Unveiling the Power of Anticancer Drug Screening: A Clinical Case Study Comparing the Effectiveness of Hollow Fiber Assay Microtube Array Membrane (MTAM-HFA) in Breast Cancer Patients
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Inclusion Criteria
2.2. Study Design
2.3. Acquisition of the Primary Breast Cancer Tissue
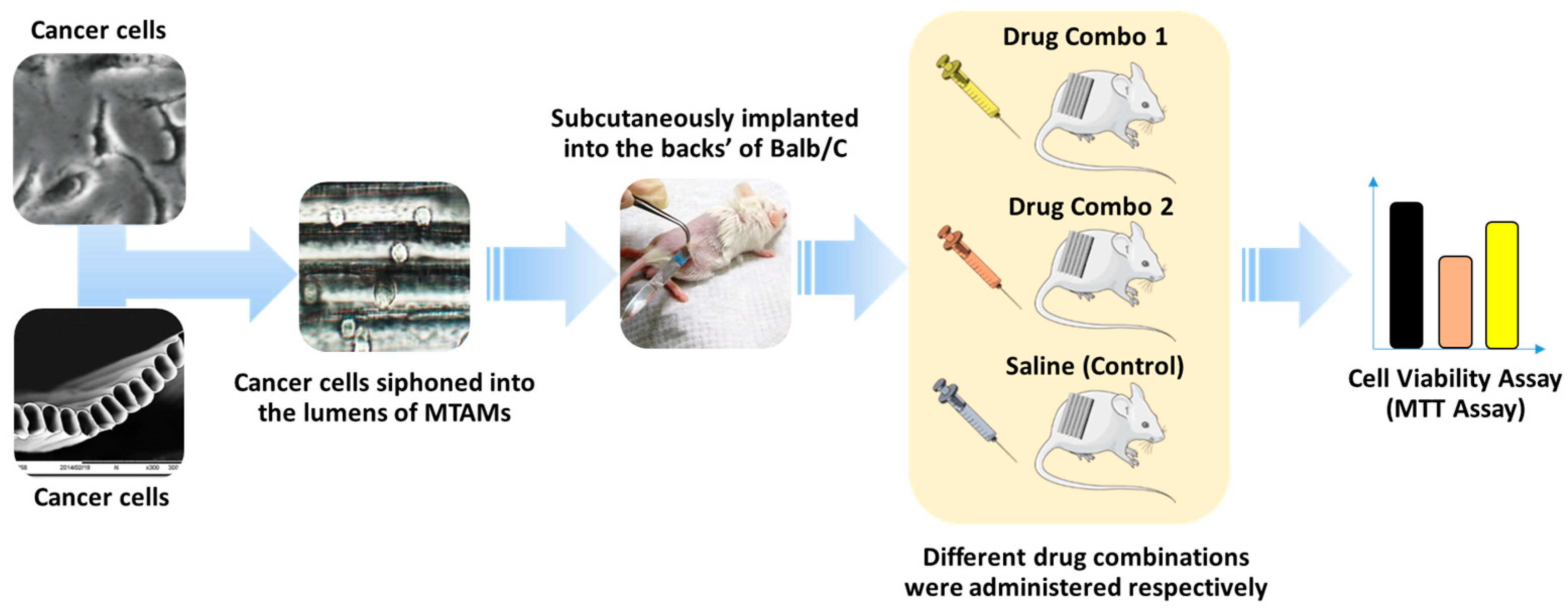
2.4. Microtube Array Membrane-Hollow Fiber Assay (MTAM-HFA)
2.4.1. Primary Breast Cancer Tissue (PBCT) Tissue Preparation
2.4.2. Microtube Array Membrane-Hollow Fiber Assay Screening
