Prevalence of Germline BRCA1/2 Variants in Ashkenazi and Non-Ashkenazi Prostate Cancer Populations: A Systematic Review and Meta-Analysis
Abstract
:Simple Summary
Abstract
1. Introduction
2. Methods
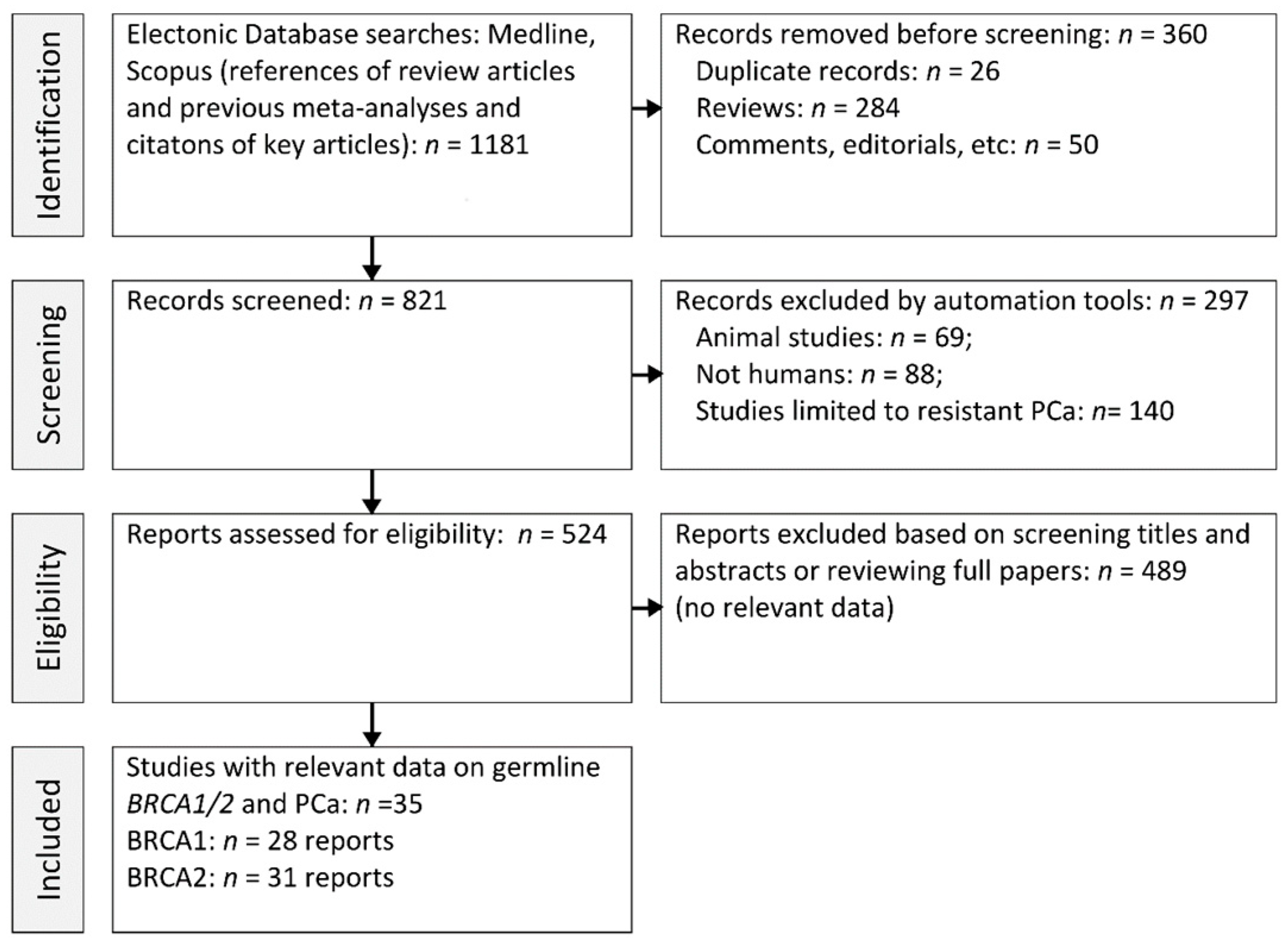
2.1. Data Retrieval
2.2. Study Selection
2.3. Data Extraction
2.4. Data Analysis
3. Results
3.1. Study Features
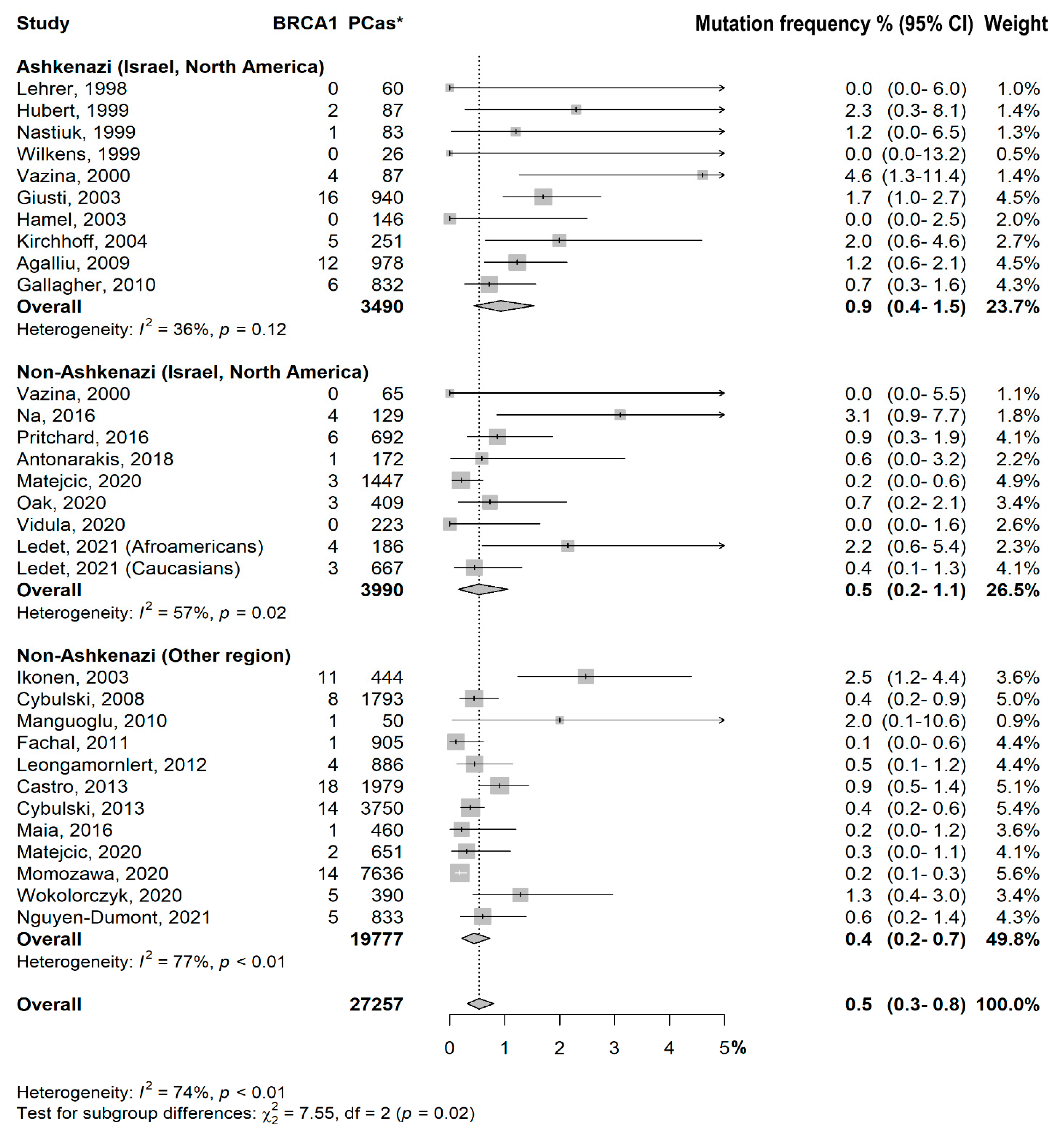
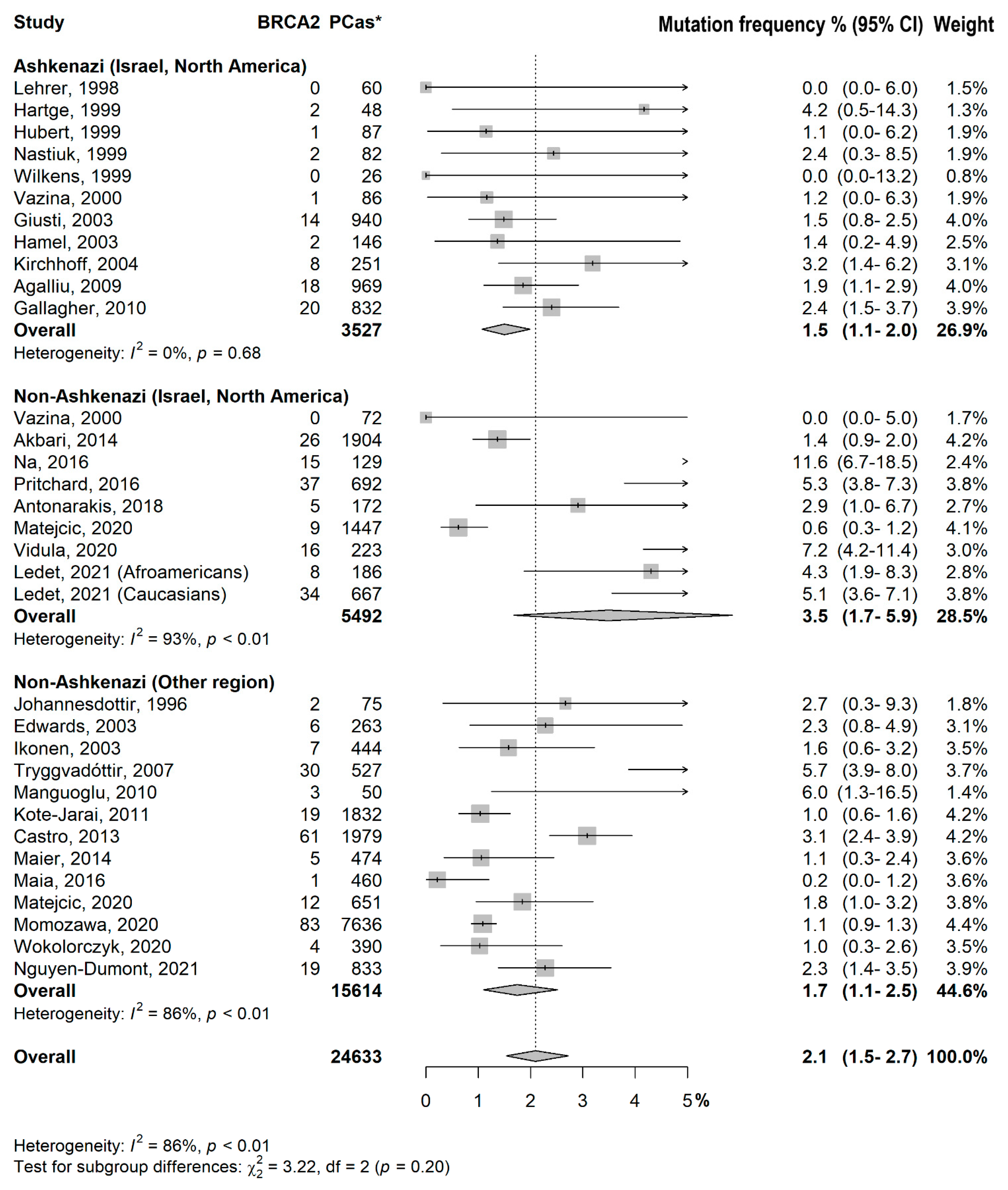
3.2. Prevalence of BRCA1/2 Mutations
- −
- Ashkenazi (Israel, North America) vs. Non-Ashkenazi (Israel, North America): p = 0.09
- −
- Ashkenazi (Israel, North America) vs. Non-Ashkenazi (Other region): p = 0.006
- −
- Non-Ashkenazi (Israel, North America) vs. Non-Ashkenazi (Other region): p = 0.50
- −
- Test for subgroup differences:
- −
- Ashkenazi (Israel, North America) vs. Non-Ashkenazi (Israel, North America): p = 0.08
- −
- Ashkenazi (Israel, North America) vs. Non-Ashkenazi (Other region): p = 0.86
- −
- Non-Ashkenazi (Israel, North America) vs. Non-Ashkenazi (Other region): p = 0.08
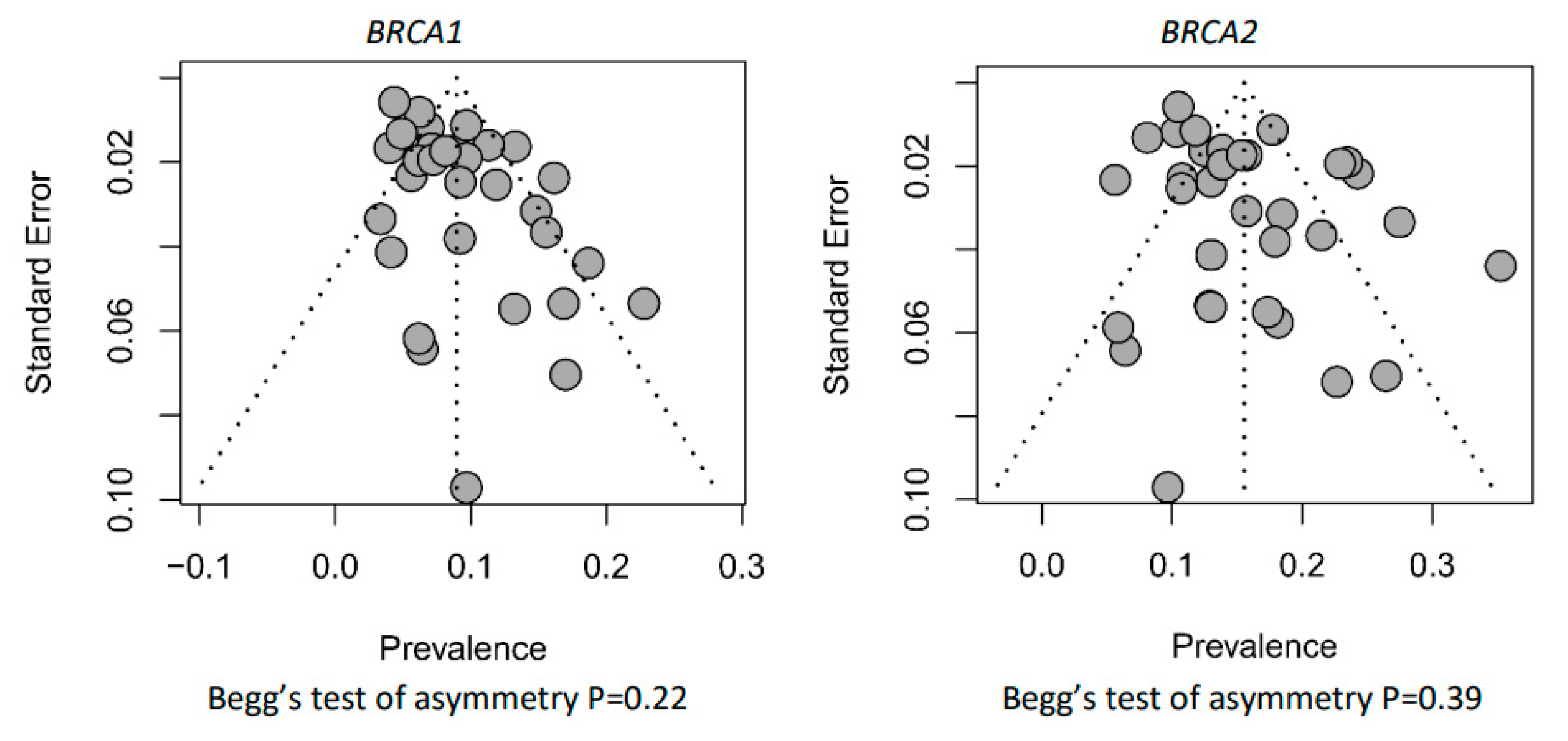
3.3. Risk of Bias
4. Discussion
4.1. Hereditary PCa in Ashkenazi Is Associated with Germline BRCA Mutation Status
4.2. BRCA2 Is Confirmed the Hallmark of Pca in Non-Ashkenazi Populations
4.3. Further Indications for PC Genetic Screening
4.4. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Parkin, D.M.; Piñeros, M.; Znaor, A.; Bray, F. Cancer statistics for the year 2020: An overview. Int. J. Cancer. 2021; in press. [Google Scholar]
- Jansson, K.F.; Akre, O.; Garmo, H.; Bill-Axelson, A.; Adolfsson, J.; Stattin, P.; Bratt, O. Concordance of tumor differentiation among brothers with prostate cancer. Eur. Urol. 2012, 62, 656–661. [Google Scholar]
- Hemminki, K. Familial risk and familial survival in prostate cancer. World J. Urol. 2012, 30, 143–148. [Google Scholar]
- Leitzmann, M.F.; Rohrmann, S. Risk factors for the onset of prostatic cancer: Age, location, and behavioral correlates. Clin. Epidemiol. 2012, 4, 1–11. [Google Scholar]
- Vidal, A.C.; Howard, L.E.; Moreira, D.M.; Castro-Santamaria, R.; Andriole, G.L.; Freedland, S.J. Does Obesity Modify the Ability of Prebiopsy Prostate Specific Antigen to Detect Prostate Cancer on Repeat Biopsy? Results from the REDUCE Study. J. Urol. 2015, 194, 52–57. [Google Scholar]
- Dickerman, B.A.; Markt, S.C.; Koskenvuo, M.; Hublin, C.; Pukkala, E.; Mucci, L.A.; Kaprio, J. Sleep disruption, chronotype, shift work, and prostate cancer risk and mortality: A 30-year prospective cohort study of Finnish twins. Cancer Causes Control 2016, 27, 1361–1370. [Google Scholar]
