Stereotactic Body Radiation Therapy (SBRT) Plus Immune Checkpoint Inhibitors (ICI) in Hepatocellular Carcinoma and Cholangiocarcinoma
Abstract
Simple Summary
Abstract
1. Introduction
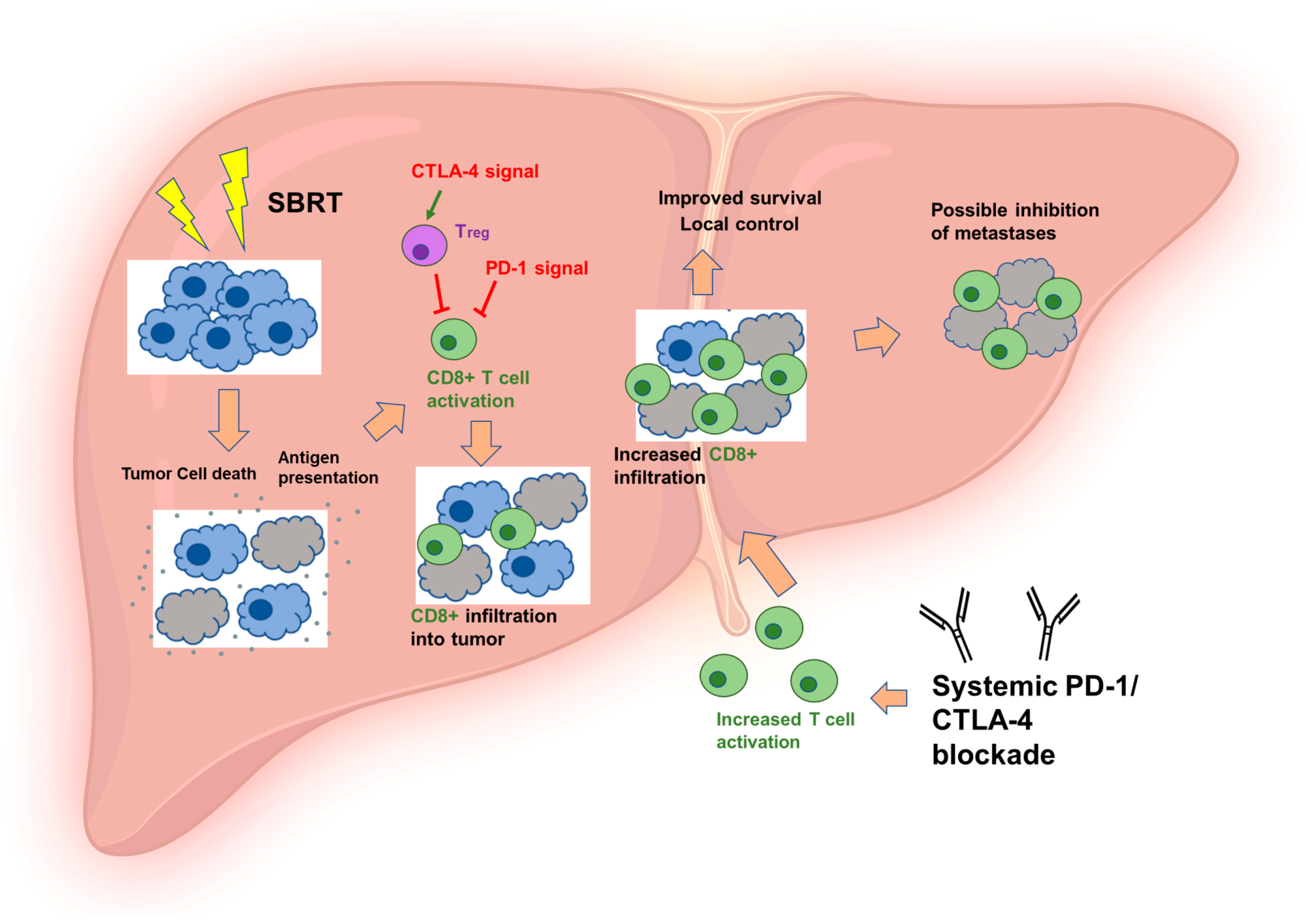
2. The Rationale of Adding ICI to SBRT
3. SBRT Plus ICI in Hepatocellular Carcinoma
Identifying Ideal HCC Patients for SBRT Plus ICI
4. SBRT Plus ICI in Cholangiocarcinoma
Identifying Ideal CCA Patients for SBRT Plus ICI
5. Future Directions
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Massarweh, N.N.; El-Serag, H.B. Epidemiology of Hepatocellular Carcinoma and Intrahepatic Cholangiocarcinoma. Cancer Control 2017, 24, 1073274817729245. [Google Scholar] [CrossRef] [PubMed]
- Howlader, N.; Noone, A.; Krapcho, M.; Miller, D.; Bishop, K.; Kosary, C.; Yu, M.; Ruhl, J.; Tatalovich, Z.; Mariotto, A.; et al. SEER Cancer Statistics Review 1975–2014; National Cancer Institute: Bethesda, MD, USA, 2017.
