome), compared to 42.5%, but this difference was not significant (
p = 0.096).
A higher percentage of respondents in Model 1 (83.5%) remembered the approximate date when they were last screened for cervical cancer than Model 2 (67.4%, p = 0.046), which is notable because only those who recalled this date (n = 1185) were asked if they had undergone self-collection for cervical cancer since March 2020. Of the 1185 respondents, a lower proportion of 22.8% in Model 1 (n = 141) versus 50.5% of respondents in Model 2 (n = 471) said that they had underdone self-collection for cervical cancer (p = 0.023) and likely performed through our study as no other programs were known to be offering HPV self-collection in these facility catchment areas during the study implementation.
Of the 612 who underwent self-collection, 29.7% in Model 1 versus 21.6% in Model 2 said that they had undergone VIA (p = 0.284), and of the 118 who said that they had undergone VIA, 71.9% versus 57.1% (p = 0.517) said they also received treatment after VIA (Secondary Outcome #1) for Models 1 and 2, respectively.
The ICC for those participants who responded to the question about ever being screened for cervical cancer was 0.080 (95% CI 0.039–0.158) at the facility level. The responses about ever being screened for cervical cancer by facility catchment area are presented in
Table A1.
3.3. Endline Household Survey Responses for Family Planning Use, Including Modern Family Planning Use (Secondary Outcome #2)
When asked about FP services, 6448 (83.1%) responded that they had ever used such services, which was not different between the 2 Models (
Table 4). Of the 6448 who had ever used FP services, 60.6% (n = 3787) had used a modern FP method since our study was implemented in March 2020, but the difference between models was not significant (58.6% vs. 62.4%,
p = 0.297). However, when asked which method they were currently using as their primary FP method (n = 3791; Secondary Outcome #2), Model 1 respondents were significantly less likely to report using a method that is considered a modern FP method (89.0%) than Model 2 respondents (96.5%,
p = 0.034), which suggests that there was confusion about the question regarding modern FP-method use since March 2020.
Among those who said that they had used a modern FP method since March 2020, 34.4% (Model 1: 32.0% vs. Model 2: 36.5%, p = 0.287) responded that it was the first time that they had used this method, and 94.2% (Model 1: 94.1% vs. Model 2: 94.3%) said that they received the method they wanted to receive. Over half (56.2%) responded that they were advised to seek a cervical cancer screening service during a FP visit (Model 1: 55.7% vs. Model 2: 56.7%, p = 0.906).
The ICC for those participants who responded to the question about their primary FP method was 0.0032 (95% CI 0.0007–0.0136) at the facility level. The current use of modern FP method by facility catchment area is presented in
Table A2.
3.4. Adjusted Results from the Endline Household Survey
For our adjusted analyses, we evaluated the effect of district, facility type (urban versus rural), and facility size (hospital versus health center) on the proportion of eligible women aged 25–50 years who had ever received CCS (
Table 5) and who were currently using a modern FP method (
Table 6).
Participants from Zomba district were more likely to have ever received CCS (0.507; 95% CI 0.445, 0.568; p < 0.001) than participants from Lilongwe District (0.311; 95% CI 0.273, 0.352), but there was no difference by closest facility type or size. Participants whose closest health facility was a hospital were more likely to be using a modern FP method (0.769; 95% CI 0.725, 0.808) than those whose closest health facility was only a health center (0.696; 95% CI 0.651, 0.738; p = 0.019), but there was no difference by district or facility type.
After adjusting for district, facility type, and facility size (
Table 7), we found that women in Model 2 were more likely to have ever received CCS than in Model 1 (adjusted OR 1.73; 95% CI 1.29, 2.33). Women in Model 2 were also more likely to be using a modern FP method (adjusted OR 1.41; 95% CI 1.01, 1.98).
4. Discussion
In our study population, the integration of HPV-based CCS with FP services at both the clinic and community levels (Model 2) led to a significantly higher proportion of eligible women ever being screened for CCS and to be currently using a modern FP method, than when these services were only integrated at the clinic level (Model 1). A significantly higher proportion of women were screened for CCS through HPV self-collection in Model 2 than in Model 1 during the study implementation. We believe that the higher proportion of women who performed HPV self-collection in Model 2 is attributable to the community-based screening performed through our project since no other programs were implementing HPV self-collection in the catchment areas of the 14 facilities surveyed.