2.5. Response Evaluation Criteria in Solid Tumors (RECIST)
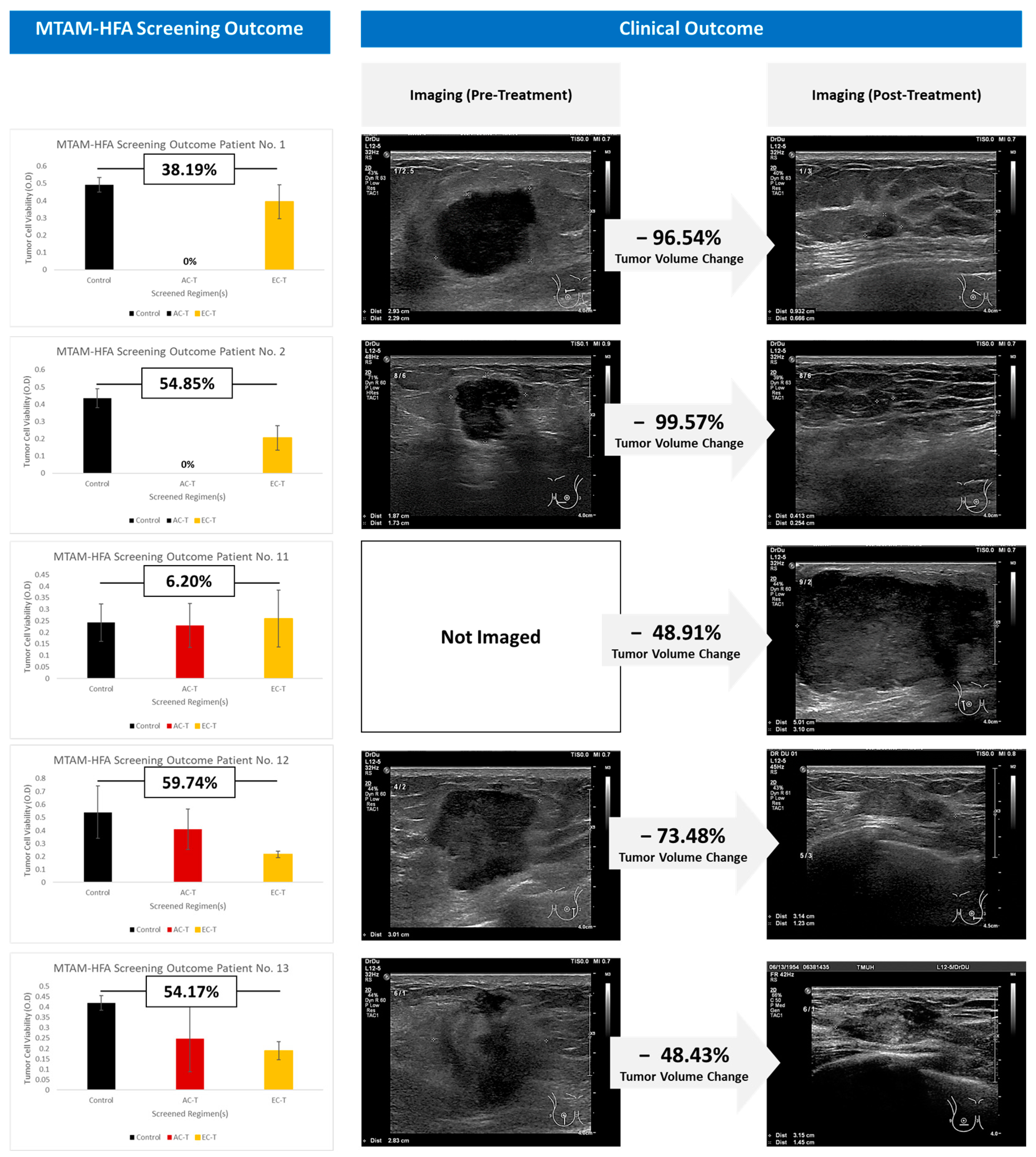
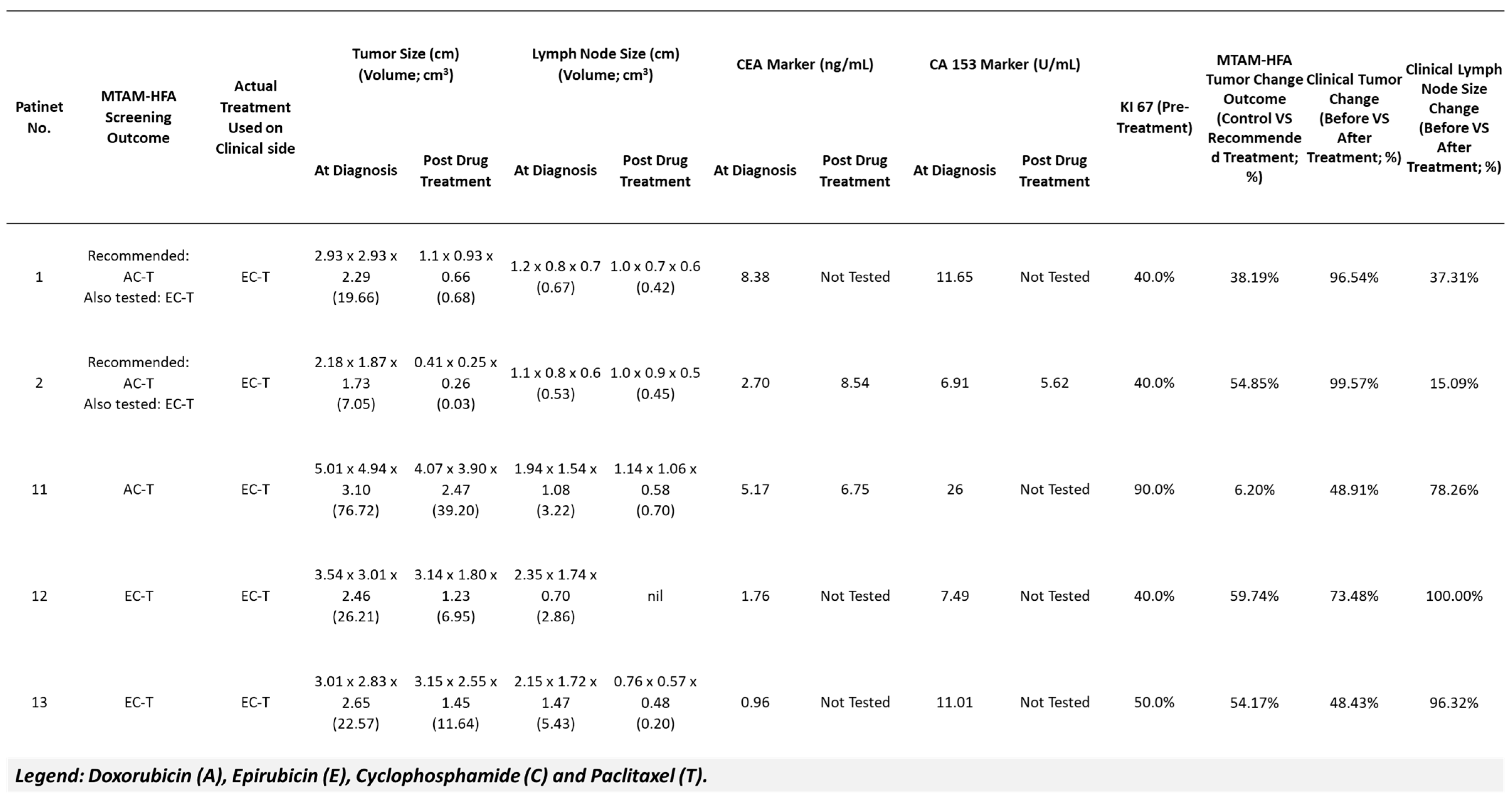
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ahmad, A. Breast cancer statistics: Recent trends. In Breast Cancer Metastasis and Drug Resistance: Challenges and Progress; Springer: Cham, Switzerland, 2019; pp. 1–7. [Google Scholar]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Azamjah, N.; Soltan-Zadeh, Y.; Zayeri, F. Global trend of breast cancer mortality rate: A 25-year study. Asian Pac. J. Cancer Prev. APJCP 2019, 20, 2015. [Google Scholar] [CrossRef] [PubMed]
- Dibden, A.; Offman, J.; Duffy, S.W.; Gabe, R. Worldwide review and meta-analysis of cohort studies measuring the effect of mammography screening programmes on incidence-based breast cancer mortality. Cancers 2020, 12, 976. [Google Scholar] [CrossRef] [PubMed]
- Trewin-Nybråten, C.B. Changing Socioeconomic Patterns of Breast Cancer Incidence, Mortality and Survival in Norway. 2023. Available online: https://www.duo.uio.no/handle/10852/100408 (accessed on 5 March 2023).