- Ouyang, G.; Liu, Q.; Wu, Y.; Liu, Z.; Lu, W.; Li, S.; Pan, G.; Chen, X. The global, regional, and national burden of gallbladder and biliary tract cancer and its attributable risk factors in 195 countries and territories, 1990 to 2017: A systematic analysis for the Global Burden of Disease Study 2017. Cancer 2021, 127, 2238–2250. [Google Scholar] [CrossRef]
- Jemal, A.; Ward, E.M.; Johnson, C.J.; Cronin, K.A.; Ma, J.; Ryerson, B.; Mariotto, A.; Lake, A.J.; Wilson, R.; Sherman, R.L.; et al. Annual Report to the Nation on the Status of Cancer, 1975–2014, Featuring Survival. J. Natl. Cancer Inst. 2017, 109, djx030. [Google Scholar] [CrossRef] [PubMed]
- Global Burden of Disease Liver Cancer, C.; Akinyemiju, T.; Abera, S.; Ahmed, M.; Alam, N.; Alemayohu, M.A.; Allen, C.; Al-Raddadi, R.; Alvis-Guzman, N.; Amoako, Y.; et al. The Burden of Primary Liver Cancer and Underlying Etiologies From 1990 to 2015 at the Global, Regional, and National Level: Results From the Global Burden of Disease Study 2015. JAMA Oncol. 2017, 3, 1683–1691. [Google Scholar] [CrossRef]
- Marrero, J.A.; Kulik, L.M.; Sirlin, C.B.; Zhu, A.X.; Finn, R.S.; Abecassis, M.M.; Roberts, L.R.; Heimbach, J.K. Diagnosis, Staging, and Management of Hepatocellular Carcinoma: 2018 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology 2018, 68, 723–750. [Google Scholar] [CrossRef]
- European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J. Hepatol. 2018, 69, 182–236. [Google Scholar] [CrossRef]
- Kokudo, N.; Takemura, N.; Hasegawa, K.; Takayama, T.; Kubo, S.; Shimada, M.; Nagano, H.; Hatano, E.; Izumi, N.; Kaneko, S.; et al. Clinical practice guidelines for hepatocellular carcinoma: The Japan Society of Hepatology 2017 (4th JSH-HCC guidelines) 2019 update. Hepatol. Res. 2019, 49, 1109–1113. [Google Scholar] [CrossRef]
- Omata, M.; Cheng, A.L.; Kokudo, N.; Kudo, M.; Lee, J.M.; Jia, J.; Tateishi, R.; Han, K.H.; Chawla, Y.K.; Shiina, S.; et al. Asia-Pacific clinical practice guidelines on the management of hepatocellular carcinoma: A 2017 update. Hepatol. Int. 2017, 11, 317–370. [Google Scholar] [CrossRef]
- Park, J.W.; Lee, J.S.; Suh, K.S.; Chung, J.W.; Seong, J.; Kim, J.H.; Kim, H.J.; Kim, H.Y.; Park, S.Y.; Shim, J.H.; et al. 2018 Korean Liver Cancer Association–National Cancer Center Korea Practice Guidelines for the Management of Hepatocellular Carcinoma. Gut Liver 2019, 13, 227–299. [Google Scholar]
- NCCN Clinical Practice Guidelines in Oncology. Available online: https://www.nccn.org/professionals/physician_gls/pdf/hepatobiliary.pdf (accessed on 20 November 2022).
- Vogel, A.; Cervantes, A.; Chau, I.; Daniele, B.; Llovet, J.M.; Meyer, T.; Nault, J.-C.; Neumann, U.; Ricke, J.; Sangro, B.; et al. Hepatocellular carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2018, 29, iv238–iv255. [Google Scholar] [CrossRef]
- Berman, Z.T.; Newton, I. Diagnosis, Staging, and Patient Selection for Locoregional Therapy to Treat Hepatocellular Carcinoma. Semin. Interv. Radiol. 2020, 37, 441–447. [Google Scholar] [CrossRef] [PubMed]
- Llovet, J.M.; Kelley, R.K.; Villanueva, A.; Singal, A.G.; Pikarsky, E.; Roayaie, S.; Lencioni, R.; Koike, K.; Zucman-Rossi, J.; Finn, R.S. Hepatocellular carcinoma. Nat. Rev. Dis. Prim. 2021, 7, 6. [Google Scholar] [CrossRef] [PubMed]
- Llovet, J.M.; Bru, C.; Bruix, J. Prognosis of hepatocellular carcinoma: The BCLC staging classification. Semin. Liver Dis. 1999, 19, 329–338. [Google Scholar] [CrossRef]
- Rim, C.H.; Kim, C.Y.; Yang, D.S.; Yoon, W.S. Comparison of radiation therapy modalities for hepatocellular carcinoma with portal vein thrombosis: A meta-analysis and systematic review. Radiother. Oncol. 2018, 129, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Finn, R.S.; Qin, S.; Ikeda, M.; Galle, P.R.; Ducreux, M.; Kim, T.Y.; Kudo, M.; Breder, V.; Merle, P.; Kaseb, A.O.; et al. Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma. N. Engl. J. Med. 2020, 382, 1894–1905. [Google Scholar] [CrossRef] [PubMed]