- La Vecchia, C.; Negri, E.; Carioli, G. Progress in cancer epidemiology: Avoided deaths in Europe over the last three decades. Eur. J. Cancer Prev. 2022, 31, 388–392. [Google Scholar]
- Santucci, C.; Carioli, G.; Bertuccio, P.; Malvezzi, M.; Pastorino, U.; Boffetta, P.; Negri, E.; Bosetti, C.; La Vecchia, C. Progress in cancer mortality, incidence, and survival: A global overview. Eur. J. Cancer Prev. 2020, 29, 367–381. [Google Scholar]
- Greenberg, S.E.; Hunt, T.C.; Ambrose, J.P.; Lowrance, W.T.; Dechet, C.B.; O’Neil, B.B.; Tward, J.D. Clinical Germline Testing Results of Men With Prostate Cancer: Patient-Level Factors and Implications of NCCN Guideline Expansion. JCO Precis. Oncol. 2021, 5, 533–542. [Google Scholar]
- Eisinger, F.; Roussel, C.; Morère, J.F.; Viguier, J. Cancer screening: Reaching the limits or terra incognita? Lessons from the EDIFICE surveys. Eur. J. Cancer Prev. 2011, 20 (Suppl. S1), S42–S44. [Google Scholar]
- Robson, M.; Dabney, M.K.; Rosenthal, G.; Ludwig, S.; Seltzer, M.H.; Gilewski, T.; Haas, B.; Osborne, M.; Norton, L.; Gilbert, F.; et al. Prevalence of recurring BRCA mutations among Ashkenazi Jewish women with breast cancer. Genet. Test. 1997, 1, 47–51. [Google Scholar]
- Rennert, G.; Dishon, S.; Rennert, H.S.; Fares, F. Differences in the characteristics of families with BRCA1 and BRCA2 mutations in Israel. Eur. J. Cancer Prev. 2005, 14, 357–361. [Google Scholar]
- Vazina, A.; Baniel, J.; Yaacobi, Y.; Shtriker, A.; Engelstein, D.; Leibovitz, I.; Zehavi, M.; Sidi, A.A.; Ramon, Y.; Tischler, L.; et al. The rate of the founder Jewish mutations in BRCA1 and BRCA2 in prostate cancer patients in Israel. Br. J. Cancer 2000, 83, 463–466. [Google Scholar]
- Matejcic, M.; Patel, Y.; Lilyquist, J.; Hu, C.; Lee, K.Y.; Gnanaolivu, R.D.; Hart, S.N.; Polley, E.C.; Yadav, S.; Boddicker, N.J.; et al. Pathogenic Variants in Cancer Predisposition Genes and Prostate Cancer Risk in Men of African Ancestry. JCO Precis. Oncol. 2020, 4, 32–43. [Google Scholar]
- Ledet, E.M.; Burgess, E.F.; Sokolova, A.O.; Jaeger, E.B.; Hatton, W.; Moses, M.; Miller, P.; Cotogno, P.; Layton, J.; Barata, P.; et al. Comparison of germline mutations in African American and Caucasian men with metastatic prostate cancer. Prostate 2021, 81, 433–439. [Google Scholar]
- Johannesdottir, G.; Gudmundsson, J.; Bergthorsson, J.T.; Arason, A.; Agnarsson, B.A.; Eiriksdottir, G.; Johannsson, O.T.; Borg, A.; Ingvarsson, S.; Easton, D.F.; et al. High prevalence of the 999del5 mutation in icelandic breast and ovarian cancer patients. Cancer Res. 1996, 56, 3663–3665. [Google Scholar]