- Chen, M.-T.; Sun, H.-F.; Zhao, Y.; Fu, W.-Y.; Yang, L.-P.; Gao, S.-P.; Li, L.-D.; Jiang, H.-L.; Jin, W. Comparison of patterns and prognosis among distant metastatic breast cancer patients by age groups: A SEER population-based analysis. Sci. Rep. 2017, 7, 1–8. [Google Scholar] [CrossRef]
- Hong, J.; Tong, Y.; He, J.; Chen, X.; Shen, K. Association between tumor molecular subtype, clinical stage and axillary pathological response in breast cancer patients undergoing complete pathological remission after neoadjuvant chemotherapy: Potential implications for de-escalation of axillary surgery. Ther. Adv. Med. Oncol. 2021, 13, 1758835921996673. [Google Scholar] [CrossRef]
- Cho, N. Molecular subtypes and imaging phenotypes of breast cancer. Ultrasonography 2016, 35, 281. [Google Scholar] [CrossRef]
- Kim, Y.-J.; Kim, J.-S.; Kim, I.A. Molecular subtype predicts incidence and prognosis of brain metastasis from breast cancer in SEER database. J. Cancer Res. Clin. Oncol. 2018, 144, 1803–1816. [Google Scholar] [CrossRef]
- Łukasiewicz, S.; Czeczelewski, M.; Forma, A.; Baj, J.; Sitarz, R.; Stanisławek, A. Breast cancer—Epidemiology, risk factors, classification, prognostic markers, and current treatment strategies—An updated review. Cancers 2021, 13, 4287. [Google Scholar] [CrossRef]
- Li, L.; Yuan, L.; Chen, X.; Wang, Q.; Tian, J.; Yang, K.; Zhou, E. Current treatments for breast cancer-related lymphoedema: A systematic review. Asian Pac. J. Cancer Prev. APJCP 2016, 17, 4875. [Google Scholar]
- Rossi, L.; Mazzara, C.; Pagani, O. Diagnosis and treatment of breast cancer in young women. Curr. Treat. Options Oncol. 2019, 20, 86. [Google Scholar] [CrossRef]
- Maruthanila, V.; Elancheran, R.; Kunnumakkara, A.; Kabilan, S.; Kotoky, J. Recent development of targeted approaches for the treatment of breast cancer. Breast Cancer 2017, 24, 191–219. [Google Scholar] [CrossRef]
- Bianchini, G.; Balko, J.M.; Mayer, I.A.; Sanders, M.E.; Gianni, L. Triple-negative breast cancer: Challenges and opportunities of a heterogeneous disease. Nat. Rev. Clin. Oncol. 2016, 13, 674–690. [Google Scholar] [CrossRef] [PubMed]
- Yin, L.; Duan, J.-J.; Bian, X.-W.; Yu, S.-C. Triple-negative breast cancer molecular subtyping and treatment progress. Breast Cancer Res. 2020, 22, 61. [Google Scholar] [CrossRef] [PubMed]
- Chang-Qing, Y.; Jie, L.; Shi-Qi, Z.; Kun, Z.; Zi-Qian, G.; Ran, X.; Hui-Meng, L.; Ren-Bin, Z.; Gang, Z.; Da-Chuan, Y. Recent treatment progress of triple negative breast cancer. Prog. Biophys. Mol. Biol. 2020, 151, 40–53. [Google Scholar] [CrossRef]
- Rosenbaum, J.N.; Weisman, P. The evolving role of companion diagnostics for breast cancer in an era of next-generation omics. Am. J. Pathol. 2017, 187, 2185–2198. [Google Scholar] [CrossRef] [PubMed]
- Coleman, W.B. Next-generation breast cancer omics. Am. J. Pathol. 2017, 187, 2130–2132. [Google Scholar] [CrossRef]
- Chakravarthi, B.V.; Nepal, S.; Varambally, S. Genomic and epigenomic alterations in cancer. Am. J. Pathol. 2016, 186, 1724–1735. [Google Scholar] [CrossRef]