- Abou-Alfa, G.K.; Chan, S.L.; Kudo, M.; Lau, G.; Kelley, R.K.; Furuse, J.; Sukeepaisarnjaroen, W.; Kang, Y.-K.; Dao, T.V.; Toni, E.N.D.; et al. Phase 3 randomized, open-label, multicenter study of tremelimumab (T) and durvalumab (D) as first-line therapy in patients (pts) with unresectable hepatocellular carcinoma (uHCC): HIMALAYA. J. Clin. Oncol. 2022, 40, 379. [Google Scholar] [CrossRef]
- Finn, R.S.; Ryoo, B.Y.; Merle, P.; Kudo, M.; Bouattour, M.; Lim, H.Y.; Breder, V.; Edeline, J.; Chao, Y.; Ogasawara, S.; et al. Pembrolizumab as Second-Line Therapy in Patients with Advanced Hepatocellular Carcinoma in KEYNOTE-240: A Randomized, Double-Blind, Phase III Trial. J. Clin. Oncol. 2020, 38, 193–202. [Google Scholar] [CrossRef]
- Zhu, A.X.; Finn, R.S.; Edeline, J.; Cattan, S.; Ogasawara, S.; Palmer, D.; Verslype, C.; Zagonel, V.; Fartoux, L.; Vogel, A.; et al. Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE-224): A non-randomised, open-label phase 2 trial. Lancet Oncol. 2018, 19, 940–952. [Google Scholar] [CrossRef]
- El-Khoueiry, A.B.; Yau, T.; Kang, Y.-K.; Kim, T.-Y.; Santoro, A.; Sangro, B.; Melero, I.; Kudo, M.; Hou, M.-M.; Matilla, A.; et al. Nivolumab (NIVO) plus ipilimumab (IPI) combination therapy in patients (Pts) with advanced hepatocellular carcinoma (aHCC): Long-term results from CheckMate 040. J. Clin. Oncol. 2021, 39, 269. [Google Scholar] [CrossRef]
- El-Khoueiry, A.B.; Sangro, B.; Yau, T.; Crocenzi, T.S.; Kudo, M.; Hsu, C.; Kim, T.Y.; Choo, S.P.; Trojan, J.; Welling, T.H.R.; et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): An open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet 2017, 389, 2492–2502. [Google Scholar] [CrossRef]
- Edeline, J.; Yau, T.; Park, J.-W.; Kudo, M.; Han, K.-H.; Mathurin, P.; Merle, P.; Finn, R.S.; Müller, T.; Taylor, F.; et al. CheckMate 459: Health-related quality of life (HRQoL) in a randomized, multicenter phase III study of nivolumab (NIVO) versus sorafenib (SOR) as first-line (1L) treatment in patients (pts) with advanced hepatocellular carcinoma (aHCC). J. Clin. Oncol. 2020, 38, 483. [Google Scholar] [CrossRef]
- Oh, D.-Y.; Ruth He, A.; Qin, S.; Chen, L.-T.; Okusaka, T.; Vogel, A.; Kim, J.W.; Suksombooncharoen, T.; Ah Lee, M.; Kitano, M.; et al. Durvalumab plus Gemcitabine and Cisplatin in Advanced Biliary Tract Cancer. NEJM Evid. 2022, 1, EVIDoa2200015. [Google Scholar] [CrossRef]
- Valle, J.; Wasan, H.; Palmer, D.H.; Cunningham, D.; Anthoney, A.; Maraveyas, A.; Madhusudan, S.; Iveson, T.; Hughes, S.; Pereira, S.P.; et al. Cisplatin plus Gemcitabine versus Gemcitabine for Biliary Tract Cancer. N. Engl. J. Med. 2010, 362, 1273–1281. [Google Scholar] [CrossRef] [PubMed]
- Marabelle, A.; Le, D.T.; Ascierto, P.A.; Di Giacomo, A.M.; De Jesus-Acosta, A.; Delord, J.-P.; Geva, R.; Gottfried, M.; Penel, N.; Hansen, A.R.; et al. Efficacy of Pembrolizumab in Patients with Noncolorectal High Microsatellite Instability/Mismatch Repair–Deficient Cancer: Results From the Phase II KEYNOTE-158 Study. J. Clin. Oncol. 2020, 38, 1–10. [Google Scholar] [CrossRef]
- Le, D.T.; Durham, J.N.; Smith, K.N.; Wang, H.; Bartlett, B.R.; Aulakh, L.K.; Lu, S.; Kemberling, H.; Wilt, C.; Luber, B.S.; et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science 2017, 357, 409–413. [Google Scholar] [CrossRef]
- Kim, R.D.; Chung, V.; Alese, O.B.; El-Rayes, B.F.; Li, D.; Al-Toubah, T.E.; Schell, M.J.; Zhou, J.-M.; Mahipal, A.; Kim, B.H.; et al. A Phase 2 Multi-institutional Study of Nivolumab for Patients With Advanced Refractory Biliary Tract Cancer. JAMA Oncol. 2020, 6, 888. [Google Scholar] [CrossRef]
- Shroff, R.T.; Kennedy, E.B.; Bachini, M.; Bekaii-Saab, T.; Crane, C.; Edeline, J.; El-Khoueiry, A.; Feng, M.; Katz, M.H.G.; Primrose, J.; et al. Adjuvant Therapy for Resected Biliary Tract Cancer: ASCO Clinical Practice Guideline. J. Clin. Oncol. 2019, 37, 1015–1027. [Google Scholar] [CrossRef]