- Lehrer, S.; Fodor, F.; Stock, R.G.; Stone, N.N.; Eng, C.; Song, H.K.; McGovern, M. Absence of 185delAG mutation of the BRCA1 gene and 6174delT mutation of the BRCA2 gene in Ashkenazi Jewish men with prostate cancer. Br. J. Cancer 1998, 78, 771–773. [Google Scholar]
- Hartge, P.; Struewing, J.P.; Wacholder, S.; Brody, L.C.; Tucker, M.A. The prevalence of common BRCA1 and BRCA2 mutations among Ashkenazi Jews. Am. J. Hum. Genet. 1999, 64, 963–970. [Google Scholar]
- Hubert, A.; Peretz, T.; Manor, O.; Kaduri, L.; Wienberg, N.; Lerer, I.; Sagi, M.; Abeliovich, D. The Jewish Ashkenazi founder mutations in the BRCA1/BRCA2 genes are not found at an increased frequency in Ashkenazi patients with prostate cancer. Am. J. Hum. Genet. 1999, 65, 921–924. [Google Scholar]
- Nastiuk, K.L.; Mansukhani, M.; Terry, M.B.; Kularatne, P.; Rubin, M.A.; Melamed, J.; Gammon, M.D.; Ittmann, M.; Krolewski, J.J. Common mutations in BRCA1 and BRCA2 do not contribute to early prostate cancer in Jewish men. Prostate 1999, 40, 172–177. [Google Scholar]
- Wilkens, E.P.; Freije, D.; Xu, J.; Nusskern, D.R.; Suzuki, H.; Isaacs, S.D.; Wiley, K.; Bujnovsky, P.; Meyers, D.A.; Walsh, P.C.; et al. No evidence for a role of BRCA1 or BRCA2 mutations in Ashkenazi Jewish families with hereditary prostate cancer. Prostate 1999, 39, 280–284. [Google Scholar]
- Edwards, S.; Meitz, J.; Eles, R.; Evans, C.; Easton, D.; Hopper, J.; Giles, G.; Foulkes, W.D.; Narod, S.; Simard, J.; et al. Results of a genome-wide linkage analysis in prostate cancer families ascertained through the ACTANE consortium. Prostate 2003, 57, 270–279. [Google Scholar]
- Giusti, R.M.; Rutter, J.L.; Duray, P.H.; Freedman, L.S.; Konichezky, M.; Fisher-Fischbein, J.; Greene, M.H.; Maslansky, B.; Fischbein, A.; Gruber, S.B.; et al. A twofold increase in BRCA mutation related prostate cancer among Ashkenazi Israelis is not associated with distinctive histopathology. J. Med. Genet. 2003, 40, 787–792. [Google Scholar]
- Hamel, N.; Kotar, K.; Foulkes, W.D. Founder mutations in BRCA1/2 are not frequent in Canadian Ashkenazi Jewish men with prostate cancer. BMC Med. Genet. 2003, 4, 7. [Google Scholar]
- Ikonen, T.; Matikainen, M.P.; Syrjäkoski, K.; Mononen, N.; Koivisto, P.A.; Rökman, A.; Seppälä, E.H.; Kallioniemi, O.P.; Tammela, T.L.; Schleutker, J. BRCA1 and BRCA2 mutations have no major role in predisposition to prostate cancer in Finland. J. Med. Genet. 2003, 40, e98. [Google Scholar]