- Xiao, Y.; Ma, D.; Yang, Y.-S.; Yang, F.; Ding, J.-H.; Gong, Y.; Jiang, L.; Ge, L.-P.; Wu, S.-Y.; Yu, Q. Comprehensive metabolomics expands precision medicine for triple-negative breast cancer. Cell Res. 2022, 32, 477–490. [Google Scholar] [CrossRef]
- Brandão, M.; Pondé, N.; Piccart-Gebhart, M. Mammaprint™: A comprehensive review. Future Oncol. 2019, 15, 207–224. [Google Scholar] [CrossRef]
- Mouttet, D.; Laé, M.; Caly, M.; Gentien, D.; Carpentier, S.; Peyro-Saint-Paul, H.; Vincent-Salomon, A.; Rouzier, R.; Sigal-Zafrani, B.; Sastre-Garau, X. Estrogen-receptor, progesterone-receptor and HER2 status determination in invasive breast cancer. Concordance between immuno-histochemistry and MapQuant™ microarray based assay. PLoS ONE 2016, 11, e0146474. [Google Scholar] [CrossRef]
- Siow, Z.R.; De Boer, R.H.; Lindeman, G.J.; Mann, G.B. Spotlight on the utility of the Oncotype DX® breast cancer assay. Int. J. Women’s Health 2018, 10, 89–100. [Google Scholar] [CrossRef] [PubMed]
- Syed, Y.Y. Oncotype DX breast recurrence Score®: A review of its use in early-stage breast cancer. Mol. Diagn. Ther. 2020, 24, 621–632. [Google Scholar] [CrossRef] [PubMed]
- Valencia, O.M.; Samuel, S.E.; Viscusi, R.K.; Riall, T.S.; Neumayer, L.A.; Aziz, H. The role of genetic testing in patients with breast cancer: A review. JAMA Surg. 2017, 152, 589–594. [Google Scholar] [CrossRef] [PubMed]
- George, A.; Riddell, D.; Seal, S.; Talukdar, S.; Mahamdallie, S.; Ruark, E.; Cloke, V.; Slade, I.; Kemp, Z.; Gore, M. Implementing rapid, robust, cost-effective, patient-centred, routine genetic testing in ovarian cancer patients. Sci. Rep. 2016, 6, 29506. [Google Scholar] [CrossRef]
- Sun, L.; Brentnall, A.; Patel, S.; Buist, D.S.; Bowles, E.J.; Evans, D.G.R.; Eccles, D.; Hopper, J.; Li, S.; Southey, M. A cost-effectiveness analysis of multigene testing for all patients with breast cancer. JAMA Oncol. 2019, 5, 1718–1730. [Google Scholar] [CrossRef]
- Migliozzi, D.; Nguyen, H.T.; Gijs, M.A. Combining fluorescence-based image segmentation and automated microfluidics for ultrafast cell-by-cell assessment of biomarkers for HER2-type breast carcinoma. J. Biomed. Opt. 2019, 24, 021204. [Google Scholar] [CrossRef]
- Jung, Y.Y.; Jung, W.H.; Koo, J.S. BRAF mutation in breast cancer by BRAF V600E mutation-specific antibody. Int. J. Clin. Exp. Pathol. 2016, 9, 1545–1556. [Google Scholar]
- Orlando, L.; Viale, G.; Bria, E.; Lutrino, E.S.; Sperduti, I.; Carbognin, L.; Schiavone, P.; Quaranta, A.; Fedele, P.; Caliolo, C. Discordance in pathology report after central pathology review: Implications for breast cancer adjuvant treatment. Breast 2016, 30, 151–155. [Google Scholar] [CrossRef]
- Tseng, C.-H.; Huang, W.-T.; Chew, C.H.; Lai, J.-K.; Tu, S.-H.; Wei, P.-L.; Lee, K.-Y.; Lai, G.-M.; Chen, C.-C. Electrospun polylactic acid (PLLA) microtube array membrane (MTAM)—An advanced substrate for anticancer drug screening. Materials 2019, 12, 569. [Google Scholar] [CrossRef]
- Huang, W.-T.; Yun, T.; Chew, C.-H.; Chen, A.; Wei, P.-L.; Lee, K.-Y.; Lee, H.-L.; Feng, P.-H.; Chiou, J.-F.; Chen, C.-M. Microtube Array Membrane Hollow Fiber Assay (MTAM-HFA)—An Accurate and Rapid Potential Companion Diagnostic and Pharmacological Interrogation Solution for Cancer Immunotherapy (PD-1/PD-L1). Biomolecules 2022, 12, 480. [Google Scholar] [CrossRef]