- Antonia, S.J.; Villegas, A.; Daniel, D.; Vicente, D.; Murakami, S.; Hui, R.; Yokoi, T.; Chiappori, A.; Lee, K.H.; de Wit, M.; et al. Durvalumab after Chemoradiotherapy in Stage III Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2017, 377, 1919–1929. [Google Scholar] [CrossRef]
- Shaverdian, N.; Lisberg, A.E.; Bornazyan, K.; Veruttipong, D.; Goldman, J.W.; Formenti, S.C.; Garon, E.B.; Lee, P. Previous radiotherapy and the clinical activity and toxicity of pembrolizumab in the treatment of non-small-cell lung cancer: A secondary analysis of the KEYNOTE-001 phase 1 trial. Lancet Oncol. 2017, 18, 895–903. [Google Scholar] [CrossRef]
- Sun, J.; Xie, L.; Feng, Y.; Qin, S. 1320TiP The efficacy and safety of stereotactic body radiation therapy (SBRT) plus toripalimab with or without bevacizumab as second-line treatment for advanced non-small cell lung cancer (NSCLC): A prospective, multicenter, open-label, phase II study. Ann. Oncol. 2021, 32, S1030. [Google Scholar] [CrossRef]
- Woeste, M.R.; Geller, A.E.; Martin, R.C.G.; Polk, H.C. Optimizing the Combination of Immunotherapy and Trans-Arterial Locoregional Therapy for Stages B and C Hepatocellular Cancer. Ann. Surg. Oncol. 2021, 28, 1499–1510. [Google Scholar] [CrossRef] [PubMed]
- Guckenberger, M.; Andratschke, N.; Alheit, H.; Holy, R.; Moustakis, C.; Nestle, U.; Sauer, O. Definition of stereotactic body radiotherapy. Strahlenther. Onkol. 2014, 190, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Manne, A.; Woods, E.; Tsung, A.; Mittra, A. Biliary Tract Cancers: Treatment Updates and Future Directions in the Era of Precision Medicine and Immuno-Oncology. Front. Oncol. 2021, 11, 768009. [Google Scholar] [CrossRef] [PubMed]
- Polistina, F.A.; Guglielmi, R.; Baiocchi, C.; Francescon, P.; Scalchi, P.; Febbraro, A.; Costantin, G.; Ambrosino, G. Chemoradiation treatment with gemcitabine plus stereotactic body radiotherapy for unresectable, non-metastatic, locally advanced hilar cholangiocarcinoma. Results of a five year experience. Radiother. Oncol. 2011, 99, 120–123. [Google Scholar] [CrossRef]
- Welling, T.H.; Feng, M.; Wan, S.; Hwang, S.Y.; Volk, M.L.; Lawrence, T.S.; Zalupski, M.M.; Sonnenday, C.J. Neoadjuvant stereotactic body radiation therapy, capecitabine, and liver transplantation for unresectable hilar cholangiocarcinoma. Liver Transplant. 2014, 20, 81–88. [Google Scholar] [CrossRef]
- Kim, J.; Byun, H.K.; Kim, T.H.; Kim, S.I.; Kim, B.K.; Kim, D.Y.; Seong, J. Liver-directed concurrent chemoradiotherapy versus sorafenib in hepatocellular carcinoma with portal vein tumor thrombus. J. Clin. Oncol. 2022, 40, 424. [Google Scholar] [CrossRef]
- Stückle, D. The 7th Asia-Pacific Primary Liver Cancer Expert Meeting (APPLE 2016). Advancing HCC Management through Multi-Disciplinary Approach. Hong Kong, SAR (China), July 8–10, 2016: Abstracts. Liver Cancer 2016, 5 (Suppl. 1), 1–94. [Google Scholar] [CrossRef]
- Brade, A.M.; Ng, S.; Brierley, J.; Kim, J.; Dinniwell, R.; Ringash, J.; Wong, R.R.; Cho, C.; Knox, J.; Dawson, L.A. Phase 1 Trial of Sorafenib and Stereotactic Body Radiation Therapy for Hepatocellular Carcinoma. Int. J. Radiat. Oncol. Biol. Phys. 2016, 94, 580–587. [Google Scholar] [CrossRef]
- Barney, B.M.; Markovic, S.N.; Laack, N.N.; Miller, R.C.; Sarkaria, J.N.; Macdonald, O.K.; Bauer, H.J.; Olivier, K.R. Increased bowel toxicity in patients treated with a vascular endothelial growth factor inhibitor (VEGFI) after stereotactic body radiation therapy (SBRT). Int. J. Radiat. Oncol. Biol. Phys. 2013, 87, 73–80. [Google Scholar] [CrossRef]
- Dillman, R.O. Cancer immunotherapy. Cancer Biother. Radiopharm. 2011, 26, 1–64. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Z. The history and advances in cancer immunotherapy: Understanding the characteristics of tumor-infiltrating immune cells and their therapeutic implications. Cell. Mol. Immunol. 2020, 17, 807–821. [Google Scholar] [CrossRef] [PubMed]