- Kirchhoff, T.; Kauff, N.D.; Mitra, N.; Nafa, K.; Huang, H.; Palmer, C.; Gulati, T.; Wadsworth, E.; Donat, S.; Robson, M.E.; et al. BRCA mutations and risk of prostate cancer in Ashkenazi Jews. Clin. Cancer Res. 2004, 10, 2918–2921. [Google Scholar]
- Tryggvadóttir, L.; Vidarsdóttir, L.; Thorgeirsson, T.; Jonasson, J.G.; Olafsdóttir, E.J.; Olafsdóttir, G.H.; Rafnar, T.; Thorlacius, S.; Jonsson, E.; Eyfjord, J.E.; et al. Prostate cancer progression and survival in BRCA2 mutation carriers. J. Natl. Cancer Inst. 2007, 99, 929–935. [Google Scholar]
- Cybulski, C.; Górski, B.; Gronwald, J.; Huzarski, T.; Byrski, T.; Debniak, T.; Jakubowska, A.; Wokołorczyk, D.; Gliniewicz, B.; Sikorski, A.; et al. BRCA1 mutations and prostate cancer in Poland. Eur. J. Cancer Prev. 2008, 17, 62–66. [Google Scholar]
- Agalliu, I.; Gern, R.; Leanza, S.; Burk, R.D. Associations of high-grade prostate cancer with BRCA1 and BRCA2 founder mutations. Clin. Cancer Res. 2009, 15, 1112–1120. [Google Scholar]
- Gallagher, D.J.; Gaudet, M.M.; Pal, P.; Kirchhoff, T.; Balistreri, L.; Vora, K.; Bhatia, J.; Stadler, Z.; Fine, S.W.; Reuter, V.; et al. Germline BRCA mutations denote a clinicopathologic subset of prostate cancer. Clin. Cancer Res. 2010, 16, 2115–2121. [Google Scholar]
- Manguoğlu, E.; Güran, S.; Yamaç, D.; Colak, T.; Simşek, M.; Baykara, M.; Akaydın, M.; Lüleci, G. Germline mutations of BRCA1 and BRCA2 genes in Turkish breast, ovarian, and prostate cancer patients. Cancer Genet. Cytogenet. 2010, 203, 230–237. [Google Scholar]
- Fachal, L.; Gómez-Caamaño, A.; Celeiro-Muñoz, C.; Peleteiro, P.; Blanco, A.; Carballo, A.; Forteza, J.; Carracedo, A.; Vega, A. BRCA1 mutations do not increase prostate cancer risk: Results from a meta-analysis including new data. Prostate 2011, 71, 1768–1779. [Google Scholar]
- Kote-Jarai, Z.; Leongamornlert, D.; Saunders, E.; Tymrakiewicz, M.; Castro, E.; Mahmud, N.; Guy, M.; Edwards, S.; O’Brien, L.; Sawyer, E.; et al. BRCA2 is a moderate penetrance gene contributing to young-onset prostate cancer: Implications for genetic testing in prostate cancer patients. Br. J. Cancer 2011, 105, 1230–1234. [Google Scholar]
- Leongamornlert, D.; Mahmud, N.; Tymrakiewicz, M.; Saunders, E.; Dadaev, T.; Castro, E.; Goh, C.; Govindasami, K.; Guy, M.; O’Brien, L.; et al. Germline BRCA1 mutations increase prostate cancer risk. Br. J. Cancer 2012, 106, 1697–1701. [Google Scholar]