- Ou, K.-L.; Chen, C.-S.; Lin, L.-H.; Lu, J.-C.; Shu, Y.-C.; Tseng, W.-C.; Yang, J.-C.; Lee, S.-Y.; Chen, C.-C. Membranes of epitaxial-like packed, super aligned electrospun micron hollow poly (l-lactic acid)(PLLA) fibers. Eur. Polym. J. 2011, 47, 882–892. [Google Scholar] [CrossRef]
- Lin, L.-C.; Shu, Y.-C.; Yang, J.-C.; Shie, H.-S.; Lee, S.-Y.; Chen, C.-C. Nano-porous poly-L-lactic acid microtube array membranes. Curr. Nanosci. 2014, 10, 227–234. [Google Scholar] [CrossRef]
- Yang, J.C.; Lee, S.Y.; Tseng, W.C.; Shu, Y.C.; Lu, J.C.; Shie, H.S.; Chen, C.C. Formation of highly aligned, single-layered, hollow fibrous assemblies and the fabrication of large pieces of PLLA membranes. Macromol. Mater. Eng. 2012, 297, 115–122. [Google Scholar] [CrossRef]
- Huang, X.-Z.; Gao, P.; Song, Y.-X.; Sun, J.-X.; Chen, X.-W.; Zhao, J.-H.; Wang, Z.-N. Efficacy of immune checkpoint inhibitors and age in cancer patients. Immunotherapy 2020, 12, 587–603. [Google Scholar] [CrossRef] [PubMed]
- Songbo, M.; Lang, H.; Xinyong, C.; Bin, X.; Ping, Z.; Liang, S. Oxidative stress injury in doxorubicin-induced cardiotoxicity. Toxicol. Lett. 2019, 307, 41–48. [Google Scholar] [CrossRef]
- Ahmad, S.; Panda, B.P.; Kohli, K.; Fahim, M.; Dubey, K. Folic acid ameliorates celecoxib cardiotoxicity in a doxorubicin heart failure rat model. Pharm. Biol. 2017, 55, 1295–1303. [Google Scholar] [CrossRef]
- Moro, N.; Dokshokova, L.; Perumal Vanaja, I.; Prando, V.; Cnudde, S.J.A.; Di Bona, A.; Bariani, R.; Schirone, L.; Bauce, B.; Angelini, A. Neurotoxic Effect of Doxorubicin Treatment on Cardiac Sympathetic Neurons. Int. J. Mol. Sci. 2022, 23, 11098. [Google Scholar] [CrossRef]
- Li, K.; Liu, W.; Zhao, Q.; Wu, C.; Fan, C.; Lai, H.; Li, S. Combination of tanshinone IIA and doxorubicin possesses synergism and attenuation effects on doxorubicin in the treatment of breast cancer. Phytother. Res. 2019, 33, 1658–1669. [Google Scholar] [CrossRef]
- Cui, L.; Huang, J.; Zhan, Y.; Qiu, N.; Jin, H.; Li, J.; Huang, H.; Li, H. Association between the genetic polymorphisms of the pharmacokinetics of anthracycline drug and myelosuppression in a patient with breast cancer with anthracycline-based chemotherapy. Life Sci. 2021, 276, 119392. [Google Scholar] [CrossRef]
- Cai, F.; Luis, M.A.F.; Lin, X.; Wang, M.; Cai, L.; Cen, C.; Biskup, E. Anthracycline-induced cardiotoxicity in the chemotherapy treatment of breast cancer: Preventive strategies and treatment. Mol. Clin. Oncol. 2019, 11, 15–23. [Google Scholar] [CrossRef]
- Khasraw, M.; Bell, R.; Dang, C. Epirubicin: Is it like doxorubicin in breast cancer? A clinical review. Breast 2012, 21, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Salvatorelli, E.; Guarnieri, S.; Menna, P.; Liberi, G.; Calafiore, A.M.; Mariggiὸ, M.A.; Mordente, A.; Gianni, L.; Minotti, G. Defective one-or two-electron reduction of the anticancer anthracycline epirubicin in human heart: Relative importance of vesicular sequestration and impaired efficiency of electron addition. J. Biol. Chem. 2006, 281, 10990–11001. [Google Scholar] [CrossRef] [PubMed]