- Demaria, S.; Kawashima, N.; Yang, A.M.; Devitt, M.L.; Babb, J.S.; Allison, J.P.; Formenti, S.C. Immune-mediated inhibition of metastases after treatment with local radiation and CTLA-4 blockade in a mouse model of breast cancer. Clin. Cancer Res. 2005, 11, 728–734. [Google Scholar] [CrossRef] [PubMed]
- Zeng, J.; See, A.P.; Phallen, J.; Jackson, C.M.; Belcaid, Z.; Ruzevick, J.; Durham, N.; Meyer, C.; Harris, T.J.; Albesiano, E.; et al. Anti-PD-1 blockade and stereotactic radiation produce long-term survival in mice with intracranial gliomas. Int. J. Radiat. Oncol. Biol. Phys. 2013, 86, 343–349. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.; Liang, H.; Burnette, B.; Beckett, M.; Darga, T.; Weichselbaum, R.R.; Fu, Y.X. Irradiation and anti-PD-L1 treatment synergistically promote antitumor immunity in mice. J. Clin. Investig. 2014, 124, 687–695. [Google Scholar] [CrossRef] [PubMed]
- Cornel, A.M.; Mimpen, I.L.; Nierkens, S. MHC Class I Downregulation in Cancer: Underlying Mechanisms and Potential Targets for Cancer Immunotherapy. Cancers 2020, 12, 1760. [Google Scholar] [CrossRef]
- Buchbinder, E.I.; Desai, A. CTLA-4 and PD-1 Pathways. Am. J. Clin. Oncol. 2016, 39, 98–106. [Google Scholar] [CrossRef]
- Friedman, D.; Baird, J.R.; Young, K.H.; Cottam, B.; Crittenden, M.R.; Friedman, S.; Gough, M.J.; Newell, P. Programmed cell death-1 blockade enhances response to stereotactic radiation in an orthotopic murine model of hepatocellular carcinoma. Hepatol. Res. 2017, 47, 702–714. [Google Scholar] [CrossRef]
- Dovedi, S.J.; Adlard, A.L.; Lipowska-Bhalla, G.; McKenna, C.; Jones, S.; Cheadle, E.J.; Stratford, I.J.; Poon, E.; Morrow, M.; Stewart, R.; et al. Acquired resistance to fractionated radiotherapy can be overcome by concurrent PD-L1 blockade. Cancer Res. 2014, 74, 5458–5468. [Google Scholar] [CrossRef]
- Wu, C.T.; Chen, W.C.; Chang, Y.H.; Lin, W.Y.; Chen, M.F. The role of PD-L1 in the radiation response and clinical outcome for bladder cancer. Sci. Rep. 2016, 6, 19740. [Google Scholar] [CrossRef]
- Young, K.H.; Baird, J.R.; Savage, T.; Cottam, B.; Friedman, D.; Bambina, S.; Messenheimer, D.J.; Fox, B.; Newell, P.; Bahjat, K.S.; et al. Optimizing Timing of Immunotherapy Improves Control of Tumors by Hypofractionated Radiation Therapy. PLoS ONE 2016, 11, e0157164. [Google Scholar] [CrossRef]
- Wu, L.; Wu, M.O.; De la Maza, L.; Yun, Z.; Yu, J.; Zhao, Y.; Cho, J.; de Perrot, M. Targeting the inhibitory receptor CTLA-4 on T cells increased abscopal effects in murine mesothelioma model. Oncotarget 2015, 6, 12468–12480. [Google Scholar] [CrossRef] [PubMed]
- Yoshimoto, Y.; Suzuki, Y.; Mimura, K.; Ando, K.; Oike, T.; Sato, H.; Okonogi, N.; Maruyama, T.; Izawa, S.; Noda, S.E.; et al. Radiotherapy-induced anti-tumor immunity contributes to the therapeutic efficacy of irradiation and can be augmented by CTLA-4 blockade in a mouse model. PLoS ONE 2014, 9, e92572. [Google Scholar] [CrossRef] [PubMed]
- Chiang, C.-L.; Chan, A.C.Y.; Chiu, K.W.H.; Kong, F.-M. Combined Stereotactic Body Radiotherapy and Checkpoint Inhibition in Unresectable Hepatocellular Carcinoma: A Potential Synergistic Treatment Strategy. Front. Oncol. 2019, 9, 1157. [Google Scholar] [CrossRef]
- Juloori, A.; Liao, C.Y.; Lemons, J.M.; Singh, A.K.; Iyer, R.; Robbins, J.R.; George, B.; Fung, J.; Pillai, A.; Arif, F.; et al. Phase I Study of Stereotactic Body Radiotherapy followed by Ipilimumab with Nivolumab vs. Nivolumab alone in Unresectable Hepatocellular Carcinoma. Int. J. Radiat. Oncol. Biol. Phys. 2020, 108, S149–S150. [Google Scholar] [CrossRef]
- Liu, X.; Yao, J.; Song, L.; Zhang, S.; Huang, T.; Li, Y. Local and abscopal responses in advanced intrahepatic cholangiocarcinoma with low TMB, MSS, pMMR and negative PD-L1 expression following combined therapy of SBRT with PD-1 blockade. J. Immunother. Cancer 2019, 7, 204. [Google Scholar] [CrossRef]