- Castro, E.; Goh, C.; Olmos, D.; Saunders, E.; Leongamornlert, D.; Tymrakiewicz, M.; Mahmud, N.; Dadaev, T.; Govindasami, K.; Guy, M.; et al. Germline BRCA mutations are associated with higher risk of nodal involvement, distant metastasis, and poor survival outcomes in prostate cancer. J. Clin. Oncol. 2013, 31, 1748–1757. [Google Scholar]
- Cybulski, C.; Wokołorczyk, D.; Kluźniak, W.; Kashyap, A.; Gołąb, A.; Słojewski, M.; Sikorski, A.; Puszyński, M.; Soczawa, M.; Borkowski, T.; et al. A personalised approach to prostate cancer screening based on genotyping of risk founder alleles. Br. J. Cancer 2013, 108, 2601–2609. [Google Scholar]
- Akbari, M.R.; Wallis, C.J.; Toi, A.; Trachtenberg, J.; Sun, P.; Narod, S.A.; Nam, R.K. The impact of a BRCA2 mutation on mortality from screen-detected prostate cancer. Br. J. Cancer 2014, 111, 1238–1240. [Google Scholar]
- Maier, C.; Herkommer, K.; Luedeke, M.; Rinckleb, A.; Schrader, M.; Vogel, W. Subgroups of familial and aggressive prostate cancer with considerable frequencies of BRCA2 mutations. Prostate 2014, 74, 1444–1451. [Google Scholar]
- Maia, S.; Cardoso, M.; Paulo, P.; Pinheiro, M.; Pinto, P.; Santos, C.; Pinto, C.; Peixoto, A.; Henrique, R.; Teixeira, M.R. The role of germline mutations in the BRCA1/2 and mismatch repair genes in men ascertained for early-onset and/or familial prostate cancer. Fam. Cancer 2016, 15, 111–121. [Google Scholar]
- Na, R.; Ye, D.; Qi, J.; Liu, F.; Lin, X.; Helfand, B.T.; Brendler, C.B.; Conran, C.; Gong, J.; Wu, Y.; et al. Race-specific genetic risk score is more accurate than nonrace-specific genetic risk score for predicting prostate cancer and high-grade diseases. Asian J. Androl. 2016, 18, 525–529. [Google Scholar]
- Pritchard, C.; Mateo, J.; Walsh, M.F.; De Sarkar, N.; Abida, W.; Beltran, H.; Garofalo, A.; Gulati, R.; Carreira, S.; Eeles, R.; et al. Inherited DNA-Repair Gene Mutations in Men with Metastatic Prostate Cancer. N. Engl. J. Med. 2016, 375, 443–453. [Google Scholar]
- Antonarakis, E.S.; Lu, C.; Luber, B.; Liang, C.; Wang, H.; Chen, Y.; Silberstein, J.L.; Piana, D.; Lai, Z.; Chen, Y.; et al. Germline DNA-repair Gene Mutations and Outcomes in Men with Metastatic Castration-resistant Prostate Cancer Receiving First-Line Abiraterone and Enzalutamide. Eur. Urol. 2018, 74, 218–225. [Google Scholar]
- Momozawa, Y.; Iwasaki, Y.; Hirata, M.; Liu, X.; Kamatani, Y.; Takahashi, A.; Sugano, K.; Yoshida, T.; Murakami, Y.; Matsuda, K.; et al. Germline Pathogenic Variants in 7636 Japanese Patients with Prostate Cancer and 12 366 Controls. J. Natl. Cancer Inst. 2020, 112, 369–376. [Google Scholar]