- Hortobagyi, G.N.; Yap, H.-Y.; Kau, S.W.; Fraschini, G.; Ewer, M.S.; Chawla, S.P.; Benjamin, R.S. A comparative study of doxorubicin and epirubicin in patients with metastatic breast cancer. Am. J. Clin. Oncol. 1989, 12, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Torti, F.M.; Bristow, M.M.; Lum, B.L.; Carter, S.K.; Howes, A.E.; Aston, D.A.; Brown Jr, B.W.; Hannigan Jr, J.F.; Meyers, F.J.; Mitchell, E.P. Cardiotoxicity of epirubicin and doxorubicin: Assessment by endomyocardial biopsy. Cancer Res. 1986, 46, 3722–3727. [Google Scholar] [PubMed]
- Boven, E.; Schlüper, H.M.M.; Erkelens, C.A.M.; Pinedo, H.M. Doxorubicin compared with related compounds in a nude mouse model for human ovarian cancer. Eur. J. Cancer Clin. Oncol. 1990, 26, 983–986. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Lv, K.; Teng, R.; Chen, J.; Xu, C.; Jin, L.; Chen, Y.; Zhao, W. Pegylated Liposomal Doxorubicin Versus Epirubicin as Adjuvant Therapy for Stage I-III Breast Cancer. Front Genet 2021, 12, 746114. [Google Scholar] [CrossRef]
- van der Zanden, S.Y.; Qiao, X.; Neefjes, J. New insights into the activities and toxicities of the old anticancer drug doxorubicin. FEBS J. 2021, 288, 6095–6111. [Google Scholar] [CrossRef]
- Armer, J.; Fu, M.; Wainstock, J.; Zagar, E.; Jacobs, L. Lymphedema following breast cancer treatment, including sentinel lymph node biopsy. Lymphology 2004, 37, 73–91. [Google Scholar] [CrossRef]
- Sharma, G.N.; Dave, R.; Sanadya, J.; Sharma, P.; Sharma, K. Various types and management of breast cancer: An overview. J. Adv. Pharm. Technol. Res. 2010, 1, 109. [Google Scholar]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tu, S.-H.; Huang, W.-T.; Chew, C.H.; Chen, A.L.; Chen, S.-T.; Chen, J.-H.; Hsieh, Y.-C.; Chen, C.-C. Unveiling the Power of Anticancer Drug Screening: A Clinical Case Study Comparing the Effectiveness of Hollow Fiber Assay Microtube Array Membrane (MTAM-HFA) in Breast Cancer Patients. Cancers 2023, 15, 2764. https://doi.org/10.3390/cancers15102764
Tu S-H, Huang W-T, Chew CH, Chen AL, Chen S-T, Chen J-H, Hsieh Y-C, Chen C-C. Unveiling the Power of Anticancer Drug Screening: A Clinical Case Study Comparing the Effectiveness of Hollow Fiber Assay Microtube Array Membrane (MTAM-HFA) in Breast Cancer Patients. Cancers. 2023; 15(10):2764. https://doi.org/10.3390/cancers15102764
Chicago/Turabian StyleTu, Shih-Hsin, Wan-Ting Huang, Chee Ho Chew, Amanda Lin Chen, Shou-Tung Chen, Jin-Hua Chen, Yi-Chen Hsieh, and Chien-Chung Chen. 2023. "Unveiling the Power of Anticancer Drug Screening: A Clinical Case Study Comparing the Effectiveness of Hollow Fiber Assay Microtube Array Membrane (MTAM-HFA) in Breast Cancer Patients" Cancers 15, no. 10: 2764. https://doi.org/10.3390/cancers15102764
APA StyleTu, S.-H., Huang, W.-T., Chew, C. H., Chen, A. L., Chen, S.-T., Chen, J.-H., Hsieh, Y.-C., & Chen, C.-C. (2023). Unveiling the Power of Anticancer Drug Screening: A Clinical Case Study Comparing the Effectiveness of Hollow Fiber Assay Microtube Array Membrane (MTAM-HFA) in Breast Cancer Patients. Cancers, 15(10), 2764. https://doi.org/10.3390/cancers15102764