- Zhao, Q.; Chen, Y.; Du, S.; Yang, X.; Chen, Y.; Ji, Y.; Zeng, Z. Integration of radiotherapy with anti-PD-1 antibody for the treatment of intrahepatic or hilar cholangiocarcinoma: Reflection from four cases. Cancer Biol. Ther. 2021, 22, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Persigehl, T.; Lennartz, S.; Schwartz, L.H. iRECIST: How to do it. Cancer Imaging 2020, 20, 2–7. [Google Scholar] [CrossRef]
- Chen, Y.; Yang, P.; Du, S.; Zhou, J.; Huang, C.; Zhu, W.; Hu, Y.; Yu, Y.; Liu, T.; Zeng, Z. A phase II study of stereotactic body radiotherapy (SBRT) combined with sintilimab in patients with recurrent or oligometastatic hepatocellular carcinoma (HCC). J. Clin. Oncol. 2022, 40, 4071. [Google Scholar] [CrossRef]
- Bujold, A.; Massey, C.A.; Kim, J.J.; Brierley, J.; Cho, C.; Wong, R.K.S.; Dinniwell, R.E.; Kassam, Z.; Ringash, J.; Cummings, B.; et al. Sequential Phase I and II Trials of Stereotactic Body Radiotherapy for Locally Advanced Hepatocellular Carcinoma. J. Clin. Oncol. 2013, 31, 1631–1639. [Google Scholar] [CrossRef]
- Shui, Y.; Yu, W.; Ren, X.; Guo, Y.; Xu, J.; Ma, T.; Zhang, B.; Wu, J.; Li, Q.; Hu, Q.; et al. Stereotactic body radiotherapy based treatment for hepatocellular carcinoma with extensive portal vein tumor thrombosis. Radiat. Oncol. 2018, 13, 188. [Google Scholar] [CrossRef]
- Munoz-Schuffenegger, P.; Barry, A.; Atenafu, E.G.; Kim, J.; Brierley, J.; Ringash, J.; Brade, A.; Dinniwell, R.; Wong, R.K.S.; Cho, C.; et al. Stereotactic body radiation therapy for hepatocellular carcinoma with Macrovascular invasion. Radiother. Oncol. 2021, 156, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Qadan, M.; Kothary, N.; Sangro, B.; Palta, M. The Treatment of Hepatocellular Carcinoma with Portal Vein Tumor Thrombosis. Am. Soc. Clin. Oncol. Educ. Book 2020, 40, 174–185. [Google Scholar] [CrossRef] [PubMed]
- Salem, R.; Lewandowski, R.J.; Mulcahy, M.F.; Riaz, A.; Ryu, R.K.; Ibrahim, S.; Atassi, B.; Baker, T.; Gates, V.; Miller, F.H.; et al. Radioembolization for hepatocellular carcinoma using Yttrium-90 microspheres: A comprehensive report of long-term outcomes. Gastroenterology 2010, 138, 52–64. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Guo, R.P.; Lai, E.C.; Zhang, Y.J.; Lau, W.Y.; Chen, M.S.; Shi, M. Transarterial chemoembolization for unresectable hepatocellular carcinoma with portal vein tumor thrombosis: A prospective comparative study. Ann. Surg. Oncol. 2011, 18, 413–420. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, Y.; Yoshida, K.; Nishimura, H.; Ejima, Y.; Miyawaki, D.; Uezono, H.; Ishihara, T.; Mayahara, H.; Fukumoto, T.; Ku, Y.; et al. Efficacy of stereotactic body radiotherapy for hepatocellular carcinoma with portal vein tumor thrombosis/inferior vena cava tumor thrombosis: Evaluation by comparison with conventional three-dimensional conformal radiotherapy. J. Radiat. Res. 2016, 57, 512–523. [Google Scholar] [CrossRef] [PubMed]
- Que, J.; Wu, H.C.; Lin, C.H.; Huang, C.I.; Li, L.C.; Ho, C.H. Comparison of stereotactic body radiation therapy with and without sorafenib as treatment for hepatocellular carcinoma with portal vein tumor thrombosis. Medicine 2020, 99, e19660. [Google Scholar] [CrossRef]
- Pollom, E.L.; Deng, L.; Pai, R.K.; Brown, J.M.; Giaccia, A.; Loo, B.W.; Shultz, D.B.; Le, Q.T.; Koong, A.C.; Chang, D.T. Gastrointestinal Toxicities With Combined Antiangiogenic and Stereotactic Body Radiation Therapy. Int. J. Radiat. Oncol. Biol. Phys. 2015, 92, 568–576. [Google Scholar] [CrossRef]
- Finn, R.S.; Qin, S.-K.; Galle, P.R.; Wang, C.; Ogburn, K.D.; Widau, R.; Zhu, A.X. 1015TiP Ramucirumab in patients with advanced hepatocellular carcinoma and elevated alpha fetoprotein following a non-sorafenib based systemic therapy: An expansion cohort of the phase III REACH-2 study. Ann. Oncol. 2020, 31, S702. [Google Scholar] [CrossRef]