- Oak, N.; Cherniack, A.D.; Mashl, R.J.; TCGA Analysis Network; Hirsch, F.R.; Ding, L.; Beroukhim, R.; Gümüş, Z.H.; Plon, S.E.; Huang, K.L. Ancestry-specific predisposing germline variants in cancer. Genome Med. 2020, 12, 51. [Google Scholar]
- Vidula, N.; Rich, T.A.; Sartor, O.; Yen, J.; Hardin, A.; Nance, T.; Lilly, M.B.; Nezami, M.A.; Patel, S.P.; Carneiro, B.A.; et al. Routine Plasma-Based Genotyping to Comprehensively Detect Germline, Somatic, and Reversion BRCA Mutations among Patients with Advanced Solid Tumors. Clin. Cancer Res. 2020, 26, 2546–2555. [Google Scholar]
- Wokołorczyk, D.; Kluźniak, W.; Huzarski, T.; Gronwald, J.; Szymiczek, A.; Rusak, B.; Stempa, K.; Gliniewicz, K.; Kashyap, A.; Morawska, S.; et al. Mutations in ATM, NBN and BRCA2 predispose to aggressive prostate cancer in Poland. Int. J. Cancer 2020, 147, 2793–2800. [Google Scholar]
- Nguyen-Dumont, T.; Dowty, J.G.; MacInnis, R.J.; Steen, J.A.; Riaz, M.; Dugué, P.A.; Renault, A.L.; Hammet, F.; Mahmoodi, M.; Theys, D.; et al. Rare Germline Pathogenic Variants Identified by Multigene Panel Testing and the Risk of Aggressive Prostate Cancer. Cancers 2021, 13, 1495. [Google Scholar]
- Corso, G.; Magnoni, F.; Veronesi, P. Points to Consider Regarding De-Escalation Surgery in High-Risk Breast Cancer. Ann. Surg. Oncol. 2022, 29, 8084–8089. [Google Scholar]
- Offit, K.; Gilewski, T.; McGuire, P.; Schluger, A.; Hampel, H.; Brown, K.; Swensen, J.; Neuhausen, S.; Skolnick, M.; Norton, L.; et al. Germline BRCA1 185delAG mutations in Jewish women with breast cancer. Lancet 1996, 347, 1643–1645. [Google Scholar]
- Kauff, N.D.; Perez-Segura, P.; Robson, M.E.; Scheuer, L.; Siegel, B.; Schluger, A.; Rapaport, B.; Frank, T.S.; Nafa, K.; Ellis, N.A.; et al. Incidence of non-founder BRCA1 and BRCA2 mutations in high risk Ashkenazi breast and ovarian cancer families. J. Med. Genet. 2002, 39, 611–614. [Google Scholar]
- Gandaglia, G.; Leni, R.; Bray, F.; Fleshner, N.; Freedland, S.J.; Kibel, A.; Stattin, P.; Van Poppel, H.; La Vecchia, C. Epidemiology and Prevention of Prostate Cancer. Eur. Urol. Oncol. 2021, 4, 877–892. [Google Scholar]
- Vietri, M.T.; D’Elia, G.; Caliendo, G.; Resse, M.; Casamassimi, A.; Passariello, L.; Albanese, L.; Cioffi, M.; Molinari, A.M. Hereditary Prostate Cancer: Genes Related, Target Therapy and Prevention. Int. J. Mol. Sci. 2021, 22, 3753. [Google Scholar]
- Cozzi, G.; Musi, G.; Ferro, M.; Cioffi, A.; de Cobelli, O.; Corso, G. Progress in prostate cancer prevention. Eur. J. Cancer Prev. 2022, 31, 554–557. [Google Scholar]