We were surprised that the proportion of women ever screened for CCS in the Model 1 facility catchment areas was only 33.1%, since a study between 2011–2015 found that 26.5% of Malawian women had been screened at least once in their lifetime by VIA [
14]. We hypothesized that we could screen 40% of eligible women in Model 1 during the study, but that was a gross overestimate. However, we still found a significant difference between models for the proportion ever screened because the proportion was so low in Model 1, and our ICC was lower than expected.
The low proportion of women ever screened in Model 1 could be due to differences in how the Malawi MoH calculated their estimates for screening [
18], a population which was more under-screened in our EHS than in their estimates, the failure to reach previously unscreened patients in Model 1, and/or misunderstanding of our survey question. In Model 2, we found that 42.5% of eligible EHS participants had ever been screened for CCS, suggesting that even if we failed to screen many new women in Model 1, we significantly increased the proportion of women ever screened in Model 2 by bringing HPV self-collection into the community.
One major related study limitation is that we did not perform a baseline household survey prior to project implementation due to timeline and financial constraints. We thought we could calculate the proportion of women screened through our study in our EHS by first asking participants if they had ever been screened for cervical cancer, then asking them if they had been screened via self-collection since our project started (in March 2020), and finally calculating the difference between those responses. However, only 66% of the EHS respondents ever-screened recalled the last date of their CCS, and only these respondents were asked about self-collection.
Therefore, we could not determine if those who did not recall their last screening date were screened through our program. In addition, some facilities reverted to VIA-based screening during the COVID-19 study pause, when they had no electricity and our solar UPS back-up systems failed (which occurred frequently, particularly at Chiwamba), and/or when we ran out of HPV self-collection kit supplies or cartridges (which we had difficulty procuring because of production and shipping delays). Those screened primarily with VIA through our study were not well-captured in our EHS, so we do not know the exact proportion of women screened by either VIA or HPV through our study and whether they received the treatment they needed if they screened positive. Finally, the data collected in the EHS was mostly by self-report and therefore subject to social desirability bias and recall bias, although the research assistants tried to verify responses in the health passport when possible.
The strengths of our study included: our cluster randomized design; implementation of our models in health facilities of different sizes, locations, and types; and strong partnership with the Malawi MoH and the district health management teams of the two districts, which enabled us to implement our project successfully, despite the many challenges that the COVID-19 pandemic brought upon our teams.
Prior studies have found that the integration of FP service provision with CCS leads to increased uptake of both services. A review published in 2017 identified six countries in SSA (Kenya, Nigeria, Tanzania, Uganda, Zambia, and Zimbabwe) that were integrating CCS with FP, although all were using VIA for primary screening and cryotherapy for treatment at that time [
19]. The review highlighted that after CCSPT was introduced in Uganda, uptake for both intrauterine devices and implants increased three-fold and half of those who received any contraceptive were also screened for cervical cancer. A few other studies about FP-CCS integration have been published since, including articles from Guinea, Cameroon, and India [
20,
21,
22], all of which found the integration to be feasible and acceptable, although data has been limited on its cost-effectiveness [
21]. The study from India focused on sex workers and was the only one that used a community-based approach, although their approach differed from ours in that VIA was used for screening [
22].
Another study from Malawi found that integrating FP and CCS into HIV care is acceptable and increased uptake of both FP and CCS among women living with HIV [
23]. It also found that their integrated services required minimal additional resources over those needed for HIV care alone and that patient flow improved. Unlike our study, it used VIA as its primary screening method. Our team is currently performing budget and cost analyses to evaluate whether the additional resources needed to perform HPV-based screening are offset by the increased sensitivity and specificity for high grade cervical dysplasia when compared to VIA-based screening. Our team has already found that the HSAs in Model 2 spent 75% more time on FP services after CCS integration through our study when compared to pre-implementation, with no significant decrease on time devoted towards other activities [
24]. Additional analyses are being performed to compare the acceptability, feasibility, and appropriateness of our two models, both for health-facility staff and women who underwent self-collection, and they will explore potential social behavior changes at the facility and community levels.