- Yau, T.; Park, J.W.; Finn, R.S.; Cheng, A.L.; Mathurin, P.; Edeline, J.; Kudo, M.; Harding, J.J.; Merle, P.; Rosmorduc, O.; et al. Nivolumab versus sorafenib in advanced hepatocellular carcinoma (CheckMate 459): A randomised, multicentre, open-label, phase 3 trial. Lancet Oncol. 2022, 23, 77–90. [Google Scholar] [CrossRef]
- Kang, J.; Nie, Q.; Du, R.; Zhang, L.; Zhang, J.; Li, Q.; Li, J.; Qi, W. Stereotactic body radiotherapy combined with transarterial chemoembolization for hepatocellular carcinoma with portal vein tumor thrombosis. Mol. Clin. Oncol. 2014, 2, 43–50. [Google Scholar] [CrossRef]
- Chiang, C.L.; Chiu, W.H.K.; Lau, W.H.V.; Chan, S.-K.; Lee, A.S.; Kong, F.-M.S.; Chan, A. Sequential trans-arterial chemoembolization and stereotactic body radiotherapy followed by immunotherapy (START-FIT) for locally advanced hepatocellular carcinoma: A single-arm, phase II trial. J. Clin. Oncol. 2022, 40, 4091. [Google Scholar] [CrossRef]
- Nakeeb, A.; Pitt, H.A. Radiation therapy, chemotherapy and chemoradiation in hilar cholangiocarcinoma. HPB 2005, 7, 278–282. [Google Scholar] [CrossRef] [PubMed]
- Oh, D.-Y.; He, A.R.; Qin, S.; Chen, L.-T.; Okusaka, T.; Vogel, A.; Kim, J.W.; Suksombooncharoen, T.; Lee, M.A.; Kitano, M.; et al. A phase 3 randomized, double-blind, placebo-controlled study of durvalumab in combination with gemcitabine plus cisplatin (GemCis) in patients (pts) with advanced biliary tract cancer (BTC): TOPAZ-1. J. Clin. Oncol. 2022, 40, 378. [Google Scholar] [CrossRef]
- Frakulli, R.; Buwenge, M.; Macchia, G.; Cammelli, S.; Deodato, F.; Cilla, S.; Cellini, F.; Mattiucci, G.C.; Bisello, S.; Brandi, G.; et al. Stereotactic body radiation therapy in cholangiocarcinoma: A systematic review. Br. J. Radiol. 2019, 92, 20180688. [Google Scholar] [CrossRef] [PubMed]
- Sebastian, N.; Miller, E.D.; Williams, T.M.; Pardo, D.A.D. Transarterial Radioembolization (TARE) vs Stereotactic Body Radiation Therapy (SBRT) in the Treatment of Unresectable Intrahepatic Cholangiocarcinoma. Int. J. Radiat. Oncol. Biol. Phys. 2018, 102, e61. [Google Scholar] [CrossRef]
- Lamarca, A.; Palmer, D.H.; Wasan, H.S.; Ross, P.J.; Ma, Y.T.; Arora, A.; Falk, S.; Gillmore, R.; Wadsley, J.; Patel, K.; et al. Second-line FOLFOX chemotherapy versus active symptom control for advanced biliary tract cancer (ABC-06): A phase 3, open-label, randomised, controlled trial. Lancet Oncol. 2021, 22, 690–701. [Google Scholar] [CrossRef] [PubMed]
| Cancer | Study | Baseline Characteristics | ICI Agent Used | Response | Toxicity |
|---|---|---|---|---|---|
| Hepatocellular cancer | Case series (n = 5) [55] | 4/5 had CP A 1/5 had CP B8 | Nivolumab * | RECIST: PR in all patients (ranging from 30–84%) mRECIST: CR in 2/5 and PR in 3/5 1-yr OS: 100% None had PD ** | ≥grade 3 toxicity (both pneumonitis and skin) in one patient No cases of radiation-induced liver disease |
| All had uHCC Single lesion Median size of tumors—9.8 cm (range: 9–16.1 cm) MVI positive—2/5 Distant metastatic disease—1/5 TACE—4/5 | |||||
| Phase I trial # (n = 14) [56] | CP A ## Adequate organ function | Nivolumab (n = 6) Ipilimumab plus nivolumab (n = 8) | iRECIST: PR—36%; SD—36%; PD—28% Ipilimumab/nivolumab group: PR—50%; SD—37.5%; PD—12.5% Nivolumab group: PR—12.5%; SD—37.5%; PD—50% | DLT in 2 patients (hepatotoxicity) | |
| uHCC ineligible to resection or transplant ### Can have multifocal or distant metastatic disease Can have TACE or ablation, but SBRT should be for a different lesion | |||||
| Cholangiocarcinoma | Case series (n = 3) [57] | Metastatic disease—1/3 Recurrent disease in 2/3 MSS, low TMB (0.98 to 3.8 muts/Mb) No systemic therapy in the first line | Pembrolizumab (n = 2) Nivolumab (n = 1) | CR in one PR in two (ranging from 41—86%) | No significant toxicity reported |