- Massari, G.; Magnoni, F.; Favia, G.; Peradze, N.; Veronesi, P.; La Vecchia, C.; Corso, G. Frequency of CDH1 Germline Mutations in Non-Gastric Cancers. Cancers 2021, 13, 2321. [Google Scholar]
- Nyberg, T.; Frost, D.; Barrowdale, D.; Evans, D.G.; Bancroft, E.; Adlard, J.; Ahmed, M.; Barwell, J.; Brady, A.F.; Brewer, C.; et al. Prostate Cancer Risks for Male BRCA1 and BRCA2 Mutation Carriers: A Prospective Cohort Study. Eur. Urol. 2020, 77, 24–35. [Google Scholar]
- Sanft, T.; Day, A.; Peterson, L.; Rodriguez, M.A.; Ansbaugh, S.; Armenian, S.; Baker, K.S.; Ballinger, T.; Broderick, G.; Demark-Wahnefried, W.; et al. NCCN Guidelines® Insights: Survivorship, Version 1.2022. Featured Updates to the NCCN Guidelines. J. Natl. Compr. Cancer Netw. 2022, 20, 1080–1090. [Google Scholar]
| Study | Country | Period | Ethnicity | BRCA1 * | Tested ** | BRCA2 * | Tested ** |
|---|---|---|---|---|---|---|---|
| Johannesdottir, 1996 [16] | Iceland | 1983-1992 | Non-Ashkenazi | - | - | 2 | 75 |
| Lehrer, 1998 [17] | USA | - | Ashkenazi | 0 | 60 | 0 | 60 |
| Hartge, 1999 [18] | USA | 1996 | Ashkenazi | - | - | 2 | 48 |
| Hubert, 1999 [19] | Israel | - | Ashkenazi | 2 | 87 | 1 | 87 |
| Nastiuk, 1999 [20] | USA | 1991–1996 | Ashkenazi | 1 | 83 | 2 | 82 |
| Wilkens, 1999 [21] | USA | - | Ashkenazi | 0 | 26 | 0 | 26 |
| Vazina, 2000 [13] | Israel | 1998 | Ashkenazi | 4 | 87 | 1 | 86 |
| Vazina, 2000 [13] | Israel | 1998 | Non-Ashkenazi | 0 | 65 | 0 | 72 |
| Edwards, 2003 [22] | UK | 1992–1999 | Non-Ashkenazi | - | - | 6 | 263 |
| Giusti, 2003 [23] | Israel | 1994–1995 | Ashkenazi | 16 | 940 | 14 | 940 |
| Hamel, 2003 [24] | Canada | 1991–2002 | Ashkenazi | 0 | 146 | 2 | 146 |
| Ikonen, 2003 [25] | Finland | 1996–1999 | Non-Ashkenazi | 11 | 444 | 7 | 444 |
| Kirchhoff, 2004 [26] | USA | 2000–2002 | Ashkenazi | 5 | 251 | 8 | 251 |
| Tryggvadóttir, 2007 [27] | Iceland | 1955–2004 | Non-Ashkenazi | - | - | 30 | 527 |
| Cybulski, 2008 [28] | Poland | 1999–2005 | Non-Ashkenazi | 8 | 1793 | - | |
| Agalliu, 2009 [29] | USA | 1998–2005 | Ashkenazi | 12 | 978 | 18 | 969 |
| Gallagher, 2010 [30] | USA | 1988–2007 | Ashkenazi | 6 | 832 | 20 | 832 |
| Manguoglu, 2010 [31] | Turkey | - | Non-Ashkenazi | 1 | 50 | 3 | 50 |
| Fachal, 2011 [32] | Spain | 2006–2009 | Non-Ashkenazi | 1 | 905 | - | - |
| Kote-Jarai, 2011 [33] | UK | - | Non-Ashkenazi | - | - | 19 | 1832 |
| Leongamornlert, 2012 [34] | UK | - | Non-Ashkenazi | 4 | 886 | - | - |