| Case series (n = 4) [58] | One unresectable ^; 2 recurrent cases; one early stage Systemic therapy used in first-line include, paclitaxel/oxaliplatin, gemcitabine/oxaliplatin, and lapatinib | Pembrolizumab (n = 2) ^^ Nivolumab (n = 2) | Resection in unresectable SD in other 3 patients | No significant toxicity reported |
| Identifier | Concurrent vs. Sequential | Phase | Investigating Arm | Comparative Arm | SBRT | Primary Outcome | Estimated Enrollment |
|---|---|---|---|---|---|---|---|
| NCT04167293 | Sequential | III | SBRT followed in 4-6 weeks by IV sintilimab every 3 weeks # | SBRT | 30–54 Gy in 3–6 Fxs | PFS at 24 weeks | 116 |
| NCT04913480 | II | Durvalumab every 2 weeks, 1 week prior to SBRT | N/A | 27.5–50 Gy in 5 Fxs | PFS at 1 year | 37 | |
| NCT04169399 | II | Toripalimab every 3 weeks, followed by SBRT within 2 weeks | N/A | 36–54 Gy in 6 Fxs | PFS in 6 months | 30 | |
| NCT04988945 | II | TACE + SBRT followed by durvalumab + tremelimumab # | N/A | Not specified | Downstaging to resection | 30 | |
| NCT03817736 | II | TACE + SBRT followed by avelumab every 2 weeks R | N/A | Not specified | Downstaging to resection | 33 S | |
| NCT04193696 | Concurrent | II | IMRT or SBRT, followed by carelizumab every 3 weeks | N/A | 20–40 Gy in 10 Fxs | ORR at 6 months | 39 |
| NCT03857815 | II | SBRT + sintilimab every 3 weeksR | N/A | Not specified * | PFS at 2 years | 30 | |
| NCT04547452 | II | SBRT + sintilimab every 3 weeks | IV sintilimab every 3 weeks | 35–80 Gy in 5–8 Fxs * | PFS at 24 weeks | 84 | |
| NCT03316872 | II | Pembrolizumab every 3 weeks, with SBRT starting day 2 | N/A | 5 Fxs over 8–15 days ** | ORR | 30 | |
| NCT03482102 | II | Durvalumab + tremelimumab every 28 days, radiation during cycle 2 *** | N/A | Not specified | ORR | 70 | |
| NCT05185531 Neo-adjuvant | I | SBRT and tislelizumab on days 1 and 22, followed by tumor resection | N/A | 8 Gy in 3 Fxs | Delay to surgery | 20 | |
| Terminated trials University of Chicago: Phase I study on SBRT + Nivolumab/Ipilimumab (NCT03203304)—poor accrual | |||||||
| Identifier | Phase | Investigating Arm | Comparative Arm | Radiation Dose | Primary Outcome | Target Accrual |
|---|---|---|---|---|---|---|
| NCT03898895 | II | IMRT/SBRT followed in 1 week by camrelizumab every 3 weeks | GC | 40 Gy as SBRT or IMRT | 2-year PFS | 184 (1:1) |
| NCT04866836 | II | IMRT/SBRT followed in 3 days by tislelizumab every 3 weeks * | N/A | ORR in 2 years | 20 | |
| NCT04708067 | I | Hypofractionated radiation followed by bintrafusp alfa every 2 weeks * | N/A | 15 fractions | Incidence of adverse events | 15 |
| Terminated trials American University of Beirut Medical Center: Phase II pilot study on SBRT + nivolumab after induction chemotherapy in cholangiocarcinoma (NCT04648319) due to poor accrual | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, J.; Diaz, D.A.; Nuguru, S.P.; Mittra, A.; Manne, A. Stereotactic Body Radiation Therapy (SBRT) Plus Immune Checkpoint Inhibitors (ICI) in Hepatocellular Carcinoma and Cholangiocarcinoma. Cancers 2023, 15, 50. https://doi.org/10.3390/cancers15010050
Jiang J, Diaz DA, Nuguru SP, Mittra A, Manne A. Stereotactic Body Radiation Therapy (SBRT) Plus Immune Checkpoint Inhibitors (ICI) in Hepatocellular Carcinoma and Cholangiocarcinoma. Cancers. 2023; 15(1):50. https://doi.org/10.3390/cancers15010050
Chicago/Turabian StyleJiang, Joanna, Dayssy Alexandra Diaz, Surya Pratik Nuguru, Arjun Mittra, and Ashish Manne. 2023. "Stereotactic Body Radiation Therapy (SBRT) Plus Immune Checkpoint Inhibitors (ICI) in Hepatocellular Carcinoma and Cholangiocarcinoma" Cancers 15, no. 1: 50. https://doi.org/10.3390/cancers15010050
APA StyleJiang, J., Diaz, D. A., Nuguru, S. P., Mittra, A., & Manne, A. (2023). Stereotactic Body Radiation Therapy (SBRT) Plus Immune Checkpoint Inhibitors (ICI) in Hepatocellular Carcinoma and Cholangiocarcinoma. Cancers, 15(1), 50. https://doi.org/10.3390/cancers15010050