| Castro, 2013 [35] | Spain | - | Non-Ashkenazi | 18 | 1979 | 61 | 1979 |
| Cybulski, 2013 [36] | Poland | 1999–2012 | Non-Ashkenazi | 14 | 3750 | - | - |
| Akbari, 2014 [37] | Canada | 1998–2010 | Non-Ashkenazi | - | - | 26 | 1904 |
| Maier, 2014 [38] | Germany | 1998–2007 | Non-Ashkenazi | - | - | 5 | 474 |
| Maia, 2016 [39] | Portugal | - | Non-Ashkenazi | 1 | 460 | 1 | 460 |
| Na, 2016 [40] | USA | - | Non-Ashkenazi | 4 | 129 | 15 | 129 |
| Pritchard, 2016 [41] | USA | - | Non-Ashkenazi | 6 | 692 | 37 | 692 |
| Antonarakis, 2018 [42] | USA | - | Non-Ashkenazi | 1 | 172 | 5 | 172 |
| Matejcic, 2020 [14] | USA | 1993–1996 | Non-Ashkenazi | 3 | 1447 | 9 | 1447 |
| Matejcic, 2020 [14] | Uganda | 1993–1996 | Non-Ashkenazi | 2 | 651 | 12 | 651 |
| Momozawa, 2020 [43] | Japan | - | Non-Ashkenazi | 14 | 7636 | 83 | 7636 |
| Oak, 2020 [44] | USA | - | Non-Ashkenazi | 3 | 409 | - | - |
| Vidula, 2020 [45] | USA | 2016–2017 | Non-Ashkenazi | 0 | 223 | 16 | 223 |
| Wokolorczyk, 2020 [46] | Poland | 2000–2017 | Non-Ashkenazi | 5 | 390 | 4 | 390 |
| Ledet, 2021 [15] | USA, black | - | Non-Ashkenazi | 4 | 186 | 8 | 186 |
| Ledet, 2021 [15] | USA, white | - | Non-Ashkenazi | 3 | 667 | 34 | 667 |
| Nguyen-Dumon, 2021 [47] | Australia | - | Non-Ashkenazi | 5 | 833 | 19 | 833 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cioffi, A.; De Cobelli, O.; Veronesi, P.; La Vecchia, C.; Maisonneuve, P.; Corso, G. Prevalence of Germline BRCA1/2 Variants in Ashkenazi and Non-Ashkenazi Prostate Cancer Populations: A Systematic Review and Meta-Analysis. Cancers 2023, 15, 306. https://doi.org/10.3390/cancers15010306
Cioffi A, De Cobelli O, Veronesi P, La Vecchia C, Maisonneuve P, Corso G. Prevalence of Germline BRCA1/2 Variants in Ashkenazi and Non-Ashkenazi Prostate Cancer Populations: A Systematic Review and Meta-Analysis. Cancers. 2023; 15(1):306. https://doi.org/10.3390/cancers15010306
Chicago/Turabian StyleCioffi, Antonio, Ottavio De Cobelli, Paolo Veronesi, Carlo La Vecchia, Patrick Maisonneuve, and Giovanni Corso. 2023. "Prevalence of Germline BRCA1/2 Variants in Ashkenazi and Non-Ashkenazi Prostate Cancer Populations: A Systematic Review and Meta-Analysis" Cancers 15, no. 1: 306. https://doi.org/10.3390/cancers15010306
APA StyleCioffi, A., De Cobelli, O., Veronesi, P., La Vecchia, C., Maisonneuve, P., & Corso, G. (2023). Prevalence of Germline BRCA1/2 Variants in Ashkenazi and Non-Ashkenazi Prostate Cancer Populations: A Systematic Review and Meta-Analysis. Cancers, 15(1), 306. https://doi.org/10.3390/cancers15010306







